Abstract
Agranulocytosis is an extremely serious, although rare, adverse effect of antithyroid drugs (ATDs), including methimazole (MMI) and propylthiouracil (PTU), in children and adolescents. There are few reports about the characteristics of ATD-induced agranulocytosis in Japanese children and adolescents. This report presents the cases of three girls with ATD-induced agranulocytosis and a retrospective analysis of 18 patients with ATD-induced agranulocytosis, whose cases had been referred to the drug manufacturer, Chugai Pharmaceutical Co., Ltd. Our 3 patients, ranging in age from 12 to 14 yr, developed ATD-induced agranulocytosis between the 15th and 57th day of ATD treatment for hyperthyroidism. Fever and sore throat were the earliest symptoms of agranulocytosis. The patients were rescued by ceasing ATD therapy and administering antibiotics, potassium iodide, glucocorticoid, immunoglobulin and granulocyte colony-stimulating factor (G-CSF). We retrospectively analyzed 18 cases of ATD-induced agranulocytosis treated with MMI in 16 cases and PTU in 2 cases. Twelve patients were treated with 20–45 mg/d MMI. Agranulocytosis developed between the 15th and 1,344th day of therapy. In conclusion, considering the risk of ATD-induced agranulocytosis, we recommend low-dose MMI therapy for treatment of Graves’ disease.
Keywords: hyperthyroidism, antithyroid drug, agranulocytosis, thyroidectomy, radioactive iodine therapy
Introduction
Graves’ disease is the most common cause of thyrotoxicosis in childhood, affecting 0.02% of children and adolescents (1). Current treatment approaches involve antithyroid medications, radioactive iodine and thyroidectomy. Antithyroid drugs (ATDs; methimazole (MMI), carbimazole and propylthiouracil (PTU)) have been widely used as a first-line therapy to treat hyperthyroidism in children and adults for more than 50 yr because they do not cause permanent hypothyroidism and limit patients’ exposure to radiation (2, 3). Remission is achieved in less than 30% of children after treatment with ATDs; thus, long-term ATD treatment or an alternative therapy is required (4).
Agranulocytosis is an extremely serious adverse effect of ATDs, occurring in 0.1% to 0.2% of medicated children and adolescents with Graves’ disease, whereas it occurs in 0.3% to 0.4% of adult patients (1). Nevertheless, there are few reports about the characteristics of ATD-induced agranulocytosis in Japanese children and adolescents. Our goals are to provide insights into ATD-induced agranulocytosis in Japanese children and adolescents. This report presents 3 cases of girls with ATD-induced agranulocytosis and includes a retrospective analysis of 18 patients (including the 3 whose reports are detailed here) with ATD-induced agranulocytosis between 1995 and 2009, whose cases were reported to Chugai Pharmaceutical Co., Ltd., the Japanese manufacturer of ATDs.
Case Report 1
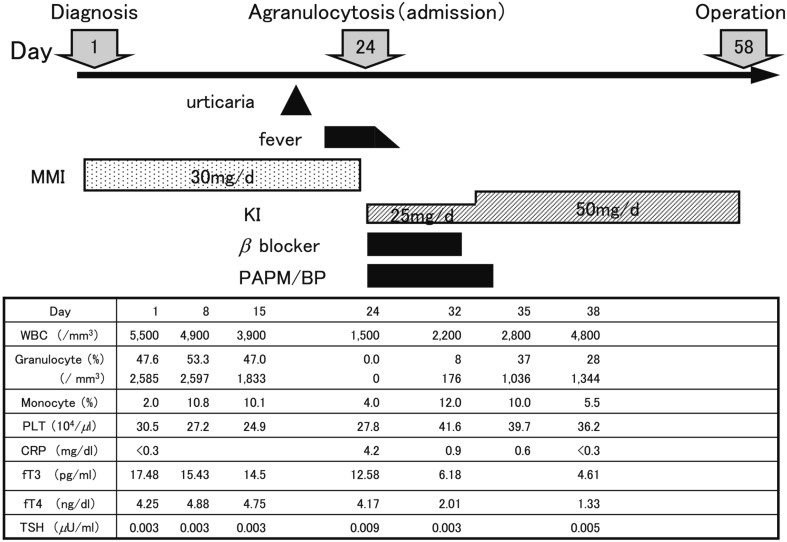
A 12-yr-old girl was referred to our department with a large diffuse goiter (Shichijo: grade III), palpitation and tremor. Laboratory investigations showed a serum free T3 level of 17.48 pg/ml (electrochemiluminescence immunoassay, ECLIA; reference value, 2.3 to 4.3 pg/ml), serum free T4 level of 4.25 ng/dl (ECLIA; reference value, 0.9 to 1.7 ng/dl), serum TSH level of <0.003 µU/ml (ECLIA; reference value,0.5 to 5.0 µU/ml) and serum TSH receptor antibody level of 50.9 IU/l (TRAb; radioreceptor assay based on a preparation of human recombinant TSH receptors; normal upper limit 1.0 IU/l). She was diagnosed with thyrotoxicosis and received MMI therapy with an initial dose of 30 mg (0.63 mg/kg) daily. She suddenly developed a high fever and sore throat on day 23 of MMI treatment and was admitted to our hospital (Fig. 1). Her white blood cell (WBC) count was 1,500/mm3 with 0% granulocytes, hemoglobin level was 12.4 g/dl, platelet count was 27.8 * 104/mm3 and serum C-reactive protein (CRP) level was 4.2 mg/dl. Following prompt discontinuation of MMI, she was treated with potassium iodide (KI), beta blocker, and antibiotics. Administration of granulocyte colony-stimulating factor (G-CSF) was refused by the parents out of concern regarding the risk of additional adverse events. The WBC count reached 2,800/mm3 with 1,036/mm3 granulocytes on the 11th day of admission. Twenty days later, total thyroidectomy was performed at Ito hospital.
Fig. 1.
Time course of the laboratory data in Case 1. PAPM/BP: panipenem/betamipron.
Case Report 2
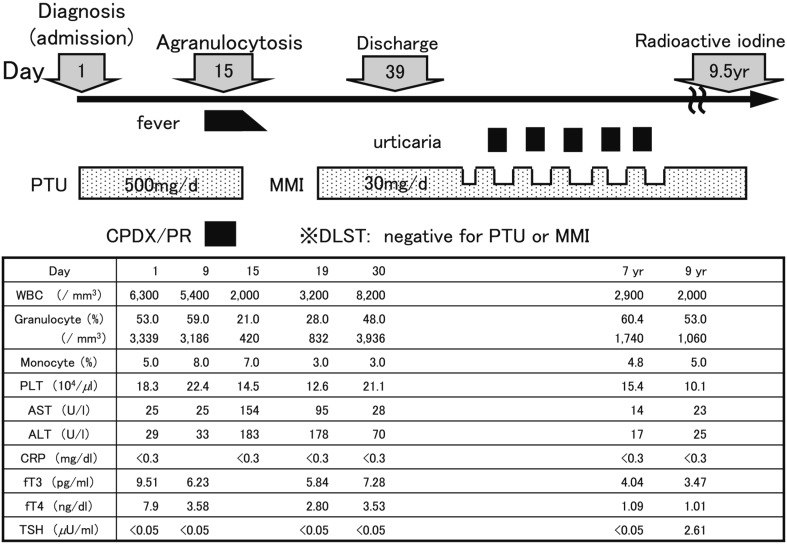
A 13-yr-old girl was referred to our department with a large diffuse goiter (Shichijo: grade III), palpitation, nervousness, increased appetite and tremor. Laboratory investigations showed a serum free T3 level of 9.51 pg/ml, serum free T4 level of 7.90 ng/dl, serum TSH level of <0.005 µU/ml and serum TRAb level of 11.6 IU/l. She was diagnosed with thyrotoxicosis and received PTU therapy with an initial dose of 500 mg (9.8 mg/kg) daily. She suddenly developed a high fever and sore throat on day 10 and was admitted to our hospital (Fig. 2). Her WBC count was 2,000/mm3 with 420/mm3 granulocytes, AST level was 154 U/l, ALT level was 183 U/l and serum CRP level was <0.3 mg/dl. PTU therapy was discontinued, and antibiotic therapy was initiated. Upon discontinuation of the medication, her fever resolved, and her WBC count reached 3,200/mm3 with 832/mm3 granulocytes. Two weeks later, she was changed to MMI therapy at a dose of 30 mg/d (0.59 mg/kg) and did not experience severe adverse events. Radioactive iodine was administered at the Ito hospital after 9.5 yr of ATD treatment.
Fig. 2.
Time course of the laboratory data in Case 2. CPDX/PR: cefpodoxime proxetil. DLST: drug-induced lymphocyte stimulation test.
Case Report 3
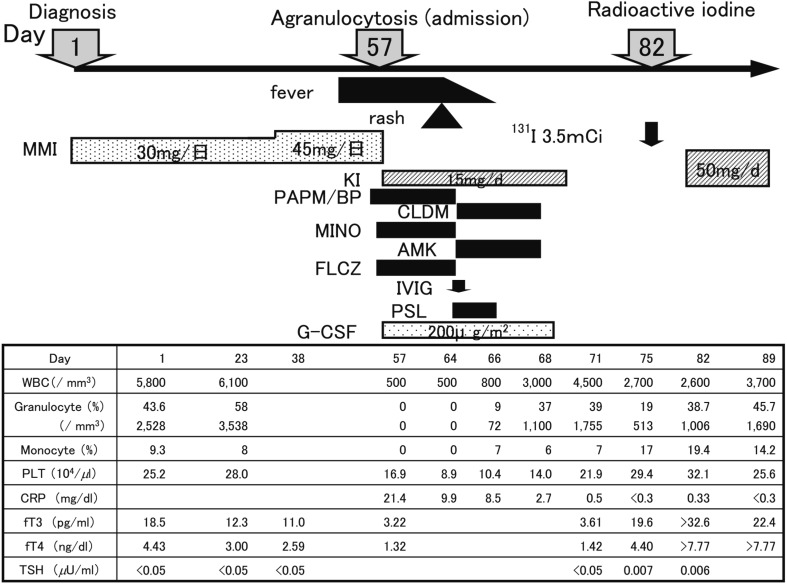
A 14-yr-old girl was referred to our department with a large diffuse goiter (Shichijo: grade III), fatigue and menstrual disturbances. Laboratory investigations showed a serum free T3 level of 18.5 pg/ml, serum free T4 level of 4.43 ng/dl, serum TSH level of <0.005 µU/ml and serum TRAb level of 89.9% (radioreceptor assay based on a preparation of the porcine thyroid membranes; normal upper limit 15%). She was diagnosed with thyrotoxicosis and received MMI therapy at an initial dose of 30 mg (0.64 mg/kg) daily. On the 42nd day after the initiation of MMI therapy, the dosage was increased to 45 mg/d (0.96 mg/kg) because the fT3 and fT4 levels remained high. On day 55 of treatment, she suddenly developed a high fever and sore throat and was admitted to our hospital (Fig. 3). Her WBC count was 500/mm3 with 0% granulocytes, platelet count was 16.9 * 104/mm3 and serum CRP level was 21.4 mg/dl. Bone marrow examination revealed maturation arrest of myeloid and erythroid cells with hyperplasia of megakaryocytic series. Following prompt discontinuation of MMI, she was treated with KI, antibiotics, G-CSF, glucocorticoid and immunoglobulin. The WBC count reached 3,000/mm3 with 1,100/mm3 granulocytes on the 11th day of admission. One month later, radioactive iodine was administered.
Fig. 3.
Time course of the laboratory data in Case 3. CLDM: clindamycin. MINO: minocycline. AMK: amikacin. FLCZ: fluconazole, IVIG: intravenous immunoglobulin. PSL: prednisolone.
Retrospective analysis of 18 cases
Eighteen patients with ATD-induced agranulocytosis referred to the drug manufacturer, Chugai Pharmaceutical Co., Ltd. between 1995 and 2009, were evaluated. Agranulocytosis was defined as a granulocyte count at or below 500/mm3. Eighteen patients were treated for Graves’ disease with MMI in 16 cases and PTU in 2 cases. The patients included 15 females and 3 males, ranging from 7 to 15 yr of age. The mean age was 12.8 ± 2.1 yr. The characteristics of these patients are summarized in Table 1.
Table 1. Clinical characteristics and outcomes of the 18 patients with ATD-induced agranulocytosis.
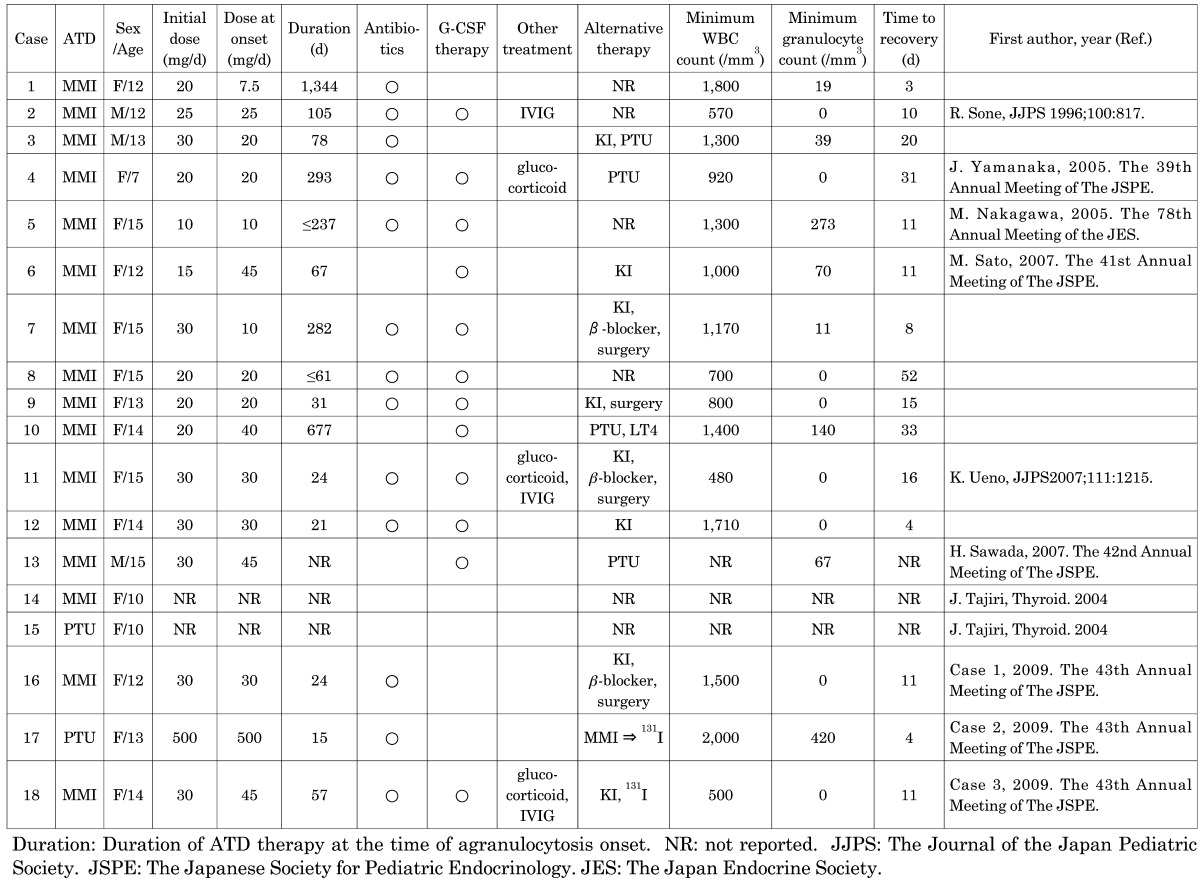
We retrospectively analyzed 15 cases of MMI-induced agranulocytosis, except case 14, for which detailed records were unavailable. The patients received MMI therapy with an initial dose of 10–30 mg daily. Three patients were treated with 7.5–10 mg/d, and 12 patients were treated with 20–45 mg/d prior to the onset of agranulocytosis. Agranulocytosis developed between the 21st and 1,344th day after initiation of MMI therapy. Agranulocytosis appeared in the first 21 to 90 d of MMI treatment in 8 cases, but in 2 cases, it occurred after 1 yr of MMI therapy. The onset of agranulocytosis in patients treated with small doses of ATD was delayed between the 237th and 1,344th day. All 15 patients were treated immediately after diagnosis of agranulocytosis. Following prompt discontinuation of MMI, they were treated with antibiotics in 12 cases, G-CSF in 12 cases, KI in 8 cases, beta blocker in 3 cases, glucocorticoid in 3 cases and immunoglobulin in 3 cases. For the 12 cases who received G-CSF therapy, the timing required for granulocyte recovery with G-CSF was between the 4th and 52nd day, and recovery without G-CSF was between the 3rd and 20th day. There was no significant difference between these groups. Granulocyte counts were elevated above 500/mm3 between the 3rd and 52nd day. Of the 11 patients with available follow-up records, thyroidectomy was performed in 4 patients, and radioactive iodine was administered to 1 patient (aged 14 yr). Four patients were changed to PTU, and 2 were changed to KI. Unlike adult cases, which are often fatal, all 18 patients survived.
Discussion
Side effects of ATD are more common in children than adults. Agranulocytosis has been reported in 0.3% of adult patients taking MMI or PTU. The incidence of agranulocytosis in children is estimated to be very low. In adults, agranulocytosis is MMI dose-dependent and rarely occurs at low doses (2). When it develops, agranulocytosis occurs within the first 90 d of therapy initiation in most cases, but this complication can occur even a year or more after treatment. Mild leucopenia (WBC counts < 4,000/mm3) is a common complication of hyperthyroidism and may be exacerbated by treatment with ATD (3, 5). Agranulocytosis is thought to be autoimmune-mediated, and antigranulocyte antibodies are found in some cases (2). Agranulocytosis should prompt discontinuation of ATD therapy.
In the 3 patients we observed, high fever and sore throat were the earliest symptoms of agranulocytosis, which is similar to the observations in another published report (6). Weekly routine monitoring of the WBC and granulocyte counts failed to predict occurrences of agranulocytosis except in case 10. Our observations raise doubts about the utility of routine monitoring of hematological profiles or liver function tests in patients receiving ATD medications. Routine monitoring of WBC counts is not recommended because of the rarity of the condition and the lack of evidence to support the notion that routine monitoring of these parameters is effective in minimizing the risk of adverse events (2, 7). The clinical importance of routine granulocyte monitoring in early detection of ATD-induced agranulocytosis remains controversial. Tajiri et al. indicated that asymptomatic patients might be detected through routine granulocyte monitoring and rescued by stopping ATD therapy and administering G-CSF (11). Therefore, clinicians should warn patients of any symptoms and signs of fever or sore throat at the beginning of therapy with ATDs, and perform routine monitoring of granulocyte counts. Once any of the symptoms occur, all patients should contact a physician immediately. ATDs should be discontinued, and close monitoring of WBC counts should be considered.
Shirooze et al. (8) and Okamura et al. (9) reported that the effectiveness of low-dose MMI for treatment of hyperthyroidism was no less than that of 30 mg MMI in adults. Tsuboi et al. (5) and Takata et al. (10) reported that the incidence of agranulocytosis with low-dose MMI therapy was significantly lower than that of the high-dose regimen in adult patients. Most medical textbooks recommend an initial starting dose of PTU of 5–10 mg/kg/d, with a maximum of 300 mg/d, whereas that of MMI is 0.5–1.0 mg/kg/d, with a maximum dose of 30 mg/d (1). In our series of 15 cases with MMI-induced agranulocytosis, 12 received MMI equal to or higher than 20 mg/d; only 3 patients were on MMI lower than 15 mg/d. In the 12 cases with a larger dosage, agranulocytosis developed between the 21st and 293rd day of MMI treatment. This finding is in agreement with previous reports as mentioned above (2, 5, 10). Our observations favor the use of low-dose MMI to reduce the risk of agranulocytosis in children with hyperthyroidism. With regard to treatment of patients with severe degrees of hyperthyroidism, large goiters or a high T3-to-T4 ratio in the serum, alternative regimens, such as a combination of a low-dose MMI plus KI, might be more desirable than a higher dose MMI to normalize thyroid function safely (11).
The administration of G-CSF may shorten the time to recovery in patients with ATD-induced agranulocytosis, and most authorities recommend using G-CSF (2, 12, 13). Our data, however, failed to support such treatment. For the 12 out of 15 patients who received G-CSF therapy, there was no difference between the time required for granulocyte recovery with G-CSF and without. Severe depression of myeloid precursors suggests a prolonged recovery time and a failure to respond to G-CSF (2), and Tajiri et al. reported that G-CSF therapy was ineffective in severe cases with granulocyte counts below 100/mm3 and symptoms (14). In one case with a minimum granulocyte count of 140/mm3 and administration of G-CSF, the recovery time from agranulocytosis was longer than 1 mo. In children, further study of the potential efficiency of G-CSF therapy in ATD-induced agranulocytosis is needed.
Because of the cross-reactivity between PTU and MMI for agranulocytosis, the use of an alternative ATD is contraindicated (2). Of the 11 cases with detailed clinical records, thyroidectomy was performed in 4 cases, and radioactive iodine was administered to 1 patient (aged 14 yr). In the remaining patients, adverse events were resolved after switching to an alternative ATD, PTU in 4 cases, raising doubts about the contraindication of use of an alternative ATD. In children, alternative treatment with surgery or radioactive iodine may be refused by the families. Although there is now an increasing tendency to advocate radioactive iodine as the treatment of choice in children because it carries a high rate of remission (3), it may be prudent to avoid radioiodine therapy in children under 18 yr of age considering the increscent risk of thyroid and nonthyroidal cancers, including those of the salivary glands, stomach and bladder.
Conclusion
On the basis of our analysis of these patients, the first and most important clinical signs of ATD-induced agranulocytosis were fever and sore throat, and routine WBC and granulocyte counts could not predict the occurrence of this adverse event. The efficacy of G-CSF treatment was not confirmed in our patients. Because data regarding the incidence of ATD-induced agranulocytosis in Japanese children is not available, a study of larger populations is needed to determine the true incidence and clarify the proper use of G-CSF. Considering the risk of serious side effects, we recommend low-dose MMI therapy for the treatment of Graves’ disease.
Acknowledgments
We appreciate the detailed information concerning 15 patients with ATD-induced agranulocytosis provided by the drug manufacturer, Chugai Pharmaceutical Co., Ltd.
References
- 1.Lafranchi S, Hanna CE. Graves’ disease in the neonatal period and childhood. In: Braverman LE, Utiger RD, editors. Werner and Ingbar’s The Thyroid 8th Edition. Philadelphia: Lippincott Williams and Wilkins; 2000. p.989–97. [Google Scholar]
- 2.Cooper DS. Antithyroid drugs. New Engl J Med 2005;352: 905–17 [DOI] [PubMed] [Google Scholar]
- 3.Rivkees SA, Sklar C, Freemark M. The management of Graves’ disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab 1998;83: 3767–76 [DOI] [PubMed] [Google Scholar]
- 4.Rivkees SA. The treatment of Graves’ disease in children. J Pediatr Endocrinol Metab 2006;19: 1095–111 [DOI] [PubMed] [Google Scholar]
- 5.Tsuboi K, Ueshiba H, Shimojo M, Ishikawa M, Watanabe N, Nagasawa K, et al. The relationship of initial methimazole dose to the incidence of methimazole-induced agranulocytosis in patients with Graves’ disease. Endocr J 2007;54: 39–43 [DOI] [PubMed] [Google Scholar]
- 6.Dai WX, Zhang JD, Zhan SW, Xu BZ, Jin H, Yao Y, et al. Retrospective analysis of 18 cases of antithyroid drug (ATD)-induced agranulocytosis. Endocr J 2002;49: 29–33 [DOI] [PubMed] [Google Scholar]
- 7.Rivkees SA, Stephenson K, Dinauer C. Adverse events associated with methimazole therapy of Graves’ disease in children. Int J Pediatr Endocrinol 2010;2010:176970. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiroozu A, Okamura K, Ikenoue H, Sato K, Nakashima T, Yoshinari M, et al. Treatment of hyperthyroidism with a small single daily dose of methimazole. J Clin Endocrinol Metab 1986;63: 125 [DOI] [PubMed] [Google Scholar]
- 9.Okamura K, Ikenoue H, Shirooze A, Sato K, Yoshinari M, Fujishima M. Reevaluation of the effects of methylmercaptoimidazole and propylthiouracil in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab 1987;65: 719–23 [DOI] [PubMed] [Google Scholar]
- 10.Takata K, Kubota K, Fukata S, Kudo T, Nishihara E, Ito M, et al. Methimazole-induced agranulocytosis in patients with Graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily. Thyroid 2009;19: 559–63 [DOI] [PubMed] [Google Scholar]
- 11.Takata K, Amino N, Kubota S, Sasaki I, Nishihara E, Kudo T, et al. Benefit of short-term iodide supplementation to antithyroid drug treatment of thyrotoxicosis due to Graves’ disease. Clin Endocrinol (Oxf) 2010;72: 845–50 [DOI] [PubMed] [Google Scholar]
- 12.Tajiri J, Noguchi S. Antithyroid drug-induced agranulocytosis: special reference to normal white blood cell count agranulocytosis. Thyroid 2004;14: 459–62 [DOI] [PubMed] [Google Scholar]
- 13.Kohno H, Yanai S, Ohno Y, Ohgami H. Treatment of methimazole-induced agranulocytosis with granulocyte colony-stimulationg factor. Clin Pediatr Endocrinol 1994;3: 31–3 [Google Scholar]
- 14.Tajiri J, Noguchi S. Antithyroid drug-induced agranulocytosis: How has granulocyte colony-stimulating factor changed therapy? Thyroid 2005;15: 292–7 [DOI] [PubMed] [Google Scholar]