Abstract
To elucidate the mechanism of insulin resistance due to insulin counterregulatory hormones (ICRHs) and evaluate ICRH secretion kinetics, ICRH concentrations were measured and correlated with blood glucose levels in 28 type 1 diabetic patients. Blood glucose was measured before bedtime. Early morning urine samples were collected the next morning before insulin injection and breakfast. Fasting blood glucose, cortisol, glucagon and HbA1c levels were measured. Growth hormone (GH), adrenaline, cortisol and C-peptide levels in morning urine samples were measured; SD scores were calculated for urine GH. The laboratory values (mean ± SD) were as follows; HbA1c of 8.1% ± 1.4%; pre-bedtime glucose of 203 ± 105 mg/dl; fasting blood glucose of 145 ± 87 mg/dl; serum cortisol of 21.6 ± 5.5 µg/dl; plasma glucagon of 98 ± 41 pg/ml; urinary GH, 27.2 ± 13.0 ng/gCr; urinary cortisol of 238 ± 197 ng/gCr; and urinary Adrenaline of 22.9 ± 21.0 ng/gCr. The mean urinary GH SD score was increased (+1.01 ± 0.70; p=0.000); the mean plasma glucagon lebel (98 ± 41 pg/ml) was not. Fasting blood glucose was positively correlated with plasma glucagon (R=0.378, p=0.0471) and negatively correlated with urinary cortisol (R=–0.476, p=0.010). Urinary adrenaline correlated positively with urinary GH (R=0.470, p=0.013) and urinary cortisol (R=0.522, p=0.004). In type 1 diabetes, GH, glucagon and cortisol hypersecretion may contribute to insulin resistance, but the mechanism remains unclear.
Keywords: cortisol, diabetic complications, growth hormone, insulin counterregulatory hormones, type 1 diabetes mellitus
Introduction
Glycemic control in type 1 diabetes is becoming easier with an increasing selection of insulin preparations and diversification of insulin therapy. However, to aim for good glycemic control, exogenous insulin in excess of physiologic insulin secretion is required. In maintaining strict glycemic control, an increased risk of hypoglycemia and an increased incidence of asymptomatic hypoglycemia are important issues.
In type 1 diabetes, recurrent hypoglycemia can impair insulin counterregulatory hormone (ICRH) responses (1), including decreased ICRH responses to nocturnal hypoglycemia (2, 3). This can lead to a further increased incidence of asymptomatic hypoglycemia and hypoglycemia-associated autonomic failure (4).
Glycemic control in type 1 diabetes worsens in adolescence. One presumed reason is because, in adolescence, increased GH secretion, which is stimulated by increased secretion of sex hormones, is involved in insulin resistance (5, 6). Therefore, HbA1c levels are about 1% higher in young type 1 diabetic patients compared with adults despite higher daily insulin doses (7).
This difficulty in control is often seen clinically, yet the mechanism of insulin resistance in type 1 diabetes remains unclear. A recent study reported that, in young type 1 diabetic patients, insulin resistance varied widely compared with adults based on sex, age and amount of adipose tissue (8). However, few studies have examined the relationship with ICRHs. To improve type 1 diabetes control in adolescence, the mechanism of insulin resistance due to ICRHs must be investigated in detail. ICRHs are involved to a varying degree in diabetic complications. Reports to date have found that growth hormone (GH) plays a role in diabetic retinopathy and nephropathy (9,10,11,12); cortisol is involved in diabetic retinopathy, autonomic neuropathy and macrovascular disease (13, 14). Insulin resistance due to ICRHs increases insulin demand, leads to unstable glycemia and increases the risk of obesity and hypoglycemia. In addition, glucagon secretion response to hypoglycemia is impaired (15, 16). Studies to date in type 1 diabetes have found that, despite higher serum insulin concentrations, insulin resistance leads to hyperglycemia.
In other words, not only insulin resistance but hepatic insulin concentration also decreases, and hepatic gluconeogenesis increases. In this study, to evaluate the secretion kinetics of ICRHs and elucidate the mechanism of insulin resistance in juvenile-onset type 1 diabetic patients, ICRH concentrations were measured and their correlations with blood glucose values were examined.
Subjects and Methods
The subjects were 28 type 1 diabetic patients (9 males, 19 females). Their mean age was 13.1 ± 3.6 yr (5.5 to 18.2 yr), and the mean disease duration was 6.0 ± 4.5 yr (0.3 to 15.1 yr; Table 1).
Table 1. Patient characteristics .
| Sex (male: female) | 9:19 |
| Age | 13.9 (5.5–18.2) yr* |
| Disease duration | 5.6 (0.3–15.1) yr* |
| HbA1c | 8.1 ± 1.4% |
| Bedtime blood glucose (day before testing) | 203 ± 105 mg/dl |
| Fasting blood glucose (day of testing) | 145 ± 87 mg/dl |
| Urinary C-peptide | |
| Positive (≥ 0.5 μg/l) | 10 patients |
| Negative (< 0.5 μg/l) | 18 patients |
Mean ± SD, *Median (range).
Blood and urine samples from the type 1 diabetic patients were collected before insulin injection and breakfast in the morning. In all patients, pre-bedtime (the day before) blood glucose was also measured.
The blood samples, after serum and plasma separation, were frozen and stored at –20C.
Blood glucose, cortisol, glucagon and HbA1c levels were measured in the fasting samples. The levels of HbA1c were measured by the JDS-standardized method, so 0.4% was added to each level to correct for the NGSP value. GH, adrenaline, cortisol and C-peptide (CPR) levels in urine samples were measured and urinary GH was evaluated by calculating SD scores using reference values by age.
For each laboratory parameter, between group comparisons were examined by the t-test. The correlations between early morning blood glucose and ICRHs, and the correlations between ICRHs themselves, were calculated using Pearson’s correlation coefficient. The urinary GH SD scores, after logarithmic transformation of age-adjusted GH reference values and urinary GH/Cr, were compared with reference values using the t-test.
The level of statistical significance (p value) was <0.05. The values shown are means ± standard deviation (SD).
This study was approved by the Ethics Committee at Yamanashi University.
Results
Patient characteristics
The characteristics of the 28 patients are shown in Table 1. The mean ± SD values were as follows; HbA1c of 8.1 ± 1.4%; pre-bedtime glucose (day before testing) of 203 ± 105 mg/dl ; fasting (day before testing ) blood glucose of 145 ± 87 mg/dl (reference range: 70 to 109 mg/dl); serum cortisol of 21.6 ± 5.5 µg/dl (reference range: 4.0 to 18.3 µg/dl); plasma glucagon of 98 ± 41 pg/ml (reference range: 40 to 180 pg/ml); urinary GH of 27.2 ± 13.0 ng/gCr; urinary cortisol of 238 ± 197 ng/gCr; and urinary adrenaline of 22.9 ± 21.0 ng/gCr. None of the patients had hypoglycemic symptoms during the nighttime while this study was being conducted.
Correlations between early morning blood glucose and insulin counterregulatory hormones
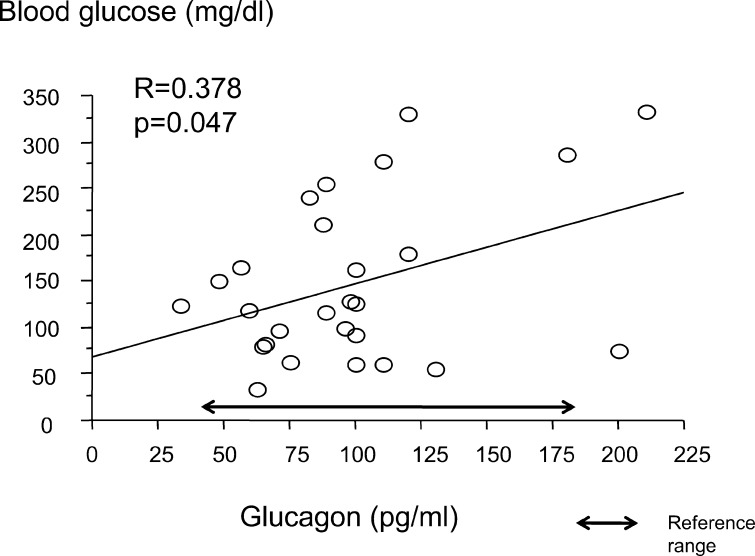
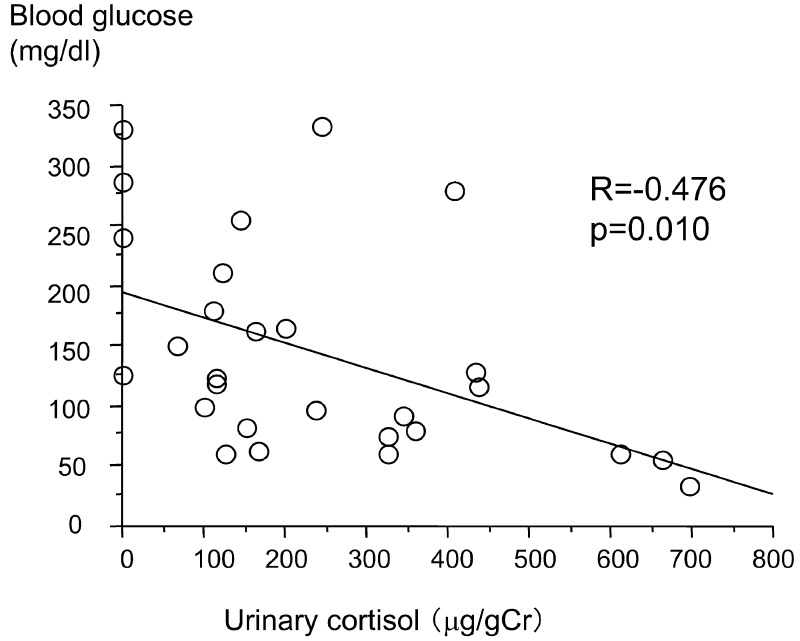
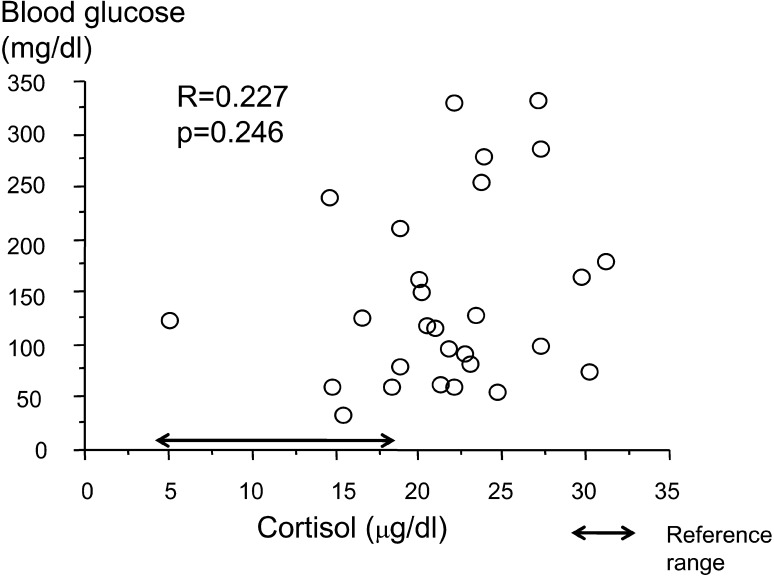
Fasting blood glucose and plasma glucagon were positively correlated (R=0.378, p=0.0471; Fig. 1). Fasting blood glucose and urinary cortisol were negatively correlated (R=–0.476, p=0.010 Fig. 2). There were no significant correlations between fasting blood glucose and serum cortisol, urinary adrenaline and urinary GH SD score. The urinary GH SD score was significantly higher than the reference value (+1.01 ± 0.70; p=0.000; Fig. 3).
Fig. 1.
Correlation between early morning blood glucose and plasma glucagon.
Fig. 2.
Correlation between early morning blood glucose and urinary cortisol.
Fig. 3.
Correlation between early morning blood glucose and serum cortisol.
Correlations between insulin counterregulatory hormones
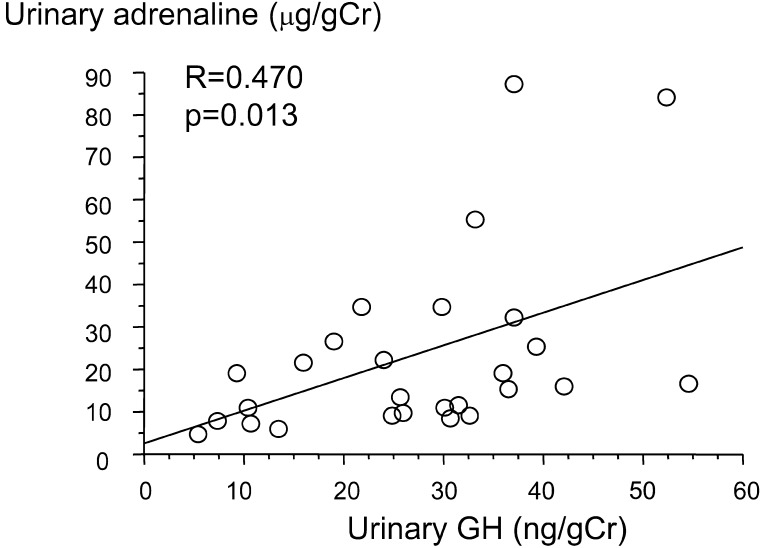
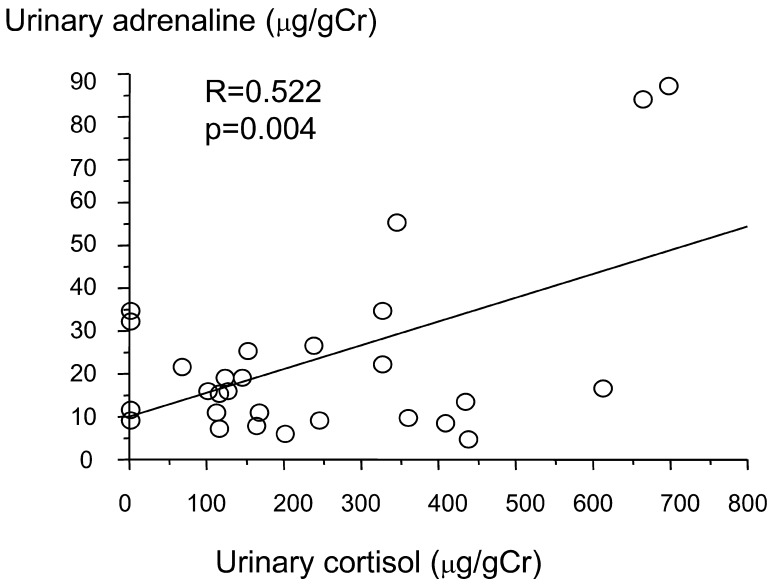
Urinary adrenaline showed positive correlations with urinary GH (R=0.470, p=0.013; Fig. 4) and urinary cortisol (R=0.522, p=0.004; Fig. 5).
Fig. 4.
Correlation between urinary adrenaline and urinary GH.
Fig. 5.
Correlation between urinary adrenaline and urinary cortisol.
Comparisons between with CPR-positive and CPR-negative subjects
The patients were divided into groups based on being above (≥0.5 µg as positive) or below the urinary CPR assay sensitivity (≤0.5 µg as negative), and their blood glucose, HbA1c and ICRHs were evaluated to compare the effect of residual endogenous insulin (Table 2). The urinary GH SD scores were +0.51 ± 0.58 in the positive group and +1.26 ± 0.63 in the negative group. Thus, the urinary GH SD score was significantly higher in the below assay sensitivity group (p=0.006). For blood glucose, HbA1c, serum cortisol, plasma glucagon, urinary cortisol and urinary adrenaline, there were no significant differences between groups. Serum cortisol was higher than the reference range in both groups, and plasma glucagon was within the reference range in both groups.
Table 2. Urinary C-peptide (CP): comparison above and below assay sensitivity.
| Above CP assay sensitivity n=10 | Below CP assay sensitivity n=18 | p value | |
| Blood glucose (mg/dl) (<110) | 109 ± 68 | 166 ± 92 | 0.076 |
| HbA1c (%) (4.5–6.2) | 7.3 ± 1.6 | 8.5 ± 1.1 | 0.065 |
| Serum cortisol (μg/dl) (3.8–18.4) | 22.3 ± 4.4 | 21.3 ± 6.0 | 0.594 |
| Glucagon (pg/ml) (40–180) | 104 ± 38 | 96 ± 43 | 0.637 |
| Urinary GH SD score | +0.51 ± 0.58 | +1.26 ± 0.63 | 0.006 |
| Urinary cortisol (μg/gCr) | 299 ± 175 | 215 ± 205 | 0.232 |
| Urinary adrenaline (μg/gCr) | 22.2 ± 23.8 | 22.6 ± 19.8 | 0.886 |
Discussion
There have been various studies on individual ICRHs in type 1 diabetes, but few comprehensive studies of ICRHs such as ours have been reported. In type 1 diabetes, especially with recurrent hypoglycemia, the action of ICRHs in response to hypoglycemic stimulation is decreased (2, 3).
Glucagon secretion in response to hypoglycemia is often insufficient in type 1 diabetes (15). However, in the present study, glucagon secretion, regardless of residual insulin secretion ability, was relatively well preserved. Moreover, the level of glucagon was positively correlated with blood glucose, suggesting that glucagon may contribute to the morning hyperglycemia. This finding may exhibit an indirect effect of insulin deficiency, which leads to hyperglycemia and glucagon secretion. Regarding GH and cortisol, the basal levels were shown to be increased in the present study. For adrenaline, no correlation with blood glucose was observed, whereas both GH and cortisol were positively correlated. This suggests these three parameters increase together, although the reason is unclear in this study. Nocturnal hypoglycemia is one of the possible stimulators. Interestingly, the fasting blood glucose level was not correlated with these three ICRHs, indicating that they do not affect morning hyperglycemia but possibly do work in short-term counterregulation reflecting at any time in the sleeping period.
Regarding GH in the present study, the results for the urinary GH SD score indicated GH hypersecretion. The GH SD scores, except in 2 patients, were ≥0, suggesting that even without hypoglycemia, GH secretion remained elevated. This GH hypersecretion is thought to be one of the factors in insulin resistance and is also a factor in the dawn phenomenon. The dawn phenomenon has been recognized in patients other than adolescents, including those with type 2 diabetes (17), who generally do not have high GH. Meanwhile, GH stimulates IGF-1 production, and IGF-1 improves insulin resistance by the insulin-like effect. The IGF-I level is not elevated in type 1 diabetes, at least the serum level, which may indicate the existence of GH resistance in the liver. We speculate that the degree of GH resistance is variable in tissues and therefore IGF-I production or the other growth factors may exist variably and serve as a deteriorating factor for diabetes complications in some specific tissues.
Serum cortisol was higher than the reference values, and there was a negative correlation between fasting blood glucose and urinary cortisol. These findings might be attributed to cortisol secretion in response to nocturnal hypoglycemia because the morning blood glucose level correlated with only urinary cortisol, and not with serum cortisol; however, an additional study of the nocturnal blood glucose level is indispensable to refer to it. In contrast to GH, which can be constantly elevated in type 1 diabetes, cortisol seems to be affected by the daily fluctuations in blood glucose level.
There have been few report about cortisol levels in type 1 diabetes a positive correlation between duration of diabetes and cortisol secretion has been reported (18).
Although the mechanism involved in the changes in the hypothalamic-pituitary-adrenal axis (HPA axis) is unclear, an association with complications has been identified (12, 13, 18). The HPA axis responds to stress due to hypoglycemic stimulation; however, this is likely to be a transient, short-term response. Cortisol is also known to have an inhibitory action on GH via negative feedback. Our results showed a state of GH hypersecretion, suggesting a lack of inhibition by cortisol action.
As for the mechanism of increased cortisol in type 1 diabetes, changes in enzymatic activity have been speculated to be involved in cortisol metabolism. The mechanism involves inhibition of 5α-reductase and 11β hydroxysteroid dehydrogenase type 2 (11β HSD2), which are involved in cortisol inactivation by direct stimulation of insulin (19). Certainly some adolescent patients with type 1 diabetes suffer from a difficulty in controlling body weight, which may be related to cortisol.
The elucidation of cortisol metabolism in diabetes may be a clue for the mechanism of the progression of although one the reason for progression of weight gain. The effects on the central nervous system of hypoglycemia are often reversible in adults, but in children who are still in the process of developing, sequelae with irreversible changes, such as decreased IQ, can occur (20). In the present study, no correlation between fasting blood glucose and urinary adrenaline was seen. Although not included in the present results, our studies to date have shown a negative correlation between blood glucose and urinary adrenaline at 2 AM, a positive correlation between blood glucose rise and urinary adrenaline from 2 AM to 4 AM and no correlation between blood glucose rise and urinary adrenaline from 4 AM to 7. Based on these results, short-term adrenaline secretion occurs in response to hypoglycemia that exceeds a certain threshold. Adrenaline likely has a transient pattern of secretion at night, which is not reflected in early morning fasting blood glucose values. Regarding glucagon secretion in type 1 diabetes, a decreased secretory response to hypoglycemia has been reported (15). However, in the present study, regardless of residual insulin secretion ability, glucagon secretion was relatively well preserved. Glucagon is essentially a hormone that is secreted from pancreatic α cells in response to hypoglycemia and is suppressed by a glucose load, but from some previous reports, glucagon contrary increases after meal in type 1 and type 2 diabetes. In addition, glucagon secretion has been reported to be influenced by residual insulin secretion ability (15, 21), by autonomic nerve dysfunction (14) and by adrenaline secretion due to autonomic nerve injury and by long disease duration (22).
In the present study patients, the mean disease duration was relatively short (6 yr), and all patients were on intensive insulin therapy. However, the relationship with autonomic nerve function and adrenaline secretion could not be fully elucidated. Regarding the positive correlation between glucagon and early morning blood glucose, glucagon is usually secreted in response to hypoglycemia, but in the present study, it was elevated in hyperglycemia.
Glucagon is one of the factors that contribute to morning hyperglycemia and/or the dawn phenomenon, and control of the glucagon level can be important to achieve good control in type 1 diabetes. GLP-1 agonist may be effective in terms of glucagon management for type 1 diabetes.
Clarifying the role of ICRHs in the pathogenesis of juvenile-onset type 1 diabetes can help to prevent hyperglycemia and hypoglycemia, stabilize, and prevent complications in these patients.
Conclusion
During insulin treatment of juvenile-onset type 1 diabetes, we examined the interaction among ICRHs and their correlation with glycemic fluctuations. In type 1 diabetes, there is hypersecretion of GH and cortisol, and this may lead to insulin resistance. In addition, this may play a role in the development of diabetic complications. However, the detailed mechanism could not be fully elucidated.
Acknowledgments
The authors would to extend their deep appreciation to Dr. Z. Yamagata and Dr. K. Suzuki of the Department of Health Sciences, School of Medicine, University of Yamanashi, and to Dr. T. Oyama of the General Clinical Research Center, Oita University Hospital, for their guidance in regard to statistical analysis in this study.
References
- 1.Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of atecedend hypoglycemia on counterregulatory responses to subsequenr exercise in type 1 diabetes. Diabetes 2003;52: 1761–9 [DOI] [PubMed] [Google Scholar]
- 2.Jones TW, Porter P, Sherwin RS, Davis EA, O’leary P, Frazer F, et al. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 1998;338: 1657–62 [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Research in Children Network (DirecNet) Study Group. Impaired overnight counterregulatory hormone responses to spontaneous hypoglycemia in children with type 1 diabetes. Pediatr Diabetes 2007;8: 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes 2003;52: 1195–203 [DOI] [PubMed] [Google Scholar]
- 5.Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med 1985;312: 1473–9 [DOI] [PubMed] [Google Scholar]
- 6.Cook JS, Hoffman RP, Stene MA, Hansen JR. Effect of maturational stage on insulin sensitivity during puberty. J Clin Endocrtinol Metab 1993;77: 725–30 [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group. Effective of intensive treatment on the development and progression of long-term complication in adolescents with onsulin-depnndent diabetes mellitus. J Peditatr 1994;125: 177–88 [DOI] [PubMed] [Google Scholar]
- 8.Szadkowska A, Pietrzak I, Mianowska B, Bodalska-Lipińska J, Keenan HA, Toporowska-Kowalska E, et al. Insulin sensitivity in Type 1 diabetic children and adolescents. Diabet Med 2008;25: 282–8 [DOI] [PubMed] [Google Scholar]
- 9.Hellstrom A, Perruzzi C, Ju M, Engstrőm E, Hård AL, Liu JL, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. PNAS 2001;98: 5804–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellström A, Engström E, Hård AL, Albertsson-Wikland K, Carlsson B, Niklasson A, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003;112: 1016–20 [DOI] [PubMed] [Google Scholar]
- 11.Landau D, Segev Y, Afargan M, Silbergeld A, Katchko L, Podshyvalov A, et al. A novel somatostatin analogue prevents early renal complications in the nonobese diabetic mouse. Kidney International 2001;60: 505–12 [DOI] [PubMed] [Google Scholar]
- 12.Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Lembo SD, et al. Cortisol secretion in patients with Type 2 diabetes. Diabetes Care 2007;30: 83–8 [DOI] [PubMed] [Google Scholar]
- 13.Tsigos C, Young RJ, White A. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab 1993;76: 554–8 [DOI] [PubMed] [Google Scholar]
- 14.Landau D, Israel E, Rivkis I, Kachko L, Schrijvers BF, Flyvbjerg A, et al. The effect of growth hormone on the development of diabetic kidney disease in rats. Nephrol Dial Transplant 2003;18: 694–702 [DOI] [PubMed] [Google Scholar]
- 15.Fukuda M, Tanaka A, Tahar Y, Ikegami H, Yamamoto Y, Kumahara Y, et al. Correlation between minimal secretory capacity of pancreatic beta-cells and stability of diabetic control. Diabetes 1988;37: 81–8 [DOI] [PubMed] [Google Scholar]
- 16.Sjöberg S, Ahrén B, Bolinder J. Residual insulin secretion is not coupled to a maintained glucagon response to hypoglycaemia in long-term type 1diabetes. J Intern Med 2002;252: 342–51 [DOI] [PubMed] [Google Scholar]
- 17.Fernández AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, et al. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 2001;15: 1926–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi K, Amemiya S, Sawanobori E, Higashida K, Ishihara T, Kobayashi K, et al. Role of IGF binding protein-1 in the dawn phenomenon and glycemic control in children and adolescents with IDDM. Diabetes Care 1997;20: 1442–7 [DOI] [PubMed] [Google Scholar]
- 19.Remer T, Maser-Gluth C, Boye KR, Hartmann MF, Heinze E, Wudy SA. Exaggerated adrenarche and altered cortisol metabolism in Type 1 diabetic children. Steroids 2006;71: 591–8 [DOI] [PubMed] [Google Scholar]
- 20.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 2001;24: 1541–6 [DOI] [PubMed] [Google Scholar]
- 21.Bergenstal RM, Polonsky KS, Pons G, Jaspan JB, Rubenstein AH. Lack of glucagon response to hypoglycemia in type 1 diabetes after long-term optimal therapy with a continuous subcutaneous insulin infusion pump. Diabetes 1983;32: 398–402 [DOI] [PubMed] [Google Scholar]
- 22.Bolli G, De Feo P, Compaqnucci P, Cartechini MG, Angeletti G, Santeusanio F, et al. Abnormal glucose counterregulation in insulin dependent diabetes mellitus: interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 1983;32: 134–41 [DOI] [PubMed] [Google Scholar]