Abstract
Background
Lactate dehydrogenase (LDH) isoenzymes are required for adenosine triphosphate production, with each of five different isoenzymes having varying proficiencies in anaerobic versus aerobic environments. With advancing pregnancy, the isoenzyme profile in uterine muscle shifts toward a more anaerobic profile, speculatively to facilitate uterine efficiency during periods of low oxygen that accompany labor contractions. Profile shifting may even occur throughout labor. Maternal serum LDH levels between 24–48 hours following delivery predominantly originate from uterine muscle, reflecting the enzymatic state of the myometrium during labor. Our purpose was to describe serum LDH isoenzymes 24–30 hours post-delivery to determine if cervical dilation rates following labor admission were associated with a particular LDH profile. We also compared differences in post-delivery LDH isoenzyme profiles between women admitted in pre-active versus established active labor.
Methods
Low-risk, nulliparous women with spontaneous labor onset were sampled (n = 91). Maternal serum LDH was measured at labor admission and 24–30 hours post-vaginal delivery. Rates of cervical dilation during the first four hours after admission were also measured. Spearman’s rho coefficients were used for association testing and t tests evaluated for group and paired-sample differences.
Results
More efficient dilation following admission was associated with decreased LDH1 (p = 0.029) and increased LDH3 and LDH4 (p = 0.017 and p = 0.017, respectively) in the post-delivery period. Women admitted in established active labor had higher relative serum levels of LDH3 (t = 2.373; p = 0.023) and LDH4 (t = 2.268; p = 0.029) and lower levels of LDH1 (t = 2.073; p = 0.045) and LDH5 (t = 2.041; p = 0.048) when compared to women admitted in pre-active labor.
Despite having similar dilatations at admission (3.4 ± 0.5 and 3.7 ± 0.6 cm, respectively), women admitted in pre-active labor had longer in-hospital labor durations (12.1 ± 4.3 vs. 5.3 ± 1.4 hours; p < 0.001) and were more likely to receive oxytocin augmentation (95.5% vs. 34.8%; p < 0.001).
Conclusions
More efficient cervical dilation following labor admission is associated with a more anaerobic maternal serum LDH profile in the post-delivery period. Since LDH profile shifting may occur throughout labor, watchful patience rather than intervention in earlier labor may allow LDH shifting within the uterus to more fully manifest. This may improve uterine efficiency during labor and decrease rates of oxytocin augmentation, thereby improving birth safety.
Keywords: Labor, Obstetric, Lactate dehydrogenase, Nullipara, Serum, Uterus
Background
The threshold for the active phase of labor is suggested to reliably begin at a “cervical dilatation of 3 to 5 cm or more, in the presence of uterine contractions” [1]. However, investigators report that these criteria do not validly describe active labor onset for a large percentage of nulliparous women with spontaneous labor onset when traditional cervical dilation expectations are used to differentiate active from earlier labor [2,3]. The clinical dilemma is that many women are inadvertently admitted prior to progressive labor (i.e., pre-active labor) yet held to dilation rate expectations of active labor [4]. It is possible that women admitted early and given interventions aimed at accelerating labor progress (e.g., oxytocin augmentation) may be disadvantaged during labor in that such intervention may interrupt the time necessary for important physiological changes within the uterine and reproductive tissues to more fully manifest. This may, in part, explain why women admitted early in labor are more prone to oxytocin augmentation and are more than twice as likely to be delivered via cesarean [5-9].
Change in the activity of the enzyme lactate dehydrogenase (LDH) within uterine muscle during pregnancy and possibly throughout labor is a key physiological adaptation that may facilitate efficient uterine activity during labor. LDH is a predominantly intracellular, cytoplasmic enzyme that catalyzes the interconversion of pyruvate and lactate [Pyruvate+NADH+H+ ↔ (L)-lactate+NAD+], a process essential for adenosine triphosphate (ATP) production. LDH is composed of two different types of polypeptide chains, commonly called ‘H’ and ‘M,’ which combine to form either homotetramer isoenzymes composed of all ‘H’ chains [LDH1 (H4)] or all ‘M’ chains [LDH5 (M4)] or heterotetramer isoenzymes composed of a mixture of ‘H’ and ‘M’ chains [LDH2 (H3M1), LDH3 (H2M2), LDH4 (H1M3)]. The profile expression of LDH isoenzymes differs between body tissues depending on typical oxygen availability, e.g., more H-subunit dominant isoenzymes are available in tissues relying on aerobic metabolism, such as the heart, while M-subunit dominant isoenzymes are more abundant in tissues using anaerobic metabolism, such as skeletal muscle and liver [10-12]. The isoenzyme profile is also capable of adaptation within body tissues in response to appropriate signals, thus ensuring the tissue consistently maintains adequate ATP production.
The majority of studies measuring LDH levels during pregnancy are from the late 1950s through the 1970s. In myometrial muscle, LDH isoenzymes shift toward a more anaerobic profile as pregnancy advances, speculatively, to better equip the uterus to contend with hypoxic episodes related to labor contractions [10,13-15]. As a result, LDH3 and/or LDH4 have been reported to be in greatest concentrations in the pregnant myometrium at term [13,16,17]. Anaerobic shifting is important because otherwise intermittent hypoxia and resultant acidosis would rapidly reduce contractile force [18-21]. The pattern and timing of LDH isoenzyme profile shifting throughout late pregnancy and labor remains largely unknown.
LDH is released from its tissue of origin and enters the general circulation when cells are broken down or damaged. Because most tissues have LDH activities that are 500–700 times greater than that found in normal serum, a significant elevation of serum LDH occurs with even small amounts of tissue breakdown [22]. While total serum measurement of LDH provides only a non-specific measure of cellular breakdown/damage, determining specific LDH isoenzyme patterns is useful in the differential diagnosis of certain pathologic states. This is possible because tissue breakdown releases the isoenzymes contained within that particular tissue, leading to a change in the serum profile measured systemically. Isoenzyme measures can assist in diagnosing pathologic processes such as myocardial infarction, liver disease, and pre-eclampsia.
During normal labor, levels of total myometrial LDH decline from levels present before labor with a disproportionate decrease in ‘M’ dominant chains over ‘H’ dominant chains [14]. This finding aligns with reports that maternal serum total LDH concentrations are higher in the postpartum period than is normally found during the pre-labor period [23-28], peaking approximately 24–48 hours after delivery [23,29]. Given the tremendous amount of work performed and stress endured by the uterus during labor coupled with rapid uterine involution following delivery, it is likely that serum LDH isoenzyme levels measured after labor, in otherwise healthy women, predominantly reflect the enzymatic profile within the uterine muscle that existed during the labor period. This has been suggested by other research teams [14,23,24]. This means that LDH isoenzyme levels measured 24–30 hours after labor may serve as a retrospective indicator of uterine preparedness for labor.
The primary objective of this study was to describe relationships between maternal serum LDH isoenzymes measured 24–30 hours post-delivery and rates of cervical dilation during the first 4 hours following hospital admission for spontaneous labor onset. We also aimed to compare differences in post-delivery LDH profiles between women admitted to the hospital in pre-active versus established active labor. We hypothesized that better uterine preparedness for labor as evidenced by more efficient labor progression would be associated with a more anaerobic post-delivery serum LDH isoenzyme profile.
Methods
A prospective study was conducted at a suburban, Midwestern hospital in the United States in which nearly 5000 women birth annually. Institutional Review Board (IRB) approval was granted (Mount Carmel IRB, study # 061130–3; The Ohio State University Biomedical IRB, protocol # 2006H0248) and written informed consents and Health Insurance Portability and Accountability Act authorizations were obtained from all women. Recruitment took place from 4/2007 – 2/2008 and was conducted by JN in the labor and delivery triage unit or in the labor room as soon after admission as possible. Approximately 70% of approached women accepted participation.
Participants were pregnant nulliparous women of low-obstetric risk (no significant medical history, absence of major pregnancy complications, e.g., pre-eclampsia or diabetes) admitted for spontaneous labor onset under criteria commonly associated with active labor onset, i.e., 3 cm to 5 cm cervical dilatation in the presence of regular uterine contractions (≥2 in a 10 minute window). Additional inclusion criteria were 18–39 years of age, 37–42 weeks gestation, singleton gestation, cephalic presentation, no identified fetal anomalies or growth issues, anticipated vaginal delivery, maternal weight <250 lbs, afebrile at study entry, and able to read and speak English. Augmentation of labor was permitted after labor admission although women undergoing labor inductions were not enrolled in the study. Care during labor was at the discretion of the labor care providers.
LDH total and isoenzyme concentrations were measured in maternal sera at two time points. The first sample was collected as near to the time of labor admission as possible with sampling occurring either concurrently with intravenous line placement, a standard facility order, or, less commonly, via a 20- or 22-gauge needle from the antecubital vein or below in the uncannulated arm. The second sample was collected 24–30 hours after vaginal delivery; typical patient discharge at this institution occurs 48 hours following vaginal birth. Each sample was collected into an 8.5 mL BD Vacutainer® serum separator tube (Reference # 367988) (Becton-Dickinson, Franklin Lakes, NJ). The blood was allowed to clot at room temperature for up 60 minutes and then centrifuged at 2500 rpm for 10 minutes. Sera were separated, immediately refrigerated, and determinations were performed within 48–72 hours after collection. Serum with visible hemolysis was discarded since the LDH released from the damaged erythrocytes might give spuriously high results. Hemolyzed samples were redrawn at the admission time point if labor had not progressed beyond the aforementioned labor onset criteria. At the post-delivery time point, hemolyzed samples were redrawn within 30 minutes of the initial sampling. For women who delivered via cesarean, post-delivery LDH samples were not collected due to the likelihood of these values being elevated secondary to surgical tissue damage. LDH measurements were made via a SYNCHRON LX System, an agarose gel electrophoresis system (Beckman Coulter, Inc., Fullerton, CA). Total LDH values were in units per liter (U/L) and the relative proportion of each LDH isoenzyme was expressed as a percentage (%). Absolute isoenzyme levels were not used because of the wide variability known to exist between individuals.
All digital cervical exams documented by labor care providers during the course of labor were transcribed post hoc from the labor record so that the average dilation slope for the first 4 hours post-admission could be determined. Since cervical exams are rarely performed at exactly 4 hours after the admission exam, slope calculations based on the exams immediately prior to and after the 4 hour time point were used to approximate dilatation at the 4 hour post-admission time point. The average dilation slope was then calculated for the first 4 hours post-admission; relationships between these rates (cm/hour) and the maternal serum LDH isoenzymes were then described for all subjects.
Finally, each subjects’ labor admission was retrospectively classified as either ‘pre-active labor’ or ‘established active labor’ based on the rate of cervical change during the first 4 hours after labor admission using a priori criteria. Between 3 cm and 5 cm dilatation, recent literature indicates that cervical dilation rates approximating >0.25 cm/hour for nulliparous women may, in some cases, indicate the potential onset of early active labor while rates ≥1 cm/hour are recognized to be indicative of established active labor [30-33]. Thus, for our study, a labor admission was classified as ‘pre-active’ when average dilation was ≤0.25 cm/hour for the first 4 hours post-admission labor or as ‘established active’ when average dilation was ≥1.0 cm/hour. Women having 4 hour post-admission dilation rates between >0.25 and <1.0 cm/hour were not included in comparisons because of the expected mix of women in pre-active and active labor in this range. Classifications of labor were determined before LDH results were available to the research team.
For data analyses, demographic variables were expressed as mean (SD) if continuous and as n (%) if categorical. Paired-analysis t tests with Bonferroni correction were used to test for differences between LDH measures at labor admission and 24–30 hours post-delivery. Spearman’s rho correlation coefficients were used to test for relationships between LDH measures and rates of cervical dilation during the first 4 hours following hospital admission because rates of dilation were not normally distributed; with a medium effect size (0.30), α = 0.05, and power = 0.80, a sample of 85 women achieving vaginal birth is required to test for correlations [34]. To test for LDH profile differences between the ‘pre-active’ and ‘established active’ labor groups, Student’s t tests were performed. We anticipated that this aim would be underpowered. P-values <0.05 were considered significant. Statistical analyses were made via SPSS Statistics 19 (IBM Corporation, Armonk, NY).
Results
Ninety-one parturients were enrolled in the study and there was no attrition. Demographics of the sample are shown in Table 1. The majority of the sample self-classified as non-Hispanic whites. Among all women enrolled in the study, 81 birthed vaginally and 10 were delivered via cesarean. Among cesarean deliveries (n = 10), six were performed in the first-stage of labor (slow labor progression = 3; non-reassuring fetal heart patterns = 3) and four in the second-stage for arrest of fetal descent.
Table 1.
Demographic variables (n = 91)
| Maternal age (yrs) |
24.9 (4.8) |
Range: 18-36 |
| Gestational age at delivery (days) |
276.1 (7.1) |
Range: 259-290 |
| Hispanic |
|
|
| Yes |
5 (5.5%) |
|
| No |
86 (94.5%) |
|
| Race |
|
|
| White |
66 (72.5%) |
|
| Black |
18 (19.8%) |
|
| Other |
7 (7.7%) |
|
| Marital status |
|
|
| Married |
41 (45.1%) |
|
| Not married |
50 (54.9%) |
|
| Body mass index (maternal) |
29.9 (4.6) |
Range: 18.0-41.7 |
| Cervical dilatation at admission (cm) |
3.6 (0.5) |
Range: 3.0-5.0 |
| Cervical effacement at admission |
|
|
| 50% – < 80% |
11 (12.1%) |
|
| ≥ 80% |
80 (87.9%) |
|
| Mode of birth |
|
|
| Vaginal |
81 (89.0%) |
|
| Cesarean |
10 (11.0%) |
|
| Membrane rupture type |
|
|
| Spontaneous |
33 (36.3%) |
|
| Amniotomy within 4 hrs of admission |
41 (45.0%) |
|
| Amniotomy at > 4 hrs after admission |
17 (18.7%) |
|
| Oxytocin augmentation |
|
|
| No |
33 (36.3%) |
|
| Yes, within 4 hrs of admission |
27 (29.7%) |
|
| Yes, at > 4 hrs after admission |
31 (34.0%) |
|
| Epidural use |
|
|
| No |
5 (5.5%) |
|
| Yes |
86 (94.5%) |
|
| In-hospital labor duration (hr)* |
8.9 (3.7) |
Range: 2.8-20.9 |
| Weight (infant) (g) |
3392.8 (460.9) |
Range: 2329-4722 |
| Length (infant) (cm) | 49.5 (2.2) | Range: 44.0-54.5 |
Mean (SD) for continuous variables; n (%) for categorical variables.
* Includes only those delivering vaginally (n = 81).
LDH samples were collected from all subjects at labor admission although four specimens hemolyzed and repeat samples were not obtained; therefore, these specimens were not included in the analyses. At 24–30 hours post-delivery, LDH values were determined in 79 subjects; subjects delivered via cesarean were not sampled and two samples were unable to be collected due to early patient discharge. In all, serum paired-samples were obtained from 75 subjects and all differences were significant with Bonferroni correction (p < 0.001) (Table 2). Specifically, post-delivery total LDH, LDH3, and LDH4 had increased over values seen at labor admission while LDH1, LDH2, and LDH5 had decreased. Figure 1 displays relative LDH isoenzyme changes from admission to 24–30 hours post-delivery.
Table 2.
Maternal serum LDH paired-sample t tests between labor admission and post-delivery samples (n = 75)
| Total (U/L) |
Isoenzyme (%) |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Labor admission |
147.59 (22.81) |
29.66 (3.13) |
30.33 (3.17) |
19.21 (2.36) |
8.74 (2.18) |
12.07 (3.88) |
| Post-delivery (24–30 hrs post) |
173.35 (30.92) |
23.89 (3.57) |
26.00 (3.30) |
27.45 (3.17) |
13.62 (3.52) |
9.05 (2.43) |
| t test value | 7.491* | 14.186* | 14.055* | 28.898* | 14.851* | 7.898* |
Values are reported as mean (SD).
Each measure was normally distributed per the Kolmogorov-Smirnov test (p > 0.05). Bonferroni correction for multiple tests was p < 0.008 (i.e., p = 0.05/6).
*p < 0.001 (2-tailed).
Figure 1.
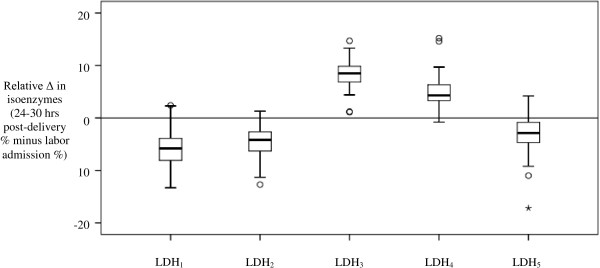
Relative change in serum LDH isoenzyme paired-samples (n = 75). p < 0.001 (2-tailed) for all isoenzyme changes.
Maternal serum LDH total and isoenzyme levels measured at labor admission held no correlation with rates of cervical dilation during the first 4 hours post-admission. However, serum LDH measures at 24–30 hours post-delivery yielded several significant relationships with post-admission cervical dilation rates. Specifically, as rates of cervical dilation during the 4 hour post-admission assessment period increased, percentage distributions of LDH1 decreased (Spearman’s rho = −0.246, p = 0.029) while LDH3 (Spearman’s rho = 0.267, p = 0.017) and LDH4 (Spearman’s rho = 0.268, p = 0.017) increased.
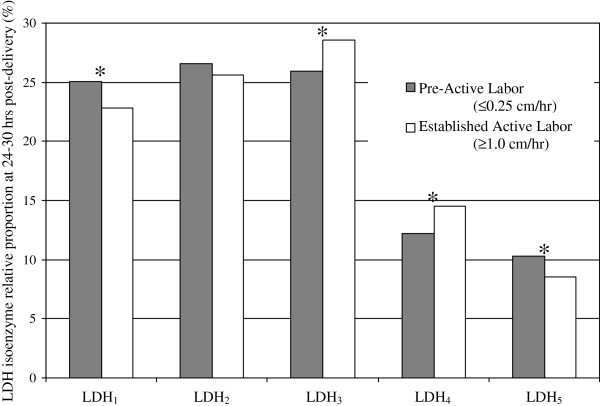
Pre-active labor (≤0.25 cm/hour) (n = 22) and established active labor (≥1.0 cm/hour) (n = 23) groups did not demonstrate significant LDH differences at baseline. However, at 24–30 hours post-delivery, several LDH isoenzyme differences emerged between those admitted in pre-active versus established active labor (Figure 2). Specifically, women admitted in established active labor had higher relative serum levels of LDH3 (t = 2.373; p = 0.023) and LDH4 (t = 2.268; p = 0.029) and lower levels of LDH1 (t = 2.073; p = 0.045) and LDH5 (t = 2.041; p = 0.048) when compared to women admitted in pre-active labor. Forty-six women had 4 hour post-admission dilation rates between >0.25 and <1.0 cm/hour; thus, they did not qualify for either the pre-active or established active labor group. This group was not compared to the others due to the nebulous nature of their labor status.
Figure 2.
Post-delivery serum LDH isoenzyme profile comparison between pre-active labor and established active labor admission groups. *p < 0.05 (2-tailed).
Despite having similar cervical dilatations at admission (3.4 ± 0.5 and 3.7 ± 0.6 cm, respectively; NS), women admitted in pre-active labor had an in-hospital labor duration of 12.1 ± 4.3 hours while those admitted in established active labor had a duration of 5.3 ± 1.4 hours (p < 0.001). The difference in in-hospital labor duration between these groups resulted from differences in the time from admission until complete dilatation; second stage durations did not differ between groups. The oxytocin augmentation rate was 95.5% among women admitted in pre-active labor and 34.8% for those admitted in established active labor (χ2 = 18.064; p < 0.001). Interestingly, all three cesareans performed in the active phase for slow labor progression followed a pre-active labor admission.
Because skeletal muscle damage can contribute to serum LHD levels, it is noteworthy that parturients with perineal episiotomy/laceration ≥2 degree (n = 54) and those with <2 degree laceration or none (n = 25) did not differ on LDH measures at 24–30 hours post-delivery.
Discussion
The significant correlations between rates of cervical dilation during the first four hours after labor admission and post-delivery LDH isoenzyme values support our hypothesis. Specifically, more efficient cervical dilation is associated with a more anaerobic LDH isoenzyme profile whereas slower labor progression is associated with a more aerobic profile. This also aligns with our finding that women admitted in progressive active labor had a more anaerobic post-delivery LDH profile as compared to women admitted in pre-active labor.
We postulate that post-delivery serum LDH profiles, sampled 24–30 hours after delivery, represent the enzymatic composition of uterine muscle surrounding the labor period since uterine muscle endures the greatest activity and stress during labor and maternal serum LDH levels peak at 24–48 hours after the tissue level event causing their release [23,29]. Others have reported that LDH anaerobic isoenzyme profile shifts occur in the human myometrium with advancing pregnancy [10,13-15] although it remains unknown when the shift is optimized. In non-pregnant states, estrogenic hormones are known to stimulate LDH activity in non-uterine tissue [35-38] with increased synthesis being predominantly of an anaerobic type [35,37,38]. In uterine muscle, positive correlations between estrogen and LDH levels have been demonstrated in non-pregnant states [39,40]. Since estrogens in maternal plasma and myometrium become more dominant with labor progression, terminating abruptly after delivery [1], we speculate that LDH shifting occurs throughout the labor continuum.
Our LDH-related findings indicate that women admitted in true active labor may have a distinct myometrial advantage in producing ATP during the hypoxic episodes that accompany uterine contractions, compared to those admitted in pre-active labor. Adverse consequences of oxytocin augmentation may be, at least partially, related to the fact that the uterine muscle isoenzyme environment may not yet be optimized for uterine contraction activity when this intervention is implemented. The significantly higher rate of oxytocin use among women admitted in pre-active labor is concerning because oxytocin, a “high-alert medication” [41], is the intervention most commonly associated with preventable adverse perinatal outcomes [42]. Receiving oxytocin at an earlier stage of labor is associated with a higher cesarean risk [9]. Incomplete LDH shifting may, in part, explain why women with spontaneous labor onset who are admitted earlier in labor (e.g. <4 vs. ≥4 cm) are more than twice as likely to be delivered via cesarean [5-9]. Rates of oxytocin supplementation and surgical birth in our small sample of women admitted in pre-active labor support these concerns.
This study was limited by a smaller than desired sample size especially in regards to testing for group differences. In addition, although uterine muscle is likely the key contributor to systemic maternal LDH measures in the peripartum period [14,23,24], other potentially contributing sources cannot be fully discounted. The placenta [17,39,43-45], skeletal muscle [14], and even intravascular hemolysis in the uterine sinusoids [23] have been suggested to be potential contributors to maternal measures. However, with the placenta being most represented by LDH4 and LDH5[16,46,47], skeletal muscle containing large concentrations of LDH5[14,22,48], and hemolysis leading the near-exclusive release of LDH1 and LDH2[22,48], one would expect that if any of these were key contributors to peripartum LDH increases, predominant increases in LDH1, LDH2, and LDH5 would have been found. Indeed, the relative measures of these three isoenzymes decreased in maternal serum between the admission and postpartum sampling time points (p < 0.001 for each). In the present study, relative and even absolute increases of LDH3 and LDH4 (p < 0.001) suggest that the uterine muscle is the most likely contributor to systemically measured LDH in the post-delivery period. These particular isoenzymes are the ones known to be in the greatest quantities in the myometrium of pregnant women [13,16,17]. Reproducing this study in a larger, more racially and/or ethnically diverse population would be a valuable scientific contribution.
Conclusions
In this study, parturients progressing most slowly after being admitted for labor appear to have LDH isoenzyme profiles that are less equipped to contend with contraction-related anaerobic conditions compared to those parturients progressing most rapidly after admittance. Although serum LDH profile assessments cannot prospectively benefit clinicians in their admission decision-making, the findings presented in this study suggest that watchful patience rather than early intervention seems prudent when there is uncertainty regarding whether active labor has begun. Such patience may allow the time needed for important physiological changes within uterine muscle to more fully manifest.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JN designed the study, conducted the study protocol, performed the statistical analyses, and drafted the manuscript. NL assisted in interpreting findings and drafting the manuscript. EC participated in the design and coordination of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Jeremy L Neal, Email: neal.167@osu.edu.
Nancy K Lowe, Email: Nancy.Lowe@ucdenver.edu.
Elizabeth J Corwin, Email: ejcorwi@emory.edu.
Acknowledgements
Funding for this study was received from the National Institute of Nursing Research [1 F31 NR010054 (Neal)] and the Sigma Theta Tau International Honor Society of Nursing: Epsilon Chapter. The authors kindly acknowledge the research support received from the nurses and managers of St. Ann’s Hospital maternity unit, Westerville, Ohio, United States.
References
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Williams Obstetrics. 23. Chicago: McGraw-Hill Companies, Inc; 2010. [Google Scholar]
- Peisner DB, Rosen MG. Transition from latent to active labor. Obstet Gynecol. 1986;68:448–451. [PubMed] [Google Scholar]
- Neal JL, Lowe NK, Ahijevych KL, Patrick TE, Cabbage LA, Corwin EJ. “Active labor” duration and dilation rates among low-risk, nulliparous women with spontaneous labor onset: a systematic review. J Midwifery Womens Health. 2010;55:308–318. doi: 10.1016/j.jmwh.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JL, Lowe NK, Patrick TE, Cabbage LA, Corwin EJ. What is the slowest-yet-normal cervical dilation rate among nulliparous women with spontaneous labor onset? J Obstet Gynecol Neonatal Nurs. 2010;39:361–369. doi: 10.1111/j.1552-6909.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailit JL, Dierker L, Blanchard MH, Mercer BM. Outcomes of women presenting in active versus latent phase of spontaneous labor. Obstet Gynecol. 2005;105:77–79. doi: 10.1097/01.AOG.0000147843.12196.00. [DOI] [PubMed] [Google Scholar]
- Holmes P, Oppenheimer LW, Wen SW. The relationship between cervical dilatation at initial presentation in labour and subsequent intervention. BJOG. 2001;108:1120–1124. doi: 10.1111/j.1471-0528.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- Impey L, Hobson J, O’Herlihy C. Graphic analysis of actively managed labor: prospective computation of labor progress in 500 consecutive nulliparous women in spontaneous labor at term. Am J Obstet Gynecol. 2000;183:438–443. doi: 10.1067/mob.2000.105899. [DOI] [PubMed] [Google Scholar]
- Rahnama P, Ziaei S, Faghihzadeh S. Impact of early admission in labor on method of delivery. Int J Gynaecol Obstet. 2006;92:217–220. doi: 10.1016/j.ijgo.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk R, Zhang J, Chan L, Grewal J. Early versus late admission to labor/delivery, labor progress and risk of caesarean section in nulliparous women. Am J Obstet Gynecol. 2008;199(6 Suppl A):S49. [Google Scholar]
- Richterich R, Schafroth P, Aebi H. A study of lactic dehydrogenase isoenzyme pattern of human tissues by adsorption-elution on Sephadex-DEAE. Clin Chim Acta. 1963;8:178–192. doi: 10.1016/0009-8981(63)90155-4. [DOI] [PubMed] [Google Scholar]
- Wróblewski F, Gregory KF. Lactic dehydrogenase isozymes and their distribution in normal tissues and plasma and in disease states. Ann N Y Acad Sci. 1961;94:912–932. doi: 10.1111/j.1749-6632.1961.tb35584.x. [DOI] [PubMed] [Google Scholar]
- Roman W. Quantitative estimation of lactate dehydrogenase isoenzymes in serum. I. Review of methods and distribution in human tissues. Enzymologia. 1969;36:189–219. [PubMed] [Google Scholar]
- Geyer H, Riebschläger M. Effect of pregnancy on cytoplasmic and mitochondrial enzymes in human and animal myometrium. Acta Endocrinol. 1974;77:368–379. doi: 10.1530/acta.0.0770368. [DOI] [PubMed] [Google Scholar]
- Makkonen M, Puhakainen E, Hänninen O, Castrén O. Lactate dehydrogenase isoenzymes in human myometrium during pregnancy and labor. Acta Obstet Gynecol Scand. 1982;61:35–37. doi: 10.3109/00016348209156948. [DOI] [PubMed] [Google Scholar]
- Makkonen M. Myometrial energy metabolism during pregnancy and normal and dysfunctional labor. Acta Obstet Gynecol Scand. 1977;71(Suppl):1–68. [PubMed] [Google Scholar]
- Hawkins DF, Whyley GA. The nature of the lactate dehydrogenase isoenzymes in human placenta and related tissues. Clin Chim Acta. 1966;13:713–719. doi: 10.1016/0009-8981(66)90136-7. [DOI] [PubMed] [Google Scholar]
- Meade BW, Rosalki SB. The origin of increased maternal serum enzyme activity in pregnancy and labour. J Obstet Gynaecol Br Commonw. 1963;70:862–868. doi: 10.1111/j.1471-0528.1963.tb04992.x. [DOI] [PubMed] [Google Scholar]
- Parratt J, Taggart M, Wray S. Abolition of contractions in the myometrium by acidification in vitro. Lancet. 1994;344:717–718. doi: 10.1016/S0140-6736(94)92209-8. [DOI] [PubMed] [Google Scholar]
- Parratt JR, Taggart MJ, Wray S. Functional effects of intracellular pH alteration in the human uterus: simultaneous measurements of pH and force. J Reprod Fertil. 1995;105:71–75. doi: 10.1530/jrf.0.1050071. [DOI] [PubMed] [Google Scholar]
- Pierce SJ, Kupittayanant S, Shmygol T, Wray S. The effects of pH change on Ca(++) signaling and force in pregnant human myometrium. Obstet Gynecol. 2003;188:1031–1038. doi: 10.1067/mob.2003.229. [DOI] [PubMed] [Google Scholar]
- Monir-Bishty E, Pierce SJ, Kupittayanant S, Shmygol A, Wray S. The effects of metabolic inhibition on intracellular calcium and contractility of human myometrium. BJOG. 2003;110:1050–1056. doi: 10.1111/j.1471-0528.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- Abraham NZ, Carty RP, DuFour R, Pincus MR. In: Henry’s Clinical Diagnosis and Management by Laboratory Methods. 21. McPherson RA, Pincus MR, editor. China: Elsevier Inc; 2007. Clinical Enzymology. [Google Scholar]
- Heimback DP, Prezyna AP. Lactic dehydrogenase in pregnancy and the puerperium. Am J Obstet Gynecol. 1960;79:108–112. doi: 10.1016/0002-9378(60)90369-0. [DOI] [PubMed] [Google Scholar]
- Konttinen A, Pyörälä T. Serum enzyme activity in late pregnancy, at delivery, and during puerperium. Scand J Clin Lab Invest. 1963;15:429–435. doi: 10.3109/00365516309079765. [DOI] [PubMed] [Google Scholar]
- Sward J, Woytoń J, Dobryszycka W, Bauer A. The influence of parturition on some serum enzyme activities. Arch Immunol Ther Exp (Warsz) 1972;20:273–275. [PubMed] [Google Scholar]
- Atuk NO, Wax SH, Word BH, McGaughey HS, Corey EL, Wood JE. Observations of the steady state of lactic dehydrogenase activity across the human placental membrane. Am J Obstet Gynecol. 1961;82:271–276. [PubMed] [Google Scholar]
- Kristensen SR, Horder M, Pedersen GT. Reference values for six enzymes in plasma from newborns and women at delivery. Scand J Clin Lab Invest. 1979;39:777–784. doi: 10.3109/00365517909108171. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Schwartz ED, Schwartz MK. Lactic acid dehydrogenase during pregnancy and puerperium. Obstet Gynecol. 1959;13:163–165. doi: 10.1097/00006250-195902000-00004. [DOI] [PubMed] [Google Scholar]
- Fylling P. Serum lactic dehydrogenase activity in umbilical cord, at the end of labor in normal women, and in uncomplicated puerperium. Scand J Clin Lab Invest. 1961;13:264–267. doi: 10.3109/00365516109137277. [DOI] [PubMed] [Google Scholar]
- Zhang J, Troendle JF, Yancey MK. Reassessing the labor curve in nulliparous women. Am J Obstet Gynecol. 2002;187:824–828. doi: 10.1067/mob.2002.127142. [DOI] [PubMed] [Google Scholar]
- Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD, Hatjis CG, Ramirez MM, Bailit JL, Gonzalez-Quintero V, Hibbard JU, Hoffman MK, Kominiarek M, Learman LA, Van Veldhuisen P, Troendle J, Reddy UM. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281–1287. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EA. Primigravid labor: a graphicostatistical analysis. Obstet Gynecol. 1955;6:567–589. doi: 10.1097/00006250-195512000-00001. [DOI] [PubMed] [Google Scholar]
- Friedman EA. Labor: Clinical evaluation and management. New York: Appleton; 1978. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, New Jersey: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Richards AH, Hilf R. Effect of estrogen administration on glucose 6-phosphate dehydrogenase and lactate dehydrogenase isoenzymes in rodent mammary tumors and normal mammary glands. Cancer Res. 1972;32:611–616. [PubMed] [Google Scholar]
- Burke RE, Harris SC, McGuire WL. Lactate dehydrogenase in estrogen-responsive human breast cancer cells. Cancer Res. 1978;38:2773–2776. [PubMed] [Google Scholar]
- Nagy I, Hirka G, Kurcz M, Anda E, Baranyai P. The role of estrogens in the regulation of lactate dehydrogenase activity and its submolecular organization in rat anterior pituitary. Endokrinologie. 1978;71:1–12. [PubMed] [Google Scholar]
- Nagy I, Hirka G, Kurcz M, Baranyai P. Changes of lactic dehydrogenase activity and combination of its subunits in rat anterior pituitary during puberty and in maturity, as well as during estrous cycle on the actue effect of estradiol and after castration. Endokrinologie. 1978;71:13–24. [PubMed] [Google Scholar]
- Hagerman DD, Wellington FM. Serum lactic dehydrogenase activity during pregnancy and in the newborn. Am J Obstet Gynecol. 1959;77:348–351. doi: 10.1016/0002-9378(59)90237-6. [DOI] [PubMed] [Google Scholar]
- Maggiulli MJ, Gustafson JC, Rector WD, Hilf R. Enzyme activities in human endometrium, myometrium and leiomyomas of the uterus. Enzyme. 1977;22:13–18. doi: 10.1159/000458501. [DOI] [PubMed] [Google Scholar]
- Institute for Safe Medication Practices ISMP’s list of high-alert medications http://www.ismp.org/tools/highalertmedications.pdf23746740
- Clark SL, Simpson KR, Knox GE, Garite TJ. Oxytocin: new perspectives on an old drug. Am J Obstet Gynecol. 2009;200:35.e1–35.e6. doi: 10.1016/j.ajog.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Little WA. Serum lactic dehydrogenase in pregnancy. Obstet Gynecol. 1959;13:152–162. doi: 10.1097/00006250-195902000-00003. [DOI] [PubMed] [Google Scholar]
- Meade BW, Rosalki SB. Lactic dehydrogenase isoenzymes in pregnancy. Lancet. 1962;1:1407. [Google Scholar]
- Dobryszycka W, Bauer A, Woyton J, Sward J. Lactate dehydrogenase in labour. I. Isoenzymes of serum, cord serum and amniotic fluid. Enzymologia. 1970;39:166–176. [PubMed] [Google Scholar]
- Wieme RJ, Van Maercke Y. The fifth (electrophoretically slowest) serum lactic dehydrogenase as an index of liver injury. Ann N Y Acad Sci. 1961;94:898–911. doi: 10.1111/j.1749-6632.1961.tb35583.x. [DOI] [PubMed] [Google Scholar]
- Bauer A, Sward J, Woyton J, Dobryszycka W. Lactate dehydrogenase in labour. II. Isoenzymes of placenta and related tissues. Enzymologia. 1970;39:177–182. [PubMed] [Google Scholar]
- Weiner H. In: Textbook of biochemistry with clinical correlations. 6. Devlin TM, editor. Hoboken, NJ: Wiley-Liss; 2006. Enzymes: classification, kinetics, and control. [Google Scholar]