Abstract
Objectives
This study sought to understand whether genetic variation at the Neuropeptide Y (NPY) locus governs secretion and stress responses in vivo as well as NPY gene expression in sympathochromaffin cells.
Background
The NPY is a potent pressor peptide co-released with catecholamines during stress by sympathetic axons. Genome-wide linkage on NPY secretion identified a LOD (logarithm of the odds ratio) peak spanning the NPY locus on chromosome 7p15.
Methods
Our approach began with genomics (linkage and polymorphism determination), extended into NPY genetic control of heritable stress traits in twin pairs, established transcriptional mechanisms in transfected chromaffin cells, and concluded with observations on blood pressure (BP) in the population.
Results
Systematic polymorphism tabulation at NPY (by re-sequencing across the locus: promoter, 4 exons, exon/intron borders, and untranslated regions; on 2n = 160 chromosomes of diverse biogeographic ancestries) identified 16 variants, of which 5 were common. We then studied healthy twin/sibling pairs (n = 399 individuals), typing 6 polymorphisms spanning the locus. Haplotype and single nucleotide polymorphism analyses indicated that proximal promoter variant ∇−880Δ (2-bp TG/—, Ins/Del, rs3037354) minor/Δ allele was associated with several heritable (h2) stress traits: higher NPY secretion (h2 = 73 ± 4%) as well as greater BP response to environmental (cold) stress, and higher basal systemic vascular resistance. Association of ∇−880Δ and plasma NPY was replicated in an independent sample of 361 healthy young men, with consistent allelic effects; genetic variation at NPY also associated with plasma NPY in another independent series of 2,212 individuals derived from Australia twin pairs. Effects of allele −880Δ to increase NPY expression were directionally coordinate in vivo (on human traits) and in cells (transfected NPY promoter/luciferase reporter activity). Promoter −880Δ interrupts a novel glucocorticoid response element motif, an effect confirmed in chromaffin cells by site-directed mutagenesis on the transfected promoter, with differential glucocorticoid stimulation of the motif as well as alterations in electrophoretic mobility shifts. The same −880Δ allele also conferred risk for hypertension and accounted for approximately 4.5/approximately 2.1 mm Hg systolic BP/diastolic BP in a population sample from BP extremes.
Conclusions
We conclude that common genetic variation at the NPY locus, especially in proximal promoter ∇−880Δ, disrupts glucocorticoid signaling to influence NPY transcription and secretion, raising systemic vascular resistance and early heritable responses to environmental stress, eventuating in elevated resting BP in the population. The results point to new molecular strategies for probing autonomic control of the human circulation and ultimately susceptibility to and pathogenesis of cardiovascular and neuropsychiatric disease states.
Keywords: genetics, glucocorticoid receptor, hypertension, neuropeptide Y
Neuropeptide Y (NPY), a 36-amino acid peptide neurotransmitter, is produced/secreted by noradrenergic neurons (both central and autonomic) as well as chromaffin cells (1). The peptide has diverse functions, including roles in immune response (2); regulation of food consumption (3); and control of heart rate, vasoconstriction, coronary blood flow, and ventricular function (4). The NPY also augments vasoconstrictor effects of noradrenergic neurons. Like epinephrine and norepinephrine, the release of NPY to the circulation occurs during sympathetic activation after environmental stress (5). Compared with the more rapid secretion and duration of catecholamine action, NPY is released by more intense and prolonged sympathetic activation, thus marking more severe stress (6). Increased NPY signaling due to elevated NPY expression in the hypothalamus might contribute to development of obesity and its related traits, Type II diabetes, and cardiovascular disease (3). The NPY is also involved in regulatory loops between the immune and adrenal systems in pathophysiological conditions wherein interleukin-1-beta secretion is activated, as in sepsis, rheumatoid arthritis, stress, or hypertension (7,8). Moreover, NPY increases the proportion of energy stored as fat and blocks nociceptive signals to the brain (9).
The effect of stress on blood pressure (BP) is thus of increasing importance. Although hypertension is not a simple consequence of stress, repeated stressors might eventuate in systemic hypertension (10). Patients with hypertension often exhibit increased sympathetic activity (11,12), and people with sympathetic overactivity tend to develop hypertension (13,14). In a rodent genetic model of stress (Rat Genome Database, Bioinformatics Research Center, Milwaukee, Wisconsin), the Npy locus lies within the confidence interval for linkage to circulating corticosterone, the rodent glucocorticoid (Stresp4). In light of the emerging biology of NPY, we undertook the present study, with genomic and computational tools to probe how heredity shapes human functional responses in the sympathetic neuroeffector junction, with NPY as a likely focal point in the pathogenesis of cardiovascular or neuropsychiatric disease.
Here we began with genomics (linkage and re-sequencing for polymorphism determination) and proceeded to phenotyping of heritable proximal traits in twin pairs. Functional studies of NPY promoter polymorphism are evolving (15), and thus we established a novel transcriptional mechanism in transfected cells, concluding with extension of the findings into disease in the population.
Methods
Subjects and clinical characterization
Subjects were volunteers from southern California (San Diego area), and each subject gave informed, written consent; the protocol was approved by the institutional review board. Recruitment procedures, definitions, and confirmation of subject diagnoses are according to previous reports. Genomic deoxyribonucleic acid of each individual was prepared from leukocytes in ethylenediaminetetraacetic acid–anticoagulated blood, with PureGene extraction columns (Gentra Biosystems, Minneapolis, Minnesota).
Polymorphism determination
See Online Methods. Characteristics of the resequenced subjects are given in Online Table 1. Details of amplicons and polymerase chain reaction primers for polymorphism determination and genotyping are given in Online Tables 2 to 4.
UCSD twin and sibling pairs
From 235 nuclear families, 399 individuals from twin and sibling (sib) pairs were recruited to conduct the following study. Zygosity was confirmed by extensive micro-satellite and single nucleotide polymorphism (SNP) genotyping, as described (16). Twins ranged in age from 15 to 84 years; 10% were hypertensive. All of the twins in these allelic/haplotype association studies were self-identified as of European (white) ancestry, to guard against the potentially artifactual effects of population stratification.
NPY replication in young American men
See Online Methods.
NPY replication in Australian twins
See Online Methods.
Primary care population derived from extremes of BP distribution
From a database of over 53,000 people in southern California, we ascertained 1,121 European-ancestry individuals, of both sexes, from the highest and lowest 5th percentiles of a primary care population (17) in BP distribution. Evaluation included physical examination, blood chemistries, hemogram, and extensive medical history questionnaire. Characteristics of the hypertensive and normotensive groups are shown as Online Table 5.
Molecular genetics
See Online Methods.
Systematic polymorphism determination at the NPY locus
See Online Methods.
Genotyping of NPY variants
See Online Methods.
Genotyping of NPY promoter variant ∇−880Δ
See Online Methods.
Human twin pair phenotyping
See Online Methods.
Biochemical
For plasma NPY measurement, see Online Methods .
Physiological (in vivo)
See Online Methods.
Cardiac and vascular function at rest
See Online Methods.
Environmental (cold) stress test
See Online Methods.
Computation
See Online Methods.
Statistical analyses
Estimates are stated as mean ± 1 SEM. Two-way analysis of variance or multivariable general linear modeling was performed in SPSS (version 17.0, SPSS, Chicago, Illinois) to evaluate the significance of single variants or interaction of variants during in vivo association studies as well as in vitro haplotype-specific NPY promoter/reporter transfections. For twin pair analyses, descriptive (genotype-specific mean ± SEM) and inferential (chi square, p value) statistics were computed across all of the twins, with generalized estimating equations in SPSS, to account for correlated trait values within each twinship, with an exchangeable correlation matrix (18). Additional permutation tests (19) on 3 × 2 contingency tables (diploid genotype vs. BP status), implemented with the Fisher exact test, were used to confirm genotype effects on the dichotomous BP trait. In addition to additive models, after inspection of clustering of the descriptive statistics (mean ± SEM), heterozygotes and minor/major allele homozygotes were grouped together as needed, to test dominant/recessive models. Meta-analysis was carried out with the command META, testing fixed effect (i.e., genotype) models in STATA (version 12, StataCorp, College Station, Texas), after individual study regression analysis in SPSS (version 17, SPSS), incorporating individual study data to derive significance as well as pooled genotype effect size (beta, or slope/allele) and its SE.
Genome-wide linkage analysis
See Online Methods.
Data transformation
See Online Methods.
Heritability of phenotype expression in vivo
See Online Methods.
Haplotypes and linkage disequilibrium
See Online Methods.
Marker-on-trait association
See Online Methods.
Promoter motif analyses
See Online Methods.
NPY promoter/luciferase reporter activity assays
See Online Methods. A schematic of NPY promoter/luciferase reporter constructions is given in Online Figure 1.
Effect of glucocorticoid and PAX6 on NPY promoter/ luciferase reporter activity
See Online Methods.
Electrophoretic mobility shift assays
See Online Methods. Oligonucleotides for electrophoretic mobility shift assay (EMSA) are given in Online Figure 6.
Results
Autonomic trait heritability in twin pairs
Basal/resting plasma concentration of NPY was under substantial genetic control, with heritability (h2) = 0.73 ± 0.04 (p = 3.1E-26) (Table 1). Heritability was also significant for resting systolic blood pressure (SBP) (h2 = 0.45 ± 0.06, p < 0.001), diastolic blood pressure (DBP) (h2 = 0.51 ± 0.06, p < 0.001), heart rate (h2 = 0.31 ± 0.07, p < 0.001), cardiac index (CI) (h2 = 0.71 ± 0.04, p < 0.001), systemic vascular resistance index (SVRI) (h2 = 0.55 ± 0.06, p < 0.001), and stroke volume index (h2 = 0.66 ± 0.05, p < 0.001). During environmental (cold) stress, heritability was also established for SBP change (h2 = 0.65 ± 0.05, p < 0.001) as well as DBP change (h2 = 0.54 ± 0.06, p < 0.001).
Table 1.
NPY Promoter Variant ∇−880 Δrs3037354: Effects on Heritable Autonomic Traits in Twins and Sibs
| Trait H2 in Twins (n = 338) | ∇−880Δ Diploid Genotype (n = 399) | |||||
|---|---|---|---|---|---|---|
| Category/Trait | Mean ± SEM | p Value | ∇/∇ (186) | ∇/Δ (175) | Δ/Δ (38) | p Value |
| Biochemical | ||||||
| Plasma NPY, pmol/l | 0.73 ± 0.04 | 3.08E-26* | 72.0 ± 3.08 | 74.3 ± 3.3 | 132.7 ± 1.6 | 4.2E-5* |
| Physiological/hemodynamic (resting) | ||||||
| SBP, mm Hg | 0.45 ± 0.06 | 2.07E-09* | 133 ± 1.2 | 132 ± 1.1 | 133 ± 1.6 | 0.658 |
| DBP, mm Hg | 0.51 ± 0.06 | 7.18E-12* | 72.6 ± 0.9 | 71.8 ± 0.8 | 71.8 ± 1.3 | 0.702 |
| HR, beats/min | 0.31 ± 0.07 | 3.94E-05* | 70.5 ± 1.0 | 68.4 ± 0.9 | 64.3 ± 1.5 | 0.002* |
| CI, l/min/m2 | 0.71 ± 0.04 | 2.51E-19* | 2.77 ± 0.05 | 2.70 ± 0.05 | 2.51 ± 0.06 | 0.004* |
| SVRI, dynes/s/cm5/m2 | 0.55 ± 0.06 | 7.70E-12* | 798.7 ± 18.8 | 845.3 ± 21 | 879.0 ± 31.3 | 0.039* |
| SVI, ml/min/m2 | 0.66 ± 0.05 | 1.81E-17* | 39.8 ± 0.4 | 39.5 ± 0.4 | 39.1 ± 0.6 | 0.576 |
| Environmental stress (cold pressor test) | ||||||
| Delta-SBP, mm Hg | 0.65 ± 0.05 | 2.60E-16* | 14.3 ± 1.5 | 15.1 ± 2.5 | 16.0 ± 2.5 | 0.786 |
| Delta-DBP, mm Hg | 0.54 ± 0.06 | 7.52E-11* | 10.4 ± 1.1 | 9.3 ± 1.0 | 14.6 ± 1.7 | 0.0072* |
Heritability (h2) was estimated from variance components in twins, with Sequential Oligogenic Linkage Analysis Routines. Descriptive statistics were computed in twins and siblings (sibs), by generalized estimating equation in SPSS (SPSS, Chicago, Illinois), with additive genetic models.
p < 0.05.
CI = cardiac index; DBP = diastolic blood pressure; HR = heart rate; “Index” = hemodynamic value (CO or SVR) normalized to body surface area (in m2); NPY = Neuropeptide Y; SBP = systolic blood pressure; SVI = stroke volume index; SVRI = systemic vascular resistance index.
Genome-wide linkage for NPY secretion in twin and sib pairs
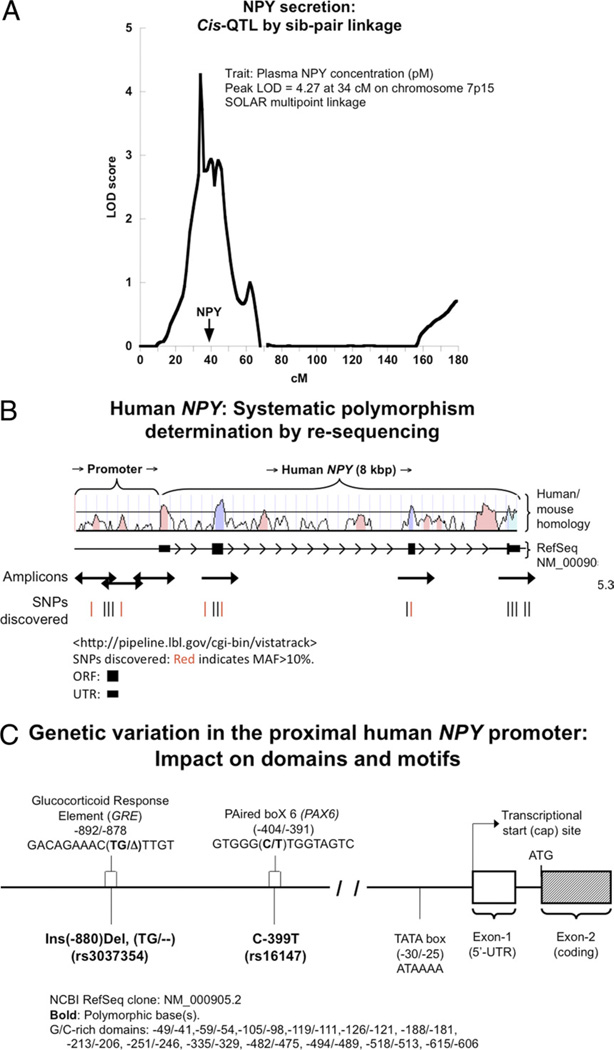
Multi point sib-pair linkage analysis of plasma NPY concentration revealed a peak logarithm of the odds score of 4.27 at 34 cmol/l on chromosome 7p15; of note, the NPY locus itself is within the linked region (Fig. 1A), suggesting that NPY itself might be a cis-QTL for NPY expression and secretion.
Figure 1. Human NPY Genetic Diversity and NPY Expression.
(A) Neuropeptide Y (NPY) secretion: cis-quantitative trait loci (QTL) by sibling (sib)-pair linkage. In a linkage study of plasma NPY concentration in sib pairs with 730 microsatellite markers, chromosome 7p15 displayed a peak logarithm of the odds (LOD) = 4.27 at 34 cmol/l, with the NPY gene as a positional candidate locus beneath the main peak. (B) Polymorphism discovery across the human NPY locus: re-sequencing strategy. There are 4 exons at the NPY locus. Five polymerase chain reaction amplicons spanned the promoter and coding regions. Human/mouse homology (VISTA Tools for Comparative Genomics) is shown for evolutionarily conserved regions, suggestive of functional importance. Red bars in the single nucleotide polymorphism (SNP) discovery panel denote polymorphisms with minor allele frequency (MAF) >20%. (C) Domains and motifs in the NPY promoter. Functional domains in the core/proximal promoter (such as the TATA box, and G/C-rich regions) were invariant in 80 people (2n = 160 chromosomes) subjected to systematic polymorphism determination by re-sequencing. Two very common polymorphisms (MAF >20%) occur in the proximal promoter: Ins−880Del and C-399T. The −880 Ins/Del variant lies in a GRE transcriptional control motif (−892/−878), whereas C-399T lies in a recognition motif for PAX6 (−402/−393). Del = deletion; Ins = insertion; NCBI = National Center for Biotechnology; SOLAR = Sequential Oligogenic Linkage Analysis Routines.
Systematic polymorphism determination across the NPY locus
In 80 individuals of 4 diverse biogeographic ancestries, we identified 14 SNPs, one 2-base insertion/deletion (Ins/Del), and 1 single-base Ins/Del, in 6 amplicons spanning a 3,219-base pair (bp) footprint over the approximately 8-kbp locus plus proximal promoter (Fig. 1B, Online Table 7). Of these, 5 polymorphisms are common (at minor allele frequency [MAF] >20%); in the proximal promoter, there is 1 common SNP (rs16147, C-399T, MAF = 47.6%) and 1 common 2-base Ins/Del (rs3037354, ∇−880Δ Ins/Del TG/—, MAF = 19.3%). We identified 2 non-synonymous SNPs: 1 in the signal peptide (rs16139; T1071C, Leu7Pro; MAF: 1.9%), and the other in exon-4 (T7493C, Met95Thr; 0.6%). Each of the 2 common polymorphisms in the NPY promoter (∇−880Δ and C—399T) potentially interrupts transcription factor binding motifs (Fig. 1C).
Pair-wise linkage disequilibrium
To visualize patterns of SNP associations, pair-wise correlations among the 5 common SNPs (MAF >20%) were quantified as linkage disequilibrium (LD) parameter D’ by Haploview (20) across the NPY locus. A single block of LD was maintained across much of locus (D’ >0.8) in both whites and Asians, whereas in blacks and Hispanics, the 2 promoter SNPs also remain in 1 block (Online Fig. 2).
NPY genetic variation and stress responses in twin and sib pairs
Here we studied stress/BP phenotypes in a sample of twin/sib pairs of European ancestry.
NPY haplotypes and heritable plasma NPY and BP changes to stress in twins
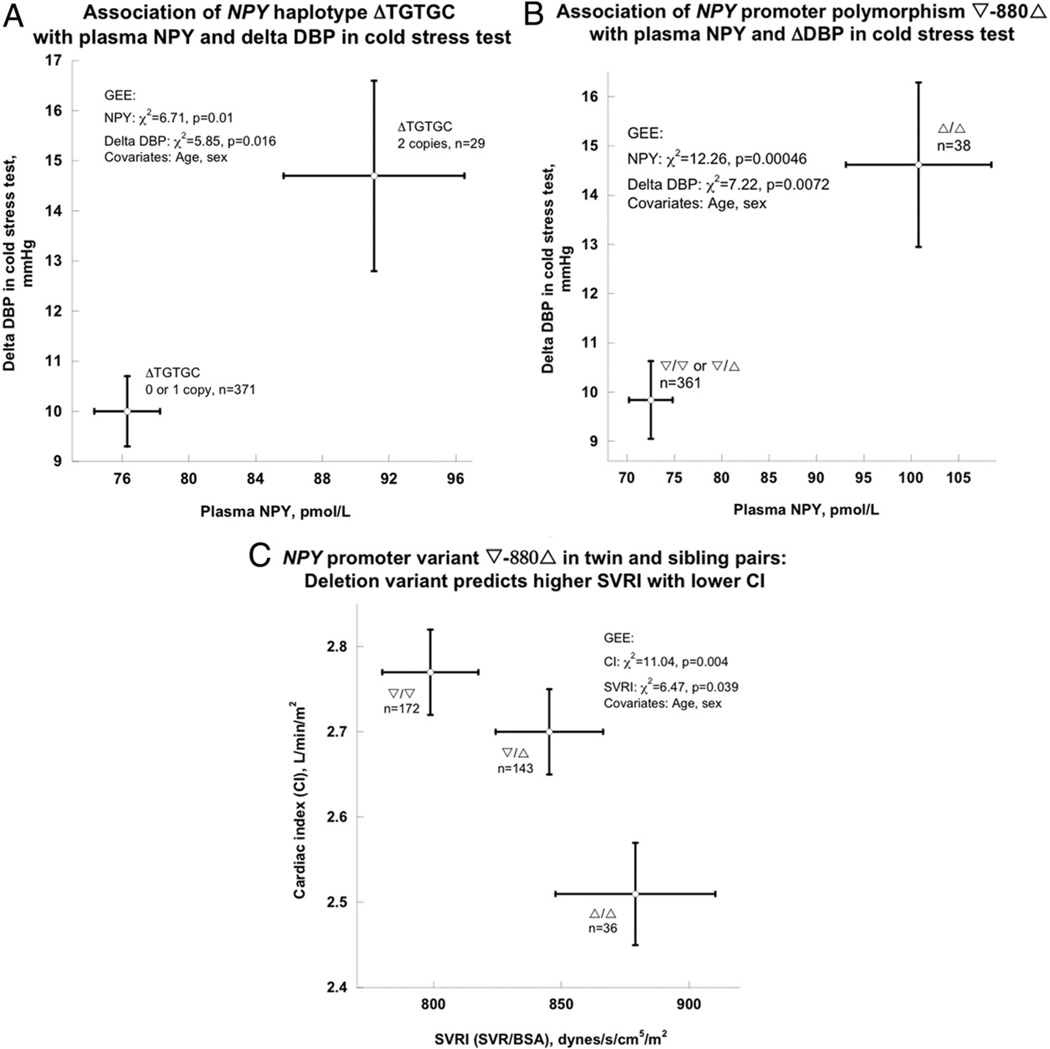
Given the relatively high degree of LD across the gene (Online Fig. 2), we began with haplotypes to span the locus, with 5 common polymorphisms (rs3037354, rs16147, rs16140, rs5573, and rs5574, MAF >20%) and 1 SNP in the signal peptide (rs16139 Leu7Pro, MAF = 2%), each in Hardy-Weinberg equilibrium (Online Tables 8 and 9), thus encompassing structural/ functional domains across the gene (promoter, exons, introns). In the twin/sib pairs, NPY displayed 3 common haplotypes (Online Table 10), among which the second-most common—ΔTGTGC—was associated with plasma NPY concentration as well as BP change during cold stress (Fig. 2A). Occurrence of haplotype ΔTGTGC elevated plasma NPY from 76.3 ± 1.97 pmol/l to 91.1 ± 5.42 pmol/l (by approximately 19%, p = 0.01), whereas ΔDBP increased from 10.0 ± 0.7 mm Hg to 14.7 ± 1.9 mm Hg (by approximately 47%, p = 0.016) (Fig. 2A).
Figure 2. Association of NPY Genetic Variation With Stress Phenotypes in the Twins and Siblings.
(A) NPY common haplotype ΔTGTGC with plasma NPY concentration and blood pressure (BP) response to stress. (B) NPY promoter polymorphism ∇−880Δ with plasma NPY as well as change in diastolic blood pressure (DBP) during environmental (cold) stress. The deletion allele was associated with higher plasma NPY and change in DBP during the cold stress test. The genetic model was Δ-allele recessive, on the basis of inspection of the marker-on-trait results. (C) ∇−880Δ with basal hemodynamic traits: systemic vascular resistance index (SVRI) or cardiac index (CI). The CI was calculated as cardiac output normalized by body surface area (BSA), and SVRI was calculated as systemic vascular resistance (SVR) normalized by BSA. The effects on the 2 traits are reciprocal. GEE = generalized estimating equation; ORF = open reading frame.
NPY promoter polymorphism ∇−880Δ: effects on heritable biochemical and physiological “intermediate” traits in twins
In the twin/sib sample, the promoter Ins/Del polymorphism within the NPY haplotype block displayed the peak effect on traits: ∇−880Δ was associated with plasma NPY as well as BP change during the cold stress test (change in DBP) (Fig. 2B, Table 1). The Δ/Δ homozygotes displayed mean NPY of 100.1 ± 7.8 pmol/l, whereas ∇ carriers (∇/Δ or ∇/∇) were diminished, at 71.8 ± 2.8 pmol/l (chi-square = 12.26, p = 0.00046). The Δ/Δ homozygotes displayed a cold-stress DBP increment of 14.6 ± 1.7 mm Hg, whereas ∇ carriers (∇/Δ or ∇/∇) exhibited a change in DBP of 9.8 ± 0.8 mm Hg (chi-square = 7.22, p = 0.0072).
Delta−880Δ exhibited reciprocal effects on basal hemodynamics: ∇/∇ homozygotes displayed the lowest SVRI (SVR/body surface area) values, at 798.7 ± 18.8 dynes/s/ cm5/m2 ; whereas Δ/Δ homozygotes had the highest SVRI values, at 879.0 ± 31.3 dynes/s/cm5/m2, thus increasing resistance by approximately 10.1% (Fig. 2C). By contrast, ∇/∇ homozygotes displayed the highest CI values, at 2.77 ± 0.05 l/min/m2; while Δ/Δ homozygotes had the lowest CI values, at of 2.51 ± 0.06 l/min/m, thus decreasing CI by approximately 9.4%. In each case (SVRI and CI), heterozygotes displayed intermediate values, suggesting an additive effect of ∇−880Δ 880A on these traits. From the distribution of p values of tests of ∇−880Δ on these correlated twin traits, we estimated (see Methods) that a p value of 0.05 corresponded to a False Discovery Rate of 0.067.
Replication of NPY promoter polymorphism ∇−880Δ effect on plasma NPY
In 361 healthy young males of the Marine Resilience Study, resting plasma NPY was significantly associated with ∇−880Δ genotype: betaADD ± SE = 7.86 ± 2.47, Padd = 1.55 × 10−3; betaDOM ± SE = 12.14 ± 3.164, Pdom = 1.47 × 10−4 (Table 2). Metaanalysis combining the twin/sib pairs and Marine Resilience Study samples in an additive model indicated allelic effects consistent in magnitude (beta) and direction (sign on slope) across groups; the overall slope of the meta-analysis regression (beta) = 7.492 with SE (of beta) = 1.769, p < 0.0001.
Table 2.
Meta-Analysis of a Human NPY Promoter Variant on NPY Secretion
| NPY RefSNP | Group | Allele_1/Allele_2 | Freq_A1 | n | Trait | Model | Effect of SNP on Trait | ||
|---|---|---|---|---|---|---|---|---|---|
| Beta (Slope/Allele) | SE of Beta | p Value | |||||||
| rs3037354 | Twins/sibs | −/TG | 29.2% | 399 | pNPY | Additive | 7.10 | 2.54 | 0.006 |
| rs3037354 | MRS Marines | −/TG | 26.0% | 361 | pNPY | Additive | 7.86 | 2.47 | 0.00155 |
| rs3037354 | Meta-analysis | −/TG | — | 760 | pNPY | Additive | 7.49 | 1.76 | <0.0001 |
Additive linear models were tested by linear regression, with effect size expressed as beta (slope/allele), ± SE of beta. Effects of rs3037354 across groups were evaluated with the META command in STATA (StataCorp, College Station, Texas), with a fixed effect (i.e., genotype) on trait (NPY) model.
A1 = allele_1 (−, less frequent allele); MRS = Marine Resilience Study; pNPY = plasma Neuropeptide Y concentration; SNP = single nucleotide polymorphism; TG = 2-base pair (bp) insertion allele; − = 2-bp deletion allele.
In a second replication, 2,212 Australian twins phenotyped for plasma NPY and subjected to genome-wide association, Ins−880Del was not typed, but nearby variant rs16139 (Exon-1, Leu7Pro) was available (hence, this was not an exact replication). By regression, T-allele beta = 0.226, SE (of beta) = 0.094; the result was significant at p = 0.016, and the directional effect of the T-allele was the same in Australia as in the haplotype bearing the T-allele in the San Diego studies (ΔTGTGC) (Fig. 2A). A separate test of Leu7Pro in the San Diego subjects confirmed its effect on plasma NPY (p = 0.034), with the same directional effect on the NPY trait (T>C).
NPY promoter motifs: computational disruption by common variants ∇−880Δ (glucocorticoid response element motif) and C-399T (PAX6 motif)
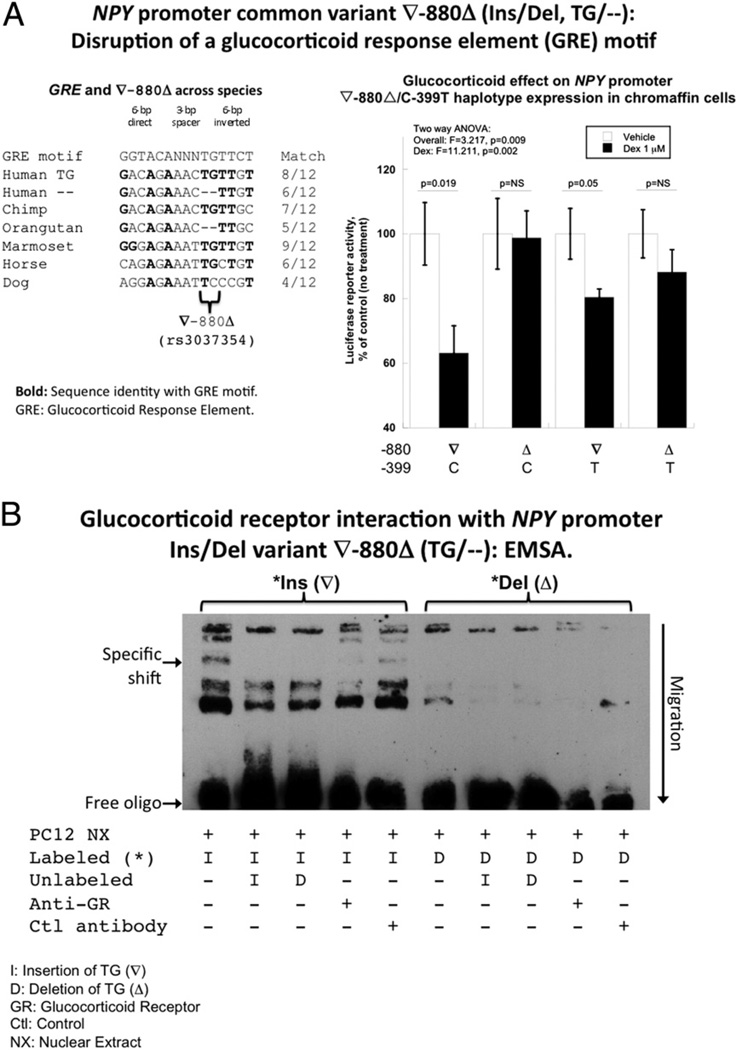
The ∇−880Δ (2-bp, TG/—, Ins/Del; rs3037354) variant lies in a region relatively conserved across species (especially in primates) with a partial match (8/12 bp) for a consensus glucocorticoid response element (GRE) motif and the insertion displaying a superior match (Figs. 1C and 3A).
Figure 3. NPY Promoter ∇−880Δ Polymorphism: GRE Motif.
(A) The Neuropeptide Y (NPY) promoter variant ∇−880Δ at the glucocorticoid response element (GRE) motif. Left panel: NPY promoter V-880A polymorphism: interspecies conservation, and consensus motifs match for GRE. Sequence alignments were done by Clustal-W, whereas motifs from TRANSFAC were explored at Chip-Mapper and JASPAR (see Methods). Right panel: Functional response: effect of exogenous glucocorticoid (dexamethasone) on NPY promoter activity in PC12 cells. Char-coal/dextran-stripped fetal bovine serum was used as the growth medium, to remove any residual glucocorticoid from serum. Dexamethasone (Dex) (or vehicle) was added 6 h after transfection. Cells were harvested at 24 h after transfection. (B) Electrophoretic mobility shift assay (EMSA) interaction of ∇−880Δ and the synthetic GRE with chromaffin cell nuclear proteins. The biotin-labeled insertion (∇) allele exhibits increased binding to nuclear proteins, as compared with the deletion (Δ) version. The binding signal for 1 band (arrow) was completely abolished by the unlabeled oligonucleotide and inhibited approximately 90% by an antibody directed against the glucocorticoid receptor (GR). ANOVA = analysis of variance.
C-399T (rs16147) also lies in a region relatively conserved across species, particularly in primates, with a partial match (9/14 bp) for a consensus PAX6 (PAired boX gene 6) motif, with the C-allele displaying a superior match (Fig. 1C, Online Fig. 3). The chimp sequence suggests that the T (less frequent)- allele is ancestral in humans. Association of C-399T (rs16147) with responses to stress or emotional challenge (15) as well as early onset atherosclerosis (21) have been reported, and C-399T lies within our NPY block of LD (Online Fig. 2) as well as our trait-associated haplotypes (Fig. 2A). However, we found no functional effects of C-399T (Online Figs. 3 and 4). A recent functional study found that the −399T (but not C-399) allele of an oligonucleotide shifted mobility during EMSA (22); that study employed nuclear protein extracts from postmortem human brain rather than a chromaffin cell line.
NPY promoter variation: coordinate directional functions in transfected cells and in vivo (twins)
In promoter/luciferase reporter plasmids transfected into PC12 cells, we studied the 2 common variants (∇−880Δ and C−399T), giving rise to 4 combinations (haplotypes), here created by site-directed mutagenesis: ∇-C, Δ-C, ∇-T, and Δ-T. Luciferase reporter basal (unstimulated) activity differed substantially across all 4 combinations (Online Fig. 5). Δ-T displayed the highest expression, with ∇-T the lowest (haplotype effect p < 0.001). Two-way analysis of variance for effects of the 2 SNP positions indicated a substantial effect for ∇−880Δ (p < 0.001), although no effect for C-399T. On either haplotypic background, the −880Δ allele increased gene expression significantly (p < 0.001): by approximately 16.2% on C-399 background and approximately 41.7% on −399T background, and the effects of the − 880A allele on NPY expression were directionally coordinate in transfected cells and in vivo (Online Fig. 6).
GRE at ∇−880Δ in the NPY promoter: characterization
NPY PROMOTER MOTIFS: COMPUTATIONAL DISRUPTION OF GRE MOTIF BY COMMON VARIANT ∇−880Δ
The ∇−880Δ (2-bp, TG/—, Ins/Del) variant lies in a region relatively conserved across species (especially in primates) with a partial match (8/12 bp) for a consensus GRE motif and the insertion displaying a superior match (Fig. 3A, left). The human ancestral allele is likely the ∇ (TG) version (i.e., the most frequent human allele), on the basis of the chimp sequence, although the Δ (—) version is recurrent in another primate, the orangutan.
PROMOTER ∇−880Δ: EXOGENOUS GLUCOCORTICOID AGONIST (DEXAMETHASONE) ON REPORTER ACTIVITY
Dexamethasone decreased transfected NPY promoter activity (p = 0.002) (Fig. 3A, right) but only in the presence of the ∇ allele at −880Δ: by 37% (p = 0.019) on ∇-C and 20% (p = 0.05) on ∇-T haplotypes. There was little or no effect of dexamethasone on haplotypes bearing the −880 Δ-allele (Δ-C or Δ-T haplotypes).
PROMOTER ∇−880Δ: EMSA AND ANTI-GLUCOCORTICOID RECEPTOR SUPER-SHIFT
During EMSA, the labeled −880 insertion (∇, TG Ins) variant was more effectively shifted by chromaffin cell nuclear proteins than the deletion (—, Δ) allele. One shifted band (see arrow in Fig. 3B) was completely displaced by the unlabeled oligonucleotides and approximately 90% diminished when an anti-glucocorticoid receptor (GR) antibody was added, suggesting specific recognition of the insertion allele by GR (Fig. 3B).
NPY GENETIC VARIATION IN SUBJECTS WITH THE MOST EXTREME BP VALUES IN THE POPULATION
Here we studied BP trait-extreme individuals of European ancestry, to enhance statistical power (23,24). As in the twin/sib pairs, we probed the 5 common polymorphisms (rs3037354, rs16147, rs16140, rs5573, and rs5574, MAF >20%) and 1 SNP in the signal peptide (rs16139 Leu7Pro, MAF = 2%), each in Hardy-Weinberg equilibrium (Online Table 11), to span the structural/functional domains across the gene (promoter, exons, introns) in this case/control study.
HAPLOTYPE ASSOCIATION WITH BP
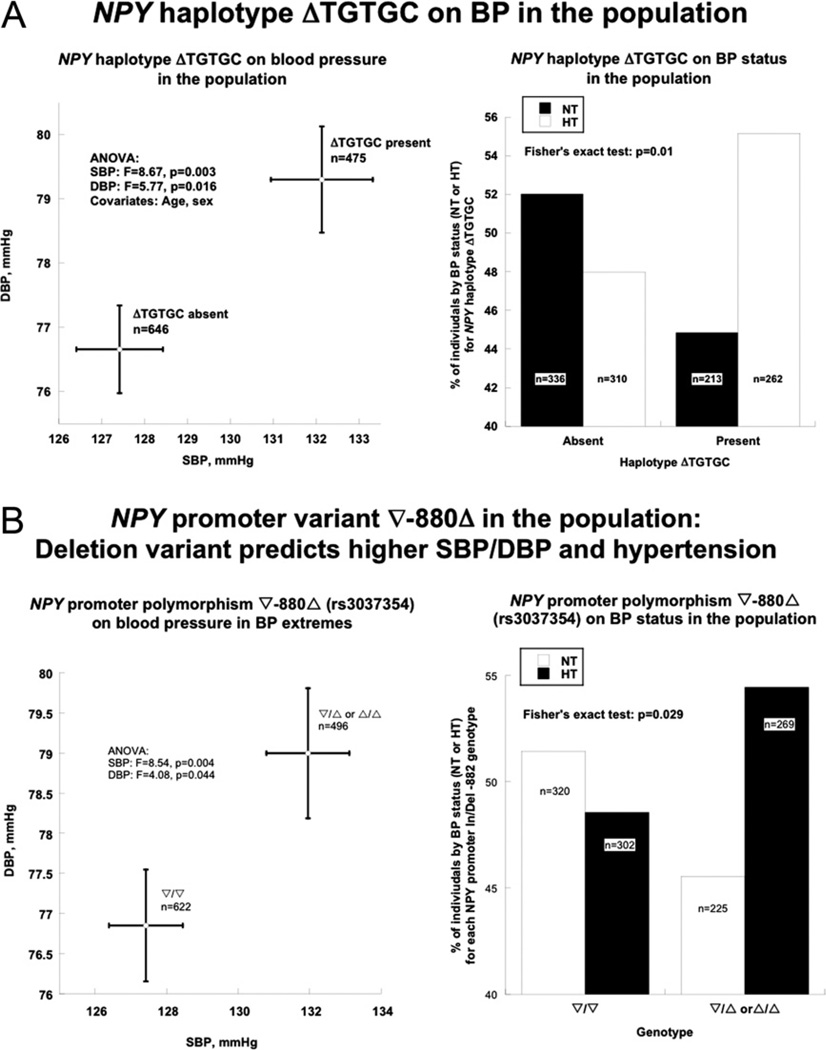
In the white BP-extreme population, NPY also displayed 3 common haplotypes (Online Table 12)—similarly as in twin/sib pairs— among which the second-most common, ΔTGTGC, was associated with the quantitative BP traits (SBP and DBP in mm Hg) as well as the dichotomous trait (higher and lower BP) (Fig. 4A). Occurrence of haplotype ΔTGTGC elevated SBP from 127.4 ± 1.0 mm Hg to 132.1 ± 1.2 mm Hg (increasing 3.7%, p = 0.003), whereas DBP increased from 76.7 ± 0.7 mm Hg to 79.3 ± 0.8 mm Hg (increasing 3.45%, p = 0.016) (Fig. 4A, left panel). Haplotype analysis on subjects dichotomized into 2 BP groups (higher vs. lower BP) indicated that individuals carrying common haplotype ΔTGTGC had a tendency to be hypertensive, with Fisher exact test p = 0.01 (Fig. 4A, right panel). The most common haplotype (∇CCTAT) did not display an effect on either the dichotomized or quantitative BP traits.
Figure 4. Association of NPY Genetic Variation With BP in the Population.
(A) NPY common haplotype ΔTGTGC with hypertension. Left: ΔTGTGC with systolic blood pressure (SBP) and DBP. Individuals who carry this haplotype display both higher SBP and DBP than those without it. Right: ΔTGTGC with BP status. Absence of this haplotype is associated with normal BP, whereas presence is associated with hypertension (p = 0.01). (B) NPY common promoter polymorphism ∇−880Δ with hypertension. Left: ∇−880Δ: effect on SBP and DBP. Subjects carrying the deletion variant display higher SBP and DBP than insertion homozygotes. Right: ∇−880Δ with BP status. The deletion version is associated with hypertension (p = 0.029). HT = hypertensives; NT = normotensives; other abbreviations as in Figures 2 and 3.
PROMOTER ∇−880Δ AND BP
Single SNP-based allele tests showed that promoter variant ∇−880Δ (TG/—, Ins/Del) yielded the maximal association with BP. In a Δ dominant/∇ recessive model, both SBP (p = 0.004) and DBP (p = 0.044) were increased in Δ carriers (ΔΔ or Δ/∇ genotype) (Fig. 4B, left panel), and such individuals also were at increased risk for hypertension, with Fisher exact test p = 0.029 (Fig. 4B, right panel).
Discussion
NPY and stress: overview
The NPY is a sympathetic co-transmitter co-stored with norepinephrine in large dense-core vesicles, which when co-released triggers vaso-constriction via its Y1 receptor (25). Compared with norepinephrine, NPY is preferentially released by intense or prolonged sympathetic activation and therefore is a marker of even more severe stress (26). Long-term elevations of NPY might exhibit both detrimental and beneficial consequences, such as resilience to and recovery from post-traumatic stress disorder or dampening the fear response, allowing individuals to perform better under extreme stress (27).
In this study, we began with genome-side linkage showing that variation at the NPY locus itself determines NPY secretion. Then systematic polymorphism determination at the gene, coupled to heritable trait phenotyping in twin pairs, suggested a role for promoter Ins/Del polymorphism ∇−880Δ in NPY secretion and autonomic traits, with replication in independent groups. Molecular biology documented that ∇−880Δ disrupted a GRE. Finally, we found that (∇−880Δ) associated with BP in the population.
Heritable control of NPY secretion by the NPY gene in twins, with replication
The NPY release is substantially under substantial genetic control (h2 = 73 ± 4%) (Table 1), and a cis-quantitative trait loci on chromosome 7p15 was identified with multipoint linkage analysis in twin and sib pairs (Fig. 1A). Subsequently, we found that common promoter variant −880Δ associated with elevated NPY secretion (Fig. 2B); this marker-on-trait association is consistent with findings of Zhou et al. (15) in which subjects with NPY haplotypes bearing allele −880Δ display higher plasma NPY, whereas haplotypes bearing allele ∇−880 associated with lower plasma NPY. Indeed, we were able to replicate the finding of NPY genetic determination on NPY secretion in vivo in 2 independent samples, with effects consistent in direction and magnitude, by meta-analysis (Table 2).
NPY gene and stress traits in twins
We found that the −880Δ allele is associated with not only NPY secretion and pressor response to environmental (cold) stress (which in longitudinal studies associated with later elevations in resting BP [28]) (Fig. 2B) but also basal hemodynamic status (systemic vascular resistance and CI) in a healthy twin sample (Fig. 2C), progressing to elevated basal SBP/DBP in population sample (Fig. 4B).
Glucocorticoids, NPY gene expression, and BP
Here we found that the insertion (∇) version of NPY promoter variant ∇−880Δ created a partial match (8/12 bp) for a GRE motif (Fig. 3A, left), and the ∇ element responded functionally to glucocorticoid (Fig. 3A, right) by binding the GR (Fig. 3B). Previous promoter studies indicated that the ∇−880Δ variant lies within a region containing a negative regulatory element, because elimination of the region between −1078/−796 bp resulted in approximately 2-fold increased transcription (29). We studied NPY transcription in PC12 (rat pheochromocytoma) cells and found that promoter activity decreased after glucocorticoid (Fig. 3A, right). This decline is consistent with previous findings that treatment with dexamethasone reduced NPY messenger ribonucleic acid accumulation in cultured human pheochromocytoma cells (30). Targeted ablation of the GR in the nervous system of transgenic mice results in a significant increase of NPY expression (31). Cortisol reduced and the glucocorticoid antagonist metyrapone increased NPY expression in fish brain (32). In an anatomically defined study, dexamethasone increased adrenal capsular NPY, with reversal by simultaneous administration of ACTH; in the medulla, however, dexamethasone decreased NPY content (33).
NPY and BP regulation
Exogenous NPY exerts either hypertensive (peripheral) or hypotensive (central) effects, depending on the site of its administration. When administered centrally, the peptide displays sympatholytic, hypotensive, and anxiolytic effects (34,35). In contrast, acute administration of NPY into the systemic circulation increases BP (36). In humans and animals, NPY secretion increases in response to cold exposure, tissue injury and ischemia, hemorrhagic shock, and treadmill exercise, particularly when combined with hypoxia (37). Elevated NPY secretion is found in a variety of conditions with sympathetic hyperactivity such as hypertension, congestive heart, and renal failure (26). Moreover, at even lower than vasoconstrictor concentration, NPY stimulates vascular smooth muscle cell proliferation (38).
Here we found that haplotypes across the NPY locus associated with not only pressor responses (Fig. 2A) but also basal SBP and DBP as well as hypertension status (Fig. 4A), and the peak effect occurred for promoter variant ∇−880Δ (Figs. 2B and 4B), at which the Δ allele was associated with higher BP.
Study strengths
We used the classical twin design in the search for trait-associated polymorphisms (39), beginning with genome-wide sib pair linkage (Fig. 1A). Multiple autonomic phenotypes were measured in the twins, permitting estimation of trait heritability as well as definition of effects of particular genetic variants at NPY on such heritable “intermediate phenotypes” (Table 1); dual documentation of heritability and association lends internal consistency to the approach. Rather than using HapMap “tagging” SNPs, we systematically scanned the NPY locus for common or unusual variants in functional gene regions (exons, untranslated regions, and promoter). This approach allowed us to associate variants in potentially active regions with physiological traits in vivo and then pursue the functional consequences of such variants in reporter assays in cella (Figs. 2 to 4). Finally, we were able to replicate our effects on NPY secretion in vivo with an additional 2 independent cohorts (Table 2).
Study limitations
Although we conducted systematic polymorphism determination in 4 diverse ethnic groups, our studies on autonomic physiology and disease were conducted only in subjects of European ancestry, because allelic association studies can be susceptible to artifactual conclusions resulting from even inapparent population admixture (40); studies of additional ethnic or population groups will be required to evaluate whether our NPY results are of more general importance in the overall population. False positive (type I) statistical errors might occur during genetic associations, because the effects of multiple genetic variants might be evaluated on traits. We guarded against such errors in several ways: we began with genome-wide linkage, and then proceeded to haplotype analysis, achieving genetic effects first on NPY secretion with genome-wide stringency (Fig. 1A) and then on population BP (Fig. 4A) without multiple testing. Moreover, we extended our effects to 3 independent subject groups; the same NPY promoter allele associated with higher basal population BP also associated with “intermediate phenotypes” for hypertension in independent groups: elevated NPY secretion, exaggerated BP response during stress, higher systemic vascular resistance and lower CI, each of which might be “intermediate” phenotypes for development of high BP in the population (Figs. 2A and 2B).
Conclusions and Perspectives
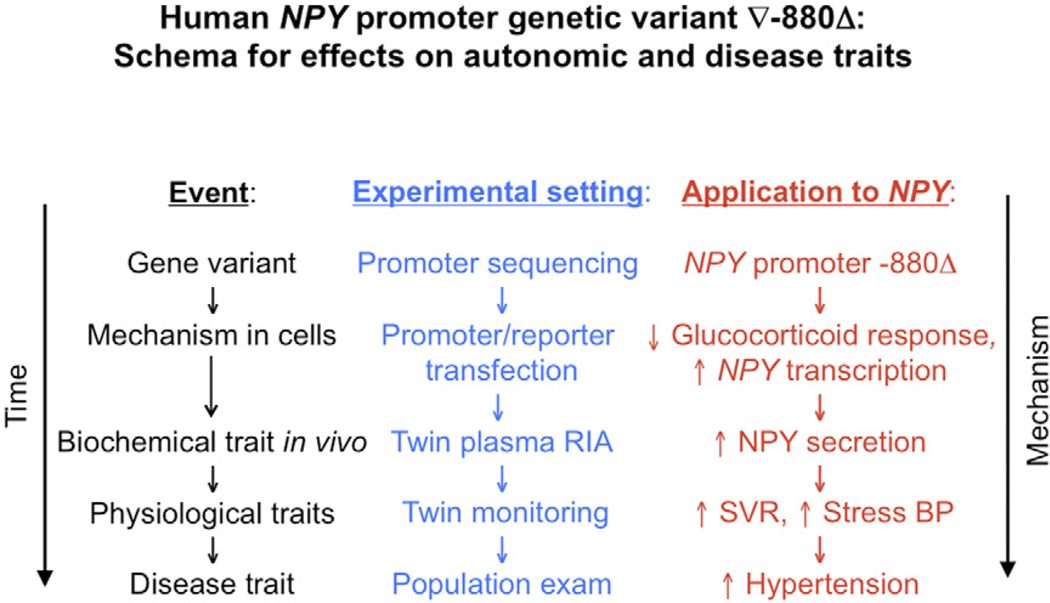
Common genetic variation at the NPY locus, particularly at promoter ∇−880Δ, alters glucocorticoid responses and so influences NPY expression as well as systemic vascular resistance, later the early heritable response to environmental stress, and ultimately resting BP in the population. Although causal inferences in this proposed chain of events are not yet established, we illustrate a proposed pathway (schematized in Fig. 5) that integrates our conceptual approach with application to the NPY system; this pathway yields testable predictions for future experimental verification. These results point to new molecular strategies for probing autonomic control of the circulation and ultimately the susceptibility to and pathogenesis of cardiovascular and neuropsychiatric disease states.
Figure 5. Schema.
NPY genetic variation: consequences for autonomic physiology and cardiovascular disease. The schematic presents a hypothetical framework integrating the overall rationale and experimental results, and suggests future questions for exploration. Abbreviations as in Figures 2 and 3.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health, Department of Veterans Affairs, and Alfonso Martin Escudero Foundation. Dr. Fung reports that she is currently employed by Amgen, and owns stock in the company, although this work was done prior to her employment, and is unassociated with this employment. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviations and Acronyms
- BP
blood pressure
- CI
cardiac index
- DBP
diastolic blood pressure
- EMSA
electrophoretic mobility shift assay
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- Ins/Del
insertion/deletion
- LD
linkage disequilibrium
- LOD
logarithm of the odds ratio
- MAF
minor allele frequency
- NPY
Neuropeptide Y
- QTL
quantitative trait locus
- SBP
systolic blood pressure
- sib
sibling
- SNP
single nucleotide polymorphism
- SOLAR
Sequential Oligogenic Linkage Analysis Routines
- SVRI
systemic vascular resistance index
- ∇/Δ
insertion/deletion (2-bp Ins/Del, TG/—, ∇−880Δ) polymorphism at human NPY promoter position −880 (rs3037354)
Footnotes
APPENDIX
For supplementary text, figures, and tables, please see the online version of this article.
REFERENCES
- 1.Takiyyuddin MA, Brown MR, Dinh TQ, et al. Sympatho-adrenal secretion in humans: factors governing catecholamine and storage vesicle peptide co-release. J Auton Pharmacol. 1994;14:187–200. doi: 10.1111/j.1474-8673.1994.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 2.Wheway J, Mackay CR, Newton RA, et al. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202:1527–1538. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Pedrazzini T, Brunner HR, Waeber B. Neuropeptide Y and cardiovascular regulation. Curr Opin Nephrol Hypertens. 1993;2:106–113. doi: 10.1097/00041552-199301000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- 6.Morris MJ, Cox HS, Lambert GW, et al. Region-specific neuropeptide Y overflows at rest and during sympathetic activation in humans. Hypertension. 1997;29:137–143. doi: 10.1161/01.hyp.29.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Rosmaninho-Salgado J, Alvaro AR, Grouzmann E, Duarte EP, Cavadas C. Neuropeptide Y regulates catecholamine release evoked by interleukin-1beta in mouse chromaffin cells. Peptides. 2007;28:310–314. doi: 10.1016/j.peptides.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Rosmaninho-Salgado J, Araujo IM, Alvaro AR, et al. Regulation of catecholamine release and tyrosine hydroxylase in human adrenal chromaffin cells by interleukin-1beta: role of neuropeptide Y and nitric oxide. J Neurochem. 2009;109:911–922. doi: 10.1111/j.1471-4159.2009.06023.x. [DOI] [PubMed] [Google Scholar]
- 9.Adrian TE, Allen JM, Bloom SR, et al. Neuropeptide Y distribution in human brain. Nature. 1983;306:584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- 10.Ruohonen ST, Savontaus E, Rinne P, et al. Stress-induced hypertension and increased sympathetic activity in mice overexpressing neuropeptide Y in noradrenergic neurons. Neuroendocrinology. 2009;89:351–360. doi: 10.1159/000188602. [DOI] [PubMed] [Google Scholar]
- 11.Schlaich MP, Lambert E, Kaye DM, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 12.Lambert E, Straznicky N, Schlaich M, et al. Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension. 2007;50:862–868. doi: 10.1161/HYPERTENSIONAHA.107.094649. [DOI] [PubMed] [Google Scholar]
- 13.Palatini P, Longo D, Zaetta V, Perkovic D, Garbelotto R, Pessina AC. Evolution of blood pressure and cholesterol in stage 1 hypertension: role of autonomic nervous system activity. J Hypertens. 2006;24:1375–1381. doi: 10.1097/01.hjh.0000234118.25401.1c. [DOI] [PubMed] [Google Scholar]
- 14.De Vogli R, Brunner E, Marmot MG. Unfairness and the social gradient of metabolic syndrome in the Whitehall II Study. J Psychosom Res. 2007;63:413–419. doi: 10.1016/j.jpsychores.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Zhu G, Hariri AR, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao F, Zhang L, Wessel J, et al. Tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis: discovery of common human genetic variants governing transcription, autonomic activity, and blood pressure in vivo. Circulation. 2007;116:993–1006. doi: 10.1161/CIRCULATIONAHA.106.682302. [DOI] [PubMed] [Google Scholar]
- 17.Waalen J, Felitti V, Gelbart T, Ho NJ, Beutler E. Prevalence of coronary heart disease associated with HFE mutations in adults attending a health appraisal center. Am J Med. 2002;113:472–479. doi: 10.1016/s0002-9343(02)01249-4. [DOI] [PubMed] [Google Scholar]
- 18.Do KA, Broom BM, Kuhnert P, et al. Genetic analysis of the age at menopause by using estimating equations and Bayesian random effects models. Stat Med. 2000;19:1217–1235. doi: 10.1002/(sici)1097-0258(20000515)19:9<1217::aid-sim421>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson D, Fan Y-A, Joe H. A remark on algorithm 643: FEXACT: an algorithm for performing Fisher’s exact test in RxC contingency tables. ACM Transactions on Mathematical Software. 1993;19:484–488. [Google Scholar]
- 20.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 21.Shah SH, Freedman NJ, Zhang L, et al. Neuropeptide Y gene polymorphisms confer risk of early-onset atherosclerosis. PLoS Genet. 2009;5:e1000318. doi: 10.1371/journal.pgen.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer WH, Lidstrom J, Sun H, et al. Human NPY promoter variation rs16147:T>C as a moderator of prefrontal NPY gene expression and negative affect. Hum Mutat. 2010;31:E1594–E1608. doi: 10.1002/humu.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schork NJ, Nath SK, Fallin D, Chakravarti A. Linkage disequilibrium analysis of biallelic DNA markers, human quantitative trait loci, and threshold-defined case and control subjects. Am J Hum Genet. 2000;67:1208–1218. doi: 10.1086/321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schork NJ. Power calculations for genetic association studies using estimated probability distributions. Am J Hum Genet. 2002;70:1480–1409. doi: 10.1086/340788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franco-Cereceda A, Liska J. Neuropeptide Y Y1 receptors in vascular pharmacology. Eur J Pharmacol. 1998;349:1–14. doi: 10.1016/s0014-2999(98)00242-8. [DOI] [PubMed] [Google Scholar]
- 26.Zukowska-Grojec Z. Neuropeptide Y. A novel sympathetic stress hormone and more. Ann N Y Acad Sci. 1995;771:219–233. doi: 10.1111/j.1749-6632.1995.tb44683.x. [DOI] [PubMed] [Google Scholar]
- 27.Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension. 1995;25:71–76. doi: 10.1161/01.hyp.25.1.71. [DOI] [PubMed] [Google Scholar]
- 29.Minth CA, Dixon JE. Regulation of the human neuropeptide Ygene. Ann N Y Acad Sci. 1990;611:99–110. doi: 10.1111/j.1749-6632.1990.tb48925.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Kahri AI, Heikkila P, Voutilainen R. Regulation of neuropeptide Y mRNA expression in cultured human pheochromocytoma cells. Eur J Endocrinol. 1999;141:431–435. doi: 10.1530/eje.0.1410431. [DOI] [PubMed] [Google Scholar]
- 31.Kellendonk C, Eiden S, Kretz O, et al. Inactivation of the GR in the nervous system affects energy accumulation. Endocrinology. 2002;143:2333–2340. doi: 10.1210/endo.143.6.8853. [DOI] [PubMed] [Google Scholar]
- 32.Doyon C, Leclair J, Trudeau VL, Moon TW. Corticotropin-releasing factor and neuropeptide Y mRNA levels are modified by glucocorticoids in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. 2006;146:126–135. doi: 10.1016/j.ygcen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Hinson JP, Renshaw D, Cruchley AT, Kapas S. Regulation of rat adrenal neuropeptide Y (NPY) content: effects of ACTH, dexameth-asone and hypophysectomy. Regul Pept. 1998;75–76:175–180. doi: 10.1016/s0167-0115(98)00065-2. [DOI] [PubMed] [Google Scholar]
- 34.Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol. 1991;260:R328–R334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura K, Tsuchihashi T, Abe I. Central cardiovascular action of neuropeptide Y in conscious rabbits. Hypertension. 2000;36:1040–1044. doi: 10.1161/01.hyp.36.6.1040. [DOI] [PubMed] [Google Scholar]
- 36.Zukowska-Grojec Z, Dayao EK, Karwatowska-Prokopczuk E, Hauser GJ, Doods HN. Stress-induced mesenteric vasoconstriction in rats is mediated by neuropeptide Y Y1 receptors. Am J Physiol. 1996;270:H796–H800. doi: 10.1152/ajpheart.1996.270.2.H796. [DOI] [PubMed] [Google Scholar]
- 37.Pernow J, Ohlen A, Hokfelt T, Nilsson O, Lundberg JM. Neuropeptide Y: presence in perivascular noradrenergic neurons and vasoconstrictor effects on skeletal muscle blood vessels in experimental animals and man. Regul Pept. 1987;19:313–324. doi: 10.1016/0167-0115(87)90173-x. [DOI] [PubMed] [Google Scholar]
- 38.Pons J, Kitlinska J, Ji H, Lee EW, Zukowska Z. Mitogenic actions of neuropeptide Y in vascular smooth muscle cells: synergetic interactions with the beta-adrenergic system. Can J Physiol Pharmacol. 2003;81:177–185. doi: 10.1139/y02-166. [DOI] [PubMed] [Google Scholar]
- 39.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 40.Knowler WC, Williams RC, Pettitt DJ, Steinberg AG. Gm3;5,13,14 and type 2 diabetes mellitus: an association in American Indians with genetic admixture. Am J Hum Genet. 1988;43:520–526. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.