Abstract
A 15-yr-old male patient with congenital adrenal hyperplasia (CAH) was referred to our department with a one year history of gradual worsening of tremors. He was diagnosed with salt-wasting 21-hydroxylase deficiency CAH at 40 d old and was started on hydrocortisone, fludrocortisone and salt. He was found to have hypertension at 8 yr of age. Detailed investigations failed to detect any cause for secondary hypertension. Physical findings on the current hospitalization objectified obesity, blood pressure of 150/80 mmHg, postural and action tremor, left cerebellar syndrome, reflex tetra pyramidal syndrome and mental decline. Brain magnetic resonance imaging (MRI) showed bilateral periventricular white matter hyperintensity that was more pronounced in the posterior regions and associated with cortico-subcortical atrophy and complete agenesis of the corpus callosum. All investigations for leukoencephalopathy were negative. A diagnosis of brain MRI abnormalities related to CAH was made, and the patient received symptomatic treatment of tremors. Our case report provides evidence of an increased frequency of brain MRI abnormalities in CAH. The literature suggests hormonal imbalance and exposure to excess exogenous glucocorticoids as main probable mechanisms. Thus, in clinical practice, CAH should be considered as one of the possible causes of brain white matter involvement associated with or without cerebral atrophy.
Keywords: congenital adrenal hyperplasia, 21-Hydroxylase deficiency, MRI, leukoencephalopathy, corticosteroid
Introduction
Congenital adrenal hyperplasia (CAH) is a rare inherited recessive disorder of adrenal steroidogenesis due, in the vast majority of cases, to deficiency of the enzyme 21-hydroxylase (21 OH). Overproduction in 17-OH progesterone and androgen as deficiency of cortisol and aldosterone could represent a harmful environment for the brain(1). Only a few studies have described brain magnetic resonance imaging (MRI) abnormalities in CAH(2,3,4). In this report, we describe a 15-yr-old male patient with salt-wasting CAH and brain MRI abnormalities. We also discuss the potential mechanisms involved in the pathogenesis of these abnormalities.
Case Report
A 15-yr-old male patient was referred to our department with a one-year history of gradual worsening of tremors. He was born at term, weighing 2,900 g, to healthy, second-degree consanguineous parents. He was diagnosed, at 40 d old, with salt-wasting 21-hydroxylase deficiency CAH based on acute dehydration, salt loss syndrome and a 17-OH progesterone level of 215 ng/ml, and he was started on hydrocortisone, fludrocortisone and salt. He was found to have hypertension at 8 yr of age and responded well to Nifedipine. Detailed investigations (renal Doppler ultrasound, adrenal CT scan, urinary metanephrine, 11-Deoxycortisol, plasma renin activity, aldosterone levels and cortisolemia) failed to detect any cause for secondary hypertension, and a diagnosis of essential hypertension was made. During follow-up, he always maintained good adherence to treatment. Two adrenal crises occurred at one and nine years of age and were precipitated by viral illnesses. The average hydrocortisone dose was 16 mg/m2 per day, and fludrocortisone was continued at a dose of 100 µg twice daily.
Physical findings on the current hospitalization were as follows: height of 158 cm (–1 SD), weight of 85 kg (>2 SD), body mass index (BMI) of 34.13 kg/m2 (greater than the 97th centile) and blood pressure of 150/80 mmHg. Neurological examination objectified postural and action tremor in upper limbs with static kinetic cerebellar syndrome more pronounced on the left, tetra pyramidal reflex syndrome and moderate mental decline. The results of an ophthalmologic were normal.
The results of biochemical investigations were as follow: basal level of 17-OH progesterone at 12 ng/ml, ACTH of 77 pg/ml (reference range: 10–50 pg/ml) and androstenedione of 2.12 nmol/l (reference range: 0.77–1.82 nmol/l).
Brain MRI showed bilateral periventricular white matter hyperintensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images, which is consistent with leukoencephalopathy (Figs. 1, 2). The lesions predominated in the posterior regions and were associated with cortico-subcortical atrophy (Figs. 1, 2) and complete agenesis of the corpus callosum (Figs. 1–3). The patient was extensively evaluated. Routine blood and urine tests were normal as well as blood tests for liver function, lactate, amino acids, ceruloplasmin, inflammatory markers and urine amino and organic acids.
Fig. 1.
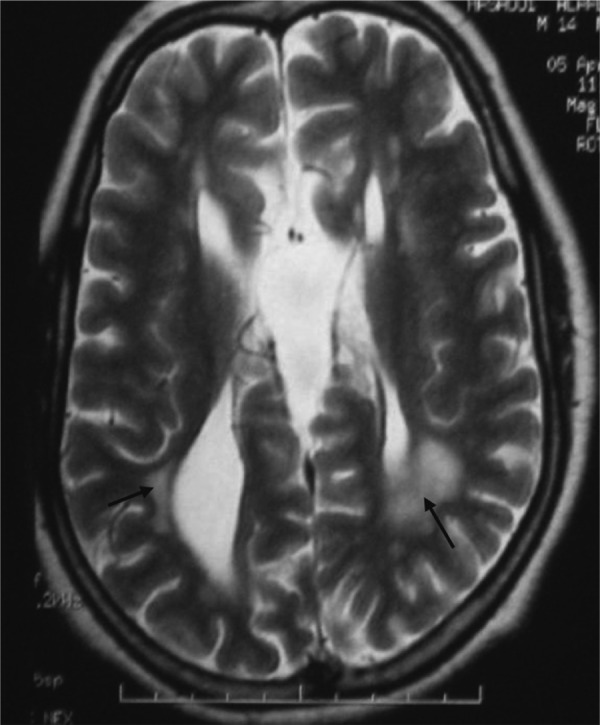
T2-weighted axial MRI sequence showing bilateral periventricular white matter hyperintensity (arrow) and cortico-subcortical atrophy.
Fig. 2.
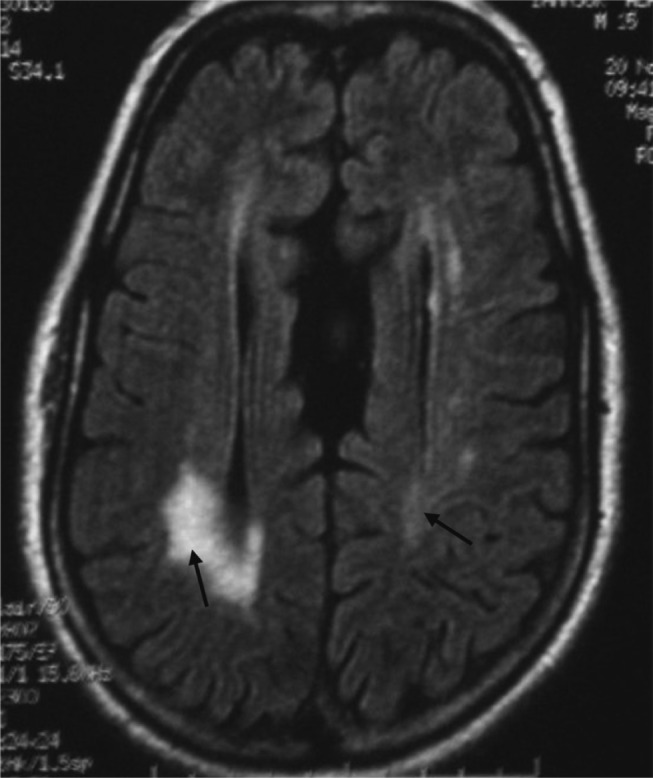
FLAIR-weighted axial MRI sequence showing bilateral periventricular white matter hyperintensity (arrow) located mainly in the posterior regions with agenesis of the corpus callosum.
Fig. 3.
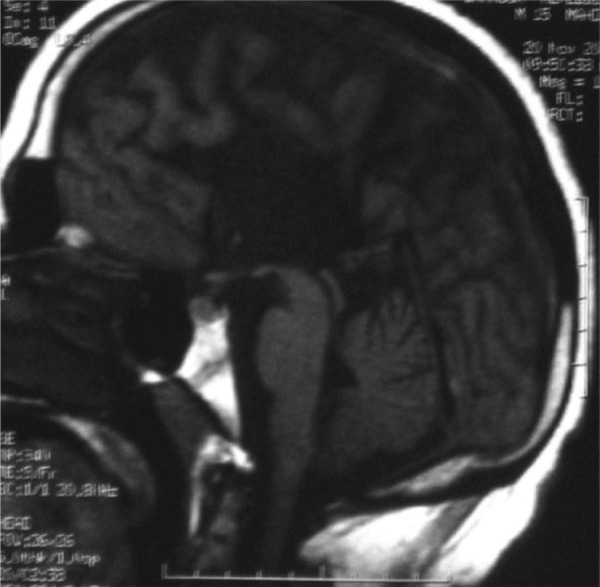
T1-weighted sagittal MRI sequence showing complete agenesis of the corpus callosum.
Lumbar puncture revealed normal CSF protein and glucose levels, and neither oligoclonal bands nor evidence of infection were detected. The serum leukocyte activity of Arylsulfatase A was normal. Autoantibodies to thyroid peroxidase and thyroglobulin as well as antinuclear antibodies were negative. The diagnosis of brain MRI abnormalities associated with CAH was made. Following administration of propranolol, the tremors decreased moderately.
Discussion
CAH is an inherited recessive disorder of adrenal steroidogenesis due to deficiency in one of the enzymes in the synthetic pathway. The most common form of CAH is caused by a 21-OH deficiency. Adrenal enzyme deficiency causes underproduction of cortisol and, in turn, hypersecretion of corticotropin. High plasma corticotropin levels lead to overproduction of intermediate metabolites, such as 17-OH progesterone, and androgens.
Past reports have suggested that brain white matter could be involved in CAH(1,2,3,4), and brain MRI abnormalities related to CAH could be detected in up to 46% of the cases(3). We reviewed the literature about brain MRI abnormalities related to CAH. Table 1 summarizes the results of major previous studies.
Table 1. Major previous reports of brain MRI abnormalities related to congenital adrenal hyperplasia.
| Authors and year | Cases | Age range | Disease phenotype | Neurologic manifestations | Main MRI findings | |
| N | % | (yr) | ||||
| Sinforiani et al., 1994 | 4 | 27 | >16 | SW: 2 SV: 2 | No | - Leukoencephalopathy- Cerebral atrophy- Ventricular dilatation |
| Nass et al., 1997 | 18 | 46.1 | 4–33 | SW: 12 SV: 6 | None except for one with known stroke | - Leukoencephalopathy- Cerebral atrophy |
| Bergamashi et al., 2006 | 10 | 45 | 16–23 | SW: 6 SV: 4 | No | Leukoencephalopathy |
| Gaudiano et al., 2010 | 3* | 100 | 27–54 | Proband: classic form Parents: heterozygotes for CYP21 mutation | Yes** | - Leukoencephalopathy- Cerebellar atrophy- Cerebral atrophy- Ventricular dilatation |
MRI: magnetic resonance imaging. SV: simple virilizing. SW: salt wasting. %: Percentage of patients with brain abnormalities in the study population. *A family with congenital adrenal hyperplasia and brain abnormalities. **Proband with postural tremor and cerebellar syndrome. The mother had blurry vision, diplopia and paresthesia, and the father had no neurologic manifestations.
MRI lesions are not specific and do not exhibit a side prevalence(2). They are often white matter changes, consistent with leukoencephalopathy, and cerebral atrophy(1,2,3,4). Disease phenotype does not seem to affect the frequency of these lesions, which may even be present in heterozygous carriers of CYP21 mutations(1, 3, 5).
In 1997, Nass et al. described some patients with CAH and white matter lesions that mimicked demyelinating diseases but without neurologic manifestations(3). Furthermore, Bergamaschi, reported a patient in 2004 with CAH and typical relapsing-remitting multiple sclerosis. This could be due to a possible link between MS susceptibility locus and the CYP21 gene, which is located on chromosome 6p21(6).
Brain MRI lesions in CAH are most often subclinical or, less frequently, associated with unremarkable neurological signs(2). Recently, it has been suggested that subclinical lesions may increase the risk of developing physical disabilities and declines in motor performance(7).
The patient described here showed neurologic signs such as tremors and left kinetic cerebellar and tetra pyramidal reflex syndrome. The diagnosis of leukoencephalopathy associated with CAH was achieved after exclusion of other possible causes including metabolic, genetic, infectious and immunological affections. Furthermore, the periventricular localization of white matter abnormalities does not argue for hypertensive Leukoencephalopathy, which is localized usually to the parietal and occipital lobes(8).
In our observation, we also reported agenesis of the corpus callosum, which, unlike atrophy, is an unusual brain abnormality in CAH. To our knowledge, this is the second case reported in the literature of corpus callosum agenesis associated with CAH(9). This association appears to be fortuitous.
The mechanisms that could explain these MRI abnormalities remain poorly understood. Several hypotheses have been suggested. Hormonal imbalance related to deficiency of cortisol and aldosterone and overproduction of 17-OH progesterone and androgen would cause destabilization of the myelin molecule leading to its degeneration(1). In addition, exogenous glucocorticoids may interfere with the process of neuronal maturation and myelination by inhibiting the proliferation of oligodendrocyte precursors(1). Thus both, overdose and discontinuous therapy have been suggested as factors favoring the emergence of brain MRI abnormalities(2).
Aldosterone has a regulating effect on the arterial structure by stimulating the proliferation of collagen and fibroblasts via direct activation of local receptors. In this regard, aldosterone deficiency could induce subclinical cerebral ischemia resulting in alteration of the cerebral arterial structure(1). This ischemia could also be indirectly promoted by glucocorticoids that induce an alteration of carbohydrate or lipid metabolism or platelet aggregability(3).
The relationship between cerebral atrophy and high doses of exogenous glucocorticoids is well established in the literature. In cases of CAH, atrophy seems to involve preferentially the temporal lobes, corpus callosum, amygdala and hippocampus(3, 10).
Our case report and review of the literature provides evidence of an increased frequency of brain MRI abnormalities in CAH. Thus, in clinical practice, when a patient presents with neuro-imaging that demonstrates diffuse white matter involvement with or without cerebral atrophy, CAH should be considered as one of the possible causes.
References
- 1.Bergamaschi R, Livieri C, Uggetti C, Candeloro E, Egitto MG, Pichiecchio A, et al. Brain white matter impairment in congenital adrenal hyperplasia. Arch Neurol 2006;63: 413–6 [DOI] [PubMed] [Google Scholar]
- 2.Sinforiani E, Livieri C, Mauri M, Bisio P, Sibilla L, Chiesa L, et al. Cognitive and neuroradiological findings in congenital adrenal hyperplasia. Psychoneuroendocrinology 1994;19: 55–64 [DOI] [PubMed] [Google Scholar]
- 3.Nass R, Heier L, Moshang T, Oberfield S, George A, New MI, et al. Magnetic resonance imaging in the congenital adrenal hyperplasia population: increased frequency of white-matter abnormalities and temporal lobe atrophy. J Child Neurol 1997;12: 181–6 [DOI] [PubMed] [Google Scholar]
- 4.Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab 2003;88: 1760–5 [DOI] [PubMed] [Google Scholar]
- 5.Gaudiano C, Malandrini A, Pollazzon M, Murru S, Mari F, Renieri A, et al. Leukoencephalopathy in 21-beta hydroxylase deficiency: report of a family. Brain Dev 2010;32: 421–4 [DOI] [PubMed] [Google Scholar]
- 6.Bergamaschi R, Livieri C, Candeloro E, Uggetti C, Franciotta D, Cosi V. Congenital adrenal hyperplasia and multiple sclerosis: is there an increased risk of multiple sclerosis in individuals with congenital adrenal hyperplasia? Arch Neurol 2004;61: 1953–5 [DOI] [PubMed] [Google Scholar]
- 7.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT , Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 2005;53: 649–54 [DOI] [PubMed] [Google Scholar]
- 8.Gümüş H, Per H, Kumandaş S, Yikilmaz A. Reversible posterior leukoencephalopathy syndrome in childhood: report of nine cases and review of the literature. Neurol Sci 2010;31: 125–31 [DOI] [PubMed] [Google Scholar]
- 9.Lajic S, Wedell A, Bui TH, Ritzén EM, Holst M. Long term somatic follow-up of prenatally treated children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 1998;83: 3872–80 [DOI] [PubMed] [Google Scholar]
- 10.Brown ES, Woolston DJ, Frol AB. Amygdala volume in patients receiving chronic corticosteroid therapy. Biol Psychiatry 2008;63: 705–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


