Abstract
Premature thelarche in later childhood (such as at 5–7 yr of age) is not always easy to distinguish from GnRH-dependent precocious puberty. In this study, a GnRH stimulation test was performed on 21 girls from 5 to 7.5 yr of age with early breast development. In 8 of 11 girls within 1 yr after thelarche, i.e., breast development, the GnRH stimulation test showed a prepubertal response, and in all 10 girls at more than 1 yr after breast development, the GnRH stimulation test showed a pubertal response. In observations of 4 girls with a prepubertal response, the GnRH stimulation test showed to a pubertal response by 1 yr or more after breast development in 3 of 4 the girls. These results indicate the possibility that almost all cases of breast development in later childhood consist of premature thelarche and that premature thelarche in later childhood may easily lead to early puberty at 1 yr or more after breast development. Careful observations are therefore recommended for at least 1 yr, even if early breast development is considered to be associated with premature thelarche in later childhood.
Keywords: Premature thelarche in later childhood, precocious puberty, GnRH stimulation test, local epidemics
Introduction
Breast development is the first step of adolescence in females, and it statistically occurs at 10 yr of age in standard Japanese girls. Girls are defined to be precocious in Japan when breast development occurs at 7.5 yr of age or under (1). The most common conditions to be diagnosed with precocious breast development are GnRHp-dependent precocious puberty and premature thelarche (2, 3). Early breast development with gonadotropin secretion equal to pubertal levels indicates GnRH-dependent precocious puberty. Premature thelarche is defined as early breast development without other pubertal maturations or an elevation of any sexual hormones. It is useful to distinguish GnRH-dependent precocious puberty from premature thelarche by evaluating the peak LH level and peak LH/FSH ratio by using a GnRH stimulation test (2, 3).
Premature thelarche usually occurs during early childhood before 2 yr of age, and breast development usually improves a few years later (4). However, it often becomes a problem in causal examinations and treatment to accurately distinguish premature thelarche from precocious puberty. This is especially true if breast development occurs in later childhood such as at 5–7 yr of age.
In this report, GnRH stimulation tests were performed on girls with breast development at 5–7.5 yr of age, and the progression of breast development was discussed by assessing the relationship between prepubertal response and pubertal response to the GnRH stimulation tests.
Participants and Methods
Twenty-five girls, who demonstrated breast development from 5 to 7.5 yr of age and, visited Okinawa Prefectural Nanbu Medical Center and Children’s Medical Center between 2006 and 2008 were selected for the current study. Of the 25 girls, 4 girls were excluded from the study for the following reasons: two girls were born with birth weights of less than 1,000 g, one girl had a past history of encephalitis, and one girl had no credible information regarding breast development. The remaining 21 girls had no exogenous estrogen intake, and had no intracranial abnormality based on the findings of magnetic resonance imaging (MRI). The clinical characteristics and laboratory data of the 21 girls are summarized in Table 1. In the 21 girls with breast development, 11 girls visited within 1 yr after the onset of breast development, while the remaining 10 girls visited more than 1 yr after the onset.
Table 1. Clinical characteristics of the 21 girls with breast development from 5 to 7.5 yr of age.
| Cases | 21 |
| Chronological age (CA) (yr) | 7.9 ± 1.0 |
| CA at breast development (yr) | 6.9 ± 0.6 |
| Bone age (BA) (yr) | 9.9 ± 1.4 |
| BA/CA ratio | 1.23 ± 0.10 |
| Height (SD) | + 0.74 ± 1.0 |
| Obesity (%) | + 14.8 ± 16.7 |
| Gestational age (w) | 38.3 ± 2.5 |
| Birth body weight (g) | 2,764 ± 716 |
| Target height (cm) | 154.9 ± 4.1 |
| Mother’s menarche (yr) | 11.6 ± 1.0 |
Data are shown as means ± SD.
The GnRH stimulation test was performed by injecting 3 µg/kg/dose (maximum dose 100 µg) of GnRH into a vein, and the serum levels of LH and FSH at 0, 30, 60, 90 and 120 min after GnRH injection were measured, respectively, by a chemiluminescent immunoassay (CLIA). Pubertal response to the GnRH stimulation test was defined as follows in this study: the peak LH level was 6 mIU/ml or over, and peak LH/FSH ratio is 0.5 or over (5). The other responses to the GnRH stimulation test were regarded as a prepubertal response. The GnRH stimulation test was started in the daytime, and there were no food restrictions in any of the cases.
Serum IGF-I, estrogen, and DHEA-S levels were measured by an immunoradiometric assay (IRMA), an electrochemiluminescence immunoassay (ECLIA), and a chemiluminescent enzyme immunoassay (CLEIA), respectively.
Bone age was estimated according to the Japanese standard Tanner-Whitehouse 2 (TW2) method through its Radius-Ulna-Short bone (RUS) system (6). An evaluation of bone age was performed by the author.
A longitudinal analysis was performed in 4 girls who showed a prepubertal response to GnRH at an initial examination. Bone age measurements and a GnRH stimulation test were performed approximately every 6 mo.
All results were expressed as means ± SD. Any correlations between two values were assessed by Pearson’s correlation coefficient. Statistical significance was assessed by either the Student’s t-test or chi-squared analysis. A p value of under 0.05 was considered to be significant.
Results
At the initial medical examination, 8 girls showed a prepubertal response to GnRH, while the remaining 13 girls showed a pubertal response to GnRH. Prepubertal responses to GnRH were significantly limited in the cases within 1 yr after the onset of breast development (Table 2A). The bone age in the cases with a prepubertal response had accelerated to a level equal to that seen in the cases with a pubertal response, and the advance in bone age was also greater than their chronological age by approximately 20% on average. No significant difference was observed between pubertal response and prepubertal response in regard to any of the clinical characteristics, while IGF-I and estrogen were significantly high in the cases with pubertal response (Table 2B).
Table 2. Comparison between the prepubertal response group and the pubertal response group.
| A | GnRH stimulation test | ||
| Prepubertal | Pubertal | p value | |
| Cases within 1 yr | 8 | 3 | <0.01 |
| Cases after 1 yr | 0 | 10 | |
| Total | 8 | 13 | |
| B | GnRH stimulation test | ||
| Prepubertal | Pubertal | p value | |
| CA at breast development (yr) | 6.9 ± 0.6 | 6.9 ± 0.7 | NS |
| BA/CA ratio | 1.19 ± 0.12 | 1.25 ± 0.08 | NS |
| Height (SD) | + 0.39 ± 1.12 | + 0.95 ± 0.90 | NS |
| Obesity (%) | + 9.2 ± 9.7 | + 18.3 ± 19.4 | NS |
| Gestational age (w) | 38.4 ± 2.1 | 38.3 ± 2.8 | NS |
| Birth body weight (g) | 2,823 ± 709 | 2,726 ± 752 | NS |
| Target height (cm) | 154.0 ± 5.2 | 155.5 ±.4 | NS |
| Mother’s menarche (yr) | 11.6 ± 1.3 | 11.5 ± 1.0 | NS |
| IGF-I (ng/ml) | 214 ± 64 | 418 ± 148 | p<0.01 |
| Estrogen (pg/ml) | 9 ± 4 | 22 ± 13 | p<0.01 |
| DHEA-S (µg/dl) | 37 ± 37 | 56 ± 25 | NS |
Data are shown as means ± SD. A: Duration from breast development, B: Clinical characteristics and laboratory data.
In the cross-sectional analysis for the GnRH stimulation test, each peak LH level and peak LH/FSH ratio had a significant correlation with the duration since breast development (Fig. 1); however, no correlations were observed in basal LH level, basal FSH level, or peak FSH level (data not shown).
Fig. 1.
Correlation of the duration from breast development with the LH peak value (left) or LH/FSH peak ratio (right) in the GnRH stimulation test. The shaded bar indicates the area of the prepubertal response in GnRH stimulation test.
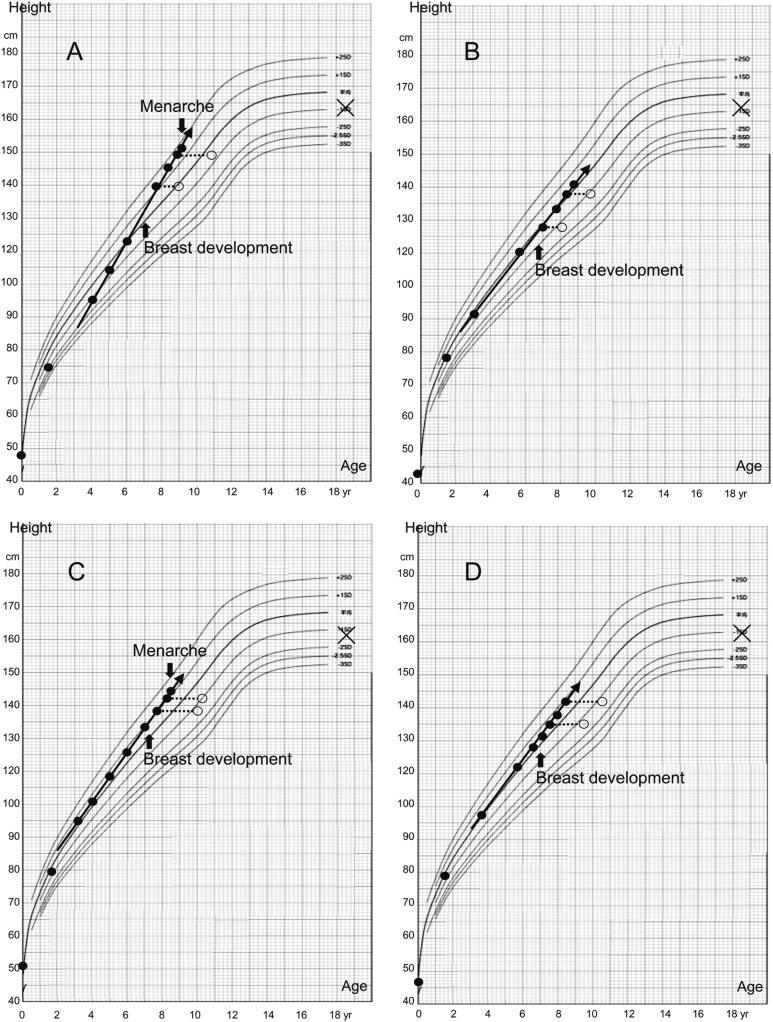
In the longitudinal analysis of 4 girls with a prepubertal response to the initial GnRH stimulation test, the result for 3 of the 4 girls changed from a prepubertal to a pubertal response at 1 yr or more after the onset of breast development. Case D did not correspond to the definition of a pubertal response to GnRH in this study, but she did show an elevation of both the peak LH level and peak LH/FSH ratio (Fig. 2). Height velocity of all 4 of the girls was greater than normal before breast development, and the height velocity did not change either before or after the onset. Similarly, the advance of bone age did not rapidly accelerate after breast development, while the bone age was initially advanced. In both Case A and Case C, early menstruation suddenly occurred at around 9 yr of age (Fig. 3).
Fig. 2.
Longitudinal study of the GnRH stimulation test in 4 girls with a prepubertal response at less than 1 yr after breast development. The shaded bar indicates the area of the prepubertal response in GnRH stimulation test.
Fig. 3.
Growth curve in 4 girls with a prepubertal response in the GnRH stimulation test at less than 1 yr before the onset of breast development (Cases A to D correspond to A–D in Fig. 2). Solid circles, open circles, Xs, and arrows indicate the height, bone age, target height, and growth velocity, respectively.
Discussion
It is important to distinguish premature thelarche from precocious puberty, because precocious puberty needs causal examinations and appropriate treatments. Idiopathic GnRH-dependent precocious puberty, the most common GnRH-dependent precocious puberty, tends to occur frequently in girls (7). Premature thelarche occurring in later childhood (such as 5–7 yr of age) may be confused with precocious puberty because it sometimes shows an advanced bone age and/or a premature growth spurt (4, 8).
In this study, 8 of 11 cases within 1 yr after the onset of breast development were considered to have premature thelarche, because the LH peak and/or LH/FSH peak ratio demonstrated a prepubertal response to the GnRH stimulation test. In another report that supports our results, 7 of 35 normal females with only breast development of Tanner stage 2 showed a prepubertal response with a peak LH level of under 6.9 IU/l in a GnRH stimulation test (2). These results mean the possibility of premature thelarche in later childhood is not rare.
Premature thelarche in later childhood may progress to a rapid progressive variant rather than to the slowly progressive variant of precocious puberty. In the longitudinal analysis of this study, the response to the GnRH stimulation test changed from prepubertal to pubertal at the boundary of 1 yr in 3 of 4 cases. This result supports the notion that premature thelarche in later childhood may easily progress to GnRH-dependent precocious puberty. In further observation of the 4 cases, 2 of the 4 cases showed early menstruation. It was previously reported that premature thelarche would rarely progress to GnRH dependent precocious puberty (9), but in other reports, it was reported that premature thelarche might sometimes accelerate the timing of puberty (10) and that premature thelarche in later childhood also has a high risk of precocious puberty (8). It must be always considered whether premature thelarche in later childhood changes to GnRH dependent precocious puberty.
The mechanism of premature thelarche in later childhood is unknown, but some reports have mentioned that exogenous estrogen (11), some foods (12) or drugs (13) might be related. Actually, several reports have implied that environmental pollutants appear to have induced local epidemics of the onset of premature thelarche in later childhood and precocious puberty (14,15,16). Recently, an activating mutation in G protein-coupled receptor (GPR) 54 was found to lead to the onset of central precocious puberty, while the initial GnRH stimulation test showed only a borderline-pubertal response like premature thelarche in later childhood (17).
According to statistics obtained from the Ministry of Health, Labour, and Welfare in Japan, the prevalence of precocious puberty in Okinawa is 4.5 times more than the Japanese standard in girls, whereas no such difference is observed in boys (Table 3). It is uncertain in Okinawa whether the high prevalence of precocious puberty is a consequence of local epidemics of premature thelarche in later childhood. Although further studies need to elucidate why the prevalence of precocious puberty is high in girls in Okinawa, premature thelarche in later childhood might be specific to Okinawa.
Table 3. Number of patients with precocious puberty, according to the statistics of the Ministry of Health, Labour, and Welfare in Japan in 2004 (modified).
| Boys | Girls | |||
| Number | Prevalence* | Number | Prevalence* | |
| Okinawa | 5 | 2.9 | 96 | 55.5 |
| Japan | 191 | 2.0 | 1,178 | 12.3 |
*Prevalence among 100,000 people under 20 yr of age.
In conclusion, for an adequate evaluation of breast development in later childhood, careful observations for 1 yr or more are recommended, even if all clinical characteristics and data are compatible with premature thelarche at first, because the response to the GnRH stimulation test can change from prepubertal to pubertal up to about 1 yr after breast development.
References
- 1.Tanaka T, Imai T. The standard of breast development in normal girls: diagnostic criteria for precocious puberty. J Child Health 2005;64: 33–8(in Japanese). [Google Scholar]
- 2.Brito VN, Batista MC, Borges MF, Latronico AC, Kohek MB, Thirone AC, et al. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab 1999;84: 3539–44 [DOI] [PubMed] [Google Scholar]
- 3.Della Manna T, Setian N, Damiani D, Kuperman H, Dichtchekenian V. Premature thelarche: identification of clinical and laboratory data for the diagnosis of precocious puberty. Rev Hosp Clin Fac Med Sao Paulo 2002;57: 49–54 [DOI] [PubMed] [Google Scholar]
- 4.Volta C, Bernasconi S, Cisternino M, Buzi F, Ferzetti A, Street ME, et al. Isolated premature thelarche and thelarche variant: clinical and auxological follow-up of 119 girls. J Endocrinol Invest 1998;21: 180–3 [DOI] [PubMed] [Google Scholar]
- 5.Ito J, Tanaka T, Horikawa R, Okada Y, Morita S, Kokaji M, et al. Clinical study on the time-resolved fluoroimmunoassay of serum luteinizing hormone and follicle stimulating hormone in children: the change of serum gonadotropins in LHRH test and night time secretion during puberty. J Jpn Pediatr Soc 1993;97: 1789–96 [Google Scholar]
- 6.Murata M. Japanese specific bone age standard on the TW2. Clin Pediatr Endocrinol 1993;2(Suppl 3): 35–41 [Google Scholar]
- 7.Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs 2007;36: 263–74 [DOI] [PubMed] [Google Scholar]
- 8.Verrotti A, Ferrari M, Morgese G, Chiarelli F. Premature thelarche: a long-term follow-up. Gynecol Endocrinol 1996;10: 241–7 [DOI] [PubMed] [Google Scholar]
- 9.Mills JL, Stolley PD, Davies J, Moshang T., JrPremature thelarche. Natural history and etiologic investigation. Am J Dis Child 1981;135: 743–5 [PubMed] [Google Scholar]
- 10.Pasquino AM, Pucarelli I, Passeri F, Segni M, Mancini MA, Municchi G. Progression of premature thelarche to central precocious puberty. J Pediatr 1995;126: 11–4 [DOI] [PubMed] [Google Scholar]
- 11.Chiabotto P, Costante L, de Sanctis C. Premature thelarche and environmental pollutants. Minerva Med 2006;97: 277–85 [PubMed] [Google Scholar]
- 12.Türkyilmaz Z, Karabulut R, Sönmez K, Can Başaklar A. A striking and frequent cause of premature thelarche in children: Foeniculum vulgare. J Pediatr Surg 2008;43: 2109–11 [DOI] [PubMed] [Google Scholar]
- 13.Bosman JM, Bax NM, Wit JM. Premature thelarche: a possible adverse effect of cimetidine treatment. Eur J Pediatr 1990;149: 534–5 [DOI] [PubMed] [Google Scholar]
- 14.Sáenz de Rodríguez CA, Bongiovanni AM, Conde de Borrego L. An epidemic of precocious development in Puerto Rican children. J Pediatr 1985;107: 393–6 [DOI] [PubMed] [Google Scholar]
- 15.Colón I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect 2000;108: 895–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou YY, Huang PC, Lee CC, Wu MH, Lin SJ. Phthalate exposure in girls during early puberty. J Pediatr Endocrinol Metab 2009;22: 69–77 [DOI] [PubMed] [Google Scholar]
- 17.Teles MG, Bianco SD, Brito VM, Trarbach EB, Kuogung W, Xu S, et al. GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 2008;358: 709–15 [DOI] [PMC free article] [PubMed] [Google Scholar]