Abstract
Drug-induced liver injury (DILI) is a common clinical entity but is underreported due to various reasons. Cyclooxygenase-2 inhibitors like Celecoxib have been proven to be associated with lesser incidence of adverse drug reactions compared to other nonsteroidal anti-inflammatory drugs (NSAID). However, Celecoxib has been rarely reported to be associated with cholestasis and hepatitis. We present a young Hispanic female presented with cholestatic liver chemistries who has been taking Celecoxib for 3 weeks. Extensive workup did not support diagnosis of viral, autoimmune, or metabolic liver diseases. Liver biopsy revealed findings suggestive of secondary sclerosing cholangitis. Imaging studies were negative for large duct involvement, and endoscopy ruled out inflammatory bowel disease. Liver chemistries normalized after cessation of medication. We recommend that physician should be aware of this rare complication when prescribing Celecoxib.
1. Introduction
Cyclooxygenase-2 (COX-2) inhibitors have been known to be associated with lower incidence of adverse events especially upper gastrointestinal complications compared to other nonsteroidal anti-inflammatory drugs (NSAID) [1]. Celecoxib, a known COX 2 inhibitor, has been reported to have beneficial effects against various malignancies in animal models [2–5]. Celecoxib has also been rarely associated with pancreatic and hepatic diseases especially acute and chronic cholestasis [6, 7]. Bile duct injury associated with drug-induced cholestasis has been reported very rarely with penicillin group of antibiotics [8, 9]. However, to the best of our knowledge, bile duct injury in association with Celecoxib has been reported only on one occasion in the literature [10]. We present a rare case of acute cholestatic hepatitis and small bile duct injury associated with Celecoxib in a young Hispanic woman.
2. Case Presentation
A 34-year-old Hispanic woman presented to the emergency room (ER) with epigastric abdominal pain of 3 days duration. Pain is burning in nature, nonradiating and started insidiously 3 days prior to ER visit, which gradually worsened. She also reported nausea but denied vomiting, bowel, or urinary symptoms. She denied any fever, skin rashes, joint pains, loss of appetite, or loss of weight. She does not have any known medical conditions. She underwent breast cyst aspiration few years back. She never used tobacco, alcohol, or recreational drugs. She is sexually active with one partner using barrier contraceptive methods. She recently travelled to Dominican Republic and did not report any illness during her visit. However, she underwent a minor gynecological procedure for abnormal Papanicolaou (Pap) smear during her visit to Dominican Republic. She is not allergic to any medications.
On initial evaluation in ER, she was found to have jaundice and minimal epigastric tenderness. She received intravenous Ranitidine, and basic labs were drawn. She was found to have abnormal liver function tests and admitted to medical floor for further workup and management. Subsequently, medical team on floor requested gastroenterology evaluation.
On initial encounter with gastroenterology team on medical floor, the patient reported that her abdominal pain has resolved. She is well built and well-nourished woman, not in distress, and noted to have icterus. Her abdominal examination is benign without any tenderness, organomegaly or clinically detectable free fluid. Her cardiovascular, respiratory, and neurological examination is grossly normal. She does not have any skin rashes and scratch marks, and musculoskeletal examination was within normal limits.
Laboratory data revealed normocytic anemia with hemoglobin of 12, white cell count of 5.8, and platelets are within normal range. Her coagulation profile, electrolytes, blood urea nitrogen, and creatinine are normal. She had elevated alanine aminotransferase (ALT) of 458 units/liter, aspartate aminotransferase (AST) of 244 units/liter, alkaline phosphatase (ALP) of 231 units/liter, and total bilirubin of 3.4 milligrams/deciliter with predominant proportion of direct bilirubin, which is 2.8 milligrams/deciliter (Table 1). Her albumin and total protein are within normal limits. She is immune to hepatitis A and tested negative for hepatitis B and hepatitis C. Abdominal sonogram has not revealed any gallstones and has been reported as having common bile duct caliber of 3 mm. Computer tomogram (CT) of abdomen has not reported any abnormality.
Table 1.
Liver chemistries.
| Day 1 | Day 4 | Day 16 | 1 month | 6 months | |
|---|---|---|---|---|---|
| Total protein1 | 7 | 7.3 | 8 | 7.6 | 7.7 |
| Albumin1 | 4.3 | 4.1 | 4.7 | 4.3 | 4.5 |
| Alanine aminotransferase2 | 458 | 402 | 147 | 10 | 12 |
| Aspartate aminotransferase2 | 244 | 177 | 40 | 13 | 15 |
| Alkaline phosphatase2 | 231 | 238 | 236 | 89 | 60 |
| Total bilirubin3 | 3.4 | 3.4 | 1.1 | 0.7 | 0.4 |
| Direct bilirubin3 | 2.8 | 2.2 | 0.4 | 0.3 | 0.1 |
1Grams/dL; 2milligrams/dL; 3units/liter.
On further questioning, she revealed that she has been taking Celecoxib since three weeks as prescribed by her gynecologist in Dominican Republic. As there is no evidence of biliary obstruction or cholangitis, initial impression was probable drug-induced liver injury. However, further laboratory tests were ordered, including anemia workup and markers to diagnose common autoimmune and metabolic liver disorders. She has been advised to stop Celecoxib. She remained stable during her hospital stay and was discharged to home. She has been scheduled to follow up in gastroenterology clinic.
On followup in gastroenterology clinic, she remained asymptomatic. Her anemia workup revealed positive hemoglobin electrophoresis for sickle cell trait. Her ceruloplasmin levels are within normal limits, and transferrin saturation is 14% ruling out Wilson disease and hemochromatosis, respectively. She has been tested negative for anti-mitochondrial, anti-smooth muscle, and anti-liver kidney microsomal antibodies and was found to have normal immunoglobulin G levels. As workup has been negative for common viral, metabolic, and autoimmune liver diseases, patient was offered CT-guided liver biopsy. The patient underwent liver biopsy, which was uneventful.
Liver biopsy findings showed periductal fibrosis and findings suggestive of primary or secondary sclerosing cholangitis (Figure 1). On subsequent followup visits, liver function tests improved and reached baseline. However, in view of liver biopsy findings suggestive of sclerosing cholangitis, magnetic retrograde cholangiopancreatogram (MRCP) has been ordered which did not reveal any large bile duct abnormalities. The patient underwent esophagogastroduodenoscopy, which has not revealed any major lesions, and the biopsy tested negative for Helicobacter pylori. She underwent colonoscopy, which has not revealed any findings suggestive of inflammatory bowel disease. Currently, the patient is asymptomatic, and her liver function tests have been normal (Table 1).
Figure 1.
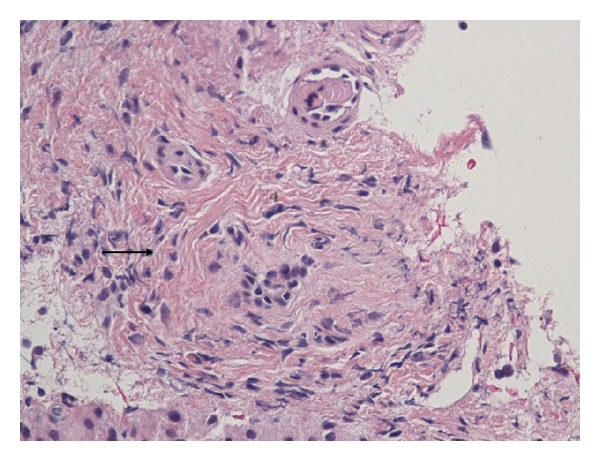
Liver biopsy portal tract and periductal fibrosis with narrowing of portal vein and it is branches.
3. Discussion
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been noted to be associated with significant incidence of adverse drug reactions [1, 11]. However, COX-2 inhibitors like Celecoxib have been noted to be on the lower end of disease spectrum of adverse drug reactions especially upper gastrointestinal disorders [1]. It has also been noted that Celecoxib has been reported to have beneficial outcomes in gastrointestinal and liver neoplasms in animal models [12, 13].
However, Celecoxib has been rarely reported to be associated with acute and chronic cholestatic hepatitis [6, 7, 10, 14–16]. In very rare instances, abnormal liver chemistries persisted up to 18 months [6], and in one case, the clinical course worsened requiring liver transplantation [10].
Our case stressed the importance of obtaining detailed history of patient's medications and supplements. Cholestatic hepatitis has been reported in association with Celecoxib in the past; however, this case has rare feature of involving small biliary ducts and pathological findings resembling small duct sclerosing cholangitis [10]. Endoscopic workup has been carried out to rule out inflammatory bowel disease, which is a common association in patients with primary sclerosing cholangitis. Patient's liver chemistries normalized after cessation of medication, which supports the diagnosis of drug-induced liver injury (Table 1).
Drug-induced liver injury is common among general and hospitalized population, but due to various reasons, it has been underreported [11, 17]. However, abnormal liver chemistries always mandate complete workup to lead to particular diagnosis, including laboratory markers of viral, metabolic, and autoimmune liver diseases and ultimately liver biopsy guided by appropriate clinical situation [18, 19]. Detailed and appropriate history of medications and supplements always plays a major role in the work up of abnormal liver chemistries [18–20], which has been proved again in this case. Timely recognition and discontinuation of offending agent may prevent life-threatening complications [10] and improve patient outcomes.
References
- 1.Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS Project) Drug Safety. 2012;35:1127–1146. doi: 10.1007/BF03261999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong H, Li X, Zhang CL, et al. Transcatheter arterial embolization followed by octreotide and celecoxib synergistically prolongs survival of rabbits with Hepatic VX2 allografts. Journal of Digestive Diseases. 2013;14(1):29–37. doi: 10.1111/1751-2980.12001. [DOI] [PubMed] [Google Scholar]
- 3.Ozturk H, Gezici A, Ozturk H. The effect of celecoxib, a selective COX-2 inhibitor, on liver ischemia/reperfusion-induced oxidative stress in rats. Hepatology Research. 2006;34(2):76–83. doi: 10.1016/j.hepres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Márquez-Rosado L, Trejo-Solís MC, García-Cuéllar CM, Villa-Treviño S. Celecoxib, a cyclooxygenase-2 inhibitor, prevents induction of liver preneoplastic lesions in rats. Journal of Hepatology. 2005;43(4):653–660. doi: 10.1016/j.jhep.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Zuo C, Li Z, Zhou X, Ouyang Y, Zhou Z, Zeng L. Inhibitory effects of cyclooxygenase-2 inhibitor celecoxib on growth and angiogenesis of human liver cancer HepG2 cell xenografts in small nude mice. Ai Zheng. 2006;25(4):414–420. [PubMed] [Google Scholar]
- 6.Chamouard P, Walter P, Baumann R, Poupon R. Prolonged cholestasis associated with short-term use of celecoxib. Gastroenterologie Clinique et Biologique. 2005;29(12):1286–1288. doi: 10.1016/s0399-8320(05)82223-7. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo-Jimenez R, Nurnberger M. Celecoxib-induced acute pancreatitis and hepatitis: a case report. Archives of Internal Medicine. 2000;160(4):553–554. doi: 10.1001/archinte.160.4.553. [DOI] [PubMed] [Google Scholar]
- 8.Eckstein RP, Dowsett JF, Lunzer MR. Flucloxacillin induced liver disease: histopathological findings at biopsy and autopsy. Pathology. 1993;25(3):223–228. doi: 10.3109/00313029309066576. [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Jang YR, Lee JW, et al. A case of amoxicillin-induced hepatocellular liver injury with bile-duct damage. The Korean Journal of Hepatology. 2011;17(3):229–232. doi: 10.3350/kjhep.2011.17.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Hajj II, Malik SM, Alwakeel HR, Shaikh OS, Sasatomi E, Kandil HM. Celecoxib-induced cholestatic liver failure requiring orthotopic liver transplantation. World Journal of Gastroenterology. 2009;15(31):3937–3939. doi: 10.3748/wjg.15.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu HM, Chen Y, Xu J, Zhou Q. Drug-induced liver injury in hospitalized patients with notably elevated alanine aminotransferase. World Journal of Gastroenterology. 2012;18:5972–5978. doi: 10.3748/wjg.v18.i41.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie H, Gao L, Chai N, et al. Potent cell growth inhibitory effects in hepatitis B virus X protein positive hepatocellular carcinoma cells by the selective cyclooxygenase-2 inhibitor celecoxib. Molecular Carcinogenesis. 2009;48(1):56–65. doi: 10.1002/mc.20455. [DOI] [PubMed] [Google Scholar]
- 13.Salcido-Neyoy ME, Sierra-Santoyo A, Beltrán-Ramírez O, Macías-Pérez JR, Villa-Treviño S. Celecoxib enhances the detoxification of diethylnitrosamine in rat liver cancer. World Journal of Gastroenterology. 2009;15(19):2345–2350. doi: 10.3748/wjg.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galan MV, Gordon SC, Silverman AL. Celecoxib-induced cholestatic hepatitis. Annals of Internal Medicine. 2001;134(3, article 254) doi: 10.7326/0003-4819-134-3-200102060-00028. [DOI] [PubMed] [Google Scholar]
- 15.O’Beirne JP, Cairns SR. Drug points: cholestatic hepatitis in association with celecoxib. British Medical Journal. 2001;323(7303, article 23) doi: 10.1136/bmj.323.7303.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grieco A, Miele L, Giorgi A, Civello IM, Gasbarrini G. Acute cholestatic hepatitis associated with celecoxib. Annals of Pharmacotherapy. 2002;36(12):1887–1889. doi: 10.1345/aph.1C110. [DOI] [PubMed] [Google Scholar]
- 17.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36(2):451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 18.Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1367–1384. doi: 10.1053/gast.2002.36061. [DOI] [PubMed] [Google Scholar]
- 19.Lichtensin GR. American gastroenterological association medical position statement: evaluation of liver chemistry tests. Gastroenterology. 2002;123(4):1364–1366. doi: 10.1053/gast.2002.36060. [DOI] [PubMed] [Google Scholar]
- 20.Barritt AS, IV, Lee J, Hayashi PH. Detective work in drug-induced liver injury: sometimes it is all about interviewing the right witness. Clinical Gastroenterology and Hepatology. 2010;8(7):635–637. doi: 10.1016/j.cgh.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


