Abstract
ABCC8 encodes the sulfonylurea receptor 1 (SUR1) subunits of the beta-cell ATP-sensitive potassium (K-ATP) channel playing a critical role in the regulation of insulin secretion, and inactivating mutations in ABCC8 cause congenital hyperinsulinism. Recently, ABCC8 inactivating mutations were reported to be involved in the development of diabetes mellitus later in life. We report a girl who was born macrosomic with transient hypoglycemia and thereafter developed diabetes mellitus accompanied by severe reactive hypoglycemia at the age of 11 yr. An OGTT (oral glucose tolerance test) revealed hyperglycemia due to poor early insulin response and subsequent hypoglycemia due to delayed prolonged insulin secretion. Hypoglycemia was improved by the combination of nateglinide, which stimulates early insulin secretion, and an alpha-glucosidase inhibitor, voglibose. Sequencing of the ABCC8 identified a compound heterozygous mutation (R1420H/F591fs604X), suggesting that this mutation may alter regulation of insulin secretion with advancing age, leading to diabetes mellitus with reactive hypoglycemia from hyperinsulinism. Therefore, long-term follow-up and periodic OGTTs are important for early detection of insulin dysregulation in congenital hyperinsulinism patients carrying the ABCC8 mutation, even though hypoglycemia resolves spontaneously during infancy. Furthermore, nateglinide may be useful therapeutically in the treatment of not only diabetes mellitus but also reactive hypoglycemia.
Keywords: ABCC8, SUR1, congenital hyperinsulinism, diabetes mellitus, reactive hypoglycemia
Introduction
The pancreatic beta-cell ATP-sensitive potassium (K-ATP) channels play a critical role in regulation of glucose-induced insulin secretion to maintain glucose homeostasis. The K-ATP channel is a hetero-octameric complex composed of two types of subunits: four sulfonylurea receptor 1 (SUR1) subunits and four inward-rectifying potassium channel pore-forming (Kir6.2) subunits encoded by the genes ABCC8 and KCNJ11, respectively. Increased blood glucose (BG) levels lead to a high ATP/ADP ratio and K-ATP channel closure in pancreatic beta cells, resulting in cell membrane depolarization, which then triggers the opening of voltage-gated calcium channels, calcium influx and a rise in intracellular calcium concentration, thereby resulting in exocytosis of insulin granules (1, 2). Inactivating mutations in ABCC8 or KCNJ11 cause congenital hyperinsulinism (CHI) characterized by hypoglycemia with inappropriate insulin secretion in infancy (3,4,5,6). CHI is usually treated by medical therapy including diazoxide and octreotide acetate. However, patients with CHI due to ABCC8/KCNJ11 mutations are not always responsive to medical treatment and sometimes require pancreatectomy, resulting in a high incidence of diabetes mellitus. On the other hand, ABCC8 inactivating mutations in nonpancreatectomized patients have recently been reported to be involved in the development of diabetes mellitus due to impaired insulin secretion later in life (7,8,9,10).
Reactive (or postprandial) hypoglycemia is a symptomatic hypoglycemia that occurs a few hours after eating (especially a high carbohydrate meal or oral glucose load), and the symptoms are improved by intake of glucose. The etiology of reactive hypoglycemia includes dumping syndrome after stomach resection, nesidioblastoma, insulinoma, impaired glucose tolerance/early diabetes mellitus, drugs or other rare mechanisms (11).
Here we described a compound heterozygous mutation in ABCC8 in a Japanese girl who was born macrosomic with transient hypoglycemia and thereafter developed diabetes mellitus accompanied by repeated reactive hypoglycemia in childhood. In addition, the patient was successfully treated by nateglinide, which stimulated early insulin secretion.
Clinical Report
The propositus was the first child of healthy, unrelated Japanese parents. She was born at 34 wk of gestation by vaginal delivery. She was large for her gestational age, with a birth weight of 3,384 g and a height of 49.0 cm, respectively. On the second day of age, she suffered severe symptomatic hypoglycemia (a blood glucose level below the detection range). She required high concentrated glucose infusion until 10 d of age. Brain MRI findings were compatible with periventricular leukomalacia. Her cousin on her father’s side, now 10 yr old, was also born macrosomic at 36 wk of gestation, with a birth weight of 4,244 g. Although he suffered hyperinsulinemic hypoglycemia during his infancy, hypoglycemia spontaneously improved without diazoxide or other medication. No other family members reported any hypoglycemic episodes or a diabetes mellitus history.
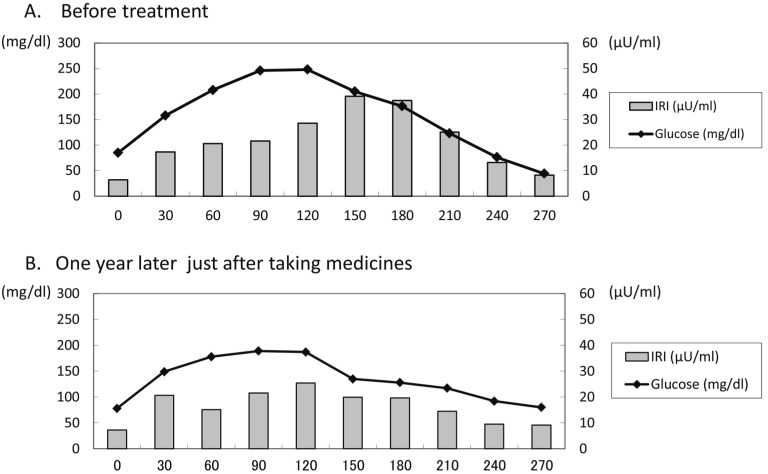
During her infancy, hypoglycemia repeatedly occurred, and glucose infusion was required each time. Although the episodes of hypoglycemia gradually decreased after 6 yr of age, she again suffered repeated symptomatic aketogenic hypoglycemia in the late morning or early evening from 10 yr of age, and the frequency was increased. She was investigated for hypoglycemia at 11 yr of age when her growth was normal, with a weight of 50.8 kg (BMI 21.6) and a height of 153.2 cm (+1.25 SD). Her physical findings were not remarkable except for mild macroglossia, and she was not mentally retarded. She developed symptomatic aketogenic hypoglycemia almost every day (BG levels were between 41–76 mg/dl) in the late morning (10:00–11:00) or early evening (16:00–17:00) and often required oral glucose or glucose infusion, although her BG level was not low at night or in the early morning (Table 1A). An oral glucose tolerance test (OGTT) revealed a high BG level at 120 min (248 mg/dl) due to poor early insulin response, which was followed by hypoglycemia due to delayed prolonged insulin secretion (BG was 44 mg/dl at 270 min) (Table 2A, Fig. 1A). The insulinogenic index was 0.15. Insulin response to a glucagon loading test was normal. Counter-regulatory hormone levels were also normal. HbA1c was elevated (6.8%, NGSP), and anti-GAD antibody and anti-insulin antibody were negative. Abdominal CT showed no obvious pancreatic lesion, and brain MRI showed no new abnormal findings except for PVL. Epileptic findings were not shown on EEG. Based on these findings, she was diagnosed with diabetes mellitus with reactive hypoglycemia.
Table 1. Circadian changes in blood glucose, serum IRI and IRI/BS.
| At breakfast | 2 h after breakfast | At lunch | 2 h after lunch | 17:00 | At dinner | 2 h after dinner | At bedtime | ||
| A One day before treatment | |||||||||
| BS (mg/dl) | 107 | 153 | 105 | 191 | 49 | — | — | 249 | |
| IRI (μU/ml) | 8 | 19.5 | 8.2 | 17.4 | 6 | — | — | — | |
| IRI/BS | 0.07 | 0.13 | 0.08 | 0.09 | 0.12 | — | — | — | |
| B One day after treatment with α-GI | |||||||||
| BS (mg/dl) | 110 | 145 | 92 | 126 | 66 | — | 136 | 170 | |
| C One day after treatment with α-GI and nateglinide | |||||||||
| BS (mg/dl) | 122 | 114 | 110 | 143 | 115 | 93 | — | — | |
| IRI (μU/ml) | 9.3 | 38.7 | 11.1 | 15.3 | 9.3 | 7 | — | — | |
| IRI/BS | 0.076 | 0.340 | 0.101 | 0.107 | 0.081 | 0.075 | — | — | |
Blood glucose levels during hypoglycemia are underlined.
Table 2. Oral glucose tolerance test.
| Time (Min) | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 270 |
| A Before treatment | ||||||||||
| BS (mg/dl) | 85 | 158 | 208 | 246 | 248 | 205 | 176 | 123 | 76 | 44 |
| IRI (μU/ml) | 6.4 | 17.3 | 20.6 | 21.6 | 28.6 | 39.1 | 37.5 | 25.1 | 13.2 | 8.2 |
| CPR (ng/ml) | 1.5 | 2.5 | 3.8 | 4.2 | 6.0 | 6.6 | 7.0 | 5.1 | 3.9 | 2.8 |
| IRI/BS | 0.075 | 0.109 | 0.099 | 0.088 | 0.115 | 0.191 | 0.213 | 0.204 | 0.174 | 0.186 |
| B After taking medicines (one year later) | ||||||||||
| BS (mg/dl) | 78 | 149 | 178 | 189 | 187 | 135 | 128 | 117 | 92 | 80 |
| IRI (μU/ml) | 7.2 | 20.6 | 15.1 | 21.5 | 25.4 | 19.9 | 19.6 | 14.5 | 9.5 | 9.1 |
| CPR (ng/ml) | 1.44 | 2.58 | 2.49 | 3.26 | 3.77 | 3.3 | 3.43 | 2.8 | 2.34 | 2.02 |
| IRI/BS | 0.092 | 0.138 | 0.085 | 0.114 | 0.136 | 0.147 | 0.153 | 0.124 | 0.103 | 0.114 |
Blood glucose, serum IRI and C-peptide (CPR) levels were measured until 270 min after glucose load. Each peak value is underlined.
Fig. 1.
Oral glucose tolerance test.
A. Before treatment, the OGTT revealed hyperglycemia (glucose level at 120 min was 248 mg/dl) due to poor early insulin secretion (insulin level at 120 min was 28.6 μU/ml), followed by hypoglycemia (glucose level at 270 min was 44 mg/dl) due to delayed prolonged insulin secretion (insulin level at 270 min was 8.2 μU/ml).
B. After treatment with α-GI and nateglinide, the peak of insulin secretion was earlier and lower compared with before treatment and hypoglycemia was improved.
We first started medical therapy with an alpha-glucosidase inhibitor (α-GI; voglibose) before each meal (0.6 mg per day) to control postprandial hyperglycemia and subsequent reactive hypoglycemia by slowing the digestion and absorption of carbohydrates and preventing prolonged insulin secretion. However, it was not effective enough for reactive hypoglycemia, although postprandial hyperglycemia was slightly improved (Table 1B). Then we added nateglinide before breakfast and lunch (30 mg per day) to control reactive hypoglycemia by stimulating early insulin secretion close to normal insulin kinetics. Nateglinide successfully decreased the frequency of hypoglycemia from almost everyday to a few times a month (Table 1C). One year later, we reexamined the OGTT just after taking nateglinide and voglibose. The peak of insulin secretion after oral glucose load was earlier and lower compared with the previous one, and hypoglycemia was improved, although hyperglycemia was still recognized (Table 2B, Fig. 1B). HbA1c fluctuated between 5.7–6.2% (NGSP). She is now 12 yr old, and her quality of life has been improved, although she occasionally suffers mild hypoglycemia and needs supplementary food.
Genetic Analysis
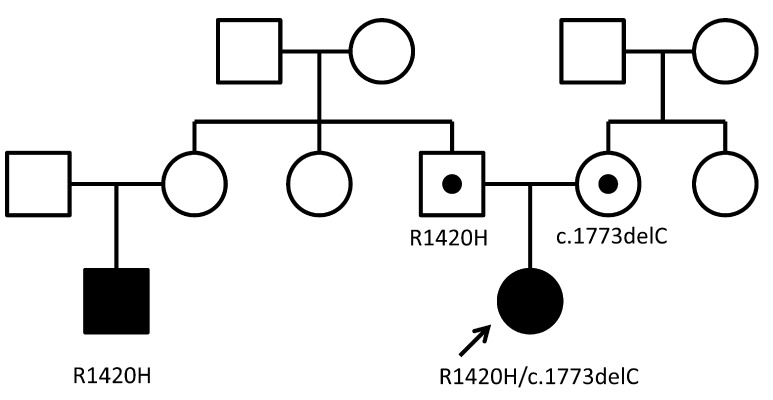
We analyzed genes involved in beta-cell functions, ABCC8, KCNJ11, and HNF4α. Genomic DNA was extracted from peripheral blood leukocytes of the patient, her parents and her cousin. Samples from the patient and her family were obtained after obtaining informed consent. All coding exons and exon/intron boundaries of ABCC8, KCNJ11 and HNF4α were PCR amplified and directly sequenced as previously reported (12). ABCC8 analysis identified a compound heterozygous mutation, c.4259G>A (p.R1420H; exon35) and c.1773delC (p.F591fs604X; exon12). No mutation was found in KCNJ11 and HNF4α. Her father and her cousin on her father’s side carried a heterozygous c.4259G>A mutation, and her mother carried a heterozygous c.1773delC mutation (Fig. 2).
Fig. 2.
Pedigree of the family.
Filled symbols represent persons with hypoglycemia. Subjects indicated by a black dot within the symbol were heterozygous for mutations but had no clinical manifestations. The patient (indicated by the arrow) carried a compound heterozygous ABCC8 mutation, c4259G>A (p.R1420H; exon 35, paternal) and c1773 delC (p.F591fs604X; exon 12, maternal). Her cousin on her father’s side carried a heterozygous c.4259G>A mutation.
Discussion
The characteristic clinical course in our case is that a patient carrying a compound heterozygous ABCC8 mutation (R1420H/F591fs604X) was born macrosomic with severe hypoglycemia in the neonatal period followed by development of diabetes mellitus accompanied by repeated reactive hypoglycemia in childhood. Although we could not determine her insulin level during hypoglycemia in the neonatal period, we presumed that she developed hyperinsulinemic hypoglycemia in the neonatal period because she was born macrosomic, suggesting intrauterine fetal hyperinsulinism.
Recently, there have been some reports of late-onset diabetes mellitus in nonpancreatectomized patients after CHI in infancy due to ABCC8 inactivated mutations. Huopio et al. reported that a heterozygous E1506K mutation in ABCC8 caused CHI in infancy, loss of insulin secretory capacity in early childhood and diabetes mellitus in middle age (7, 13). Vieira et al. also reported a patient carrying the same mutation who developed gestational diabetes and diabetes mellitus in adulthood after mild hypoglycemia in infancy (10). On the other hand, Abdulhadi-Atwan et al. reported diabetes mellitus development at 10.5 yr after mild hypoglycemia during infancy in a patient carrying a heterozygous R370S mutation in ABCC8 (8). Gussinyer et al. reported development of glucose metabolism impairment from glucose intolerance to insulin-dependent diabetes mellitus in 3 nonpancreatectomized patients with compound heterozygous ABCC8 mutations (9). All 3 patients presented persistent hyperinsulinemic hypoglycemia in infancy unresponsive to diazoxide, and OGTTs were performed. Patient 1 had a diabetic OGTT response with hypoglycemic episodes at 22 yr of age, followed by overt insulin-dependent diabetes mellitus without hypoglycemia at the age of 25 yr. In Patient 2, who was 17 yr of age, diabetic OGTT responses and hyperglycemic and hypoglycemic episodes were observed from 10 yr of age despite normal HbA1C values. In Patient 3, who was 24 yr of age, intolerant OGTT responses were observed from 16 yr of age. Furthermore, there is a report that the acute insulin response to glucose was blunted in nonpancreatectomized children with diffuse hyperinsulinism, suggesting the increased risk of diabetes mellitus later in life (14). However, the precise mechanism of transition with age from hyperinsulinemic hypoglycemia to hypoinsulinemic hyperglycemia due to ABCC8 mutation is not known.
In K-ATP channel KO mice (Kir6.2−/− and SUR1−/−) or transgenic mice expressing a dominant-negative form of Kir6.2, hypoglycemia with hyperinsulinemia occurs in neonates followed by hyperglycemia with insulin secretory failure in adults (15,16,17,18). It was also shown that disruption of the K-ATP channel induced elevation of the basal calcium concentration and apoptosis in beta cells (15, 19). Therefore, it has been speculated that the chronic elevation of the intracellular calcium concentration due to mutant K-ATP channel closure induces apoptosis in pancreatic beta cells, resulting in loss of beta-cell mass and decreased insulin secretion (18, 20). Indeed persistent increased beta-cell apoptosis was also observed in pancreases from CHI patients (21).
The patient we described carried a compound heterozygous ABCC8 mutation, c4259G>A (R1420H; exon 35, paternal) and c1773 delC (F591fs604X; exon 12, maternal). R1420 is located in the second nucleotide binding fold (NBF2) of SUR1, and homozygous missense mutation R1420C is reported in Japanese CHI siblings (22). Although they were born macrosomic and developed hypoglycemia soon after birth, hypoglycemia resolve spontaneously after 3 mo of age. Functional analysis of the mutant R1420C SUR1 showed lower expression of the mutant channel and reduced affinity of NFB2 for MgADP that might lead to the enhanced insulin secretion (23). Although the substituted amino acid was different and the mutation was compound heterozygous in our case, the R1420H mutation may also impair K-ATP channel activity. However, it is unknown why the patient’s father is healthy and her cousin developed hypoglycemia in infancy despite carrying the same heterozygous mutation. On the other hand, we presume that the F591fs604X mutation from the maternal allele is also pathogenic because it is a frameshift mutation leading to truncated protein, which might be nonfunctional.
Our patient developed diabetes mellitus accompanied by repeated reactive hypoglycemia, and it was hard to control not only hyperglycemia but also reactive hypoglycemia. Reactive hypoglycemia usually occurred in the late morning or early evening, and not at night. Not only taking supplementary food for reactive hypoglycemia and subsequently dinner but also the secretions of counter-regulatory hormones triggered by hypoglycemia might prevent reactive hypoglycemia after dinner. The OGTT before treatment showed that hyperglycemia developed due to delayed early insulin response to an elevation of blood glucose levels and that reactive hypoglycemia developed due to delayed suppression of insulin secretion in response to a fall in blood glucose levels. Therefore, it is suggested that the response to glucose metabolic change was impaired in the mutant K-ATP channel. In our case, we could not control reactive hypoglycemia with only an alpha-glucosidase inhibitor, which is described as effective for reactive hypoglycemia (24, 25). However, the combination of α-GI and nateglinide could obviously decrease episodes of reactive hypoglycemia, although this combination has not been reported to be effective for reactive hypoglycemia. Nateglinide is a D-phenylalanine derivative antihyperglycemic drug that stimulates early insulin secretion, and it has a shorter duration of action than sulfonylurea. Considering its action, it has been expected that nateglinide is effective for diabetes mellitus with reactive hypoglycemia by inducing early insulin secretion and preventing delayed prolonged insulin secretion (26). In fact, we could control not only hyperglycemia but also reactive hypoglycemia with the combination of α-GI and nateglinide.
In conclusion, our case showed that a SUR1 mutation in CHI could alter regulation of insulin secretion with advancing age, leading to diabetes mellitus with reactive hypoglycemia from hyperinsulinism. Therefore, we think that long-term follow-up and periodic OGTTs are important for early detection of insulin dysregulation in CHI patients carrying a SUR1 mutation, even though hypoglycemia resolves spontaneously without pancreatectomy during infancy. Furthermore, nateglinide may be useful therapeutically in the treatment of reactive hypoglycemia with impaired glucose tolerance/early diabetes mellitus.
References
- 1.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 1984;312: 446–8 [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest 2005;115: 2047–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas PM, Cote GJ, Wohilk N, Haddad B, Mathew PM, Rabl W, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 1995;268: 426–9 [DOI] [PubMed] [Google Scholar]
- 4.Thomas PM, Wohllk N, Huang E, Kuhnle U, Rabl W, Gagel RF, et al. Inactivation of the first nucleotide-binding fold of the sulfonylurea receptor, and familial persistent hyperinsulinemic hypoglycemia of infancy. Am J Hum Genet 1996;59: 510–8 [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas P, Yuyang Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet 1996;5: 1809–12 [DOI] [PubMed] [Google Scholar]
- 6.Flanagan SE, Clauin, Bellanné-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, et al. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat 2009;30: 170–80 [DOI] [PubMed] [Google Scholar]
- 7.Huopio H, Otonkoski T, Vauhkonen I, Reimann F, Ashcroft FM, Laakso M. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet 2003;361: 301–7 [DOI] [PubMed] [Google Scholar]
- 8.Abdulhadi-Atwan M, Bushman J, Tornovsky-Babaey S, Perry A, Abu-Libdeh A, Glaser B, et al. Novel de novo mutation in sulfonylurea receptor 1 presenting as hyperinsulinism in infancy followed by overt diabetes in early adolescence. Diabetes 2008;57: 1935–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gussinyer M, Clemente M, Cebrián R, Yeste D, Albisu M, Carrascosa A. Glucose intolerance and diabetes are observed in the long-term follow-up of nonpancreatectomized patients with persistent hyperinsulinemic hypoglycemia of infancy due to mutations in the ABCC8 gene. Diabetes Care 2008;31: 1257–9 [DOI] [PubMed] [Google Scholar]
- 10.Vieira TC, Bergamin CS, Gurgel LC, Moisés RS. Hyperinsulinemic hypoglycemia evolving to gestational diabetes and diabetes mellitus in a family carrying the inactivating ABCC8 E1506K mutation. Pediatr Diabetes 2010;11: 505–8 [DOI] [PubMed] [Google Scholar]
- 11.Brun JF, Fedou C, Mercier J. Postprandial reactive hypoglycemia. Diabetes Metab 2000;26: 337–51 [PubMed] [Google Scholar]
- 12.Yorifuji T, Kawakita R, Nagai S, Sugimine A, Doi H, Nomura A, et al. Molecular and clinical analysis of Japanese patients with persistent congenital hyperinsulinism: predominance of paternally inherited monoallelic mutations in the KATP channel genes. J Clin Endocrinol Metab 2011;96: E141–5 [DOI] [PubMed] [Google Scholar]
- 13.Huopio H, Reimann F, Ashfield R, Komulainen J, Lenko HL, Rahier J, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest 2000;106: 897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimberg A, Ferry RJ, Kelly A, Koo-McCoy S, Polonsky K, Glaser B, et al. Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutations. Diabetes 2001;50: 322–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miki T, Tashiro F, Iwanaga T, Nagashima K, Yoshitomi H, Aihara H, et al. Abnormalities of pancreatic islets by targeted expression of adominant-negative KATP channel. Proc Natl Acad Sci USA 1997;94: 11969–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K (ATP) channel-independent regulation of insulin secretion. J Biol Chem 2000;275: 9270–7 [DOI] [PubMed] [Google Scholar]
- 17.Remedi MS, Rocheleau JV, Tong A, Patton BL, McDaniel ML, Piston DW, et al. Hyperinsulinism in mice with heterozygous loss of KATP channels. Diabetologia 2006;49: 2368–78 [DOI] [PubMed] [Google Scholar]
- 18.Nichols CG, Koster JC, Remedi MS. β-cell hyperexcitability: from hyperinsulinism to diabetes. Diabetes Obes Metab 2007;9: 81–8 [DOI] [PubMed] [Google Scholar]
- 19.Miki T, Iwanaga T, Nagashima K, Ihara Y, Seino S. Roles of ATP-sensitive K+channels in cell survival and differentiation in the endocrine pancreas. Diabetes 2001;50: S48–51 [DOI] [PubMed] [Google Scholar]
- 20.Efanova IB, Zaitsev SV, Zhivotovsky B, Köhler M, Efendić S, Orrenius S, et al. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J Biol Chem 1998;273: 33501–7 [DOI] [PubMed] [Google Scholar]
- 21.Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 2000;49: 1325–33 [DOI] [PubMed] [Google Scholar]
- 22.Tanizawa Y, Matsuda K, Matsuo M, Ohta Y, Ochi N, Adachi M, et al. Genetic analysis of Japanese patients with persistent hyperinsulinemic hypoglycemia of infancy nucleotide-binding fold-2 mutation impairs cooperative binding of adenine nucleotides to sulfonylurea receptor 1. Diabetes 2000;49: 114–20 [DOI] [PubMed] [Google Scholar]
- 23.Matsuo M, Trapp S, Tanizawa Y, Kioka N, Amachi T, Oka Y, et al. Functional analysis of a mutant sulfonylurea receptor, SUR1-R1420C, that is responsible for persistent hyperinsulinemic hypoglycemia of infancy. J Biol Chem 2000;275: 41184–91 [DOI] [PubMed] [Google Scholar]
- 24.Peter S. Acarbose and idiopathic reactive hypoglycemia. Horm Res 2003;60: 166–7 [DOI] [PubMed] [Google Scholar]
- 25.Tamura Y, Araki A, Chiba Y, Horiuchi T, Mori S, Hosoi T. Postprandial reactive hypoglycemia in an oldest-old patient effectively treated with low-dose acarbose. Endocr J 2006;53: 761–71 [DOI] [PubMed] [Google Scholar]
- 26.Mori Y. Postprandial hypoglycemia. Nihon Rinsho 2006;28: 224–9 [PubMed] [Google Scholar]