Abstract
In vitro and animal model studies have indicated that oxidative stress from exposure to excess glucose and fatty acids impairs insulin signaling. However, few clinical studies have examined the association between oxidative stress and insulin action, particularly in non-diabetic individuals. The objective of this study was to examine the association between insulin sensitivity and protein carbonyls, a systemic marker of oxidative stress, in healthy individuals, and to determine if the magnitude of the relationship differed in African Americans (AA), who are at elevated risk for type 2 diabetes, relative to European Americans (EA). Subjects were 53 normal-glucose-tolerant women (25 AA, mean BMI 28.4 ± 6.2, range BMI???; mean age 33.1 ± 13.5, range age????; 28 EA mean BMI 26.2 ± 5.9, range BMI???; mean age 31.6 ± 12.4, range age??? ) . Insulin sensitivity was determined using an intravenous glucose tolerance test incorporating [6,6-2H2]-glucose, and a two-compartment mathematical model. Multiple linear regression results indicated that insulin sensitivity was independently positively associated with protein carbonyls in AA (r = 0.33, P<0.05) but not EA (r = ??P=0.945), after adjusting for %body fat. In contrast, %body fat was significantly positively associated with insulin sensitivity in EA (r = 0.29, P<0.05) but not AA (r = ??P=0.196). Protein carbonyls were associated with free fatty acids (FFA) in AA (r = 0.58, P<0.01) but not EA (r = −0.11, P=0.59). When subjects were divided based on median levels of fasting glucose and FFA, those with higher glucose/FFA concentrations had a significantly greater concentration of circulating protein carbonyls compared to those with lower glucose/FFA concentrations (P<0.05). These results suggest that oxidative stress independently contributes to insulin sensitivity among AA women. Further, this association in AA may be mediated by circulating FFA and/or glucose.
Keywords: oxidative stress, protein carbonyls, insulin sensitivity, ethnicity
Introduction
Type 2 diabetes is associated with both insulin resistance and decreased insulin secretion 1-3. There is considerable evidence demonstrating that hyperglycemia and/or elevated free fatty acids (FFA) may increase the production of reactive oxygen species (ROS) 4, 5. An increase in glucose/FFA induced production of ROS may create a persistent imbalance between the formation and adequate removal (via antioxidant defenses) of ROS, leading to oxidative stress. Convincing evidence, both in vivo 6-8 and in vitro 9, 10, has shown that oxidative stress may play a critical role in the pathogenesis of type 2 diabetes.
Elevated ROS production without a concomitant increase in scavenging from antioxidant defense mechanisms can alter the redox balance within the cell, leading to oxidative damage to proteins, lipids, and nucleic acids. The mitochondria respiratory chain is thought to represent the major source of ROS formation 11; however NADPH oxidases are also known to contribute to ROS production 12. A few recent clinical trials have shown associations between systemic oxidative stress and insulin resistance, evaluated by homeostasis model assessment, in both diabetic and pre-diabetic individuals 13, 14. The mechanism through which ROS elicits its deleterious effects on insulin signaling is thought to be the activation of multiple serine/threonine kinase pathways 15, 16. Several studies have shown that oxidative stress is often present before diabetic complications become clinically evident 5, 17; therefore it is important to assess the relationship between oxidative stress and insulin sensitivity in non-diabetic individuals.
Oxidative stress has been implicated in the etiology of several chronic diseases, including type 2 diabetes, atherosclerosis, hypertension, and cancer 12, 18-21. It has also been well documented that African Americans (AA) are at a disproportionately higher risk for developing many of these oxidative stress-related conditions 20, 22, 23. Whether oxidative stress plays a larger role in determining insulin sensitivity within populations at elevated risk for type 2 diabetes, such as AA, is currently not known. Therefore, the objectives of this study were: 1) to examine the association between insulin sensitivity and a systemic marker of oxidative stress in a group of healthy women; 2) to determine if the relationship between oxidative stress and insulin sensitivity differed with ethnic background; and 3) to test the hypothesis that higher concentrations of circulating glucose and free fatty acids (FFA) would be associated with elevated levels of oxidative stress.
Methods
Participants
Participants were 53 pre- and postmenopausal women. 28 were European-American; 25 were African-American(25 AA, mean BMI 28.4 ± 6.2, range BMI???; mean age 33.1 ± 13.5, range age????; 28 EA mean BMI 26.2 ± 5.9, range BMI???; mean age 31.6 ± 12.4, range age??? ). Exclusion criteria were type 1 or type 2 diabetes, polycystic ovary disease, disorders of glucose or lipid metabolism, use of medication that could affect body composition or glucose metabolism (including anti-hypertensive medication, oral contraceptives, and postmenopausal hormone replacement therapy), use of tobacco, alcohol consumption in excess of 400 grams per week, history of hypoglycemic episodes, and a medical history that counter-indicated inclusion in the study. All subjects had normal glucose tolerance (a 2-hr oral glucose test was used during initial screening to assess glucose tolerance; individuals with 2-hr glucose > 140 mg/dl were excluded). Women were queried regarding their menstrual cycles, and were classified as postmenopausal if they had not had a cycle in the past 12 months. Serum FSH was used to verify postmenopausal status (FSH>35 IU/ml). Participants were informed of the experimental design, and written consent was obtained. The study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham (UAB).
Protocol
All testing was done on an in-patient basis at UAB’s General Clinical Research Center (GCRC). Participants were provided with a list of common foods and their carbohydrate content and were asked to consume at least 250 grams carbohydrates for 3 days prior to admission. Subjects came to the GCRC the evening prior to testing. While at the GCRC, participants were given a standard meal consisting of 50% energy from carbohydrate, 30% energy from fat, and 20% energy from protein. No food was consumed for 12 hours prior to intravenous glucose tolerance testing, which was performed at 7:00 a.m. the following morning. After completion of the glucose tolerance test, subjects were given a late breakfast/lunch.
Intravenous glucose tolerance test (IVGTT)
Insulin sensitivity was determined during an intravenous glucose tolerance test (IVGTT). Flexible catheters were placed in the antecubital spaces of both arms. Three blood samples were taken over a 15 min period to determine basal glucose and insulin (the average of the values was used for basal concentrations). At time zero, glucose (50% dextrose, 270 mg/kg, plus [6,6-2H2]glucose, 30 mg/kg) was given intravenously. Insulin (0.02 units/kg) was infused over a 5-min period from 20-25 min post glucose injection. Blood samples (2.0 ml) were collected at the following times (min) relative to glucose administration: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, 180, 210, 240, 300. Serum was stored at −85°C until analysis.
Laboratory analyses
Concentrations of total glucose and insulin were analyzed in the Core Laboratory of the GCRC (now Center for Clinical and Translational Science; CCTS), Nutrition Obesity Research Center (NORC), and Diabetes Research and Training Center (DRTC). Glucose was measured in 10 μl of sera using an Ektachem DT II System (Johnson and Johnson Clinical Diagnostics). This analysis had a mean intra-assay coefficient of variation (CV) of 0.61%, and a mean inter-assay CV of 1.45%. Insulin was measured by RIA (Linco Research Inc., St. Charles, MO; now Millipore Corporation, Billerica, MA); assay sensitivity was 3.35 μIU/ml; mean intra-assay CV was 3.49%; and mean interassay CV was 5.57%. Analysis of labeled glucose was conducted by gas chromatography mass spectrometry. Serum samples were deproteinized, evaporated, and prepared with N,O-bis[Trimethylsilyl]trifluoroacetamide (BSTFA) and 1% trimethylchlorosilane (TMSC). Derivatives were analyzed on an Agilent 6890 gas chromatograph coupled to a 5973 mass spectrometer autotuned in Electron Impact mode. This analysis uses a standard curve prepared with in-house control serum samples. M+0 and M+2 ions were monitored. Total area counts were used to calculate mole fractions.
Protein Carbonyl Assay
Prior to analysis, all serum samples were assayed for protein concentration based on the methods of Bradford 24 and adjusted to 4 mg·mL−1 protein using a phosphate buffer. Protein carbonyls, a measure of protein oxidation, were analyzed in duplicate in 50 μl of sera using a commercially available ELISA kit (NWK-PCK01).The intra- and interassay coefficients of variation were 2.7 % and 5%. The lower detection limit of the assay was 0.1 nmol/mg.
Estimates of insulin sensitivity
Disposal-insulin sensitivity (SID) was assessed using a 2-compartment model from serum concentrations of glucose, insulin and 6,6 d2-glucose concentrations, as previously described 25, 26. Briefly, the stable isotope of glucose (6,6 d2-glucose) was incorporated in the IVGTT in order to segregate glucose disposal from hepatic glucose production, since 6,6 d2-glucose is only utilized, not produced, by the body . The two-compartment model describes the kinetics of glucose both in the circulation and in a remote compartment (e.g., interstitial space), that exchanges slowly with the first compartment. Insulin action on glucose disposal occurs in the remote glucose pool. SID, which measures how much insulin is able to increase glucose utilization, can be easily derived by model parameters.
Body composition and fat distribution
Body composition was determined by dual-energy X-ray absorptiometry (Lunar Prodigy; (GE Healthcare Lunar, Madison, WI). Subjects were scanned in light clothing while lying flat on their backs with arms at their sides. Intra-abdominal adipose tissue (IAAT) was analyzed by computed tomography scanning 27, 28 with a HiLight/Advantage Scanner (General Electric, Milwaukee) located in the UAB Department of Radiology. Subjects were scanned in the supine position with arms stretched above their heads. A 5mm scan at the level of the umbilicus (approximately the L4-L5 intervertebral space) was taken. Scans were analyzed for cross-sectional area (cm2) of adipose tissue using the density contour program with Hounsfield units for adipose tissue set at −190 to −30. All scans were analyzed by the same individual. The CV for repeat cross-section analysis of scans among 40 subjects in our laboratory is less than 2% 28.
Statistical analysis
Descriptive characteristics (mean ± SD) are presented by ethnic group. Between-group differences were determined using ANOVA. Data were analyzed using SAS version 9.2 (Carey, NC). Serum- and model-derived variables, fat mass, and IAAT were log transformed prior to analyses to ensure a normal distribution.
Multiple linear regression analysis was used to identify the independent associations of SID with protein carbonyls and %fat or IAAT within each group. Standardized regression coefficients were determined for each independent variable.
Pearson correlation analysis was used to examine the association of protein carbonyls with FFA and glucose within each ethnic group.
To determine if women with relatively high concentrations of glucose and/or FFA differed from those with relatively low concentrations of glucose and/or FFA regarding concentrations of protein carbonyls, subjects (combined EA and AA) were divided into high glucose/high fat, high glucose/low fat, low glucose/high fat, or low glucose/low fat groups based on the median concentration of FFA (0.542 mEq/L) and glucose (92 mg/dL) in the entire sample. The four groups then were compared using a t-test.
Results
Descriptive statistics are presented in Table 1 by ethnic group. There were no statistically significant differences for any variables between ethnic groups, however it must be noted that SID approached significance (P = 0.07).
Table 1.
Descriptive and Study variables for 25 AA and 28 EA women
| Variables | AA | EA | P |
|---|---|---|---|
| Age (yrs) | 33.1 ± 13.5 | 31.6 ± 12.4 | 0.72 |
| Height (cm) | 165.4 ± 5.5 | 162.5 ± 20.8 | 0.50 |
| Weight (kg) | 77.6 ± 16.3 | 72.1 ± 16.0 | 0.23 |
| BMI (kg.m2) | 28.4 ± 6.2 | 26.2 ± 5.9 | 0.18 |
| Body Fat (%) | 38.5 ± 11.0 | 37.5 ± 7.9 | 0.70 |
| IAAT (cm2) | 68.0 ± 52.3 | 72.3 ± 43.7 | 0.75 |
| SAAT (cm2) | 310.6 ± 191.4 | 296.2 ± 166.8 | 0.78 |
| Free Fatty Acids (mEq/L) | 0.55 ± 0.16 | 0.53 ± 0.18 | 0.64 |
| Glucose (mg/dl) | 90.3 ± 7.1 | 91.4 ± 5.9 | 0.53 |
| Insulin (μIu/ml) | 12.4 ± 4.0 | 10.4 ± 4.7 | 0.13 |
| AIRg (μIu/ml × 10min) | 1052.6 ± 587.9 | 515.8 ± 396.4 | 0.001 |
| DI | 732.8 ± 396.4 | 420.3 ± 328.6 | 0.003 |
| Protein Carbonyl (nmol/mg) | 0.607 ± 0.32 | 0.615 ± 0.34 | 0.93 |
| SID | 6.8 ± 5.3 | 10.3 ± 7.6 | 0.07 |
IAAT, intra-abdominal adipose tissue; SAAT, superficial subcutaneous abdominal adipose tissue; AIRg, acute insulin response to glucose; DI, disposition index; SID, disposal specific insulin sensitivity.
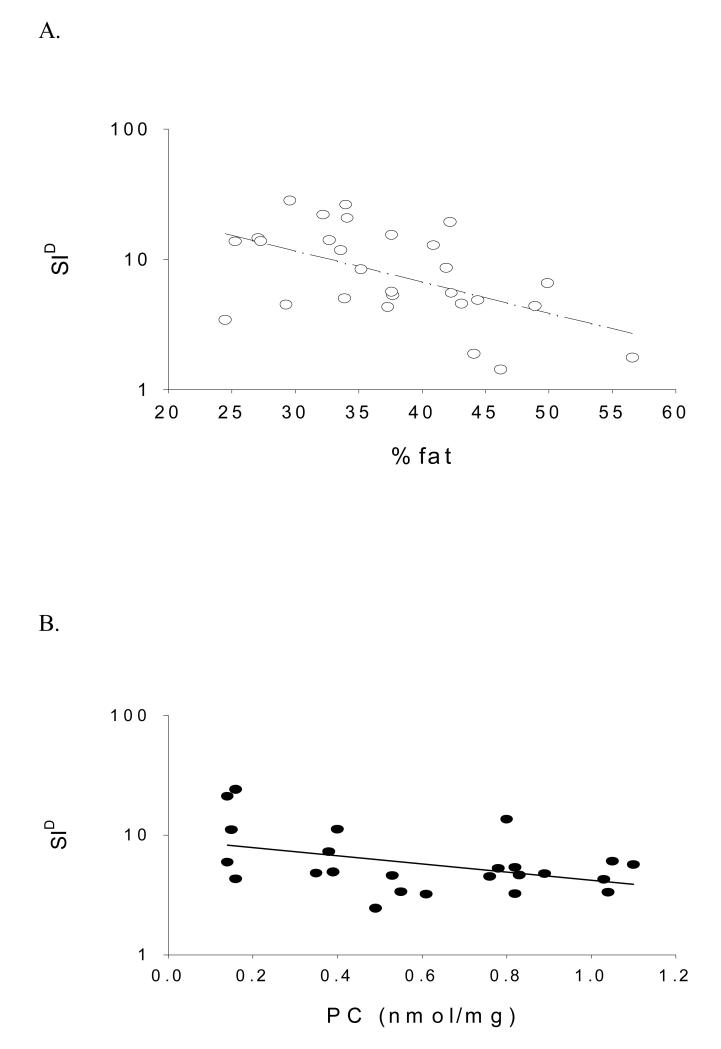
In multiple linear regression analysis, SID was independently associated with protein carbonyl concentration among AA women (P < 0.05) irrespective if %fat or IAAT were included in the model. Neither %fat nor IAAT was significantly associated with SID in AA. In EA women, SID was independently associated with %fat (P < 0.01) or IAAT (P < 0.05). Protein carbonyls were not independently associated with SID among EA. These data are presented in Table 2 and Figure 1.
Table 2.
A. Multiple Linear Regression Model of SID with Protein Carbonyl and %Fat
| Ethnicity | Independent Variables | Standardized β | P |
|---|---|---|---|
| European American | % Fat | −0.54 | 0.004 |
| Model R2=0.29 | Protein Carbonyl | 0.01 | 0.945 |
| African American | % Fat | −0.24 | 0.196 |
| Model R2=0.33 | Protein Carbonyl | −0.47 | 0.019 |
|
B. Multiple Linear Regression Model of SID with Protein Carbonyl and IAAT | |||
|---|---|---|---|
| Ethnicity | Independent Variables | Standardized β | P |
| European American | IAAT | −0.46 | 0.019 |
| Model R2=0.23 | Protein Carbonyl | 0.010 | 0.602 |
| African American | IAAT | 0.14 | 0.510 |
| Model R2=0.26 | Protein Carbonyl | −0.54 | 0.018 |
Boldface values indicate P < 0.05
IAAT = intra-abdominal adipose tissue.
Figure 1.
A. SID significantly associated with %fat in EA women (P < 0.01; adjusted for PC). B. SID significantly associated with PC in AA women (P < 0.05; adjusted for %fat).
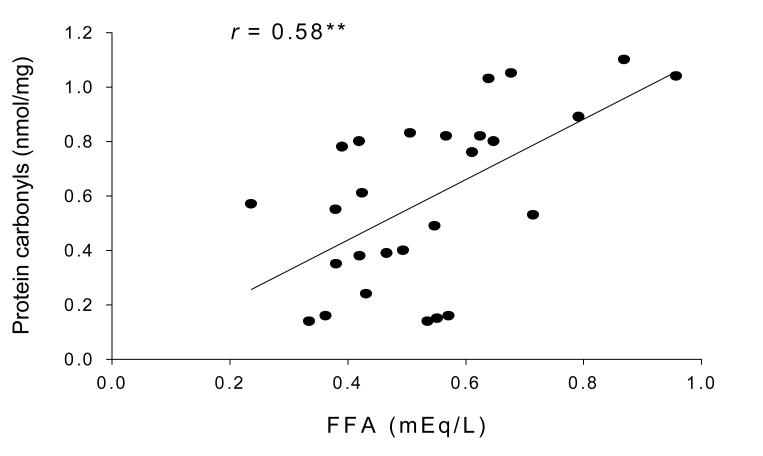
In Pearson correlation analysis, protein carbonyl concentration was significantly correlated with FFA in AA (r = 0.58, P<0.01) (Figure 2). No significant correlations were observed between protein carbonyls and FFA concentrations in EA women (r = −0.11, P=0.59).
Figure 2.
Pearsons partial correlation analysis showed protein carbonyl concentration was significantly correlated with FFA in AA (r = 0.58, P<0.01).
In subgroups based on median concentrations of glucose and FFA, subjects with high concentrations of both glucose and FFA had higher concentrations of protein carbonyls than those with low concentration of both glucose and FFA (P<0.05; Figure 3).
Figure 3.
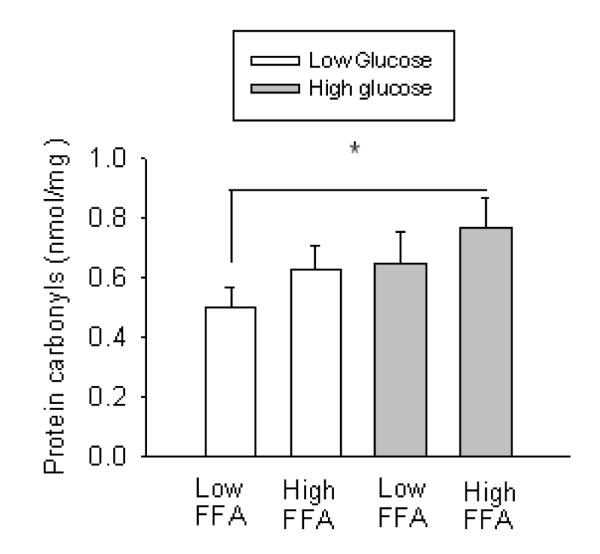
Subjects with higher glucose/FFA concentrations had a significantly greater concentration of circulating protein carbonyls compared to those with lower glucose/FFA concentrations (P<0.05).
Discussion
The purpose of this study was to examine the association between insulin sensitivity and a systemic marker of oxidative stress in a group of healthy women, and to determine if the relationship between oxidative stress and insulin sensitivity differed with ethnicity. The main findings were that: 1) protein carbonyls were associated with SID among AA women, while %fat and IAAT were associated with SID among EA women, 2) protein carbonyls were positively associated with FFA concentration among AA but not EA women and 3) women with higher glucose/FFA concentrations had a significantly greater concentration of circulating protein carbonyls compared to those with lower glucose/FFA concentrations. Our measure of insulin sensitivity was specific to glucose disposal, and thereby reflects primarily skeletal muscle glucose uptake. These observations suggest that oxidative stress may contribute to skeletal muscle insulin resistance among AA women, which may be mediated by circulating FFA and/or glucose.
Among potential factors that may contribute to the pathogenesis of type 2 diabetes, oxidative stress is thought to be an important underlying mechanism that leads to both insulin resistance and beta cell dysfunction 29. While the majority of data regarding oxidative stress and insulin sensitivity have been performed in vitro, several recent clinical trials have shown associations between oxidative stress and decreased insulin sensitivity 13, 14. In this study oxidative stress was independently associated with SID only among AA women. These findings suggest that oxidative stress may have a greater impact in the etiology of decreased insulin sensitivity in AA as compared to EA women.
The mechanism relating protein carbonyls to insulin sensitivity is not known, but may be related to the production of ROS within skeletal muscle. Previous investigations have shown associations between skeletal muscle mitochondrial dysfunction, ROS, and reduced insulin sensitivity 30-33. The pathophysiology of ROS-induced insulin resistance involves a complex network of insulin signaling pathways. The primary stress pathways thought to be activated by ROS production are the nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK) pathways 34-36. These stress activated pathways are thought to decrease insulin sensitivity by increasing serine phosphorylation while subsequently decreasing tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1) 15, 36, 37.
The reason why the association between protein carbonyls and SID differed with ethnicity is not clear. However, we previously have shown that reduced muscle mitochondrial function among AA women may explain part of the ethnic differences in insulin sensitivity 38. Additionally, Ballinger et al (personal communication) have assessed mitochondrial function in human endothelial cells (from cord blood of AA and EA donors) and found a significantly reduced mitochondrial reserve capacity among AA as compared to EA individuals. These experiments demonstrate a distinct difference in mitochondrial phenotype between AA and EA and suggest that increases in bioenergetic demand may reduce the bioenergetic reserve capacity to a greater extent in AA, rendering them more susceptible to production of ROS. Therefore, potential differences in mitochondrial phenotypes between AA and EA women may, in part, explain the ethnic differences for associations between oxidative stress and SID. Future studies should incorporate skeletal muscle mitochondria measures, biomarkers of oxidative stress, and SID in order to explore these potential ethnic physiological differences.
In addition to potential physiological phenotype differences between AA and EA, dietary and nutritional factors must also be considered. Clinical trials have shown that antioxidants (vitamins E, C, and glutathione) can improve insulin sensitivity in insulin resistant and/or diabetic patients 39-41. Several studies have revealed that AA consume fewer daily fruits and vegetables and tend to have lower blood levels of antioxidant nutrients as compared to EA 42-44. Given this information it seems plausible that a reduced dietary antioxidant intake in addition to a reduced mitochondrial functional capacity may render AA more vulnerable to oxidative stress associated diseases. Therefore, future studies should explore potential behavioral and dietary interventions that lead to improvements in both endogenous and exogenous antioxidant concentrations.
To further explore the relationship between glucose and FFA concentrations with levels of oxidative stress, we divided our subjects based on median levels of fasting glucose and FFA concentrations. We found that those with higher glucose/FFA concentrations had a significantly greater concentration of circulating protein carbonyls compared to those with lower glucose/FFA concentrations. Our findings are in agreement with several previous studies 4, 7, 15, 33 that have shown the ability of glucose and FFA to induce oxidative stress. Paolisso et al 4 demonstrated that infusion of FFA in healthy subjects caused an increase in oxidative stress. Additionally, they showed that type 2 diabetic patients demonstrated an inverse correlation between fasting plasma FFA concentration and the ratio of reduced/oxidized glutathione (one of the major endogenous antioxidants 4. To our knowledge, this is the first in vivo human study to demonstrate a relationship with elevated glucose/FFA concentrations and a systemic biomarker of oxidative stress in a non-diabetic population. This is an important finding since oxidative stress is triggered by elevations in both glucose and FFA, and has been linked to the activation of several stress pathways that lead to reduced insulin sensitivity 29. Further study is warranted in order to better understand the mechanisms through which oxidative stress triggered by glucose/FFA in non-diabetic individuals contributes to the progression of type 2 diabetes.
Strengths of this study included robust measures of body composition, body fat distribution, a systemic biomarker of oxidative stress, and the use of a disposal-specific insulin sensitivity measure. Limitations included the relatively small sample size, the cross-sectional nature of the study, and measurement of only a single biomarker of oxidative stress. Additionally, our results are limited to a population of healthy women. Future research should include men and women of different ethnic background, and various stages of insulin resistance to better understand the contribution of oxidative stress to development of type 2 diabetes.
In conclusion, results from this study demonstrate an independent association between oxidative stress and insulin sensitivity in AA but not EA women, as well as an association between oxidative stress and circulating FFA that was specific to AA. Whether the higher prevalence of many metabolic diseases in AA vs EA (e.g., hypertension, type 2 diabetes) is related to aspects of greater oxidative damage within AA is an intriguing possibility that deserves further research.
Acknowledgements
This work was supported by 2T32DK062710-07, R01DK58278, M01-RR-00032, UL 1RR025777, P30-DK56336, and P60DK079626. Maryellen Williams and Cindy Zeng conducted laboratory analyses; Tena Hilario served as project coordinator; Crystal Douglas and Jeannine Lawrence provided support with subject recruitment and data entry.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
Disclosure: The authors have nothing to disclose.
References
- 1.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 2.Henry RR. Insulin resistance: from predisposing factor to therapeutic target in type 2 diabetes. Clin Ther. 2003;25(Suppl B):B47–63. doi: 10.1016/s0149-2918(03)80242-4. [DOI] [PubMed] [Google Scholar]
- 3.Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature. 2001;414:788–91. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- 4.Paolisso G, Giugliano D. Oxidative stress and insulin action: is there a relationship? Diabetologia. 1996;39:357–63. doi: 10.1007/BF00418354. [DOI] [PubMed] [Google Scholar]
- 5.Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 6.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 7.Paolisso G, D’Amore A, Volpe C, et al. Evidence for a relationship between oxidative stress and insulin action in non-insulin-dependent (type II) diabetic patients. Metabolism. 1994;43:1426–9. doi: 10.1016/0026-0495(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49:27–9. doi: 10.1016/s0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 9.Robinson R, Robinson LJ, James DE, Lawrence JC. Glucose transport in L6 myoblasts overexpressing GLUT1 and GLUT4. J Biol Chem. 1993;268:22119–26. [PubMed] [Google Scholar]
- 10.Maddux BA, See W, Lawrence JC, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes. 2001;50:404–10. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 12.Rueckschloss U, Duerrschmidt N, Morawietz H. NADPH oxidase in endothelial cells: impact on atherosclerosis. Antioxid Redox Signal. 2003;5:171–80. doi: 10.1089/152308603764816532. [DOI] [PubMed] [Google Scholar]
- 13.Meigs JB, Larson MG, Fox CS, Keaney JF, Vasan RS, Benjamin EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes Care. 2007;30:2529–35. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- 14.Park K, Gross M, Lee DH, et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32:1302–7. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–6. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 18.Kang DH. Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues. 2002;13:540–9. doi: 10.1097/00044067-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–39. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 20.American Cancer Society . Cancer facts and figures for African Americans 2005-2006. 2006. Atlanta, Ga: 2006. [Google Scholar]
- 21.Simic MG. DNA markers of oxidative processes in vivo: relevance to carcinogenesis and anticarcinogenesis. Cancer Res. 1994;54:1918s–23s. [PubMed] [Google Scholar]
- 22.Gower B, Ard J, Hunter G, Fernandez J, Ovalle F. Elements of the metabolic syndrome: association with insulin sensitivity and effects of ethnicity. Metab Syndr Relat Disord. 2007;5:77–86. doi: 10.1089/met.2006.0027. [DOI] [PubMed] [Google Scholar]
- 23.Minor DS, Wofford MR, Jones DW. Racial and ethnic differences in hypertension. Curr Atheroscler Rep. 2008;10:121–7. doi: 10.1007/s11883-008-0018-y. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Toffolo G, Cobelli C. The hot IVGTT two-compartment minimal model: an improved version. Am J Physiol Endocrinol Metab. 2003;284:E317–21. doi: 10.1152/ajpendo.00499.2001. [DOI] [PubMed] [Google Scholar]
- 26.Vicini P, Caumo A, Cobelli C. The hot IVGTT two-compartment minimal model: indexes of glucose effectiveness and insulin sensitivity. Am J Physiol. 1997;273:E1024–32. doi: 10.1152/ajpendo.1997.273.5.E1024. [DOI] [PubMed] [Google Scholar]
- 27.Kekes-Szabo T, Hunter GR, Nyikos I, Nicholson C, Snyder S, Berland L. Development and validation of computed tomography derived anthropometric regression equations for estimating abdominal adipose tissue distribution. Obes Res. 1994;2:450–7. doi: 10.1002/j.1550-8528.1994.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 28.Goran M, Kaskoun M, Shuman W. Intra-abdominal adipose tissue in young children. Int J Obes Relat Metab Disord. 1995;19:279–83. [PubMed] [Google Scholar]
- 29.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 30.Razak F, Anand SS, Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vasc Med. 2004;9:223–4. doi: 10.1191/1358863x04vm568xx. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 32.Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–81. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed AK, Bierhaus A, Schiekofer S, Tritschler H, Ziegler R, Nawroth PP. The role of oxidative stress and NF-kappaB activation in late diabetic complications. Biofactors. 1999;10:157–67. doi: 10.1002/biof.5520100211. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–53. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 36.Nishikawa T, Kukidome D, Sonoda K, et al. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res Clin Pract. 2007;77(Suppl 1):S161–4. doi: 10.1016/j.diabres.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 37.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–93. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirikul B, Gower BA, Hunter GR, Larson-Meyer DE, Newcomer BR. Relationship between insulin sensitivity and in vivo mitochondrial function in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E724–8. doi: 10.1152/ajpendo.00364.2005. [DOI] [PubMed] [Google Scholar]
- 39.Hirai N, Kawano H, Hirashima O, et al. Insulin resistance and endothelial dysfunction in smokers: effects of vitamin C. Am J Physiol Heart Circ Physiol. 2000;279:H1172–8. doi: 10.1152/ajpheart.2000.279.3.H1172. [DOI] [PubMed] [Google Scholar]
- 40.Caballero B. Vitamin E improves the action of insulin. Nutr Rev. 1993;51:339–40. doi: 10.1111/j.1753-4887.1993.tb03761.x. [DOI] [PubMed] [Google Scholar]
- 41.Paolisso G, Di Maro G, Pizza G, et al. Plasma GSH/GSSG affects glucose homeostasis in healthy subjects and non-insulin-dependent diabetics. Am J Physiol. 1992;263:E435–40. doi: 10.1152/ajpendo.1992.263.3.E435. [DOI] [PubMed] [Google Scholar]
- 42.Subar AF, Heimendinger J, Patterson BH, Krebs-Smith SM, Pivonka E, Kessler R. Fruit and vegetable intake in the United States: the baseline survey of the Five A Day for Better Health Program. Am J Health Promot. 1995;9:352–60. doi: 10.4278/0890-1171-9.5.352. [DOI] [PubMed] [Google Scholar]
- 43.Ford ES, Schleicher RL, Mokdad AH, Ajani UA, Liu S. Distribution of serum concentrations of alpha-tocopherol and gamma-tocopherol in the US population. Am J Clin Nutr. 2006;84:375–83. doi: 10.1093/ajcn/84.1.375. [DOI] [PubMed] [Google Scholar]
- 44.Watters JL, Satia JA, Kupper LL, Swenberg JA, Schroeder JC, Switzer BR. Associations of antioxidant nutrients and oxidative DNA damage in healthy African-American and White adults. Cancer Epidemiol Biomarkers Prev. 2007;16:1428–36. doi: 10.1158/1055-9965.EPI-06-1030. [DOI] [PubMed] [Google Scholar]