Abstract
Molecular and non-invasive imaging are rapidly emerging fields in preclinical cancer drug discovery. This is driven by the need to develop more efficacious and safer treatments, the advent of molecular-targeted therapeutics, and the requirements to reduce and refine current preclinical in vivo models. Such bioimaging strategies include MRI, PET, single positron emission computed tomography, ultrasound, and optical approaches such as bioluminescence and fluorescence imaging. These molecular imaging modalities have several advantages over traditional screening methods, not least the ability to quantitatively monitor pharmacodynamic changes at the cellular and molecular level in living animals non-invasively in real time. This review aims to provide an overview of non-invasive molecular imaging techniques, highlighting the strengths, limitations and versatility of these approaches in preclinical cancer drug discovery and development.
Keywords: non-invasive imaging, cancer pharmacology, bioluminescence, ultrasound, PET, MRI
Introduction
Despite advances in cancer treatment, response of many tumour types is suboptimal and in many cases not curative. Therefore, new treatments or better targeting of current treatments for cancer therapy is of the upmost importance. As knowledge of molecular systems and pathways expands and improves, development of novel agents that are directed to specific molecular targets has become forefront. This approach aims to increase selective toxicity to cancer cells, reduce the likelihood of therapeutic resistance, and limit patient morbidity commonly associated with chemotherapy. The development and improvement of these ‘molecular targeted’ therapies is a major goal of current anticancer drug development. Analytical tools that enable the assessment of new therapies targeted specifically to individual pathways/molecules in living mammals are already benefiting researchers, with the potential to provide a lot more information in the future.
Limitations of current preclinical in vivo models in cancer drug development
In vitro studies can be used to give basic information on the toxicity of a drug against cancer cell lines, to evaluate drug-target interactions, and to define biochemical and gene expression pathways (Massoud and Gambhir, 2003). These studies do not however answer questions regarding clinical response in the whole body system (de Jong and Maina, 2010). Ultimately, the value of laboratory and preclinical studies depends on their capacity to accurately predict clinical response as a surrogate to humans due to the ethical and practical concerns this raises (de Jong and Maina, 2010). Over the past few decades, animal models have played a key part in revealing many biochemical and physiological processes involved in the onset of cancer and its development in living organisms (de Jong and Maina, 2010). The primary objectives of these animal models are firstly to mimic the human disease as closely as possible, and secondly to proficiently test new therapies (Liao et al., 2007). In vivo models allow target-orientated drug screening and can yield pharmacokinetic and pharmacodynamic information, both of which are clinically relevant. However, despite animal models giving valuable information regarding drug efficacy, there are several factors that should be considered when extrapolating mouse data to the clinical testing, a topic succinctly reviewed by de Jong and Maina (2010). One such example is the use of subcutaneous tumour xenograft models, a tumour environment that does not accurately mimic the clinical situation.
Subcutaneous tumour xenograft models are traditionally used as a front line screen for assessing therapeutic efficacy and drug-target interactions, the response that has been determined via calliper-based measurements, and monitoring the lifespan of animals (Suggitt and Bibby, 2005; Zhang et al., 2007). This strategy has proved successful for several agents now in the clinic, including trastuzumab for treatment of HER2-overexpressing breast cancer (Baselga et al., 1998; Vogel et al., 2002), melphalan in the treatment of rhabdomyosarcoma (Horowitz et al., 1988), and vorinostat for treatment of cutaneous T-cell lymphoma (Kelly et al., 2003; Marks and Breslow, 2007). Despite these successes and this strategy providing high-quality information on therapeutics, several limitations exist, which impinge upon the utility of this approach for molecular-targeted therapeutics. Firstly, these models do not fully recapitulate the tumour cellular heterogeneity of the clinical tumour environment. Secondly, significant efforts and advances are being made with molecular-targeted agents against tumour vasculature, including vascular-disrupting and anti-angiogenic agents. Therapeutic strategies such as these may exert their cytostatic effect very efficiently, but since they are not cytotoxic will not necessarily lead to a decrease in tumour size (Cai et al., 2006). Therefore, monitoring tumour size using callipers could underestimate the effect of these agents, give little information regarding the internal structure of the tumour, and thus provide inconclusive information regarding their efficacy.
A major issue with the use of calliper-measured subcutaneous xenograft tumour models is their lack of applicability for evaluation of metastatic disease (Suggitt and Bibby, 2005). Metastasis is the leading cause of death among cancer patients so the development of robust model systems for front line drug discovery in this area is an urgent requirement. Although preclinical models exist involving both spontaneous and experimentally induced metastatic tumours, these are often time-consuming and labour-intensive methodologies being non-amenable to calliper-based measurements and requiring termination of multiple animals for tumour load determination (Grosios et al., 1999). Consequently, there is a need for improved preclinical models or strategies that translate and represent the clinical situation.
Molecular imaging as a tool for preclinical cancer pharmacology studies
Molecular imaging is a rapidly emerging field with a multitude of characteristics that make it useful for the drug discovery process. Technically, it has many advantages over traditional techniques as it is rapid, can be high throughput, is non-invasive and is less labour intensive than pathology- and chemistry-based assays (Massoud and Gambhir, 2007). It allows imaging to be performed at the cellular and molecular level in living animals, in real-time and with a truly quantitative outcome (Laxman et al., 2002; Massoud and Gambhir, 2007; Wessels et al., 2007). With regard to the requirement to replace, refine and reduce (the 3Rs) the use of animals in research, molecular imaging can be used to follow tumour development or therapeutic effect over a period of time in the same animal, thereby reducing the number of animals being used while simultaneously improving the data set as each animal serves as its own control (Contag and Ross, 2002; Fomchenko and Holland, 2006). A further benefit of sequentially studying tumour pathogenesis in one animal is that information regarding the possible behaviour of a tumour in humans, especially regarding metastasis and therapy response, is more achievable.
Earlier detection and characterization of disease are additional benefits of bioimaging, as is the information that can be obtained which is specific to particular molecular events, e.g., evaluation of therapeutic interactions (Contag and Ross, 2002; Laxman et al., 2002). In vivo, bioimaging can be used for target and therapeutic validation in a dynamic environment (Gwyther and Schwartz, 2008; Laxman et al., 2002). It also allows quicker assessment of drug/target interactions and identification of therapeutic efficacy prior to any macroscopic or phenotypic changes (Massoud and Gambhir, 2007; Morse et al., 2007). Clinically, this is important as efficacy of a treatment can be determined earlier and if necessary the treatment regime can be altered (Morse et al., 2007). Some types of bioimaging also have the potential to image specific intracellular pathways, a feature that has previously been illusive (Laxman et al., 2002).
Imaging modalities in preclinical cancer drug discovery: advantages and limitations
There are seven main types of bioimaging; MRI, PET, single positron emission computed tomography (SPECT), CT, ultrasound (US) and optical imaging [including bioluminescence imaging (BLI) and fluorescence imaging]. All of these techniques demonstrate merit during drug development, with each of them having advantages and disadvantages (Table 1).
Table 1.
Advantages and disadvantages of imaging modalities for preclinical cancer drug discovery
| Modality | Advantages | Disadvantages |
|---|---|---|
| MRI | •High spatial resolution | •Low sensitivity |
| •Good soft tissue contrast | •Relatively long acquisition time | |
| •Provides both anatomical and functional information | •Requires expensive equipment | |
| PET | •Provides biochemical information | •Limited anatomical information |
| •High sensitivity | •Requires specialized equipment | |
| •Three-dimensional imaging | •Requires radio-nucleotide facilities | |
| •Can monitor changes in tumour metabolism and drug biodistribution | •Requires expensive equipment | |
| SPECT | •Potential to detect multiple probes simultaneously in contrast to PET | •Lower sensitivity than PET |
| CT | •High-sensitivity anatomical imaging | •Lower resolution |
| •Provides three-dimensional image | •Limited functional information | |
| •Poor soft tissue contrast | ||
| •Requires expensive equipment | ||
| Ultrasound | •Good resolution | •Inability to image through bone |
| •Provides both anatomical and functional information | ||
| •Fast and portable technique | ||
| •Relatively inexpensive | ||
| •Amenable to smaller research laboratories | ||
| Optical (BLI and fluorescent) | •Wide applicability | •Requires genetic manipulation of investigated cells |
| •Simultaneously monitor several molecular events | •Provides limited anatomical information | |
| •Relatively inexpensive | •Reduced sensitivity with increased imaging depth | |
| •Amenable to smaller research laboratories |
MRI
MRI is based on the absorption and emission of energy in the radio frequency range of the electromagnetic spectrum, and the fact that the body is primarily composed of hydrogen-rich fat and water. When induced by a strong magnetic field, the hydrogen nuclei emit a nuclear magnetic resonance signal that is determined by the direction of the spin induced. MRI gives high spatial resolution of about 50 μm3 (Czernin et al., 2006) and good soft tissue contrast. MRI is a very versatile technique and is widely used in small animal studies to address tumour physiology and quantification of tumour volume (Fomchenko and Holland, 2006). However, MRI has low sensitivity and a relatively long acquisition time (up to 1 h), thereby limiting its utility for preclinical drug development studies (Lyons, 2005). Its ability to amalgamate anatomical and functional information provides great insights into disease processes, including cancer (Hasegawa et al., 2010). Improvements in the methodology for MRI are constant (Schroder et al., 2006), including the use of higher magnetic fields and enhancing contrast agents, which has led to an improvement in the sensitivity of MRI (Hasegawa et al., 2010).
MRI has been used to successfully report efficacy of suicide gene therapy in delaying tumour growth in a mouse model of orthotopic glioma (Breton et al., 2010) and to demonstrate complete infiltration of intraprostatic injected gene therapies in preclinical mouse models of prostate cancer (Kassouf et al., 2007). More recently, MRI has been used with drug-containing liposomes, either gadolinium labelled or paramagnetic, to facilitate image-supervised therapeutic delivery and subsequent monitoring of efficacy (Grange et al., 2010; Strijkers et al., 2010). Advancements in technology have now led to more sensitive quantitative MRI techniques, such as high-field MRI and dynamic contrast-enhanced MRI (DCE-MRI), which are even more valuable for research into tumour vasculature and the effects of drugs.
DCE-MRI
DCE-MRI is a quantitative method of investigating the structure and function of tumour microvasculature and microcirculation (O'Connor et al., 2007; Ali et al., 2010). Advantages of this technique are that in addition to initial anatomical MRI information, data are subsequently acquired every few seconds over a period of 5–10 min providing dynamic detail of features such as blood flow (O'Connor et al., 2007). DCE-MRI has been used to characterize tumour angiogenesis (Brix et al., 2010), and has the potential to aid investigation of anti-angiogenic and vascular-disrupting therapeutics, although it is not restricted to such agents. Recently, DCE-MRI has been used to show co-treatment with a folate-linked liposomal doxorubicin and a TGFβ-receptor-1 inhibitor (A-83-01) enhanced the therapeutic effect of doxorubicin, indicated by DCE-MRI monitored tumour leakage of the gadolinium-liposome complex (Taniguchi et al., 2010). Furthermore, DCE-MRI demonstrated pancreatic xenograft tumour response to the hypoxia-inducible factor (HIF)-1α inhibitor PX-478 before any anatomical changes were recorded (Schwartz et al., 2010).
In general, MRI, although constrained by acquisition time and sensitivity, can be used preclinically to help develop new therapies, both cytotoxic and target molecule specific, for a broad range of cancers. This strategy does address the requirement for a refinement of usage and reduction in animal numbers according to the 3Rs. In addition, MRI also allows tumour response to be followed in the same animal, therefore improving the power of a study despite fewer animals being used. A drawback to the use of MRI in preclinical studies is the cost of equipment for smaller labs where in vivo work is done.
PET
PET produces a three-dimensional (3D) image of functional processes in the body, measuring biochemical function rather than structure, and thus provides a crucial insight into cancer biology and pharmacology (Zaidi and Prasad, 2009). Mechanistically, a probe comprising a metabolically active molecule (such as glucose or water) incorporating a γ-ray emitting radioisotope is introduced into animal and its uptake and metabolism monitored. The commonest probe is 18-fluorodeoxyglucose, of which uptake indicates glucose metabolism and thus the enhanced glycolysis associated with malignancy, enabling differentiation between malignant and benign tissue (Vansteenkiste, 2002; Otsuka et al., 2007). Unfortunately, due to the requirement of a cyclotron for radio-nucleotide production and dedicated synthetic chemical equipment, PET systems are generally limited to research laboratories associated with a clinical centre. However, where they are used, PET has strong potential for translational research from small animals to humans (Laforest and Liu, 2008). Additionally, PET has the advantage over other imaging modalities in that it also permits evaluation of changes in tumour metabolism and proliferation, drug biodistribution, and pharmacokinetics, all of which aid assessment to drug efficacy (Avril and Propper, 2007).
A huge variety of cancer treatments have been investigated using PET, including treatment of malignant gliomas using bevacizumab and irinotecan (Chen et al., 2007), defining optimal dose of mTOR inhibitors (Cejka et al., 2009), screening novel HDAC inhibitors (Leyton et al., 2006), evaluating ovarian tumour response to the antivascular agent AVE8062 and taxanes (Kim et al., 2007), and determining the therapeutic effect of the EGFR tyrosine kinase inhibitor lapatinib (Diaz et al., 2010). In addition, radiotracers to indicate changes in receptor expression following therapeutic interventions have also been studied (Kramer-Marek et al., 2009), as was the case with the pharmacodynamics of C75, a fatty acid synthase inhibitor and emerging target for anticancer therapy (Lee et al., 2007a).
The ability to use PET to determine drug and target distribution in cancer cells has been used to monitor whether yttrium-90 spheres, a novel treatment for advanced liver cancer, are preferentially delivered to tumour cells following injection into the hepatic arteries (Tehranipour et al., 2007). Furthermore, this application of PET has been adopted to determine tumour specificity of the JAA-f11 antibody on the survival of mice with metastatic 4T1 breast tumours (Rittenhouse-Olson, 2007) and to confirm that the sigma-2 receptor was a valid target for cancer drug development through preferential expression in tumour tissue but not normal tissues (Kashiwagi et al., 2007).
One problem reported for PET in preclinical studies was the heterogeneity of glucose uptake in different areas of a tumour, resulting in a lack of correlation between PET and standard calliper measurements, evidenced with enzastaurin, a novel protein kinase C-β II inhibitor (Pollok et al., 2009). Despite this potential issue for experimental reproducibility, the use of PET preclinically is a viable option as it allows treatment optimization, which is one of the main goals of preclinical studies prior to clinical trial. However, as with MRI, the equipment necessary for imaging small animals is expensive and not available to the majority of laboratories.
SPECT
SPECT is an imaging technique that detects low energy γ-rays arising from radioisotope decay and has resolution of 1–2 mm. An advantage of this technique over PET is the capacity to detect multiple probes simultaneously. Conversely, SPECT has lower sensitivity and therefore requires higher amounts of probe (Lyons, 2005). A detailed comparison of PET versus SPECT methodologies is provided by Rahmim and Zaidi (2008).
In cancer drug development, SPECT has been used to investigate the dosage effects of the human monoclonal antibody hu3S193, targeted to the Lewis-Y (Krug et al., 2007), and using Tc-99 m tagged VEGF-C to analyse VEGF-induced signalling pathways and changes in VEGF-receptor expression in response to the anti-angiogenic agent PTK787 (Ali et al., 2010). Similarly, small high-affinity anti-HER2 molecules have been investigated as suitable tracers of SPECT visualization of HER2-expressing tumours (Ahlgren et al., 2009), with the benefit of also permitting assessment of treatment-induced effects on HER2 expression.
New probes are continually being engineered to optimize current and develop new methods that may help in targeted cancer drug development. For instance, activity of MMP-14, a hallmark of cancer metastasis, has been probed using a technetium-99 m SPECT marker developed to be ‘activatable’ by MMP-14 in vivo, a strategy which may facilitate development of targeted molecular therapies (Watkins et al., 2009).
CT
CT is a medical imaging method whereby contrast agents are administered intravenously, and then digital geometry processing is used to generate 3D images from a series of two-dimensional (2D) X-ray scans. CT is the most commonly used tool for clinical assessment of the structural features of cancer and along with MRI is the modality of choice to monitor tumour response to therapy (Torigian et al., 2007). The benefits of CT include its ability to separate anatomical structures at different depths within the body, making it more useful than standard X-rays. It is inherently high contrast; therefore, even extremely small differences in tissue density can be distinguished. Images created by CT scans can be manipulated so that they can be viewed in different planes.
Conventional CT imaging used in the clinic is not suitable for small animal studies due to differences in species size and modality requirements; therefore, dedicated systems were developed for preclinical studies (Paulus et al., 2000; Schambach et al., 2010). This micro-CT modality offers higher resolution volumetric imaging of the anatomy of living small animals and has proved useful in monitoring the preclinical response of bone metastatic deposits to radiofrequency ablation (Proschek et al., 2008; Schambach et al., 2010) and small molecule inhibitors of heat-shock proteins (Kang et al., 2010). The greatest utility of micro-CT is detection of metastases, demonstrated through its ability to detect micrometastatic pheochromocytoma tumours in the livers of a nude mouse model, providing a tool for evaluating treatment strategies for this cancer (Ohta et al., 2006). Similarly, micro-CT has been employed preclinically to measure bone and tumour volumes in the evaluation of new treatment options for prostate cancer (Morgan et al., 2008; Kang et al., 2010), non-invasive real-time monitoring of lung cancer, and treatment response (Fushiki et al., 2009), and to follow colorectal tumorigenesis through the use of micro-CT colonography (Durkee et al., 2008).
Several studies have now demonstrated that micro-CT has significant potential for preclinical anticancer drug development. However, there still remain several issues regarding this technique, including imaging resolution, which often falls short of the intended 100 μm objective due to scan quality and reader ability (Durkee et al., 2008), and the inherently poor contrast between different soft tissues (Prajapati and Keller, 2011). In preclinical studies, resolution is vitally important, as the commonest use of CT is to monitor micrometastatic deposits, which may be impossible to detect unless scans of exceptional quality are achieved (Schambach et al., 2010). Consequently, higher resolution often involves exposure to a higher dose of radiation, which in itself results in issues regarding exposure levels, although advances in technology have decreased this somewhat (Prajapati and Keller, 2011; Schambach et al., 2010). Taking this into account, although CT is a viable imaging technique for preclinical cancer pharmacology studies, it does have pitfalls and limitations and is generally more expensive than other optical imaging techniques.
High-frequency US in preclinical cancer drug discovery
US uses sound waves greater than 20 000 Hz generated by pulse/echo transducers and integrated by signal processing software to produce grey scale images. The first high-frequency (HF) US instrument specifically designed for micro-imaging of the mouse was described 10 years ago (Foster et al., 2002). Since then, US has developed a clear and growing role in preclinical imaging and drug development due to the significant advantages it has over other preclinical imaging modalities in that it is relatively inexpensive, fast, portable, easy to use, works in real time and does not involve ionizing radiation. Several systems are currently available for preclinical imaging and these have recently been compared for versatility and performance (Moran et al., 2011). In addition to anatomical imaging, US also lends itself to functional imaging with Doppler US or US contrast agents allowing qualitative and quantitative assessment of tumour blood flow and perfusion and tumour angiogenesis.
Anatomical imaging using HF-US
Small animal imaging uses HF-US in the range of 25–50 MHz for anatomical imaging giving spatial resolution of 90 × 30 μm (Turnbull et al., 1995), allowing high-definition imaging of mouse organs such as liver, kidney, eyes and heart (Graham et al., 2005; Jolly et al., 2005; Sun et al., 2008). HF-US is applicable for imaging mouse colon and measuring colon wall thickness in vivo (Abdelrahman et al., 2012) and for imaging ovarian structures in mice (Jaiswal et al., 2009). HF-US has also been used to study tumour growth and development in different genetically engineered mouse models (GEMMs) and in human cancer cell xenografts where grey scale resolution allows for the differentiation of subtle changes in anatomy and monitoring of changes in disease pathology (Figure 1). This is particularly important when considering spontaneously arising tumours in GEMM. Zhao et al. (2010) used HF-US to determine pre-leukaemic changes and splenomegaly in a transgenic model of acute myeloid leukaemia (Zhao et al., 2010). In conditional Kras Trp53 mutant mice, HF-US was the modality of choice for non-invasive detection of pancreatic tumours (Olive and Tuveson, 2006). In this context, HF-US is particularly useful in pre-screening cohorts for presence of tumours prior to randomization and drug treatment, thereby reducing the number of animals used and refining experimental protocols.
Figure 1.
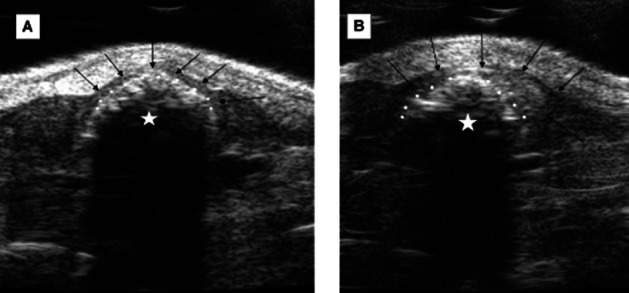
(HF-US) imaging of the proximal colon of an ApcMin/+ mouse. The VisualSonics Vevo770 system has a resolution of 30 μm allowing identification of normal and pathological colon. (A) shows normal colon where an even thickness of colon wall surrounds the fecal pellet. The wall thickness was measured as 0.18 mm (measurement not shown). (B) shows an adjacent section of colon where an adenoma had formed. This was identified in vivo as a significant thickening of the colon wall. The maximum wall thickness was measured as 0.87 mm (measurement not shown). Black arrows show the outer colon wall, white circles delineate the border between fecal pellet and the inner colon wall, and white stars indicate the fecal pellet.
Measurement of tumour size in longitudinal studies is an important readout in anti-cancer drug discovery, and 3D HF-US has been shown to be more accurate, precise and reproducible than manual caliper measurement (Ayers et al., 2010). This is particularly important when assessing small irregular shaped tumours (Cheung et al., 2005) and xenograft tumours that are often irregularly shaped. Furthermore, as 3D HF-US can also be used to determine tumour volume and burden within organs, it can facilitate longitudinal studies to assess responses to novel and existing drugs in GEMM and orthotopic models (Singh et al., 2010).
As described earlier, although subcutaneous tumour xenografts are traditionally used as a front line screen for assessing therapeutic efficacy, they do not accurately recapitulate the clinical location or environment (Suggitt and Bibby, 2005). To address the deficiencies of subcutaneous models, orthotopic tumour xenografts are increasingly being explored for increased clinical relevance (Suggitt and Bibby, 2005). Anatomical imaging in real time using HF-US provides the basis for image-guided injection, thus facilitating production of orthotopic tumour models. For example, US-guided cell injections were used to create clinically relevant models of human pancreatic cancer (Huynh et al., 2011). Similarly, US-image-guided injection of syngeneic colonic carcinoma cells was used to create a robust mouse model of liver metastasis without the need for invasive surgery (Hawcroft et al., 2012).
A limitation of US is that it cannot be used to image bone, which reflects US waves, making US unsuitable for analysis of brain and bone tumours. It has also been suggested with US that the imaging and observations from the technique are largely operator dependent with discrepancies observed between studies, a feature that can however be controlled by development of robust imaging protocols (Abdelrahman et al., 2012).
HF-US in functional and molecular imaging
In addition to its ease of use and high resolution in anatomical imaging, US can be used to assess tumour angiogenesis by qualitative and quantitative assessment of tumour blood flow using Doppler US, or addition of a US contrast agent or microbubbles (MBs). This is increasingly important given the development of anti-angiogenic therapies and the consequent need for non-invasive strategies for continued tumour assessment and monitoring of drug pharmacodynamics.
Doppler US detects blood flow by the Doppler shift frequency, with both 2D and 3D power Doppler US (PD-US) being capable of monitoring and imaging blood flow velocities in murine tumour models (Goertz et al., 2002). One such example was the use of 3D PD-US to monitor angiogenic therapeutic response in a GEMM of prostate cancer (Xuan et al., 2007). However, a limitation of PD-US in this context is caused by the signal-to-noise ratio and presence of imaging artefacts, which make accurate determination of tumour blood flow difficult and slow-moving blood undetectable. This may be important when assessing overall tumour blood flow in experimental tumours.
A further type of US with potential for preclinical cancer pharmacology studies is contrast-enhanced HF-US which uses gas-filled phospholipid MBs of 2–7 μm in size. The small size of these MBs makes them true vascular tracers as they are restricted to the vascular network and can readily pass through the lungs, unlike contrast agents from other imaging modalities. MBs are hyperechoic, producing bright signals on the grey scale image which can be artificially coloured to produce images of tumour blood flow (Figure 2A). Relative tumour blood flow can be quantitated using time intensity curves (Figure 2B) or destruction replenishment imaging giving relative rates of blood flow and maximum perfusion. Using this methodology, therapeutic response was detected earlier than determination of tumour volume in an orthotopic model of breast cancer, with the area under the time intensity curve and peak intensity correlating to treatment efficacy (Hoyt et al., 2010). Furthermore, the effect of discontinuation of the anti-angiogenic therapy bevacizumab was also evaluated by contrast-enhanced US in an orthotopic model of renal cancer (Guibal et al., 2010). This study identified a quantitative change in tumour perfusion between those continuously treated with bevacizumab and those in which treatment was interrupted. Moreover, contrast-enhanced US was shown to be more sensitive at detecting therapeutic response than histological assessment of microvessel density (Guibal et al., 2010).
Figure 2.
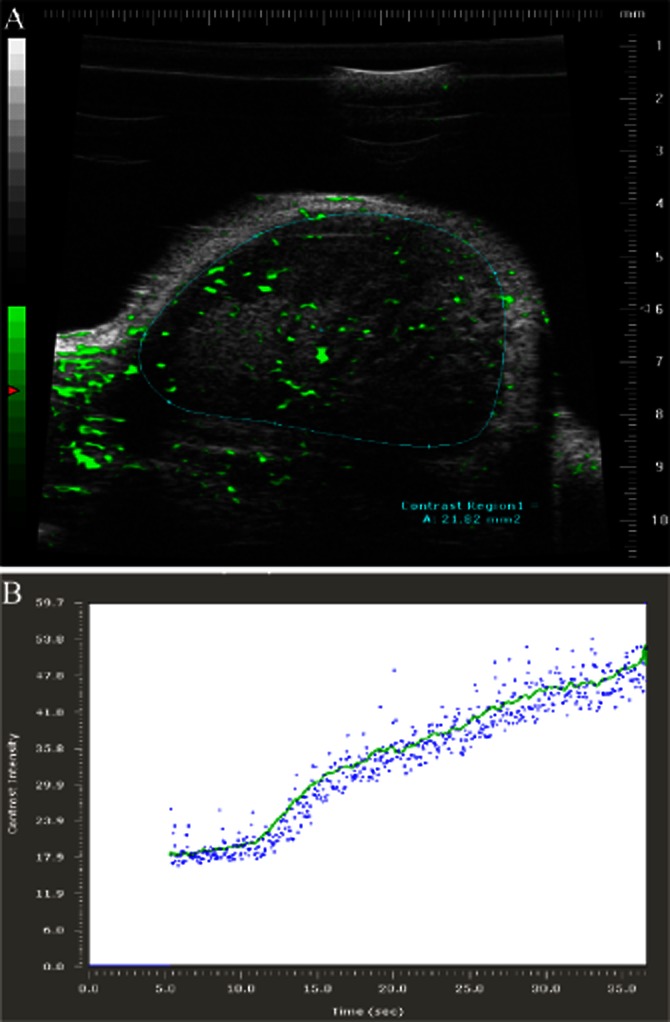
Contrast-enhanced HF-US imaging. The use of microbubble contrast agents with HF-US imaging protocols allows the visualization and relative quantification of tumour blood flow and perfusion. (A) shows a contrast-enhanced HF-US image of an SW480 human colorectal cancer xenograft where microbubbles are coloured green and the region of interest (tumour) is delineated by the blue line. By imaging the contrast agent over time, both qualitative and quantitative data on tumour vascularity and tumour blood flow, respectively, can be obtained. (B) shows a wash-in time intensity curve where the contrast intensity in arbitrary units is plotted against time in seconds. Analysis of these curves provides values for the maximum intensity of the contrast agent or relative perfusion and the maximum relative rate of tumour blood flow.
Recently, contrast-enhanced US has been adapted to simultaneously monitor in vivo pharmacodynamics, achieved through surface modification of MBs to include targeting moieties to vascular biomarkers. Targeted-contrast-enhanced HF-US imaging has been used for in vivo assessment of aνβ3 integrin, endoglin (CD105) and VEGFR2 levels in mouse models of breast, ovarian and pancreatic cancers (Deshpande et al., 2011). This relatively new approach combining the sensitivity of molecular imaging with low-cost, easy-to-use US imaging has the potential to enhance and accelerate preclinical drug development.
Optical imaging in preclinical cancer drug discovery
The term optical imaging encompasses preclinical approaches using either luminescence or fluorescence detection as a means for evaluating drug activity, molecular events or biological activity of potential drug targets. These techniques typically require less equipment and expertise and are more cost effective than the methodologies described above, making them more suitable techniques for smaller laboratories. This system relies upon detection of either a fluorescent or luminescent event and provides no results regarding the physical characteristics or pathology of the tissue or animal, requiring the overlay of the detected events on an anatomical image of the animal or tissue.
The use of optical imaging for monitoring biological changes is now a well-established methodology within drug discovery and pharmacology (Fomchenko and Holland, 2006). Early in the drug discovery process, in vitro investigations are performed on cancer cell lines, and here optical imaging can be used to determine the therapeutic or pharmacological effect on cancer cell lines treated with different compounds (Loo et al., 2007), to investigate transcription factor activity (Weiss et al., 2010), or to study specific protein expression following drug treatment (Sakoguchi-Okada et al., 2007). Furthermore, mechanistic information can be obtained through the use of tagged proteins or promoter-driven reporter expression within these experimental models. In preclinical cancer pharmacology, there are now a wide range of cancer cells available that express optical imaging proteins or probes, representing all the major human solid tumour types.
A downside to the use of optical imaging is that the target or investigated cells require genetic modification to express either luciferase or a fluorescent protein prior to their use, making it unlikely that such an approach will ever translate to the clinic. Nevertheless, the high selectivity, specificity and applicability of this approach remain a valuable tool for preclinical evaluation of cancer therapeutics and their acceleration and progression into the clinic.
BLI
The principle of BLI relies upon detection of photons emitted from the oxygen-mediated conversion of luciferin to oxyluciferin by cells genetically modified to express luciferase (Figures 3 and 4), a process that does not necessitate an external light source (Gould and Subramani, 1988). Several luciferase genes have now been identified and cloned from various natural sources, with the most common ones for imaging purposes being ATP-dependent luciferase isolated from the North American firefly (Photonis pyralis) and ATP-independent luciferase from the anthozoan sea pansy (Renilla reniformis) (Gould and Subramani, 1988; Snoeks et al., 2010). Firefly luciferase catalyses D-luciferin to give a flash of green light at 562 nm, whereas Renilla luciferase catalyses coelenterazine to generate blue luminescence with a wavelength centred at 482 nm (Bhaumik et al., 2004). This lack of cross-reactivity between firefly and Renilla luciferase substrates also means that the BLI system can be utilized for dual-label and target imaging (Figure 4).
Figure 3.
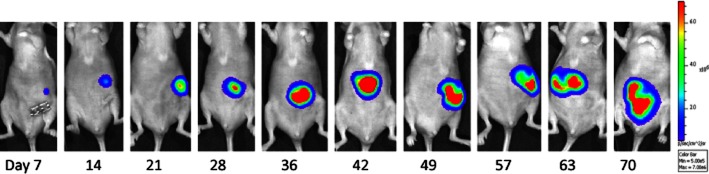
Bioluminescence imaging of orthotopic tumour growth. Mice were orthotopically implanted on the caecum with DLD1-1 colorectal cancer cells engineered to express firefly luciferase (under control of the Simian Virus-40 promoter). At weekly intervals, the mice were injected with D-luciferin and imaged using an IVIS-50 system (Caliper Life Sciences, Runcorn, UK). The image shown depicts the growth of the orthotopic tumour over time.
Figure 4.
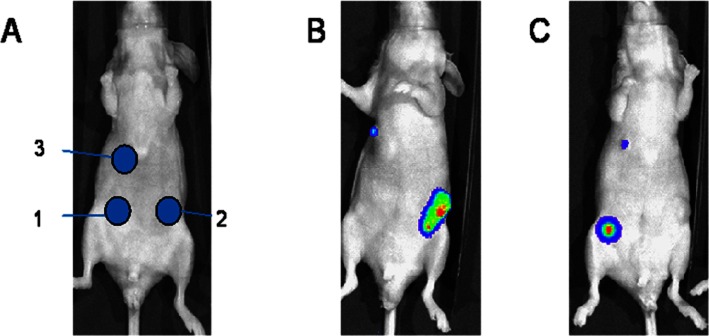
Potential for dual bioluminescence imaging of tumours in vivo. (A) Mice bearing subcutaneous tumours engineered to express either firefly luciferase (tumour site 1, FLuc), Renilla luciferase (tumour site 2, RLuc) or both luciferase systems (tumour site 3). (B) Bioluminescence image following injection of coelenterazine (substrate for Renilla luciferase) with light detected from tumours 2 and 3. (C) The same mouse imaged 4 h later following injection with D-luciferin (substrate for firefly luciferase) with light detected from tumours 1 and 3. Neither of the substrates showed any cross-reactivity with the alternative luciferase enzyme, and tumour 3 (a mixture of both cell types) emitted a signal when both substrates were administered, supporting the potential for dual BLI imaging in preclinical cancer pharmacology studies. All images were collected using the IVIS-50 system.
Anatomical BLI
The most common use of BLI in the preclinical development of drugs is to monitor any change in tumour volume following treatment of xenograft (subcutaneous or orthotopic) tumours in rodents, usually mice (Figures 3 and 4). This system is underpinned by the fact that luciferase-driven photon emission can be detected externally, even when the cells are located several millimetres below the skin (Lyons, 2005; Moriyama et al., 2008). In drug efficacy studies, a decrease in luminescence is attributed to the cytotoxic effects of the drugs as a result of either induction of cell death or a reduction in cell metabolic ability. In terms of this application of BLI, there have been an extensive number of preclinical cancer pharmacology studies across a wide range of tumour cell types, including brain, breast, and lung carcinoma (Kemper et al., 2006; Grozio et al., 2008; Lim et al., 2009; Ozawa and James, 2010; Zeng et al., 2010; Holzmuller et al., 2011), sarcomas (Rousseau et al., 2010; Wang et al., 2010), and multiple myeloma (Jia et al., 2010). Importantly, in terms of the utility of BLI to cancer pharmacology, it is now established that neither luciferase itself nor BLI affects tumour growth in vitro or in vivo (Tiffen et al., 2010).
Preclinical models of metastasis for assessment of new therapeutic strategies are extremely hard to develop and there are limited systems that adequately represent the human disease. Unlike conventional preclinical tumour models, BLI has the capability to detect micrometastatic disease and has been reliably demonstrated to detect as few as 500 cells in vivo at specific anatomical sites (Troy et al., 2004). This thereby permits tumour dissemination and appearance of micrometastatic disease to be tracked temporally and non-invasively. Similarly, using BLI, spontaneous tumour growth can also be monitored using genetically modified murine models which can provide vital information regarding tumour pathogenesis and treatment strategies (Hawes and Reilly, 2010). In this context, there are a plethora of studies that have used BLI as a means to monitor tumour metastases and others that have utilized these models to evaluate the treatment of metastatic disease across a range of tumour types (Nogawa et al., 2005; Cordero et al., 2010; Drake et al., 2010; McNally et al., 2010; Shelton et al., 2010; Takahashi et al., 2010; Vikis et al., 2010; Wang et al., 2010; Zhang et al., 2010).
Although there is compelling evidence provided by a multitude of studies confirming a strong correlation between photon emission and tumour burden, a number of studies have now suggested that the intensity of this signal can plateau or even decline during tumour progression (Dickson et al., 2007; Jurczok et al., 2008). One explanation for this phenomenon is the increased degree of necrotic tissue in these advanced tumours, which despite not being biochemically active and metabolizing luciferin still contributes to the tumour mass, providing a disparity between tumour size and bioluminescence output (Jurczok et al., 2008). This increase in the proportion of necrotic tissue and reduced gross luciferase activity in these tumours is supported by histological studies (Jurczok et al., 2008). In addition, this increase in necrotic tissue within the tumour is also related to an increase in subtumoural hypoxia, which impacts upon luciferase activity that is an oxygen-dependent process (Gould and Subramani, 1988). Another issue affecting the viability of BLI for cancer pharmacology studies is the location of the tumour within the animal. Although BLI can detect tumours and cells located a distance below the skin (Lyons, 2005; Moriyama et al., 2008), signal attenuation has been noted in deeper tumours (Kang et al., 2006) and dominant signals produced by one organ have been shown to mask a weaker signal produced by another (Nogawa et al., 2005). Taken together, despite appearing minor in terms of the major successes of BLI, these limitations to this strategy must be borne in mind when evaluating advanced or larger tumours, and multifocal metastatic models.
BLI of pharmacodynamics
In light of the evolution of molecular-targeted cancer therapeutics and the need to extract as much information from in vivo studies as possible, the most significant advantage and potential for BLI is the assessment of molecular-target interactions and drug pharmacodynamics. This approach has now been demonstrated in several studies with general success. Induction of tumour apoptosis simultaneously with retardation of tumour growth with a panel of chemotherapeutics was observed in a non-invasive preclinical in vivo study (Scabini et al., 2011). Induction of apoptosis was demonstrated using a conjugate of luciferin and the caspase 3/7 substrate Z-DEVD, and tumour cells engineered to express luciferase. Upon apoptosis induction, the conjugated substrate is cleaved by the caspases and releases luciferin, which is converted by the luciferase-expressing tumour cells to produce the bioluminescent signal (Scabini et al., 2011). Similarly, a caspase-3-activated luciferase-reporter strategy was used to demonstrate that concomitant therapy with 5-fluorouracil and tumour necrosis factor α-related apoptosis-inducing ligand enhances apoptotic activity in vivo, resulting in a significantly greater antitumour response (Lee et al., 2007b). In another iteration of this approach, BLI was utilized to show that inhibition of N-linked glycosylation activity reduces receptor tyrosine kinase activity in tumour cells and is a novel therapeutic strategy for targeting tumours resistant to epidermal growth factor inhibitors (Contessa et al., 2010). In this study, the BLI approach was also applied to determine safe and efficacious in vivo dosing of tunicamycin, which blocks N-glycan precursor biosynthesis (Contessa et al., 2010). Recently, BLI has also been used to evaluate tumour glycolysis pathways and demonstrate that lactate but not pyruvate concentrations correlate with tumour response to fractionated irradiation, an in vivo observation that was not predicted using in vitro assays (Sattler et al., 2010).
One area of preclinical cancer pharmacology where BLI has proved highly useful is the assessment of agents targeting angiogenesis or disrupting existing tumour vasculature (Zhao et al., 2008; Angst et al., 2010; Snoeks et al., 2010; Sun et al., 2010a). Unlike the majority of chemotherapeutic approaches that are cytotoxic and induce tumour regression, those strategies that target the tumour blood supply normally induce a cytostatic response and subsequent tumour necrosis. Consequently, palpation of these treated tumours would not indicate tumour shrinkage and may prove misleading in terms of a response. Use of BLI for assessment of tumour response to these agents provides much greater information regarding tumour response. Evidence for this application is succinctly reviewed elsewhere (Zhao et al., 2008; Snoeks et al., 2010).
Despite showing good results in vitro and in conventional in vivo tumour models, many cancer therapeutics fail to progress into the clinic as a consequence of poor bioavailability, drug resistance and target selectivity (Jones et al., 2006; Wender et al., 2007). Identifying and predicting the potential for these limitations preclinically has often proved difficult, involving ex vivo drug and tissue analysis or extensive pharmacokinetic studies. Over recent years, BLI strategies have been utilized in preclinical pharmacology assessments to address many of these issues with specific agents. The potential for modulating the multidrug resistance gene and subsequently P-glycoprotein expression in vivo, and thus chemotherapeutic response, has been demonstrated using BLI with Renilla luciferase (Jeon et al., 2010). Bioavailability and tumour drug delivery have been monitored using BLI (Cirstoiu-Hapca et al., 2010; Peng et al., 2010). Similarly, luciferin-transporter conjugates have been used as tools for real-time determination of drug uptake into cells and tumours in vivo (Jones et al., 2006; Wender et al., 2007). Furthermore, activity of the metabolic enzyme cytochrome P450 3A4 has been monitored in vivo using BLI (Weisheng et al., 2005), an important factor when considering pharmacokinetics, metabolism and clearance of a novel anticancer agent.
Fluorescence imaging
In agreement with BLI, fluorescence optical imaging requires either modification of the target cells to express a specific fluorescent protein or introduction of a fluorescently tagged reporter construct into the animal. The principle of fluorescence imaging is similar to BLI in that it relies upon detection of emitted photons; but in contrast to BLI, this does not require addition of an exogenous substrate and relies upon excitation of the fluorophore using a light source or laser. There are now a vast number of fluorescent proteins, both natural and engineered, with a wide variety of emitted colours across the visible spectrum from blue to far red. The utility of these fluorescent proteins for in vivo preclinical cancer pharmacology studies has been shown in many studies representing all areas of the preclinical cancer drug discovery and pharmacology process, including drug efficacy, monitoring of tumour growth, and detection and analysis of angiogenesis and metastatic tumour spread (Liu et al., 2007).
Relative to BLI, fluorescence imaging has lower sensitivity and a higher background signal due to the requirement of an external illumination source to facilitate fluorescent emission. This high background being due to biomolecules having intrinsic fluorescence and the fact that cells and tissues can both quench and scatter emitted light. Consequently, this can limit the depth of penetration of light as the emitted light is absorbed and scattered by various tissues, including haemoglobin (Tung, 2004). As such, important criteria for selection of fluorescent proteins for in vivo molecular imaging studies are the required signal intensity, photostability and potential for tumour autofluorescent interference. The use of fluorescent signals with excitation and emission wavelengths within the near-infrared (NIR; 650–900 nm) region of the spectrum has now proven highly applicable in this context. Lower background signals and noise are observed with NIR due principally to the fact that it is poorly absorbed by haemoglobin and lipids (Weissleder and Pittet, 2008). This therefore improves contrast between target and background tissues and allows much greater in vivo tissue penetration and detection (Weissleder and Pittet, 2008). Despite these apparent limitations, fluorescence imaging has proved invaluable in extending our understanding of cancer biology, drug efficacy and preclinical cancer pharmacology.
One major benefit of fluorescence imaging over other imaging strategies has been the ability to simultaneously monitor several processes or molecular events within the same cell or preclinical tumour model, facilitated by the wide range of fluorescent proteins with differential emission spectra. For example, the role, involvement, and interactions between tumour cells and the host cellular environment were conclusively addressed using GFP-expressing transgenic mice transplanted with tumour cells engineered to express red fluorescent protein (Hoffman, 2009). Such an approach permitted the distinction between host and tumour cells, the involvement of specific cell types in tumour development such as macrophages, lymphocytes and fibroblasts (Hoffman, 2009). Using this model, the effects of cancer drug upon each of these cell types were also examined (Hoffman, 2009). A similar approach was also utilized for the evaluation of novel anti-angiogenic compounds (Amoh et al., 2006; Dunphy et al., 2009). In the initial study, human pancreatic tumour cells expressing red fluorescent protein were injected intrasplenically into mice expressing GFP under the control of the nestin promoter, a marker of blood vessel formation, with this model then being used to simultaneously visualize and quantify nascent angiogenesis and the effects of gemcitabine (Amoh et al., 2006). In the latter strategy, GFP expression was driven by the Tie2 promoter, an endothelial specific promoter (Dunphy et al., 2009).
Quantum dots (QDs)
One advance made within the area of fluorescence imaging is the development of QDs, small nanocrystals (1–10 nm) made of inorganic semiconductor materials (Bentolila et al., 2009). QDs exhibit several properties that make them suited for preclinical imaging; the emission wavelength can be precisely tuned and can range from ultraviolet to NIR, they are very bright and photostable, and they have a wide absorption band but a narrow emission band making them ideal for multiplexed analysis. The relatively large surface area of QDs also allows their utilization with other contrast agents, permitting the use in multimodality imaging strategies (Bentolila et al., 2009). Consequently, because of these beneficial properties and the lack of detrimental effects upon cellular proliferation or tumourigenicity of cancer cells in vivo, QDs are becoming popular for in vivo monitoring of cancer cell behaviour and growth (Sun et al., 2007; 2010b; Bentolila et al., 2009; Tavares et al., 2011).
The nanoscale structure and versatility of QDs has also provided the potential for the development of multifunctional theragnostics, whereby the QD is utilized as both a tumour-imaging marker and an indicator of drug delivery (Nie et al., 2007; Choi et al., 2010; Jain, 2011). The in vivo feasibility of this approach was demonstrated preclinically by QDs targeted to prostate-specific membrane antigen and integrin ανβ3, which bound to the surface of prostate and melanoma cells respectively (Choi et al., 2010). The potential for using QDs as ‘markers’ for tumour-selective drug delivery and evaluation of therapeutic response was recently demonstrated preclinically (Savla et al., 2011). Using QDs linked to doxorubicin and bioconjugated to an aptamer for the mucin-1 tumour marker, the targeting and delivery of doxorubicin to ovarian cancer was shown (Savla et al., 2011). This approach whereby QDs are developed to evaluate imaging and delivery of therapeutic agents has the potential to significantly refine and increase the utility of preclinical cancer pharmacology studies and their translation to the clinic.
Multimodal imaging in preclinical cancer pharmacology
Although all of the techniques discussed above are proficient in their own right, very often these imaging techniques are combined to allow the best aspects of different techniques to be used in parallel and to gain as much information as possible from the same animal. Combination of modalities providing both functional and anatomical information has proved particularly advantageous, such as MRI/BLI, SPECT/CT and PET/CT (Lyons, 2005; van Dalen et al., 2007; McCann et al., 2009; Mulder et al., 2009; Nam et al., 2010). With regard to preclinical cancer pharmacology, there are now a large number of studies in which multimodal imaging has been utilized, of which several studies are described below.
Combination of MRI with optical imaging has been used preclinically to produce a 3D brain image, allowing the morphology, physiology and chemotherapeutic response of glioma to be determined non-invasively (Kang et al., 2006; McCann et al., 2009). For instance, efficacy of the HIF-1 inhibitor 2-methoxyestradiol against glioma was determined in an orthotopic glioma model using BLI and MRI to monitor HIF-1 activity and tumour size respectively (Kang et al., 2006). Several other preclinical approaches monitoring tumour response and molecular interactions following chemotherapy have also been reported (Medarova et al., 2009), including visualization and monitoring of tumour angiogenesis (Mulder et al., 2009), temporal determination of optimal prodrug administration for enzyme-prodrug therapy (Li et al., 2008), and combination therapy of chemo- and immunotherapy against pancreatic cancer (Kim et al., 2008). In the current era of molecular-targeted personalized therapeutics, MRI has been applied alongside fluorescence imaging to indicate expression and activity of the epidermal growth factor receptor in orthotopic glioma, thereby improving the potential for evaluating new and existing treatments for this tumour type (Davis et al., 2010).
The combination of MicroSPECT and CT imaging has proved very informative in preclinical pharmacology studies through provision of tumour metabolic capacity within the framework of anatomical structures. In addition, this multimodal strategy demonstrated beneficial utility through its ability to monitor retention, pharmacokinetics, distribution and excretion of therapeutics, including prostate cancer immunotherapy (Chang et al., 2007), distribution of liposome-based drug carriers (Bao et al., 2006), and the evaluation of antibodies as putative therapeutics for tumour-lymph angiogenesis (Zehnder-Fjallman et al., 2007). SPECT/CT has also been used to optimize biodistribution of bombesin analogues, with the potential of using them to target gastrin-releasing-peptide receptor-positive tumours (Garcia Garayoa et al., 2007; Mendoza-Sanchez et al., 2011). In this sense, information from such studies can be used to demonstrate whether the pharmacokinetic properties of the drug are optimal or whether improvements need to be made.
SPECT and MRI have been applied to evaluate a variety of therapies including the monitoring of changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in a rat glioma model (Ali et al., 2010), and to demonstrate the capacity of AC133+ progenitor cells as a breast cancer cell-targeted gene delivery system (Rad et al., 2009).
As a consequence of its ability to provide information non-invasively regarding tumour viability and metabolic activity, PET imaging has been combined with many of the other imaging modalities. One of the most commonly utilized approaches is the use of PET with CT, which allows anatomical localization and size to also be monitored (Otsuka et al., 2007). PET/CT imaging has been shown to be a reliable preclinical tool for the early detection of response to molecular-targeted therapeutics such as the kinase inhibitor erlotinib in head and neck cancers, and as such a surrogate marker for predicting tumour response (Vergez et al., 2010). PET/CT imaging also has significant utility for the detection and monitoring of tumour development, progression and response in preclinical models (Walter et al., 2010). In this context, PET/CT imaging of a genetically engineered mouse model of lung carcinoma has proved valuable in determining whether the clinical efficacy of phosphoinositide 3-kinase inhibitors is restricted to malignancies with specific mutations in this signalling pathway (Engelman et al., 2008).
Incorporation of a third or fourth technique into a multimodal imaging strategy (BLI, MRI and PET) has also been suggested to have potential for preclinical cancer pharmacology studies (Mouchess et al., 2006; Deroose et al., 2007; Hwang do et al., 2009; Xie et al., 2010). Using a trimodality imaging strategy of BLI/Micro-CT/MRI, the effects of zoledronic acid upon tumour progression and bone resorption were evaluated in a neuroblastoma xenograft tumour model (Mouchess et al., 2006). In this study, BLI increased concomitantly with detectable osteolytic lesions and also reflected tumour growth inhibition by zoledronic acid. Bone loss was quantified using micro-CT, and MRI allowed assessment of tumour cells both within the bone marrow cavity and as distant metastases (Mouchess et al., 2006). This simultaneous multimodal strategy thereby allowed a detailed analysis of the tumour and host tissue response. In another study, greater quantification of primary and metastatic tumour burden in mice was achieved using PET/BLI combined with CT (Deroose et al., 2007). The use of a trimodality fusion reporter gene, which allows detection by fluorescence, BLI and PET, combined with CT, gave improved sensitivity and allowed molecular signals to be analysed in the context of anatomical structures. PET/CT combination was advantageous as it allowed localization of lesions not observed by CT due to poor contrast resolution and not seen by PET because of high background signal (Deroose et al., 2007).
More recently, greater imaging capability for use in preclinical cancer pharmacology studies has been achieved through combination of molecular imaging with nanoparticles. Nanoparticles capable of concurrent fluorescence, bioluminescence, BRET, PET and MR imaging have been developed and provide further advantages through cellular uptake, the ability to track tumour dissemination and therapeutic response in vivo, and their amenability for molecular targeting (Hwang do et al., 2009; Xie et al., 2010).
Conclusion
Non-invasive and molecular imaging strategies are now well-established and powerful methodologies used to evaluate drug efficacy and safety in preclinical cancer pharmacological studies. Through advances in this area, we have a much greater understanding of tumour development at the molecular level, and have made significant advances in our ability to monitor or predict therapeutic response. Furthermore, molecular imaging in preclinical studies is increasingly more important in light of the clinical progression towards personalized medicine, with treatment being tailored to a specific tumour protein, gene profile or genetic polymorphism. In this context, preclinical disease models that can give information regarding specific targets will be hugely beneficial.
The increasing availability of new multifunctional imaging probes, BLI reporter systems, and more sensitive equipment in combination with more clinically relevant and improved animal models of human cancers is likely to have increasing impact on the development of new therapeutics and subsequent improved clinical response. The advent of molecular bioimaging approaches and advances in this area is further improving the impact of preclinical cancer pharmacology studies, not least through the additional ability to dynamically monitor drug pharmacodynamics and drug-target interactions non-invasively. The most promising advances have been made through the combination of several approaches into a single multimodal imaging strategy, with the ability now to utilize tri- and quadruple-modality approaches within a single animal. Finally, detection of metastatic disease and treatment response against these lesions is an essential requirement for preclinical pharmacological studies, requiring sensitive methodology and clinically applicable disease models. The significant improvements in non-invasive methodologies, multimodal imaging capabilities, and greater detection sensitivity coupled with better metastatic disease models and the ability to monitor response in the same animal over time are now permitting the previously elusive evaluation of metastatic tumour therapeutic response to be evaluated in much greater depth and better translation of therapeutic approaches to the clinic.
Acknowledgments
All images of mice shown in this manuscript were obtained from studies conducted in accordance with institutional guidelines, with all procedures carried out under a United Kingdom Home Office Project License, following UKCCCR guidelines (Workman et al., 2010).
This work was supported by Cancer Research UK, the Engineering and Physical Sciences Research Council and Yorkshire Cancer Research. We thank Professor Mike Bibby for his helpful and constructive advice.
Glossary
- BLI
bioluminescence imaging
- DCE-MRI
dynamic contrast-enhanced MRI
- HF-US
high-frequency ultrasound
- MB
microbubbles
- PD-US
power Doppler ultrasound
- QD
quantum dot
- SPECT
single positron emission computed tomography
- US
ultrasound
Conflict of interest
No potential conflicts of interest.
References
- Abdelrahman MA, Marston G, Hull MA, Markham AF, Jones PF, Evans JA, et al. High-frequency ultrasound for in vivo measurement of colon wall thickness in mice. Ultrasound Med Biol. 2012;38:432–442. doi: 10.1016/j.ultrasmedbio.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Ahlgren S, Wallberg H, Tran TA, Widstrom C, Hjertman M, Abrahmsen L, et al. Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J Nucl Med. 2009;50:781–789. doi: 10.2967/jnumed.108.056929. [DOI] [PubMed] [Google Scholar]
- Ali MM, Janic B, Babajani-Feremi A, Varma NR, Iskander AS, Anagli J, et al. Changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in rat glioma. PLoS One. 2010;5:e8727. doi: 10.1371/journal.pone.0008727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Nagakura C, Maitra A, Moossa AR, Katsuoka K, Hoffman RM, et al. Dual-color imaging of nascent angiogenesis and its inhibition in liver metastases of pancreatic cancer. Anticancer Res. 2006;26:3237–3242. [PubMed] [Google Scholar]
- Angst E, Chen M, Mojadidi M, Hines OJ, Reber HA, Eibl G. Bioluminescence imaging of angiogenesis in a murine orthotopic pancreatic cancer model. Mol Imaging Biol. 2010;12:570–575. doi: 10.1007/s11307-010-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril N, Propper D. Functional PET imaging in cancer drug development. Future Oncol. 2007;3:215–228. doi: 10.2217/14796694.3.2.215. [DOI] [PubMed] [Google Scholar]
- Ayers GD, McKinley ET, Zhao P, Fritz JM, Metry RE, Deal BC, et al. Volume of preclinical xenograft tumors is more accurately assessed by ultrasound imaging than manual caliper measurements. J Ultrasound Med. 2010;29:891–901. doi: 10.7863/jum.2010.29.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao A, Phillips WT, Goins B, Zheng X, Sabour S, Natarajan M, et al. Potential use of drug carried-liposomes for cancer therapy via direct intratumoral injection. Int J Pharm. 2006;316:162–169. doi: 10.1016/j.ijpharm.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- Bentolila LA, Ebenstein Y, Weiss S. Quantum dots for in vivo small-animal imaging. J Nucl Med. 2009;50:493–496. doi: 10.2967/jnumed.108.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, Lewis XZ, Gambhir SS. Optical imaging of Renilla luciferase, synthetic Renilla luciferase, and firefly luciferase reporter gene expression in living mice. J Biomed Opt. 2004;9:578–586. doi: 10.1117/1.1647546. [DOI] [PubMed] [Google Scholar]
- Breton E, Goetz C, Kintz J, Accart N, Aubertin G, Grellier B, et al. In vivo preclinical low-field MRI monitoring of tumor growth following a suicide-gene therapy in an orthotopic mice model of human glioblastoma. C R Biol. 2010;333:220–225. doi: 10.1016/j.crvi.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Brix G, Griebel J, Kiessling F, Wenz F. Tracer kinetic modelling of tumour angiogenesis based on dynamic contrast-enhanced CT and MRI measurements. Eur J Nuc Med Mol Imag. 2010;37(Suppl. 1):S30–S51. doi: 10.1007/s00259-010-1448-7. [DOI] [PubMed] [Google Scholar]
- Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006;5:2624–2633. doi: 10.1158/1535-7163.MCT-06-0395. [DOI] [PubMed] [Google Scholar]
- Cejka D, Kuntner C, Preusser M, Fritzer-Szekeres M, Fueger BJ, Strommer S, et al. FDG uptake is a surrogate marker for defining the optimal biological dose of the mTOR inhibitor everolimus in vivo. Br J Cancer. 2009;100:1739–1745. doi: 10.1038/sj.bjc.6605076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Hsu WC, Wang CY, Jan ML, Tsai TH, Lee TW, et al. Longitudinal microSPECT/CT imaging and pharmacokinetics of synthetic luteinizing hormone-releasing hormone (LHRH) vaccine in rats. Anticancer Res. 2007;27:3251–3258. [PubMed] [Google Scholar]
- Chen W, Delaloye S, Silverman DH, Geist C, Czernin J, Sayre J, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- Cheung AM, Brown AS, Hastie LA, Cucevic V, Roy M, Lacefield JC, et al. Three-dimensional ultrasound biomicroscopy for xenograft growth analysis. Ultrasound Med Biol. 2005;31:865–870. doi: 10.1016/j.ultrasmedbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Choi HS, Liu W, Liu F, Nasr K, Misra P, Bawendi MG, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstoiu-Hapca A, Buchegger F, Lange N, Bossy L, Gurny R, Delie F. Benefit of anti-HER2-coated paclitaxel-loaded immuno-nanoparticles in the treatment of disseminated ovarian cancer: therapeutic efficacy and biodistribution in mice. J Control Release. 2010;144:324–331. doi: 10.1016/j.jconrel.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Contag CH, Ross BD. It's not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16:378–387. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- Contessa JN, Bhojani MS, Freeze HH, Ross BD, Rehemtulla A, Lawrence TS. Molecular imaging of N-linked glycosylation suggests glycan biosynthesis is a novel target for cancer therapy. Clin Cancer Res. 2010;16:3205–3214. doi: 10.1158/1078-0432.CCR-09-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero AB, Kwon Y, Hua X, Godwin AK. In vivo imaging and therapeutic treatments in an orthotopic mouse model of ovarian cancer. J Vis Exp. 2010;42:2125. doi: 10.3791/2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernin J, Weber WA, Herschman HR. Molecular imaging in the development of cancer therapeutics. Annu Rev Med. 2006;57:99–118. doi: 10.1146/annurev.med.57.080904.190431. [DOI] [PubMed] [Google Scholar]
- van Dalen JA, Vogel WV, Corstens FH, Oyen WJ. Multi-modality nuclear medicine imaging: artefacts, pitfalls and recommendations. Cancer Imaging. 2007;7:77–83. doi: 10.1102/1470-7330.2007.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SC, Samkoe KS, O'Hara JA, Gibbs-Strauss SL, Payne HL, Hoopes PJ, et al. MRI-coupled fluorescence tomography quantifies EGFR activity in brain tumors. Acad Radiol. 2010;17:271–276. doi: 10.1016/j.acra.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroose CM, De A, Loening AM, Chow PL, Ray P, Chatziioannou AF, et al. Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging. J Nucl Med. 2007;48:295–303. [PMC free article] [PubMed] [Google Scholar]
- Deshpande N, Ren Y, Foygel K, Rosenberg J, Willmann JK. Tumor angiogenic marker expression levels during tumor growth: longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology. 2011;258:804–811. doi: 10.1148/radiol.10101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R, Nguewa PA, Parrondo R, Perez-Stable C, Manrique I, Redrado M, et al. Antitumor and antiangiogenic effect of the dual EGFR and HER-2 tyrosine kinase inhibitor lapatinib in a lung cancer model. BMC Cancer. 2010;10:188. doi: 10.1186/1471-2407-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PV, Hamner B, Ng CY, Hall MM, Zhou J, Hargrove PW, et al. In vivo bioluminescence imaging for early detection and monitoring of disease progression in a murine model of neuroblastoma. J Pediatr Surg. 2007;42:1172–1179. doi: 10.1016/j.jpedsurg.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Drake JM, Danke JR, Henry MD. Bone-specific growth inhibition of prostate cancer metastasis by atrasentan. Cancer Biol Ther. 2010;9:607–614. doi: 10.4161/cbt.9.8.11112. [DOI] [PubMed] [Google Scholar]
- Dunphy MP, Entenberg D, Toledo-Crow R, Larson SM. In vivo microcartography and subcellular imaging of tumor angiogenesis: a novel platform for translational angiogenesis research. Microvasc Res. 2009;78:51–56. doi: 10.1016/j.mvr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee BY, Mudd SR, Roen CN, Clipson L, Newton MA, Weichert JP, et al. Reproducibility of tumor volume measurement at microCT colonography in living mice. Acad Radiol. 2008;15:334–341. doi: 10.1016/j.acra.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- Foster FS, Zhang MY, Zhou YQ, Liu G, Mehi J, Cherin E, et al. A new ultrasound instrument for in vivo microimaging of mice. Ultrasound Med Biol. 2002;28:1165–1172. doi: 10.1016/s0301-5629(02)00567-7. [DOI] [PubMed] [Google Scholar]
- Fushiki H, Kanoh-Azuma T, Katoh M, Kawabata K, Jiang J, Tsuchiya N, et al. Quantification of mouse pulmonary cancer models by microcomputed tomography imaging. Cancer Sci. 2009;100:1544–1549. doi: 10.1111/j.1349-7006.2009.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Garayoa E, Schweinsberg C, Maes V, Ruegg D, Blanc A, Blauenstein P, et al. New [99mTc]bombesin analogues with improved biodistribution for targeting gastrin releasing-peptide receptor-positive tumors. Q J Nucl Med Mol Imaging. 2007;51:42–50. [PubMed] [Google Scholar]
- Goertz DE, Yu JL, Kerbel RS, Burns PN, Foster FS. High-frequency Doppler ultrasound monitors the effects of antivascular therapy on tumor blood flow. Cancer Res. 2002;62:6371–6375. [PubMed] [Google Scholar]
- Gould SJ, Subramani S. Firefly luciferase as a tool in molecular and cell biology. Anal Biochem. 1988;175:5–13. doi: 10.1016/0003-2697(88)90353-3. [DOI] [PubMed] [Google Scholar]
- Graham KC, Wirtzfeld LA, MacKenzie LT, Postenka CO, Groom AC, MacDonald IC, et al. Three-dimensional high-frequency ultrasound imaging for longitudinal evaluation of liver metastases in preclinical models. Cancer Res. 2005;65:5231–5237. doi: 10.1158/0008-5472.CAN-05-0440. [DOI] [PubMed] [Google Scholar]
- Grange C, Geninatti-Crich S, Esposito G, Alberti D, Tei L, Bussolati B, et al. Combined delivery and magnetic resonance imaging of neural cell adhesion molecule-targeted doxorubicin-containing liposomes in experimentally induced Kaposi's sarcoma. Cancer Res. 2010;70:2180–2190. doi: 10.1158/0008-5472.CAN-09-2821. [DOI] [PubMed] [Google Scholar]
- Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer. 1999;81:1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Paleari L, Catassi A, Servent D, Cilli M, Piccardi F, et al. Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: a promising prospective in anti-cancer drug development. Int J Cancer. 2008;122:1911–1915. doi: 10.1002/ijc.23298. [DOI] [PubMed] [Google Scholar]
- Guibal A, Taillade L, Mule S, Comperat E, Badachi Y, Golmard JL, et al. Noninvasive contrast-enhanced US quantitative assessment of tumor microcirculation in a murine model: effect of discontinuing anti-VEGF therapy. Radiology. 2010;254:420–429. doi: 10.1148/radiol.09090728. [DOI] [PubMed] [Google Scholar]
- Gwyther SJ, Schwartz LH. How to assess anti-tumour efficacy by imaging techniques. Eur J Cancer. 2008;44:39–45. doi: 10.1016/j.ejca.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Furukawa T, Saga T. Molecular MR imaging of cancer gene therapy: ferritin transgene reporter takes the stage. Magn Reson Med Sci. 2010;9:37–47. doi: 10.2463/mrms.9.37. [DOI] [PubMed] [Google Scholar]
- Hawcroft G, Volpato M, Marston G, Ingram N, Perry SL, Cockbain AJ, et al. The omega-3 polyunsaturated fatty acid eicosapentaenoic acid inhibits mouse MC-26 colorectal cancer cell liver metastasis via inhibition of PGE2-dependent cell motility. Br J Pharmacol. 2012;166:1724–1737. doi: 10.1111/j.1476-5381.2012.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes JJ, Reilly KM. Bioluminescent approaches for measuring tumor growth in a mouse model of neurofibromatosis. Toxicol Pathol. 2010;38:123–130. doi: 10.1177/0192623309357075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RM. Imaging cancer dynamics in vivo at the tumor and cellular level with fluorescent proteins. Clini Exp Metas. 2009;26:345–355. doi: 10.1007/s10585-008-9205-z. [DOI] [PubMed] [Google Scholar]
- Holzmuller R, Mantwill K, Haczek C, Rognoni E, Anton M, Kasajima A, et al. YB-1 dependent virotherapy in combination with temozolomide as a multimodal therapy approach to eradicate malignant glioma. Int J Cancer. 2011;129:1265–1276. doi: 10.1002/ijc.25783. [DOI] [PubMed] [Google Scholar]
- Horowitz ME, Etcubanas E, Christensen ML, Houghton JA, George SL, Green AA, et al. Phase II testing of melphalan in children with newly diagnosed rhabdomyosarcoma: a model for anticancer drug development. J Clin Oncol. 1988;6:308–314. doi: 10.1200/JCO.1988.6.2.308. [DOI] [PubMed] [Google Scholar]
- Hoyt K, Warram JM, Umphrey H, Belt L, Lockhart ME, Robbin ML, et al. Determination of breast cancer response to bevacizumab therapy using contrast-enhanced ultrasound and artificial neural networks. J Ultrasound Med. 2010;29:577–585. doi: 10.7863/jum.2010.29.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh AS, Abrahams DF, Torres MS, Baldwin MK, Gillies RJ, Morse DL. Development of an orthotopic human pancreatic cancer xenograft model using ultrasound guided injection of cells. PLoS One. 2011;6:e20330. doi: 10.1371/journal.pone.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang do W, Ko HY, Kim SK, Kim D, Lee DS, Kim S. Development of a quadruple imaging modality by using nanoparticles. Chemistry. 2009;15:9387–9393. doi: 10.1002/chem.200900344. [DOI] [PubMed] [Google Scholar]
- Jain KK. Advances in the field of nanooncology. BMC Med. 2011;8:83. doi: 10.1186/1741-7015-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal RS, Singh J, Adams GP. High-resolution ultrasound biomicroscopy for monitoring ovarian structures in mice. Reprod Biol Endocrinol. 2009;7:69. doi: 10.1186/1477-7827-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YH, Bae SA, Lee YJ, Lee YL, Lee SW, Yoon GS, et al. Evaluation of the reversal of multidrug resistance by MDR1 ribonucleic acid interference in a human colon cancer model using a Renilla luciferase reporter gene and coelenterazine. Mol Imaging. 2010;9:343–350. [PubMed] [Google Scholar]
- Jia D, Koonce NA, Halakatti R, Li X, Yaccoby S, Swain FL, et al. Repression of multiple myeloma growth and preservation of bone with combined radiotherapy and anti-angiogenic agent. Radiat Res. 2010;173:809–817. doi: 10.1667/RR1734.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Jeanny JC, Behar-Cohen F, Laugier P, Saied A. High-resolution ultrasonography of subretinal structure and assessment of retina degeneration in rat. Exp Eye Res. 2005;81:592–601. doi: 10.1016/j.exer.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Jones LR, Goun EA, Shinde R, Rothbard JB, Contag CH, Wender PA. Releasable luciferin-transporter conjugates: tools for the real-time analysis of cellular uptake and release. J Am Chem Soc. 2006;128:6526–6527. doi: 10.1021/ja0586283. [DOI] [PubMed] [Google Scholar]
- de Jong M, Maina T. Of mice and humans: are they the same? – Implications in cancer translational research. J Nucl Med. 2010;51:501–504. doi: 10.2967/jnumed.109.065706. [DOI] [PubMed] [Google Scholar]
- Jurczok A, Fornara P, Soling A. Bioluminescence imaging to monitor bladder cancer cell adhesion in vivo: a new approach to optimize a syngeneic, orthotopic, murine bladder cancer model. BJU Int. 2008;101:120–124. doi: 10.1111/j.1464-410X.2007.07193.x. [DOI] [PubMed] [Google Scholar]
- Kang BH, Siegelin MD, Plescia J, Raskett CM, Garlick DS, Dohi T, et al. Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer. Clin Cancer Res. 2010;16:4779–4788. doi: 10.1158/1078-0432.CCR-10-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Cho HT, Devi S, Zhang Z, Escuin D, Liang Z, et al. Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res. 2006;66:11991–11997. doi: 10.1158/0008-5472.CAN-06-1320. [DOI] [PubMed] [Google Scholar]
- Kashiwagi H, McDunn JE, Simon PO, Jr, Goedegebuure PS, Xu J, Jones L, et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Mol Cancer. 2007;6:48. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassouf W, Brown GA, Shetty A, Hazle JD, Stafford RJ, Rosser CJ, et al. An in vivo orthotopic canine model to evaluate distribution of intraprostatic injectate: implications for gene therapy and drug delivery for prostate cancer. Urology. 2007;70:822–825. doi: 10.1016/j.urology.2007.06.637. [DOI] [PubMed] [Google Scholar]
- Kelly WK, Richon VM, O'Connor O, Curley T, MacGregor-Curtelli B, Tong W, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Can Res. 2003;9:3578–3588. [PubMed] [Google Scholar]
- Kemper EM, Leenders W, Kusters B, Lyons S, Buckle T, Heerschap A, et al. Development of luciferase tagged brain tumour models in mice for chemotherapy intervention studies. Eur J Cancer. 2006;42:3294–3303. doi: 10.1016/j.ejca.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Kim H, Morgan DE, Buchsbaum DJ, Zeng H, Grizzle WE, Warram JM, et al. Early therapy evaluation of combined anti-death receptor 5 antibody and gemcitabine in orthotopic pancreatic tumor xenografts by diffusion-weighted magnetic resonance imaging. Cancer Res. 2008;68:8369–8376. doi: 10.1158/0008-5472.CAN-08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Ravoori M, Landen CN, Kamat AA, Han LY, Lu C, et al. Antitumor and antivascular effects of AVE8062 in ovarian carcinoma. Cancer Res. 2007;67:9337–9345. doi: 10.1158/0008-5472.CAN-06-4018. [DOI] [PubMed] [Google Scholar]
- Kramer-Marek G, Kiesewetter DO, Capala J. Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and (18)F-labeled affibody molecules. J Nucl Med. 2009;50:1131–1139. doi: 10.2967/jnumed.108.057695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug LM, Milton DT, Jungbluth AA, Chen LC, Quaia E, Pandit-Taskar N, et al. Targeting Lewis Y (Le(y)) in small cell lung cancer with a humanized monoclonal antibody, hu3S193: a pilot trial testing two dose levels. J Thorac Oncol. 2007;2:947–952. doi: 10.1097/JTO.0b013e3181560dcc. [DOI] [PubMed] [Google Scholar]
- Laforest R, Liu X. Image quality with non-standard nuclides in positron emission tomography. Q J Nucl Med Mol Imaging. 2008;52:151–158. [PubMed] [Google Scholar]
- Laxman B, Hall DE, Bhojani MS, Hamstra DA, Chenevert TL, Ross BD, et al. Noninvasive real-time imaging of apoptosis. Proc Natl Acad Sci USA. 2002;99:16551–16555. doi: 10.1073/pnas.252644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Orita H, Gabrielson K, Alvey S, Hagemann RL, Kuhajda FP, et al. FDG-PET for pharmacodynamic assessment of the fatty acid synthase inhibitor C75 in an experimental model of lung cancer. Pharm Res. 2007a;24:1202–1207. doi: 10.1007/s11095-007-9264-x. [DOI] [PubMed] [Google Scholar]
- Lee KC, Hamstra DA, Bhojani MS, Khan AP, Ross BD, Rehemtulla A. Noninvasive molecular imaging sheds light on the synergy between 5-fluorouracil and TRAIL/Apo2L for cancer therapy. Clin Cancer Res. 2007b;13:1839–1846. doi: 10.1158/1078-0432.CCR-06-1657. [DOI] [PubMed] [Google Scholar]
- Leyton J, Alao JP, Da Costa M, Stavropoulou AV, Latigo JR, Perumal M, et al. In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3'-deoxy-3'-[18F]fluorothymidine positron emission tomography. Cancer Res. 2006;66:7621–7629. doi: 10.1158/0008-5472.CAN-05-3962. [DOI] [PubMed] [Google Scholar]
- Li C, Penet MF, Winnard P, Jr, Artemov D, Bhujwalla ZM. Image-guided enzyme/prodrug cancer therapy. Clin Cancer Res. 2008;14:515–522. doi: 10.1158/1078-0432.CCR-07-1837. [DOI] [PubMed] [Google Scholar]
- Liao CP, Zhong C, Saribekyan G, Bading J, Park R, Conti PS, et al. Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. Cancer Res. 2007;67:7525–7533. doi: 10.1158/0008-5472.CAN-07-0668. [DOI] [PubMed] [Google Scholar]
- Lim E, Modi KD, Kim J. In vivo bioluminescent imaging of mammary tumors using IVIS spectrum. J Vis Exp. 2009;26:1210. doi: 10.3791/1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ding Y, Xie W, Li Z, Bai X, Li X, et al. An imageable metastatic treatment model of nasopharyngeal carcinoma. Clin Cancer Res. 2007;13:3960–3967. doi: 10.1158/1078-0432.CCR-07-0089. [DOI] [PubMed] [Google Scholar]
- Loo WT, Sasano H, Chow LW. Effects of capecitabine and vinorelbine on cell proliferation, metabolism and COX2 and p16 expression in breast cancer cell lines and solid tumour tissues. Biomed Pharmacother. 2007;61:596–600. doi: 10.1016/j.biopha.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Lyons SK. Advances in imaging mouse tumour models in vivo. J Pathol. 2005;205:194–205. doi: 10.1002/path.1697. [DOI] [PubMed] [Google Scholar]
- McCann CM, Waterman P, Figueiredo JL, Aikawa E, Weissleder R, Chen JW. Combined magnetic resonance and fluorescence imaging of the living mouse brain reveals glioma response to chemotherapy. NeuroImage. 2009;45:360–369. doi: 10.1016/j.neuroimage.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally LR, Welch DR, Beck BH, Stafford LJ, Long JW, Sellers JC, et al. KISS1 over-expression suppresses metastasis of pancreatic adenocarcinoma in a xenograft mouse model. Clin Exp Metastasis. 2010;27:591–600. doi: 10.1007/s10585-010-9349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- Massoud TF, Gambhir SS. Integrating noninvasive molecular imaging into molecular medicine: an evolving paradigm. Trends Mol Med. 2007;13:183–191. doi: 10.1016/j.molmed.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Medarova Z, Rashkovetsky L, Pantazopoulos P, Moore A. Multiparametric monitoring of tumor response to chemotherapy by noninvasive imaging. Cancer Res. 2009;69:1182–1189. doi: 10.1158/0008-5472.CAN-08-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Sanchez AN, Ferro-Flores G, Ocampo-Garcia BE, Morales-Avila E, de M Ramírez F, De Leon-Rodriguez LM, et al. Lys3-bombesin conjugated to 99mTc-labelled gold nanoparticles for in vivo gastrin releasing peptide-receptor imaging. J Biomed Nanotechnol. 2011;6:375–384. doi: 10.1166/jbn.2010.1132. [DOI] [PubMed] [Google Scholar]
- Moran CM, Pye SD, Ellis W, Janeczko A, Morris KD, McNeilly AS, et al. A comparison of the imaging performance of high resolution ultrasound scanners for preclinical imaging. Ultrasound Med Biol. 2011;37:493–501. doi: 10.1016/j.ultrasmedbio.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TM, Pitts TE, Gross TS, Poliachik SL, Vessella RL, Corey E. RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate. 2008;68:861–871. doi: 10.1002/pros.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama EH, Niedre MJ, Jarvi MT, Mocanu JD, Moriyama Y, Subarsky P, et al. The influence of hypoxia on bioluminescence in luciferase-transfected gliosarcoma tumor cells in vitro. Photochem Photobiol Sci. 2008;7:675–680. doi: 10.1039/b719231b. [DOI] [PubMed] [Google Scholar]
- Morse DL, Raghunand N, Sadarangani P, Murthi S, Job C, Day S, et al. Response of choline metabolites to docetaxel therapy is quantified in vivo by localized (31)P MRS of human breast cancer xenografts and in vitro by high-resolution (31)P NMR spectroscopy of cell extracts. Magn Reson Med. 2007;58:270–280. doi: 10.1002/mrm.21333. [DOI] [PubMed] [Google Scholar]
- Mouchess ML, Sohara Y, Nelson MD, Jr, DeCLerck YA, Moats RA. Multimodal imaging analysis of tumor progression and bone resorption in a murine cancer model. J Comput Assist Tomogr. 2006;30:525–534. doi: 10.1097/00004728-200605000-00030. [DOI] [PubMed] [Google Scholar]
- Mulder WJ, Castermans K, van Beijnum JR, Oude Egbrink MG, Chin PT, Fayad ZA, et al. Molecular imaging of tumor angiogenesis using alphavbeta3-integrin targeted multimodal quantum dots. Angiogenesis. 2009;12:17–24. doi: 10.1007/s10456-008-9124-2. [DOI] [PubMed] [Google Scholar]
- Nam T, Park S, Lee SY, Park K, Choi K, Song IC, et al. Tumor targeting chitosan nanoparticles for dual-modality optical/MR cancer imaging. Bioconjug Chem. 2010;21:578–582. doi: 10.1021/bc900408z. [DOI] [PubMed] [Google Scholar]
- Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- Nogawa M, Yuasa T, Kimura S, Kuroda J, Sato K, Segawa H, et al. Monitoring luciferase-labeled cancer cell growth and metastasis in different in vivo models. Cancer Lett. 2005;217:243–253. doi: 10.1016/j.canlet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- O'Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189–195. doi: 10.1038/sj.bjc.6603515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S, Lai EW, Morris JC, Bakan DA, Klaunberg B, Cleary S, et al. MicroCT for high-resolution imaging of ectopic pheochromocytoma tumors in the liver of nude mice. Int J Cancer. 2006;119:2236–2241. doi: 10.1002/ijc.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Morita N, Yamashita K, Nishitani H. FDG-PET/CT for cancer management. J Med Invest. 2007;54:195–199. doi: 10.2152/jmi.54.195. [DOI] [PubMed] [Google Scholar]
- Ozawa T, James CD. Establishing intracranial brain tumor xenografts with subsequent analysis of tumor growth and response to therapy using bioluminescence imaging. J Vis Exp. 2010;41:1986. doi: 10.3791/1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MJ, Gleason SS, Kennel SJ, Hunsicker PR, Johnson DK. High resolution X-ray computed tomography: an emerging tool for small animal cancer research. Neoplasia. 2000;2:62–70. doi: 10.1038/sj.neo.7900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010;16:5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok KE, Lahn M, Enas N, McNulty A, Graff J, Cai S, et al. In vivo Measurements of Tumor Metabolism and Growth after Administration of Enzastaurin Using Small Animal FDG Positron Emission Tomography. J Oncol. 2009;2009:596560. doi: 10.1155/2009/596560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati SI, Keller C. Contrast enhanced vessel imaging using microCT. J Vis Exp. 2011;47:2377. doi: 10.3791/2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschek D, Mack MG, Kurth AA, Proschek P, Martin B, Hansmann ML, et al. Radiofrequency ablation of experimental bone metastases in nude rats. Anticancer Res. 2008;28:879–885. [PubMed] [Google Scholar]
- Rad AM, Iskander AS, Janic B, Knight RA, Arbab AS, Soltanian-Zadeh H. AC133+ progenitor cells as gene delivery vehicle and cellular probe in subcutaneous tumor models: a preliminary study. BMC Biotech. 2009;9:28. doi: 10.1186/1472-6750-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29:193–207. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Olson K. Jaa-f11: extending the life of mice with breast cancer. Expert Opin Biol Ther. 2007;7:923–928. doi: 10.1517/14712598.7.7.923. [DOI] [PubMed] [Google Scholar]
- Rousseau J, Escriou V, Perrot P, Picarda G, Charrier C, Scherman D, et al. Advantages of bioluminescence imaging to follow siRNA or chemotherapeutic treatments in osteosarcoma preclinical models. Cancer Gene Ther. 2010;17:387–397. doi: 10.1038/cgt.2009.89. [DOI] [PubMed] [Google Scholar]
- Sakoguchi-Okada N, Takahashi-Yanaga F, Fukada K, Shiraishi F, Taba Y, Miwa Y, et al. Celecoxib inhibits the expression of survivin via the suppression of promoter activity in human colon cancer cells. Biochem Pharmacol. 2007;73:1318–1329. doi: 10.1016/j.bcp.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Sattler UG, Meyer SS, Quennet V, Hoerner C, Knoerzer H, Fabian C, et al. Glycolytic metabolism and tumour response to fractionated irradiation. Radiother Oncol. 2010;94:102–109. doi: 10.1016/j.radonc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011;153:16–22. doi: 10.1016/j.jconrel.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Scabini M, Stellari F, Cappella P, Rizzitano S, Texido G, Pesenti E. In vivo imaging of early stage apoptosis by measuring real-time caspase-3/7 activation. Apoptosis. 2011;16:198–207. doi: 10.1007/s10495-010-0553-1. [DOI] [PubMed] [Google Scholar]
- Schambach SJ, Bag S, Schilling L, Groden C, Brockmann MA. Application of micro-CT in small animal imaging. Methods. 2010;50:2–13. doi: 10.1016/j.ymeth.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science. 2006;314:446–449. doi: 10.1126/science.1131847. [DOI] [PubMed] [Google Scholar]
- Schwartz DL, Bankson JA, Lemos R, Jr, Lai SY, Thittai AK, He Y, et al. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol Cancer Ther. 2010;9:2057–2067. doi: 10.1158/1535-7163.MCT-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton LM, Huysentruyt LC, Seyfried TN. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int J Cancer. 2010;127:2478–2485. doi: 10.1002/ijc.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Lima A, Molina R, Hamilton P, Clermont AC, Devasthali V, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- Snoeks TJ, Lowik CW, Kaijzel EL. ‘In vivo’ optical approaches to angiogenesis imaging. Angiogenesis. 2010;13:135–147. doi: 10.1007/s10456-010-9168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijkers GJ, Kluza E, Van Tilborg GA, van der Schaft DW, Griffioen AW, Mulder WJ, et al. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis. 2010;13:161–173. doi: 10.1007/s10456-010-9165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin Cancer Res. 2005;11:971–981. [PubMed] [Google Scholar]
- Sun A, Hou L, Prugpichailers T, Dunkel J, Kalani MA, Chen X, et al. Firefly luciferase-based dynamic bioluminescence imaging: a noninvasive technique to assess tumor angiogenesis. Neurosurgery. 2010a;66:751–757. doi: 10.1227/01.NEU.0000367452.37534.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Yang K, Zheng G, Li Z, Cao Y. Study on effect of peptide-conjugated near-infrared fluorescent quantum dots on the clone formation, proliferation, apoptosis, and tumorigenicity ability of human buccal squamous cell carcinoma cell line BcaCD885. Int J Nanomedicine. 2010b;5:401–405. doi: 10.2147/ijn.s10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhu MQ, Fu K, Lewinski N, Drezek RA. Lead sulfide near-infrared quantum dot bioconjugates for targeted molecular imaging. Int J Nanomedicine. 2007;2:235–240. [PMC free article] [PubMed] [Google Scholar]
- Sun L, Xu X, Richard WD, Feng C, Johnson JA, Shung KK. A high-frame rate duplex ultrasound biomicroscopy for small animal imaging in vivo. IEEE Trans Biomed Eng. 2008;55:2039–2049. doi: 10.1109/TBME.2008.919110. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ohkohchi N, Yasunaga M, Kuroda J, Koga Y, Kenmotsu H, et al. Detailed distribution of NK012, an SN-38-incorporating micelle, in the liver and its potent antitumor effects in mice bearing liver metastases. Clin Cancer Res. 2010;16:4822–4831. doi: 10.1158/1078-0432.CCR-10-1467. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Kawano K, Minowa T, Sugino T, Shimojo Y, Maitani Y. Enhanced antitumor efficacy of folate-linked liposomal doxorubicin with TGF-beta type I receptor inhibitor. Cancer Sci. 2010;10:2207–2213. doi: 10.1111/j.1349-7006.2010.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares AJ, Chong L, Petryayeva E, Algar WR, Krull UJ. Quantum dots as contrast agents for in vivo tumor imaging: progress and issues. Anal Bioanal Chem. 2011;399:2331–2342. doi: 10.1007/s00216-010-4010-3. [DOI] [PubMed] [Google Scholar]
- Tehranipour N, AL-Nahhas A, Canelo R, Stamp G, Woo K, Tait P, et al. Concordant F-18 FDG PET and Y-90 bremsstrahlung scans depict selective delivery of Y-90-microspheres to liver tumors: confirmation with histopathology. Clin Nuc Med. 2007;32:371–374. doi: 10.1097/01.rlu.0000259568.54976.bd. [DOI] [PubMed] [Google Scholar]
- Tiffen JC, Bailey CG, Ng C, Rasko JE, Holst J. Luciferase expression and bioluminescence does not affect tumor cell growth in vitro or in vivo. Mol Cancer. 2010;9:299. doi: 10.1186/1476-4598-9-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigian DA, Huang SS, Houseni M, Alavi A. Functional imaging of cancer with emphasis on molecular techniques. CA Cancer J Clin. 2007;57:206–224. doi: 10.3322/canjclin.57.4.206. [DOI] [PubMed] [Google Scholar]
- Troy T, Jekic-McMullen D, Sambucetti L, Rice B. Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol Imaging. 2004;3:9–23. doi: 10.1162/15353500200403196. [DOI] [PubMed] [Google Scholar]
- Tung CH. Fluorescent peptide probes for in vivo diagnostic imaging. Biopolymers. 2004;76:391–403. doi: 10.1002/bip.20139. [DOI] [PubMed] [Google Scholar]
- Turnbull DH, Starkoski BG, Harasiewicz KA, Semple JL, From L, Gupta AK, et al. A 40–100 MHz B-scan ultrasound backscatter microscope for skin imaging. Ultrasound Med Biol. 1995;21:79–88. doi: 10.1016/0301-5629(94)00083-2. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste JF. Imaging in lung cancer: positron emission tomography scan. Eur Resp Journal. 2002;35:49s–60s. doi: 10.1183/09031936.02.00252402. [DOI] [PubMed] [Google Scholar]
- Vergez S, Delord JP, Thomas F, Rochaix P, Caselles O, Filleron T, et al. Preclinical and clinical evidence that Deoxy-2-[18F]fluoro-D-glucose positron emission tomography with computed tomography is a reliable tool for the detection of early molecular responses to erlotinib in head and neck cancer. Clin Cancer Res. 2010;16:4434–4445. doi: 10.1158/1078-0432.CCR-09-2795. [DOI] [PubMed] [Google Scholar]
- Vikis HG, Jackson EN, Krupnick AS, Franklin A, Gelman AE, Chen Q, et al. Strain-specific susceptibility for pulmonary metastasis of sarcoma 180 cells in inbred mice. Cancer Res. 2010;70:4859–4867. doi: 10.1158/0008-5472.CAN-09-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- Walter MA, Hildebrandt IJ, Hacke K, Kesner AL, Kelly O, Lawson GW, et al. Small-animal PET/CT for monitoring the development and response to chemotherapy of thymic lymphoma in Trp53-/- mice. J Nucl Med. 2010;51:1285–1292. doi: 10.2967/jnumed.109.073585. [DOI] [PubMed] [Google Scholar]
- Wang S, Ren W, Liu J, Lahat G, Torres K, Lopez G, et al. TRAIL and doxorubicin combination induces proapoptotic and antiangiogenic effects in soft tissue sarcoma in vivo. Clin Cancer Res. 2010;16:2591–2604. doi: 10.1158/1078-0432.CCR-09-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins GA, Jones EF, Scott Shell M, VanBrocklin HF, Pan MH, Hanrahan SM, et al. Development of an optimized activatable MMP-14 targeted SPECT imaging probe. Biorg Med Chem. 2009;17:653–659. doi: 10.1016/j.bmc.2008.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisheng Z, Min C, West DB, Purchio AF. Visualizing drug efficacy in vivo. Mol Imaging. 2005;4:88–90. doi: 10.1162/15353500200505109. [DOI] [PubMed] [Google Scholar]
- Weiss MS, Penalver Bernabe B, Bellis AD, Broadbelt LJ, Jeruss JS, Shea LD. Dynamic, large-scale profiling of transcription factor activity from live cells in 3D culture. PLoS One. 2010;5:e14026. doi: 10.1371/journal.pone.0014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PA, Goun EA, Jones LR, Pillow TH, Rothbard JB, Shinde R, et al. Real-time analysis of uptake and bioactivatable cleavage of luciferin-transporter conjugates in transgenic reporter mice. Proc Natl Acad Sci USA. 2007;104:10340–10345. doi: 10.1073/pnas.0703919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels JT, Busse AC, Mahrt J, Dullin C, Grabbe E, Mueller GA. In vivo imaging in experimental preclinical tumor research – a review. Cytometry A. 2007;71:542–549. doi: 10.1002/cyto.a.20419. [DOI] [PubMed] [Google Scholar]
- Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, et al. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan JW, Bygrave M, Jiang H, Valiyeva F, Dunmore-Buyze J, Holdsworth DW, et al. Functional neoangiogenesis imaging of genetically engineered mouse prostate cancer using three-dimensional power Doppler ultrasound. Cancer Res. 2007;67:2830–2839. doi: 10.1158/0008-5472.CAN-06-3944. [DOI] [PubMed] [Google Scholar]
- Zaidi H, Prasad R. Advances in multimodality molecular imaging. J Med Phys. 2009;34:122–128. doi: 10.4103/0971-6203.54844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder-Fjallman AH, Marty C, Halin C, Hohn A, Schibli R, Ballmer-Hofer K, et al. Evaluation of anti-VEGFR-3 specific scFv antibodies as potential therapeutic and diagnostic tools for tumor lymph-angiogenesis. Oncol Rep. 2007;18:933–941. [PubMed] [Google Scholar]
- Zeng Q, Yang Z, Gao YJ, Yuan H, Cui K, Shi Y, et al. Treating triple-negative breast cancer by a combination of rapamycin and cyclophosphamide: an in vivo bioluminescence imaging study. Eur J Cancer. 2010;46:1132–1143. doi: 10.1016/j.ejca.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Yan Z, Zhang Q, Kuszpit K, Zasadny K, Qiu M, et al. PF-03732010: a fully human monoclonal antibody against P-cadherin with antitumor and antimetastatic activity. Clin Cancer Res. 2010;16:5177–5188. doi: 10.1158/1078-0432.CCR-10-1343. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Chen TB, Bednar B, Connolly BM, Hargreaves R, Sur C, et al. Optical imaging of tumor cells in hollow fibers: evaluation of the antitumor activities of anticancer drugs and target validation. Neoplasia. 2007;9:652–661. doi: 10.1593/neo.07421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Richer E, Antich PP, Mason RP. Antivascular effects of combretastatin A4 phosphate in breast cancer xenograft assessed using dynamic bioluminescence imaging and confirmed by MRI. FASEB J. 2008;22:2445–2451. doi: 10.1096/fj.07-103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YZ, Lu CT, Zhou ZC, Jin Z, Zhang L, Sun CZ, et al. Enhancing chemotherapeutic drug inhibition on tumor growth by ultrasound: an in vivo experiment. J Drug Target. 2010;19:154–160. doi: 10.3109/10611861003801834. [DOI] [PubMed] [Google Scholar]


