Abstract
Background
The extent to which the prognosis for AIDS and death of patients initiating highly active antiretroviral therapy (HAART) continues to be affected by their characteristics at the time of initiation (baseline) is unclear.
Methods
We analyzed data on 20,379 treatment-naive HIV-1–infected adults who started HAART in 1 of 12 cohort studies in Europe and North America (61,798 person-years of follow-up, 1844 AIDS events, and 1005 deaths).
Results
Although baseline CD4 cell count became less prognostic with time, individuals with a baseline CD4 count <25 cells/µL had persistently higher progression rates than individuals with a baseline CD4 count >350 cells/µL (hazard ratio for AIDS = 2.3, 95% confidence interval [CI]: 1.0 to 2.3; mortality hazard ratio = 2.5, 95% CI: 1.2 to 5.5, 4 to 6 years after starting HAART). Rates of AIDS were persistently higher in individuals who had experienced an AIDS event before starting HAART. Individuals with presumed transmission by means of injection drug use experienced substantially higher rates of AIDS and death than other individuals throughout follow-up (AIDS hazard ratio = 1.6, 95% CI: 0.8 to 3.0; mortality hazard ratio = 3.5, 95% CI: 2.2 to 5.5, 4 to 6 years after starting HAART).
Conclusions
Compared with other patient groups, injection drug users and patients with advanced immunodeficiency at baseline experience substantially increased rates of AIDS and death up to 6 years after starting HAART.
Keywords: AIDS, CD4 counts, highly active antiretroviral therapy, HIV, prognosis, substance abuse (intravenous)
The prognosis of HIV-1–infected patients has been dramatically improved by highly active antiretroviral therapy (HAART).1–5 Such improvements have been observed in all patient groups, including patients who are severely immunosuppressed at the time that they start therapy. Nonetheless, patients’ characteristics at the time of initiation of HAART are strongly associated with subsequent rates of AIDS and death.6 For example, there is a strong inverse association between the CD4 cell count at the time of starting therapy and subsequent rates of AIDS and death. Patients who experience an AIDS-defining event before starting therapy and patients infected by means of injection drug use (IDU) also have higher subsequent morbidity and mortality than other patients.6 Recent analyses of the Swiss HIV Cohort Study suggest that HAART reduced rates of AIDS and death by approximately 60% in patients infected by means of IDU, although the reduction was less than in patients infected by means of other routes.5
It is important to identify whether these higher rates of clinical events and death persist as patients’ time on HAART increases. Little information is available because of the low rates at which these events occur and because HAART has been in widespread use for only 10 years, so that many patients have been treated for much shorter periods. We used the data of the Antiretroviral Therapy Cohort Collaboration (ART Cohort Collaboration) to examine the extent to which patient characteristics at initiation of HAART remain prognostic with increasing time since initiation of therapy.
METHODS
The ART Cohort Collaboration is an international collaboration between the investigators of 12 cohort studies of HIV-1–infected patients from Europe and North America. The collaboration has been described in detail elsewhere,6–8 and further information is available on the Internet (http://www.art-cohort-collaboration.org). Briefly, prospective cohort studies were eligible for participation if they had enrolled at least 100 HIV-1–infected patients aged ≥16 years who had not previously received antiretroviral treatment and who had started antiretroviral therapy with a combination of at least 3 drugs, including nucleoside reverse transcriptase inhibitors (NRTIs), protease inhibitors (PIs), and/or nonnucleoside reverse transcriptase inhibitors (NNRTIs). Patients with a baseline HIV-1 RNA level <1000 copies/mL at initiation of therapy were excluded because they might not have been treatment naive. The data set analyzed here was assembled during 2004 and included data from 12 cohorts: the AIDS Therapy Evaluation Project, Netherlands (ATHENA), the Agence Nationale de la Recherche sur SIDA et le hepatitis virale (ANRS) CO3 Aquitaine Cohort, the British Columbia Center for Excellence in HIV, Collaborations in HIV Outcomes Research (CHORUS), the EuroSIDA study, the Frankfurt HIV Cohort, the ANRS CO4 French Hospital Database on HIV (FHDH), the Italian Cohort of Antiretroviral-Naive Patients (ICONA), the Köln/Bonn Cohort, the Royal Free Hospital Cohort, the South Alberta Clinic Cohort, and the Swiss HIV Cohort Study (SHCS). All cohorts provided anonymized data on a predefined set of demographic, laboratory, and clinical variables, which were then pooled and analyzed centrally. Updated estimates of the probability of progression to AIDS/death and of mortality up to 5 years after starting HAART according to baseline patient characteristics, based on the data set analyzed here, were published recently.9
We considered progression to (1) an AIDS-defining disease (based on the clinical part of the 1993 US Centers for Disease Control and Prevention revision of the AIDS case definition) and (2) death from any cause. In all analyses, we used an “intent-to-continue-treatment” approach, and thus ignored changes to treatment regimen, including treatment interruptions and terminations. We measured time from the start of HAART to the date the endpoints occurred. In patients free of events, the same censoring strategy was used for all cohorts: for the endpoint AIDS, follow-up was censored on the date of the most recent follow-up visit, and for the endpoint mortality, follow-up was censored on the date the patient was last known to be alive.
Based on previous results, we considered 5 prognostic variables in the analyses presented here: presumed mode of transmission (by means of IDU or other), CD4 count (6 categories, from <25 to ≥350 cells/µL), plasma HIV-1 RNA level (<100,000 or ≥100,000 copies/mL), clinical AIDS before initiation (yes or no), and age (16 to 29, 30 to 39, 40 to 49, or ≥50 years). We used Weibull proportional hazards models stratified by cohort to estimate hazard ratios for the association of these variables with each endpoint. Schoenfeld residuals were used to test the proportional hazards assumption (test for trend in the hazard ratio with time). Changes in the effect of baseline covariates with time were examined by splitting follow-up time into 4 intervals: <1 year, 1 to 1.99 years, 2 to 3.99 years, and 4 to 6 years. Analyses were censored at 6 years after starting HAART.
When there was evidence of nonproportional hazards for a covariate (eg, presumed mode of transmission by means of IDU, AIDS diagnosis before starting therapy, baseline CD4 cell count), we estimated the rate of each endpoint over time using kernel density smoothing of the Nelson-Aalen cumulative hazard function, which was differentiated to give the estimated hazard function.10 We also derived bias-corrected bootstrap confidence intervals (CIs)11 for the estimated hazard functions, using 2000 bootstrap replications.
RESULTS
Detailed characteristics of the 20,379 patients included in the combined data set have been published elsewhere.9 The median month of initiation of therapy was February 1999 (interquartile range [IQR]: November 1997 to September 2000). Median age at initiation was 36 (IQR: 31 to 43) years, 4737 patients (23%) had a diagnosis of AIDS before initiating HAART, and 3231 (16%) had a presumed mode of transmission by means of IDU. The median CD4 count at initiation was 224 (IQR: 91 to 368) cells/µL. Most patients (17,976 [88%]) started on a HAART regimen containing 3 drugs: 1376 (7%) of initial regimens included NRTIs only, 13,378 (66%) included NRTIs plus a PI, and 4891 (24%) included NRTIs plus an NNRTI; 390 (2%) patients were on a 3-class regimen. A further 344 (2%) of patients were on other combinations: PIs and NNRTIs with no NRTIs (n = 10), with the fusion inhibitor T20 (n = 212) or with tenofovir (n = 122).
During 61,798 person-years of follow-up, 1844 patients developed at least 1 AIDS event and 1005 patients died. Of 656 patients who died with no new AIDS diagnosis, 307 had been diagnosed with AIDS before starting HAART. The median duration of follow-up was 3.0 (IQR: 1.5 to 4.5) years. During the first year of follow-up, there were 1219 AIDS events (incidence rate per year = 0.075, 95% CI: 0.071 to 0.079) and 386 deaths (incidence rate per year = 0.020, 95% CI: 0.019 to 0.023) over 18,847 person-years. Corresponding figures during 1 to 1.99 years after starting HAART were 263 AIDS events (incidence rate per year = 0.021, 95% CI: 0.018 to 0.023) and 223 deaths (incidence rate per year = 0.015, 95% CI: 0.013 to 0.017) over 15,175 person-years; during 2 to 3.99 years there were 274 AIDS events (incidence rate per year = 0.017, 95% CI: 0.015 to 0.019) and 288 deaths (incidence rate per year = 0.014, 95% CI: 0.013 to 0.016) over 20,342 person-years; and during 4 to 6 years there were 74 AIDS events (incidence rate per year = 0.013, 95% CI: 0.010 to 0.017) and 105 deaths (incidence rate per year = 0.015, 95% CI: 0.012 to 0.018) over 7208 person-years.
A total of 6838 individuals (23%) were still being followed at the end of the fourth year after they started HAART, whereas only 791 individuals (4%) were followed for more than 6 years. Most patients who did not experience an AIDS event or die were still being followed at the time that data were combined for analyses: a total of 3600 patients (18%) had had no clinic visit or event for 1 year and were considered lost to follow-up.
Patients with presumed transmission by means of IDU or with an AIDS diagnosis before starting therapy experienced substantially higher progression rates than patients without these characteristics. Consistent with previous analyses,6 the most strongly prognostic variable in multivariable analyses was the baseline CD4 cell count. In contrast with earlier results,6 baseline HIV-1 RNA level was weakly associated with progression rates after allowing for other variables (hazard ratios comparing baseline HIV-1 RNA level ≥100,000 copies/mL with baseline level <100,000 copies/mL were 1.33 [95% CI: 1.20 to 1.47] for AIDS and 1.11 [95% CI: 0.96 to 1.27] for death). Patients’ gender did not seem prognostic (hazard ratios comparing women with men = 1.00 [95% CI: 0.89 to 1.12] for AIDS and 0.93 [95% CI: 0.79 to 1.09] for death) and was not considered further.
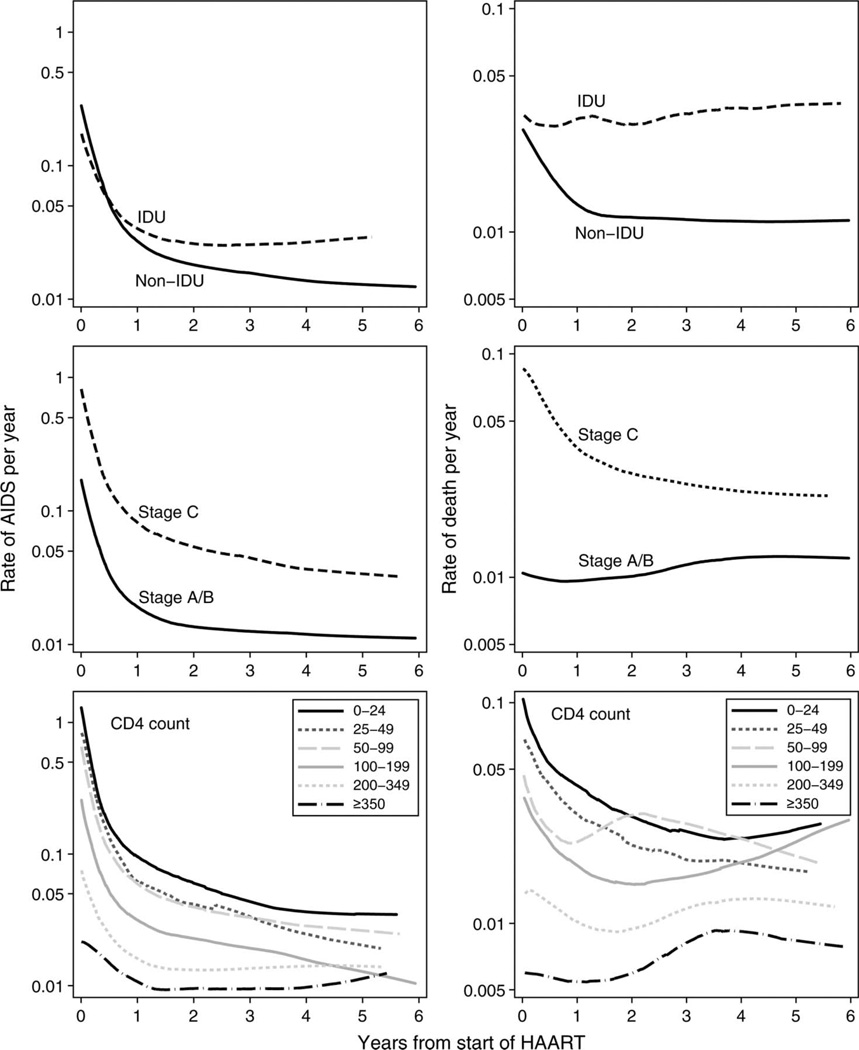
Figure 1 shows rates of AIDS and death over time (estimated by smoothing of the cumulative hazard function) separately for patients whose presumed mode of transmission was and was not by means of IDU, patients with and without an AIDS diagnosis before starting therapy, and categories of baseline CD4 cell count. Note that these rates are not adjusted for other prognostic variables and that the rate at each time is based on patients who have survived to that time (eg, the rate of AIDS at 1 year is calculated among individuals who did not experience an AIDS event during their first year on HAART). Graphs that include bootstrapped 95% CIs are available on request from the authors. There were sharp declines in the rate of AIDS during the first year on HAART. Mortality rates also declined during the first year on HAART, although these declines were less marked in patients whose presumed mode of transmission was by means of IDU, who were free of AIDS at initiation of HAART, or whose baseline CD4 count was >200 cells/µL. The differences between progression rates comparing individuals with presumed transmission by means of IDU with other individuals widened with increasing length of follow-up. Individuals who were free of AIDS at initiation of therapy or whose baseline CD4 counts were >350 cells/µL had the lowest progression rates throughout follow-up, whereas individuals whose baseline CD4 counts were <25 cells/µL experienced the highest rates of AIDS throughout follow-up.
FIGURE 1.
Estimated rates of progression to AIDS (left panels) or death (right panels) from baseline to 6 years after starting HAART, according to baseline CD4 cell count, clinical stage and presumed transmission group. Rates were estimated by smoothing and then differentiating the cumulative hazard function.
Table 1 shows adjusted hazard ratios for AIDS for the 4 follow-up time periods. Rates of AIDS in individuals with presumed transmission by means of IDU were similar to those in other individuals during the first year after HAART but were approximately double those of other individuals from 1 to 6 years after starting HAART (P = 0.00003 in test for proportional hazards based on Schoenfeld residuals). There was weak evidence (P = 0.04 from test for proportional hazards) that hazard ratios for the association of age with rates of AIDS changed with time. During the first year on HAART, rates of AIDS were slightly lower in the youngest age group, but there was little evidence of any association of age with rates of AIDS from 1 year onward. Rates of AIDS were at least twice as high throughout follow-up in individuals who had experienced an AIDS event before initiation of therapy compared with individuals who were free of AIDS at initiation, with little evidence that this changed over time. There was clear evidence that hazard ratios for CD4 cell count changed over time (P < 0.001 from test for proportional hazards). Individuals who started therapy with a CD4 count <25 cells/µL had persistently higher rates of AIDS throughout follow-up, although CD4 cell count hazard ratios declined with increasing time on HAART. The graded association between baseline CD4 cell count and rates of AIDS was apparent until 4 years after initiation of HAART. Beyond 1 year after starting HAART, there was little evidence of an association of baseline HIV RNA level with rates of AIDS.
TABLE 1.
Hazard Ratios for Progression to AIDS for Different Time Periods Measured From Initiation of HAART (Mutually Adjusted for Presumed Transmission by Means of IDU, Age at Initiation of Therapy, AIDS Before Initiation of Therapy, CD4 Cell Count at the Time of Initiation, and HIV-1 RNA Level at the Time of Initiation and Stratified on Cohort)
| Patient Characteristic at Time of initiation of HAART |
Time Since Initiation of Therapy | |||
|---|---|---|---|---|
| <1 y | 1 to 1.99 y | 2 to 3.99 y | 4 to 6 y | |
| Presumed transmission by means of IDU* | 1.1 (0.9 to 1.2) | 1.9 (1.4 to 2.5) | 2.0 (1.5 to 2.7) | 1.6 (0.8 to 3.0) |
| Age (y) | ||||
| 16 to 29 (reference group) | 1 | 1 | 1 | 1 |
| 30 to 39 | 1.3 (1.1 to 1.5) | 1.2 (0.8 to 1.7) | 0.8 (0.6 to 1.2) | 0.7 (0.4 to 1.3) |
| 40 to 49 | 1.4 (1.1 to 1.7) | 1.0 (0.7 to 1.6) | 0.7 (0.5 to 1.1) | 0.8 (0.4 to 1.6) |
| ≥50 | 1.4 (1.1 to 1.7) | 1.3 (0.8 to 2.1) | 0.9 (0.6 to 1.5) | 0.7 (0.3 to 1.8) |
| AIDS before initiation* | 2.4 (2.1 to 2.7) | 3.4 (2.5 to 4.6) | 3.8 (2.8 to 5.3) | 2.3 (1.2 to 4.4) |
| CD4 count (cells/µL) | ||||
| <25 | 10.0 (7.8 to 13.0) | 3.7 (2.2 to 6.1) | 2.4 (1.5 to 3.8) | 2.3 (1.0 to 5.2) |
| 25 to 49 | 7.6 (5.8 to 10.0) | 4.3 (2.6 to 7.2) | 2.0 (1.1 to 3.5) | 1.2 (0.4 to 3.5) |
| 50 to 99 | 6.5 (5.0 to 8.5) | 3.6 (2.2 to 5.8) | 2.9 (1.9 to 4.5) | 1.2 (0.5 to 3.0) |
| 100 to 199 | 3.4 (2.6 to 4.3) | 2.5 (1.6 to 4.0) | 2.0 (1.3 to 2.9) | 0.7 (0.3 to 1.6) |
| 200 to 349 | 1.6 (1.3 to 2.2) | 1.5 (1.0 to 2.3) | 1.4 (1.0 to 2.1) | 1.0 (0.6 to 2.0) |
| >350 (reference group) | 1 | 1 | 1 | 1 |
| HIV-1 RNA <100,000 copies/mL* | 0.7 (0.6 to 0.8) | 0.8 (0.6 to 1.1) | 1.0 (0.8 to 1.3) | 0.8 (0.5 to 1.3) |
Compared with patients without this characteristic.
Mortality hazard ratios are displayed in Table 2. Hazard ratios for individuals with presumed transmission by means of IDU, compared with other individuals, tended to increase with time (P = 0.01 from test for proportional hazards) and were 3.5 (95% CI: 2.2 to 5.5) 4 to 6 years after initiation of therapy. There was a clear graded association of age group with mortality, with little evidence (P = 0.26 from test for proportional hazards) that mortality hazard ratios for age changed with time. Mortality hazard ratios comparing individuals who did and did not experience an AIDS event before initiation of therapy declined with time (P < 0.0001 from test for proportional hazards), from 3.8 (95% CI: 3.0 to 4.8) in the first year after starting HAART to 1.2 (95% CI: 0.7 to 1.9) 4 to 6 years after starting HAART. Mortality hazard ratios for CD4 cell count diminished with time (P = 0.03 from test for proportional hazards), but mortality rates in individuals with a baseline CD4 count <25 cells/µL remained elevated, compared with individuals with a baseline CD4 count >350 cells/µL, throughout follow-up. Surprisingly, the highest mortality hazard in the period from 4 to 6 years after starting HAART was in individuals with a baseline CD4 count from 100 to 199 cells/µL. In this last period, however, CIs for each CD4 cell count group were wide, and we think that chance is the most likely explanation for this finding. Individuals with a baseline CD4 count >350 cells/µL experienced lower mortality rates than other individuals throughout follow-up, although given the width of CIs, it was unclear whether this group had a persistent advantage compared with individuals whose baseline CD4 count was 200 to 349 cells/µL. There was little evidence of an association of baseline levels of HIV-1 RNA with mortality.
TABLE 2.
Mortality Hazard Ratios for Different Time Periods Measured From Initiation of HAART (Mutually Adjusted for Presumed Transmission by Means of IDU, Age at Initiation of Therapy, AIDS Before Initiation of Therapy, CD4 Cell Count at the Time of Initiation, and HIV-1 RNA Level at the Time of Initiation and Stratified on Cohort)
| Patient Characteristic at Time of Initiation of HAART |
Time Since Initiation of Therapy | |||
|---|---|---|---|---|
| <1 y | 1 to 1.99 y | 2 to 3.99 y | 4 to 6 y | |
| Presumed transmission by means of IDU* | 1.9 (1.5 to 2.4) | 3.6 (2.7 to 4.9) | 2.7 (2.1 to 3.5) | 3.5 (2.2 to 5.5) |
| Age (y) | ||||
| 16 to 29 | 1 | 1 | 1 | 1 |
| 30 to 39 | 1.1 (0.8 to 1.5) | 1.1 (0.7 to 1.8) | 1.5 (1.0 to 2.3) | 1.7 (0.8 to 3.7) |
| 40 to 49 | 1.4 (1.0 to 2.1) | 1.6 (1.0 to 2.6) | 1.8 (1.2 to 2.9) | 2.2 (1.0 to 5.0) |
| ≥50 | 2.8 (1.9 to 4.0) | 2.6 (1.5 to 4.4) | 3.4 (2.1 to 5.4) | 4.9 (2.2 to 11.1) |
| AIDS before initiation* | 3.8 (3.0 to 4.8) | 1.9 (1.4 to 2.6) | 1.8 (1.4 to 2.4) | 1.2 (0.7 to 1.9) |
| CD4 count (cells/µL) | ||||
| <25 | 3.8 (2.5 to 6.0) | 4.9 (2.9 to 8.5) | 1.7 (1.1 to 2.7) | 2.5 (1.2 to 5.5) |
| 25 to 49 | 3.2 (2.0 to 5.2) | 3.4 (1.8 to 6.2) | 1.8 (1.1 to 3.0) | 1.4 (0.5 to 3.9) |
| 50 to 99 | 2.1 (1.3 to 3.4) | 3.6 (2.1 to 6.2) | 2.2 (1.4 to 3.2) | 2.1 (1.0 to 4.4) |
| 100 to 199 | 2.8 (1.8 to 4.2) | 2.2 (1.3 to 3.8) | 1.3 (0.9 to 1.9) | 2.6 (1.4 to 4.7) |
| 200 to 349 | 1.8 (1.2 to 2.8) | 1.6 (0.9 to 2.7) | 1.2 (0.8 to 1.7) | 1.5 (0.8 to 2.7) |
| ≥350 (reference group) | 1 | 1 | 1 | 1 |
| HIV-1 RNA <100,000 copies/mL* | 1.0 (0.8 to 1.2) | 0.8 (0.6 to 1.1) | 0.8 (0.6 to 1.1) | 1.0 (0.7 to 1.6) |
Compared with patients without this characteristic.
DISCUSSION
Based on a collaborative analysis of data from 12 cohort studies in Europe and North America, we found that the presumed mode of transmission of HIV, a diagnosis of AIDS before starting therapy, and the CD4 cell count before starting therapy remain prognostic for AIDS and death for a number of years after starting HAART.
Patients with presumed transmission by means of IDU form a diverse group, in whom some of the beneficial effects of HAART may be offset by competing causes of death that are not related to immunodeficiency, including overdose, liver-related death, suicide, and trauma.12–14 In contrast to the other transmission groups, mortality rates did not decline in these patients. Their mortality rate was nevertheless lower than what would have been expected without HAART, because in the absence of treatment, progression rates increase with time.15 Hepatitis coinfection is much more likely in patients with presumed transmission by means of IDU, in whom liver-related death is much more common.16 Poorer adherence to HAART may also have contributed to the higher mortality rate observed in patients with presumed transmission by means of IDU. For example, in British Columbia, injection drug users had markedly lower rates of plasma HIV-1 RNA suppression and, among patients who achieved suppression, injection drug users had higher rates of subsequent virologic rebound. This was explained by lower levels of adherence, as measured by the ratio of dispensed to required medication during follow-up.17 A French survey of causes of death in the HAART era found that, overall, 47% of deaths were related to AIDS, whereas among injection drug users, only 16% of deaths were related to AIDS.18 Similarly, a study of heroin-related deaths in Italy showed that the percentage of deaths attributable to AIDS rose from 3% in 1985 to 42% in 1996, only to decrease to 17% in 1998.19
Patients initiating HAART experience substantial increases in CD4 cell counts regardless of the CD4 cell count at initiation, with slightly greater average increases in patients with low CD4 cell counts at initiation.20–22 In the Swiss HIV Cohort Study21,23 and a Spanish cohort,24 the likelihood that patients achieved a CD4 count >500 cells/µL up to 5 years after starting HAART was inversely associated with baseline CD4 cell count, and in gay men enrolled in the Multicenter AIDS Cohort Study, the CD4 cell count increased during the first 2 years of therapy but reached a plateau thereafter.25 Poorer outcomes in patients starting HAART at low CD4 cell counts, compared with those starting at higher CD4 cell counts, are therefore likely to be explained by the lower CD4 cell counts experienced by these patients after initiation of HAART. Furthermore, data from EuroSIDA indicate that a low CD4 cell count nadir may be associated with higher rates of clinical progression, even after recovery of CD4 cell counts after initiation of HAART.26 Future updates of the ART Cohort Collaboration data should help to clarify whether the disadvantage associated with starting HAART at low CD4 cell counts may eventually disappear.
Our results may have been affected by confounding. Factors that predispose individuals to present late in the course of their HIV infection (eg, low socioeconomic status, predisposition to poorer adherence, comorbidity) may explain part of the higher risk of progression observed in these patients. Patients’ race/ethnic group was not sufficiently well recorded to allow consideration of this factor in analyses. The fact that hazard ratios comparing CD4 cell count groups at the end of follow-up are larger for mortality than for AIDS is compatible with the existence of such confounding. Conversely, differences in prognosis between CD4 cell count groups might be underestimated if those starting HAART with high CD4 cell counts did so because they were at higher risk of progression.
Only 23% of patients were still being followed at the end of the fourth year after they started HAART. Because all cohorts continued to recruit patients during the study period, it was inevitable that a substantial proportion of patients were followed up for shorter periods. Most patients were still being followed up at the time that data were combined for analyses. Therefore, we do not believe that informative censoring is likely to be an important source of bias.
Our findings should be generalizable to patients starting antiretroviral therapy in developed countries. Our database included patients from many countries in Europe and from North America, who were treated in different settings. The range of patients was broad; men and women as well as teenagers and elderly people were included, and the major exposure categories were well represented. The large number of patients and events allowed us to compare progression rates among patient groups defined by levels of several prognostic factors in different time periods after starting HAART. Hazard ratios for some comparisons of interest were imprecisely estimated, however. For example, there is great interest in comparing progression among patients who start HAART with CD4 cell counts of 200 to 349 cells/µL with progression among patients with CD4 cell counts of 350 to 500 cells/µL, although such comparisons are problematic because of the need to take “lead times” (the time spent off therapy but free of AIDS by individuals who delay initiation) into account.27,28 Rates of AIDS and death in these groups were low (mortality rate of 1 per 100 person-years or less), and the number of patients being followed declined with increasing time on HAART. In consequence, there were too few events in the later follow-up periods to allow hazard ratios comparing these 2 groups to be precisely estimated.
In previous analyses6,7 of data from the ART Cohort Collaboration, we used a combined endpoint of AIDS or death, whereas in the analyses presented here, we separated these 2 outcomes and found striking differences between AIDS and mortality hazard ratios as well as differences in the patterns of change in these hazard ratios over time. A potential disadvantage of analyses using an endpoint of AIDS alone is that death occurring in patients who are severely immunosuppressed is likely to be associated with HIV and to have similar etiologies to AIDS events, even if no AIDS-defining event was recorded before death. The focus of the analyses reported here is on comparisons of rates of clinical events and death in different patient groups in different time periods rather than on the cumulative probabilities of these events, however. Because the current data set does not contain information on immunodeficiency at the time of death, or on causes of death, we cannot investigate this issue further. In future updates of the ART Cohort Collaboration data set, we plan to collect additional data, which should allow the separation of deaths that do and do not seem to be related to immunodeficiency.
In conclusion, we found that rates of AIDS and death were persistently higher in patients infected by means of IDU and that although the prognostic value of baseline CD4 cell count and a prior AIDS diagnosis declined with time, patients who were severely immunodeficient when they started therapy experienced higher rates of AIDS and death up to 6 years later. Our results may strengthen the case for screening for HIV,29,30 because delaying treatment until patients are severely immunosuppressed has long-term disadvantages. The strikingly poorer outcomes seen in the patients who are most immunosuppressed at the time of starting HAART also have worrying implications for the rollout of antiretroviral therapy in developing countries, particularly in sub-Saharan Africa, where severe resource limitations may mean that treatment is restricted to patients with clear clinical evidence of immunosuppression, with the consequence that the potential benefits of therapy are not fully realized.31
ACKNOWLEDGMENTS
The authors are grateful to all patients, doctors, and study nurses who were involved in the participating cohort studies. They thank Paula Braitstein and Francois Dabis for helpful comments on a previous version of the manuscript.
The Antiretroviral Therapy Cohort Collaboration is supported by UK Medical Research Council grant number G0100221. Sources of funding of individual cohorts include the Agence Nationale de la Recherche sur SIDA et le hepatitis virale CO3 and CO4; the Institut National de la Santé et de la Recherche Médicale; the French, Italian, and Swiss Ministries of Health; the Stichting HIV Monitoring; the European Commission; the British Columbia and Alberta Governments; the Michael Smith Foundation for Health Research; the Canadian Institutes of Health Research; and unrestricted grants from GlaxoSmithKline, Roche, and Boehringer-Ingelheim.
APPENDIX
ART Cohort Collaboration
Writing Committee
Jonathan A. C. Sterne, Margaret May, Caroline Sabin, Andrew Phillips, Dominique Costagliola, Geneviève Chêne, Amy C. Justice, Frank de Wolf, Robert Hogg, Manuel Battegay, Antonella D’Arminio Monforte, Gerd Fätkenheuer, Schlomo Staszewski, John Gill, Matthias Egger
Steering Committee
Jordi Casabona, Geneviève Chêne, Dominique Costagliola, Francxois Dabis, Antonella D’Arminio Monforte, Frank de Wolf, Matthias Egger, John Gill, Robert Hogg, Amy Justice, Mari Kitahata, Bruno Ledergerber, Catherine Leport, Jens Lundgren, Margaret May, Andrew Phillips, Peter Reiss, Michael Saag, Caroline Sabin, Gerd Fätkenheuer, Schlomo Staszewski, Jonathan Sterne, Ian Weller
Data Managers
Margaret May, Brenda Beckthold, Benita Yip, Brenda Dauer, Jenifer Fusco, Emilie Lanoy, Martin Rickenbach, Valerie Lavignolle, Ard van Sighem, Edwige Pereira, Patrizio Pezzotti, Andrew Phillips, Caroline Sabin, Norbert Schmeisser
Members of the 12 Study Groups
FHDH ANRS CO4 (61 sites)
Scientific Committee: Dr. E. Billaud, Pr. F. Boué, D. Costagliola, Dr. X. Duval, Dr. C. Duvivier, Dr. P. Enel, Dr. S. Fournier, Dr. J. Gasnault, Dr. C. Gaud, Dr. J. Gilquin, Dr. S. Grabar, Dr. M. A. Khuong, Pr. J. M. Lang, M. Mary-Krause, Pr. S. Matheron, Pr. M. C. Meyohas, Pr. G. Pialoux, Dr. I. Poizot-Martin, Dr. C. Pradier, Pr. E. Rouveix, Pr. D. Salmon-Ceron, Pr. A. Sobel, Dr. P. Tattevin, Dr. H. Tissot-Dupont, Dr. Y. Yasdanpanah. DMI2 Coordinating Center, French Ministry of Health: Dr. E. Aronica, Dr. V. Tirard-Fleury, I. Tortay. Statistical Analysis Center, Institut National de la Santé et de la Recherche Médicale (INSERM) EMI 0214: Dr. S. Abgrall, D. Costagliola, Dr. S. Grabar, M. Guiguet, E. Lanoy, H. Leneman, L. Lièvre, M. Mary-Krause, V. Potard, Dr. S. Saidi. CISIH Paris area: CISIH de Bichat-Claude Bernard (Hôpital Bichat-Claude Bernard: Pr. S. Matheron, Pr. J. L. Vildé, Pr. C. Leport, Pr. P. Yeni, Pr. E. Bouvet, C. Gaudebout, Pr. B. Crickx, Dr. C. Picard-Dahan), CISIH de Paris-Center Ouest (Hôpital Européen Georges Pompidou: Pr. L. Weiss, D. Tisne-Dessus; GH Tarnier-Cochin: Pr. D. Sicard, Pr. D. Salmon; Hôpital Saint-Joseph: Dr. J. Gilquin, Dr. I. Auperin; Hôpital Necker adultes: Dr. J. P. Viard, Dr. L. Roudière), CISIH de Paris-Sud (Hôpital Antoine Béclère: Pr. F. Boué, Dr. R. Fior; Hôpital de Bicêtre: Pr. J. F. Delfraissy, Dr. C. Goujard; Hôpital Henri Mondor: Dr. Ph. Lesprit, C. Jung; Hôpital Paul Brousse), CISIH de Paris-Est (Hôpital Saint-Antoine: Pr. M. C. Meyohas, Dr. J. L. Meynard, Dr. O. Picard, N. Desplanque; Hôpital Tenon: Pr. J. Cadranel, Pr. C. Mayaud, Pr. G. Pialoux, Pr. W. Rozenbaum), CISIH de Pitié-Salpétrière (GH Pitié-Salpétrière: Pr. F. Bricaire, Pr. C. Katlama, Pr. S. Herson, Dr. A. Simon), CISIH de Saint-Louis (Hôpital Saint-Louis: Pr. J. M. Decazes, Pr. J. M. Molina, Pr. J. P. Clauvel, Dr. L. Gerard; GH Lariboisière-FernandWidal: Dr. P. Sellier, Dr. M. Diemer), CISIH 92 (Hôpital Ambroise Paré: Dr. C. Dupont, H. Berthé, Pr. P. Saïag; Hôpital Louis Mourier: Dr. E. Mortier, C. Chandemerle; Hôpital Raymond Poincaré: Dr. P. de Truchis), CISIH 93 (Hôpital Avicenne: Dr. M. Bentata, P. Honoré; Hôpital Jean Verdier: S. Tassi, Dr. V. Jeantils; Hôpital Delafontaine: Dr. D. Mechali, B. Taverne). CISIH outside Paris area: CISIH Auvergne-Loire (CHU de Clermont-Ferrand: Dr. H. Laurichesse, Dr. F. Gourdon; CHRU de Saint-Etienne: Pr. F. Lucht, Dr. A. Fresard); CISIH de Bourgogne-Franche Comté (CHRU de Besançon; CHRU de Dijon; CH de Belfort: Dr. J.P. Faller, P. Eglinger; CHRU de Reims); CISIH de Caen (CHRU de Caen: Pr. C. Bazin, Dr. R. Verdon), CISIH de Grenoble (CHU de Grenoble), CISIH de Lyon (Hôpital de la Croix-Rousse: Pr. D. Peyramond, Dr. A. Boibieux; Hôpital Edouard Herriot: Pr. J. L. Touraine, Dr. J. M. Livrozet; Hôtel-Dieu: Pr. C. Trepo, Dr. L. Cotte), CISIH de Marseille (Hôpital de la Conception: Dr. I. Ravaux, Dr. H. Tissot-Dupont; Hôpital Houphouët-Boigny: Pr. J. P. Delmont, Dr. J. Moreau; Institut Paoli Calmettes: Pr. J. A. Gastaut; Hôpital Sainte-Marguerite: Dr. I. Poizot-Martin, Pr. J. Soubeyrand, Dr. F. Retornaz; CHG d’Aix-En-Provence: Dr. P. A. Blanc, Dr. T. Allegre; Center pénitentiaire des Baumettes: Dr. A. Galinier, Dr. J. M. Ruiz; CH d’Arles; CH d’Avignon: Dr. G. Lepeu; CH de Digne Les Bains: Dr. P. Granet-Brunello; CH de Gap: Dr. L. Pelissier, Dr. J. P. Esterni; CH de Martigues: Dr. M. Nezri, Dr. R. Cohen-Valensi; CHI de Toulon: Dr. A. Laffeuillade, Dr. S. Chadapaud), CISIH de Montpellier (CHU de Montpellier: Pr. J. Reynes; CHG de Nîmes), CISIH de Nancy (Hôpital de Brabois: Pr. T. May, Dr. C. Rabaud), CISIH de Nantes (CHRU de Nantes: Pr. F. Raffi, Dr. E. Billaud), CISIH de Nice (Hôpital Archet 1: Dr. C. Pradier, Dr. P. Pugliese; CHG Antibes Juan les Pins), CISIH de Rennes (CHU de Rennes: Pr. C. Michelet, Dr. C. Arvieux), CISIH de Rouen (CHRU de Rouen: Pr. F. Caron, Dr. F. Borsa-Lebas), CISIH de Strasbourg (CHRU de Strasbourg: Pr. J. M. Lang, Dr. D. Rey, Dr. P. Fraisse; CH de Mulhouse), CISIH de Toulouse (CHU Purpan: Pr. P. Massip, Dr. L. Cuzin, Pr. E. Arlet-Suau, Dr. M. F. Thiercelin Legrand; Hôpital la Grave; CHU Rangueil), CISIH de Tourcoing-Lille (CH Gustave Dron; CH de Tourcoing: Dr. Y. Yasdanpanah), CISIH de Tours (CHRU de Tours; CHU Trousseau). CISIH overseas: CISIH de Guadeloupe (CHRU de ointe-à-Pitre), CISIH de Guyane (CHG de Cayenne: Dr. M. Sobesky, Dr. R. Pradinaud), CISIH de Martinique (CHRU de Fort-de-France), CISIH de La Réunion (CHD Félix Guyon: Dr. C. Gaud, Dr. M. Contant)
ICONA (47 sites)
Ancona: M. Montroni, G. Scalise, M. C. Braschi, A. Riva; Aviano (PN): U. Tirelli, R. Cinelli; Bari: G. Pastore, N. Ladisa, G. Minafra; Bergamo: F. Suter, C. Arici; Bologna: F. Chiodo, V. Colangeli, C. Fiorini, O. Coronado; Brescia: G. Carosi, G.P. Cadeo, C. Torti, C. Minardi, D. Bertelli; Busto Arsizio: G. Rizzardini, S. Melzi; Cagliari: P. E. Manconi, P. Piano; Catanzaro: L. Cosco, A. Scerbo; Chieti: J. Vecchiet, M. D’Alessandro; Como: D. Santoro, L. Pusterla; Cremona: G. Carnevale, P. Citterio; Cuggiono: P. Viganò, M. Mena; Ferrara: F. Ghinelli, L. Sighinolfi; Firenze: F. Leoncini, F. Mazzotta, M. Pozzi, S. Lo Caputo; Foggia: G. Angarano, B. Grisorio, A. Saracino, S. Ferrara; Galatina (LE): P. Grima, P. Tundo; Genova: G. Pagano, G. Cassola, A. Alessandrini, R. Piscopo; Grosseto: M. Toti, S. Chigiotti; Latina: F. Soscia, L. Tacconi; Lecco: A. Orani, P. Perini; Lucca: A. Scasso, A. Vincenti; Macerata: F. Chiodera, P. Castelli; Mantova: A. Scalzini, L. Palvarini; Milano: M. Moroni, A. Lazzarin, A. Cargnel, G.M. Vigevani, L. Caggese, A. d’Arminio Monforte, D. Repetto, A. Galli, S. Merli, C. Pastecchia, M. C. Moioli; Modena: R. Esposito, C. Mussini; Napoli: N. Abrescia, A. Chirianni, C. M. Izzo, M. Piazza, M. De Marco, R. Viglietti, E. Manzillo, S. Nappa; Palermo: A. Colomba, V. Abbadessa, T. Prestileo, S. Mancuso; Parma: C. Ferrari, P. Pizzaferri; Pavia: G. Filice, L. Minoli, R. Bruno, S. Novati; Perugia: F. Baldelli, M. Tinca; Pesaro: E. Petrelli, A. Cioppi; Piacenza: F. Alberici, A. Ruggieri; Pisa: F. Menichetti, C. Martinelli; Potenza: C. De Stefano, A. La Gala; Ravenna: G. Ballardini, E. Rizzo; Reggio Emilia: G. Magnani, M. A. Ursitti; Rimini: M. Arlotti, P. Ortolani; Roma: R. Cauda, F. Dianzani, G. Ippolito, A. Antinori, G. Antonucci, S. D’Elia, P. Narciso, N. Petrosillo, V. Vullo, A. De Luca, A. Bacarelli, M. Zaccarelli, R. Acinapura, P. De Longis, A. Brandi, M. P. Trotta, P. Noto, M. Lichtner, M. R. Capobianchi, F. Carletti, E. Girardi, P. Pezzotti, G. Rezza; Sassari: M. S. Mura, M. Mannazzu; Torino: P. Caramello, G. Di Perri, M. L. Soranzo, G. C. Orofino, I. Arnaudo, M. Bonasso; Varese: P. A. Grossi, C. Basilico; Verbania: A. Poggio, G. Bottari; Venezia: E. Raise, F. Ebo; Vicenza: F. De Lalla, G. Tositti; Taranto: F. Resta, K. Loso; London, UK: A. Cozzi Lepri
SHCS (7 sites)
M. Battegay, E. Bernasconi, J. Böni, H. Bucher, Ph. Bürgisser, S. Cattacin, M. Cavassini, R. Dubs, M. Egger, L. Elzi, P. Erb, K. Fantelli, M. Fischer, M. Flepp, A. Fontana, P. Francioli (President of the SHCS, Center Hospitalier Universitaire Vaudois, Lausanne), H. Furrer (Chairman of the Clinical and Laboratory Committee), M. Gorgievski, H. Günthard, B. Hirschel, L. Kaiser, C. Kind, Th. Klimkait, U. Lauper, B. Ledergerber, M. Opravil, F. Paccaud, G. Pantaleo, L. Perrin, J.-C. Piffaretti, M. Rickenbach (Head of Data Center), C. Rudin (Chairman of the Mother and Child Substudy), P. Schmid, J. Schüpbach, R. Speck, A. Telenti, A. Trkola, P. Vernazza (Chairman of the Scientific Board), R. Weber, S. Yerly
ATHENA (25 sites)
Treating physicians (*site coordinating physicians): Dr. W. Bronsveld*, Dr. M. E. Hillebrand-Haverkort (Alkmaar); Dr. J. M. Prins*, Dr. J. C. Bos, Dr. J. K. M. Eeftinck Schattenkerk, Dr. S. E. Geerlings, Dr. M. H. Godfried, Prof. dr. J. M. A. Lange, Dr. F. C. van Leth, Dr. S. H. Lowe, Dr. J. T. M. van der Meer, Dr. F. J. B. Nellen, Dr. K. Pogány, Dr. T. van der Poll, Dr. P. Reiss, Dr. Th.A. Ruys, Dr. S. Sankatsing, Dr. R. Steingrover, Dr. G. van Twillert, Dr. M. van der Valk, Dr. M. G. A. van Vonderen, Dr. S. M. E Vrouenraets, Dr. M. van Vugt, Dr. F. W. M. N. Wit (Amsterdam); Prof. Dr. T. W. Kuijpers, Dr. D. Pajkrt, Dr. H. J. Scherpbier, Emmakinderziekenhuis (Amsterdam); Dr. A. van Eeden*, Onze Lieve Vrouwe Gasthuis (Amsterdam); Dr. J. H. 10 Veen*, Dr. P. S. van Dam, Dr. J. C. Roos, Onze Lieve Vrouwe Gasthuis (Amsterdam); Dr. K. Brinkman*, Dr. P. H. J. Frissen, Dr. H. M. Weigel, Onze Lieve Vrouwe Gasthuis (Amsterdam); Dr. J. W. Mulder*, Dr. E. C. M. van Gorp, Dr. P. L. Meenhorst, Dr. A. T. A. Mairuhu, Slotervaart Ziekenhuis (Amsterdam); Dr. J. Veenstra*, (Amsterdam); Prof. Dr. S. A. Danner*, Dr. M. A. Van Agtmael, Dr. F. A. P. Claessen, Dr. R. M. Perenboom, Dr. A. Rijkeboer, Dr. M. van Vonderen (Amsterdam); Dr. C. Richter*, Dr. J. van der Berg, Dr. R. van Leusen (Arnhem); Dr. R. Vriesendorp*, Dr. F. J. F. Jeurissen (Westeinde-Den Haag); Dr. R. H. Kauffmann*, Dr. E. L. W. Koger (Leyenburg-Den Haag); Dr. B. Bravenboer* (Catharina Ziekenhuis-Eindhoven); Dr. C. H. H. ten Napel*, Dr. G. J. Kootstra (Medisch Spectrum Twente-Enschede); Dr. H. G. Sprenger*, Dr. W. M. A. J. Miesen, Dr. R. Doedens, Dr. E. H. Scholvinck (Groningen); Dr. R. W. ten Kate* (Kennemer Gasthuis-Haarlem); Dr. D. P. F. van Houte*, Dr. M. Polee (Zuid); Dr. F. P. Kroon*, Prof. Dr. van den Broek, Prof. Dr. J. T. van Dissel, Dr. E. F. Schippers (Leiden); Dr. G. Schreij*, Dr. S. van de Geest, Dr. A. Verbon (Maastricht); Dr. P. P. Koopmans*, Dr. M. Keuter, Dr. F. Post, Dr. A. J. A. M. van der Ven (Nijmegen); Dr. M. E. van der Ende*, Dr. I. C. Gyssens, Dr. M. van der Feltz, Dr. J. G. den Hollander, Dr. S. de Marie, Dr. J. L Nouwen, Dr. B. J. A. Rijnders, Dr. T. E. M. S. de Vries (Rotterdam); Dr. G. Driessen, Prof. dr. R. de Groot, Dr. N. Hartwig (Rotterdam); Dr. J. R. Juttmann*, Dr. C. van de Heul, Dr. M. E. E. van Kasteren (St. Elisabeth-Tilburg); Dr. M. M. E. Schneider* (until October 2004), Dr. M. J. M. Bonten, Dr. J. C. C. Borleffs, Dr. P. M. Ellerbroek, Prof. Dr. I. M. Hoepelman*, Dr. C. A. J. J. Jaspers, Dr. I. Schouten, Dr. C. A. M. Schurink (Utrecht); Dr. S. P. M. Geelen, Dr. T. F.W. Wolfs (Utrecht); Dr. W. L. Blok*, Dr. A. A. Tanis (Vlissingen); Dr. P. H. P. Groeneveld*, Isala Klinieken-Zwolle. Virologists: Dr. N. K. T. Back, Dr. M. E. G. Bakker, Prof. Dr. B. Berkhout, Dr. S. Jurriaans (Amsterdam); Dr. Th. Cuijpers (Amsterdam); Dr. P. J. G. M. Rietra, Dr. K. J. Roozendaal (Amsterdam); Dr.W. Pauw, Dr. A. P. van Zanten, Dr. P. H. M. Smits (Amsterdam); Dr. B. M. E. von Blomberg, Dr. P. Savelkoul (Amsterdam); Dr. C. M. A. Swanink (Arnhem); Dr. P. F. H. Franck, Dr. A. S. Lampe, HAGA (Leyenburg-Den Haag); Dr. C.L. Jansen (Westeinde-Den Haag); Dr. R. Hendriks (Streeklaboratorium Twente-Enschede); Dr. J. Schirm, Dr. C. A. Bennw (Groningen); Dr. D. Veenendaal (Haarlem); Dr. H. Storm, Dr. J. Weel, Dr. J. H. van Zeijl (Leeuwarden); Prof. Dr. A. C. M. Kroes, Dr. H. C. J. Claas (Leiden); Prof. Dr. C. A. M. V. A. Bruggeman, Drs. V. J. Goossens (Maastricht); Prof. Dr. J. M. D. Galama, Dr. W. J. G. Melchers, Mevr. Y. A. G. Poort (Nijmegen); Dr. G. J. J. Doornum, Dr. M. G. Niesters, Prof. Dr. A. D. M. E. Osterhaus, Dr. M. Schutten (Rotterdam); Dr. A. G. M. Buiting, Mevr. C. A. M. Swaans (Tilburg); Dr. C. A. B. Boucher, Dr. R. Schuurman (Utrecht); Dr. E. Boel, Dr. A. F. Jansz (Veldhoven)
Multicenter Study Group on EuroSIDA (73 sites)
Argentina: (M. Losso, National Coordinator [NC]), A. Duran, Buenos Aires. Austria: (N. Vetter, NC), Vienna. Belarus: (I. Karpov, NC), A. Vassilenko, Minsk. Belgium: (N. Clumeck, NC), S. DeWit, B. Poll, Brussels; R. Colebunders, Antwerp. Czech Republic: (L. Machala, NC), H. Rozsypal, Prague; D. Sedlacek, Plzen. Denmark: (J. Nielsen, NC), J. Lundgren, T. Benfield, O. Kirk, Copenhagen; J. Gerstoft, T. Katzenstein, A.-B. E. Hansen, P. Skinhøj, Copenhagen; C. Pedersen, Odense. Estonia: (K. Zilmer, NC), Tallinn. France: (C. Katlama, NC), J.-P. Viard, P.-.M Girard, Paris; T. Saint-Marc, P. Vanhems, Lyon; C. Pradier, Nice; F. Dabis, Bordeaux. Germany: M. Dietrich, C. Manegold, Hamburg; J. van Lunzen, H.-J. Stellbrink, Hamburg; S. Staszewski, M. Bickel, Frankfurt; F.-D. Goebel, Munich; G. Fätkenheuer, Cologne; J. Rockstroh, Bonn; R. Schmidt, Hannover. Greece: (J. Kosmidis, NC), P. Gargalianos, H. Sambatakou, J. Perdios, G. Panos, A. Filandras, E. Karabatsaki, Athens. Hungary: (D. Banhegyi, NC), Budapest. Ireland: (F. Mulcahy, NC), Dublin. Israel: (I. Yust, NC), D. Turner, M. Burke, Tel Aviv; S. Pollack, G. Hassoun, Haifa; Z. Sthoeger, Rehovot; S. Maayan, Jerusalem. Italy: (A. Chiesi, NC), Rome; R. Esposito, R. Borghi, Modena; C. Arici, Bergamo; R. Pristera, Bolzano; F. Mazzotta, A. Gabbuti, Firenze; V. Vullo, M. Lichtner, Rome; A. Chirianni, E. Montesarchio, Presidio Ospedaliero A. D. Cotugno, Napoli; G. Antonucci, F. Iacomi, P. Narciso, M. Zaccarelli, Rome; A. Lazzarin, R. Finazzi, A. D’Arminio Monforte, Milan. Latvia: (L. Viksna, NC), Riga. Lithuania: (S. Chaplinskas, NC), Vilnius. Luxembourg: (R. Hemmer, NC), T. Staub, Luxembourg. Netherlands: (P. Reiss, NC), Amsterdam. Norway: (J. Bruun, NC), A. Maeland, V. Ormaasen, Oslo. Poland: (B. Knysz, NC), J. Gasiorowski, Medical University, Wroclaw; A. Horban, Warsaw; D. Prokopowicz, A. Wiercinska-Drapalo, Bialystok; A. Boron-Kaczmarska, M. Pynka, Szczecin; M. Beniowski, E. Mularska, Chorzow; H. Trocha, Gdansk. Portugal: (F. Antunes, NC), E. Valadas, Lisbon; K. Mansinho, Lisbon; F. Matez, Lisbon. Romania: (D. Duiculescu, NC), Dr. Victor Babes, Bucarest; A. Streinu-Cercel, Bucarest. Russia: E. Vinogradova, A. Rakhmanova, St Petersburg. Serbia and Montenegro (D. Jevtovic, NC), Belgrade. Slovakia: (M. Mokráš, NC), D. Staneková, Bratislava. Spain: (J. González-Lahoz, NC), M. Sánchez-Conde, T. García-Benayas, L. Martin-Carbonero, V. Soriano, Madrid; B. Clotet, A. Jou, J. Conejero, C. Tural, Badalona; J. M. Gatell, J. M. Miró, Barcelona. Sweden: (A. Blaxhult, NC), Solna; A. Karlsson, Stockholm; P. Pehrson, Huddinge. Switzerland: (B. Ledergerber, NC), R. Weber, Zürich; P. Francioli, A. Telenti, Lausanne; B. Hirschel, V. Soravia-Dunand, Geneve; H. Furrer, Bern. Ukraine: (E. Kravchenko, NC), N. Chentsova, Kyiv. United Kingdom: (S. Barton, NC), London; A.M. Johnson, D. Mercey, London; A. Phillips, M. A. Johnson, A. Mocroft, London; M. Murphy, London; J. Weber, G. Scullard, London; M. Fisher, Brighton; R. Brettle, Edinburgh. Virology group: C. Loveday, B. Clotet (Central Coordinators) plus ad hoc virologists from participating sites in the EuroSIDA Study. Steering Committee: Francisco Antunes, Anders Blaxhult, Nathan Clumeck, Jose Gatell, Andrzej Horban, Anne Johnson, Christine Katlama, Bruno Ledergerber (Chair), Clive Loveday, Andrew Phillips, Peter Reiss, Stefano Vella. Coordinating Center Staff: J. Lundgren (Project Leader), I. Gjørup, O. Kirk, N. Friis-Moeller, A. Mocroft, A. Cozzi-Lepri, W. Bannister, D. Mollerup, D. Podlevkareva, C. Holkmann Olsen, J. Kjær
CHORUS (4 sites)
Stephen Raffanti, Douglas Dieterch, Amy Justice, Stephen Becker, Anthony Scarsella, Gregory Fusco, Bernard Most, Rukmini Balu, Rashida Rana, Robin Beckerman, Theodore Ising, Jennifer Fusco, Renae Irek, Bernadette Johnson, Ashwin Hirani, Edwin DeJesus, Gerald Pierone, Philip Lackey, Chip Irek, Alison Johnson, John Burdick, Saul Leon, Joseph Arch
Frankfurt HIV Cohort (1 site)
Schlomo Staszewski, Eilke B. Helm, Amina Carlebach, Axel Müller, Annette Haberl, Gabi Nisius, Tessa Lennemann, Carsten Rottmann, Timo Wolf, Christoph Stephan, Markus Bickel, Manfred Mösch, Peter Gute, Leo Locher, Thomas Lutz, Stephan Klauke, Gabi Knecht (Clinical Group); Hans W. Doerr, Martin Stürmer (Virology Group); Brenda Dauer (Scientific Advisor); Nils von Hentig (Pharmacology Group); Beverly Jennings (Data Management)
Aquitaine Cohort ANRS CO3 (6 sites)
Scientific Committee: J. Beylot, G. Chêne, F. Dabis, M. Dupon, M. Longy-Boursier, J. L. Pellegrin, J. M. Ragnaud, R. Salamon. Methodological Coordination: F. Dabis, G. Chêne, R. Thiébaut, C. Lewden, S. Lawson-Ayayi. Medical Coordination: M. Dupon, P. Mercié, J. F. Moreau, P. Morlat, J. L. Pellegrin, J. M. Ragnaud, N. Bernard, D. Lacoste, D. Malvy, D. Neau. Data Management and Analysis: M. J. Blaizeau, M. Decoin, S. Delveaux, C. Hannapier, S. Labarrère, V. Lavignolle-Aurillac, B. Uwamaliya-Nziyumvira, G. Palmer, D. Touchard, E. Balestre, A. Alioum, H. Jacqmin-Gadda, R. Thiébaut. Participating Physicians: Bordeaux University Hospital: J. Beylot, P. Morlat, N. Bernard, M. Bonarek, F. Bonnet, B. Coadou, P. Gellie, D. Lacoste, C. Nouts, M. Dupon, F. Bocquentin, H. Dutronc, S. Lafarie, M. Longy-Boursier, P. Mercié, A. Aslan, D. Malvy, T. Pistonne, P. Thibaut, R. Vatan, J. M. Ragnaud, D. Chambon, C. De La Taille, C. Cazorla, D. Neau, A. Ocho, J. L. Pellegrin, J. F. Viallard, O. Caubet, C. Cipriano. E. Lazaro. P. Couzigou, L. Castera. H. Fleury, M.E. Lafon, B. Masquelier, I. Pellegrin, D. Breilh, J. F. Moreau, P. Blanco; Dax Hospital: P. Loste, L. Caunègre; Bayonne Hospital: F. Bonnal, S. Farbos, M. Ferrand; Libourne Hospital: J. Ceccaldi, S. Tchamgoué; Mont de Marsan Hospital: S. De Witte; Villeneuve sur Lot Hospital: E. Buy
HAART Observational Medical Evaluation and Research, British Columbia Center for Excellence in HIV/AIDS (96 sites)
Chris Alexander, Rolando Barrios, Paula Braitstein, Zabrina Brumme, Keith Chan, Helen Cote, Nada Gataric, Josie Geller, Silvia Guillemi, P. Richard Harrigan, Marrianne Harris, Robert Hogg, Ruth Joy, Adrian Levy, Julio Montaner, Val Montessori, Anita Palepu, Elizabeth Phillips, Peter Phillips, Natasha Press, Mark Tyndall, Evan Wood, Benita Yip
Royal Free Centre for HIV Medicine
Clinical: S. Bhagani, P. Byrne, A. Carroll, Z. Cuthbertson, A. Dunleavy, A. M. Geretti, B. Heelan, M. Johnson, S. Kinloch-de Loes, M. Lipman, S. Madge, N. Marshall, D. Nair, G. Nebbia, B. Prinz, L. Swaden, M. Tyrer, M. Youle. Data management: C. Chaloner, H. Grabowska, J. Holloway, J. Puradiredja, D. Ransom, R. Tsintas. Biostatistics/Epidemiology: W. Bannister, L. Bansi, A. Cozzi-Lepri, Z. Fox, E. Harris, T. Hill, F. Lampe, R. Lodwick, A. Mocroft, A. Phillips, J. Reekie, C. Sabin, C. Smith. Laboratory: E. Amoah, C. Booth, G. Clewley, A. Garcia Diaz, B. Gregory, W. Labbett, F. Tahami, M. Thomas
South Alberta Clinic (1 site)
John Gill, Ron Read
Köln/Bonn Cohort (2 sites)
G. Fatkenheuer, J. Rockstroh, N. Schmeisser, K. Voigt, J. C. Wasmuth, A. Wohrmann
Footnotes
See Appendix for Writing Committee and members of study group.
REFERENCES
- 1.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 2.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg RS, Yip B, Kully C, et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ. 1999;160:659–665. [PMC free article] [PubMed] [Google Scholar]
- 4.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 5.Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 6.Egger M, May M, Chêne G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 7.Chêne G, Sterne JA, May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 8.May M, Royston P, Egger M, et al. Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2004;23:2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 9.May M, Sterne JA, Sabin C, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J-L, et al. Smoothing hazard rate. In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics. 2nd ed. Chichester, UK: Wiley; 2005. pp. 4986–4997. [Google Scholar]
- 11.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Prins M, Hernandez AI, Brettle RP, et al. Pre-AIDS mortality from natural causes associated with HIV disease progression: evidence from the European Seroconverter Study among injecting drug users. AIDS. 1997;11:1747–1756. doi: 10.1097/00002030-199714000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 14.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 15.Vermund SH. Rising HIV-related mortality in young Americans. JAMA. 1993;269:3034–3035. [PubMed] [Google Scholar]
- 16.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 17.Wood E, Montaner JS, Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- 18.Lewden C, Salmon D, Morlat P, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. doi: 10.1093/ije/dyh307. [DOI] [PubMed] [Google Scholar]
- 19.Quaglio G, Talamini G, Lechi A, et al. Study of 2708 heroin-related deaths in north-eastern Italy 1985–98 to establish the main causes of death. Addiction. 2001;96:1127–1137. doi: 10.1046/j.1360-0443.2001.96811276.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith CJ, Sabin CA, Youle MS, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004;190:1860–1868. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 22.Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/µL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 24.Garcia F, de Lazzari E, Plana M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr. 2004;36:702–713. doi: 10.1097/00126334-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 25.Tarwater PM, Margolick JB, Jin J, et al. Increase and plateau of CD4 T-cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:168–175. doi: 10.1097/00126334-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 26.Miller V, Mocroft A, Reiss P, et al. Relations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: results from the EuroSIDA study. Ann Intern Med. 1999;130:570–577. doi: 10.7326/0003-4819-130-7-199904060-00005. [DOI] [PubMed] [Google Scholar]
- 27.Cole SR, Li R, Anastos K, et al. Accounting for leadtime in cohort studies: evaluating when to initiate HIV therapies. Stat Med. 2004;23:3351–3363. doi: 10.1002/sim.1579. [DOI] [PubMed] [Google Scholar]
- 28.Phillips AN, Lepri AC, Lampe F, et al. When should antiretroviral therapy be started for HIV infection? Interpreting the evidence from observational studies. AIDS. 2003;17:1863–1869. doi: 10.1097/00002030-200309050-00004. [DOI] [PubMed] [Google Scholar]
- 29.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 30.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–595. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 31.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]