Abstract
IL-22, a member of the IL-10 cytokine family, has recently gained significant attention as a protective agent in murine models of diseases driven by epithelial injury. Like its biochemical and functional sibling IL-10, IL-22 elicits cellular activation primarily by engaging the STAT3 signalling pathway. Exclusively produced by leukocytes, but targeting mostly cells of epithelial origin, IL-22 has been proposed as a specialized cytokine messenger acting between leukocytic and non-leukocytic cell compartments. A lack of response in leukocytes to IL-22 mirrors tightly controlled IL-22 receptor expression and probably explains the apparent lack of instant adverse effects after systemic IL-22 administration to mice. Anti-apoptotic, pro-proliferative and pro-regenerative characteristics the major biological properties of this cytokine. Specifically, application of IL-22 is associated with tissue protection and/or regeneration in murine models of infection/microbe-driven inflammation at host/environment interfaces, ventilator-induced lung injury, pancreatitis and liver damage. Overall, preclinical studies would support therapeutic administration of seemingly well-tolerated recombinant IL-22 for treatment of an array of acute diseases manifested in epithelial tissues. However, the feasibility of prolonged administration of this cytokine is expected to be restricted by the tumourigenic potential of the IL-22/STAT3 axis. IL-22, moreover, apparently displays an inherent context-specific capacity to amplify distinct aspects of autoimmune inflammation. Here, the prospects, expectations and restrictions of IL-22 administration in tissue-protective therapy are discussed.
Keywords: tissue protective therapy, IL-22, STAT3, epithelial cells, inflammation, immunopharmacology, biologicals
Introduction
Cell death and loss of tissue integrity is regarded a major pillar of pathophysiology determining the course of disease in acute and chronic inflammation. As a result of direct infection, infection-related collateral tissue damage, sterile inflammation or drug-induced toxicity, danger-associated molecular patterns (DAMPs or alarmins), normally located in the intracellular compartment or fixed in the extracellular matrix, are released from dying cells into the extracellular space. Among others, DAMPs include heat shock proteins, hyaluronic acid fragments, biglycan, high mobility group box-1, uric acid and nucleic acids. Once released, these molecules serve as ligands for Toll-like receptors thereby initiating or enhancing inflammatory mechanisms usually associated with activation of innate immunity. This concept of DAMP release and recognition is essential to the perpetuation and amplification of inflammatory pathogenesis (Gallucci and Matzinger, 2001; Rock and Kono, 2008; Goh and Midwood, 2012; Lukens et al., 2012; Pradeu and Cooper, 2012) and has been proposed as a promising target for pharmacological intervention and drug development. In this context, therapeutic approaches using tissue-preserving biological agents, including recombinant IL-22, may have the ability to break cell death-driven vicious cycles that would otherwise perpetuate pathological inflammation.
IL-22 is a member of the IL-10 cytokine family with pronounced tissue-protective properties that recently became a major focus of cytokine biology and related translational research (Wolk et al., 2010; Sonnenberg et al., 2011; Gao, 2012; Kronenberger et al., 2012). Because of its ability to strengthen homeostatic epithelial barrier functions as well as overall tissue robustness and stress resistance, therapeutic administration of IL-22 may open the way to novel strategies for the treatment of injury-driven pathogenic inflammation. In the present review, prospects, expectations and limitations related to use of IL-22 as a tissue-protective biological treatment are assessed in the context of pulmonary, intestinal, pancreatic and hepatic inflammatory disorders.
Biochemical properties of IL-22
IL-22, formerly known as IL-10-related T–cell-derived inducible factor (Dumoutier et al., 2000; Xie et al., 2000), displays crucial properties that define this cytokine as a member of the IL-10 family. Those characteristics include structural features such as bundle-forming clustering of α-helices as well as activation of the transcription factor STAT3 as dominant means of signal transduction and coincide with a 25% overall amino acid identity between IL-22 and its cytokine sibling IL-10. Interestingly, both cytokines share the IL-10 receptor chain-2 as one of two receptor chains, which combines with either IL-10 receptor chain-1 or IL-22 receptor chain-1, to generate functional heterodimeric receptors for IL-10 or IL-22, respectively (Aujla and Kolls, 2009; Wolk et al., 2010; Ouyang et al., 2011). Subsequent to receptor ligation and activation of the JAK1/Tyk2/STAT3 pathway, IL-22 acts primarily by modulating gene expression profiles in target cells. Accordingly, prototypic STAT3-inducible genes such as LPS-binding protein (Wolk et al., 2007) or suppressor of cytokine signalling-3 are up-regulated under the influence of IL-22 (Nagalakshmi et al., 2004; Brand et al., 2006; Hoegl et al., 2011).
Although the STAT3 pathway must be regarded as the principal and dominant mechanism of cellular activation by IL-22, this cytokine is able to engage additional means of signal transduction. The capacity of IL-22 to mediate some moderate activation of STAT1, in addition to STAT3, has been appreciated early on (Dumoutier et al., 2000; Nagalakshmi et al., 2004; Ziesche et al., 2007). This feature of IL-22 biology is greatly enhanced under the influence of type I IFN (Bachmann et al., 2013). Given the pro-inflammatory characteristics of STAT1 (Paludan, 2000), this interaction with type I IFN probably underlies some specific pro-inflammatory functions of IL-22 (Mühl, 2013). IL-22 likewise activates, albeit to a rather variable degree, pro-proliferative MAPK pathways (Eyerich et al., 2010), mostly ERK 1/2, in a range of cell types, among others, rat and human hepatoma cells (Lejeune et al., 2002; Radaeva et al., 2004; Brand et al., 2007), human colon carcinoma cells (Brand et al., 2006; Fukui et al., 2011), myofibroblasts (Andoh et al., 2005), hepatic stellate cells (Kong et al., 2012) as well as primary keratinocytes (Sestito et al., 2011) and immortalized human keratinocytes (HaCaT cells; Zhang et al., 2012). Besides that, activation of pro-survival Akt (PKB) by IL-22 has been reported for human colon carcinoma cells (Brand et al., 2006; Fukui et al., 2011), primary hepatocytes (Brand et al., 2007) as well as for primary keratinocytes and synovial fibroblasts (Mitra et al., 2012). Notably, activation of the key pro-inflammatory transcription factor NF-κB is generally not associated with IL-22 biological activity (Eyerich et al., 2010), with a few exceptions. For example, in contrast to reports on human primary keratinocytes (Wolk et al., 2006) and DLD1 colon carcinoma cells (Ziesche et al., 2007), IL-22 activates the NF-κB pathway in human SW404 colon carcinoma cells (Fukui et al., 2011) and myofibroblasts (Andoh et al., 2005).
IL-22 in health and disease
The biological activity of IL-22 is largely determined by two principal features that in combination set the basis for quite unique properties of this cytokine in physiology and pathophysiology. First, IL-22 acts primarily on non-leukocytic cells and characteristically not on leukocytes. This property is based on selective expression of the IL-22 receptor chain-1 on non-leukocytic cells, in particular on cells of epithelial origin such as keratinocytes, intestinal or lung epithelial cells, and hepatocytes (Wolk et al., 2004; 2010; Aujla and Kolls, 2009; Sonnenberg et al., 2011). In addition, the IL-22 receptor chain-1 is expressed on hepatic stellate cells (Kong et al., 2012) and on synovial fibroblasts (Ikeuchi et al., 2005) and intestinal sub-epithelial myofibroblasts (Andoh et al., 2005). With regard to the pancreas, IL-22 receptor chain-1 has been detected on human insulin-expressing beta-cells and glucagon-expressing alpha-cells, but not on acinar cells (Shioya et al., 2008). In contrast, pancreatic acinar cells of murine origin respond to stimulation with IL-22 (Aggarwal et al., 2001). In contrast to the IL-22 receptor chain-1, IL-10 receptor chain-2, the second component of the heterodimeric IL-22 receptor, is ubiquitous (Ouyang et al., 2011). Secondly, IL-22 is exclusively produced by leukocytes, primarily activated Th1, Th17, Th22 and CD8+ T cells, γδ T-cells, dendritic cells, NK cells (Colonna, 2009; Duhen et al., 2009; Eyerich et al., 2009; Pickert et al., 2009; Bachmann et al., 2010; Wolk et al., 2010; Ouyang et al., 2011), invariant NK T-cells (Paget et al., 2012) and a range of NK-like cells recently named as innate lymphoid cells (Colonna, 2009; Koyasu and Moro, 2012). This spectrum of producer and target cells suggests that IL-22 is a specific messenger between leukocytic and non-leukocytic cell compartments. Despite this rather diverse pattern of leukocyte subsets capable of producing IL-22, some common parameters determining production of this cytokine have emerged. These are chiefly IL-1 (Dinarello, 2011) and IL-23, which in some cases synergize to induce IL-22 (Bachmann et al., 2010; Hughes et al., 2010; Marijnissen et al., 2011; Paget et al., 2012; Shaw et al., 2012). As expected, in light of IL-22 being exclusively produced by leukocytes, increased levels of local or systemic IL-22 have been consistently detected in numerous human diseases associated with overt immunoactivation. Such conditions may be infection- or microbe-driven, as seen in Mycobacterium tuberculosis infection (Matthews et al., 2011), sepsis (Bingold et al., 2010) and inflammatory bowel diseases (Andoh et al., 2005; Brand et al., 2006; Schmechel et al., 2008) or be unrelated to obvious infections, as in autoimmune inflammation, including rheumatoid arthritis (Ikeuchi et al., 2005; Leipe et al., 2011) and psoriasis (Wolk et al., 2006; Boniface et al., 2007; Nakajima et al., 2011). In addition, production of IL-22 is enhanced in patients with chronic hepatitis and liver cirrhosis of diverse aetiology (Jiang et al., 2011; Kang et al., 2012; Kronenberger et al., 2012).
Of particular interest are the interactions between IL-22 and other inflammatory cytokines including Th17-/Th22-related cytokines, especially IL-17 and TNFα (Lowes et al., 2008; Eyerich et al., 2009; Korn et al., 2009). In fact, IL-22 cooperates with IL-17 or TNFα for efficient induction of antimicrobial peptides and chemokines (Liang et al., 2006; Aujla et al., 2008; Eyerich et al., 2011) as well as IL-36γ, IL-6 and granulocyte colony-stimulating factor (Aujla et al., 2008). This may not only be relevant for effective initiation of host defence. Notably, because of this IL-17A/IL-22 synergism, the presence or absence of IL-17A can determine the net pro- or anti-inflammatory and protective functions of IL-22 in lethal airway inflammation induced by high-dose bleomycin (Sonnenberg et al., 2010b). If also applicable to other pathological conditions, this observation should be highly relevant when considering IL-22 for use as a tissue-protective therapy.
The functions of IL-22 in vivo mirror elemental roles of STAT3 in cell physiology. Among the cellular tasks connected with STAT3, anti-apoptosis and proliferation are particularly crucial (Jarnicki et al., 2010; Wang et al., 2012a). IL-22 up-regulates STAT3-inducible Bcl-2 and/or Bcl-XL (Radaeva et al., 2004; Zhang et al., 2008; Sonnenberg et al., 2010a; Curd et al., 2012; Kong et al., 2012) as well as cyclin D1 and/or c-Myc (Radaeva et al., 2004; Kong et al., 2012), all of which are prototypic parameters relating to anti-apoptosis and proliferation. These pro-survival proteins, along with the activation of ERK 1/2 (Wortzel and Seger, 2011) and Akt (X Zhang et al., 2011a), are likely to form the cellular basis for tissue protective properties of IL-22, as observed in pathophysiological situations, driven by epithelial cell death. Figure 1 summarizes the cellular signalling engaged by IL-22 in relation to the specific functional properties of this cytokine.
Figure 1.
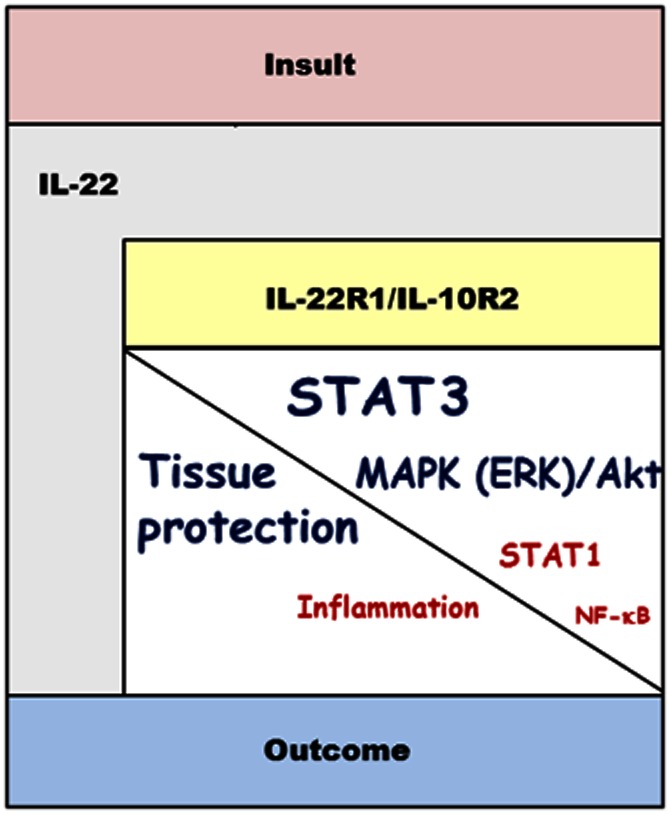
Structure chart-like illustration of IL-22 signalling related to cytokine function. Regardless of whether endogenously produced in response to an epithelial insult or provided in the course of tissue-protective therapy, IL-22 will activate its heterodimeric IL-22R1/IL-10R2 receptor, which is predominantly expressed at the liver and host/environment interfaces. Subsequent to receptor activation, signal transduction mechanisms are engaged that are greatly dominated by STAT3 along with activation of MAPK and Akt pathways. This specific profile of cellular activation mediates proliferation and anti-apoptosis, finally increasing tissue robustness and stress resistance. Through these pathways, IL-22 is capable of mediating tissue protection in the context of various pathogenic conditions. However, a latent pro-inflammatory role of IL-22 related to activation of STAT1 and NF-κB may counteract the therapeutic potential of this cytokine in a context-specific manner. IL-10R2, IL-10 receptor chain-2; IL-22R1, IL-22 receptor chain-1.
The biological activity of IL-22 is controlled by its specific endogenous antagonist, IL-22 binding protein (IL-22BP). This protein is encoded by a unique gene locus and displays 34% amino acid identity compared with the extracellular domain of IL-22 receptor chain-1, but lacks its transmembrane and intracellular domains. In fact, IL-22BP is functionally regarded as a soluble, neutralizing decoy receptor for IL-22. As IL-22BP displays higher affinity towards IL-22 as compared with the IL-22 receptor complex, it is supposed to control IL-22 biological activity in vivo (Dumoutier et al., 2001; Kotenko et al., 2001; Xu et al., 2001; Huber et al., 2012). Notably, in terms of the clinical IL-22 administration, individual IL-22BP levels may affect the therapeutic efficacy of this cytokine in patients.
Modulation of IL-22 bioactivity, achieved either by application of neutralizing antibodies or by using knockout mice, exacerbates symptoms in a range of disease models. This is well-documented for liver injury induced by concanavalin A (Radaeva et al., 2004; Zenewicz et al., 2007) or by the combination of LPS plus d-galactosamine (Radaeva et al., 2004; Marks et al., 2009). IL-22 neutralization likewise impairs proliferation and recovery in experimental hepatectomy (Ren et al., 2010) and aggravates cerulein-induced pancreatitis in the context of enhanced IL-22 biological activity under the influence of aryl hydrocarbon receptor ligands (Xue et al., 2012). Using knockout mice, tissue-protective effects of endogenous IL-22 were also identified in influenza A virus infections in mice. In this model, sufficient production of IL-22 early during infection stabilized lung epithelial integrity (Kumar et al., 2013; Paget et al., 2012). Actually, IL–22-knockout mice displayed a more severe course of disease (Kumar et al., 2013). The notion that endogenous IL-22 acts protectively in the lung extends to murine allergic asthma, where application of neutralizing antibodies worsens established disease (Besnard et al., 2011). A further study on experimental allergy likewise documents exacerbation of airway constriction and inflammation in IL–22-knockout mice (Taube et al., 2011). Finally, IL–22-deficient mice display increased pathology in experimental graft versus host disease (Hanash et al., 2012) and murine heart transplant rejection (Kapessidou et al., 2008) and likewise show impaired thymic regeneration in response to total body irradiation. In accord with a tissue-protective activity of IL-22, authors of the latter study demonstrated that the cytokine was pivotal for thymic epithelial proliferation and survival, subsequent to the thymic insult (Dudakov et al., 2012).
In addition to these anti-apoptotic and pro-proliferative mechanisms, IL-22 mediates further protection at host/environment interfaces by enhancing mucus production and, moreover, by activating more specific means of antibacterial host defence. IL-22 may, thus, also have an anti-infective indication. Of note, killing by antibacterial peptides such as β-defensins, lipocalin and RegIIIβ/γ or by inducible NO synthase-derived NO are candidate effector mechanisms likely to be utilised by IL–22-dependent control of infection- or microbe-associated tissue injury and inflammation (Aujla and Kolls, 2009; Blaschitz and Raffatellu, 2010; Mühl et al., 2011; Sonnenberg et al., 2011; Eddens and Kolls, 2012). However, it should be noted that inducible NO synthase (Mühl et al., 2000) and β-defensins (Niyonsaba et al., 2007) also have an inherent potential to serve pro-inflammatory functions. Therefore, overt induction of both these systems may, under some conditions, interfere with the protective potential of IL-22.
Specifically, neutralization of IL-22 increases mortality in murine Klebsiella pneumonia infection (Aujla et al., 2008). Moreover, deficiency of IL-22 bioactivity correlates with increased disease severity in murine intestinal Citrobacter rodentium infection (Zheng et al., 2008) and experimental colitis subsequent to dextran sulfate sodium-associated epithelial injury (Sugimoto et al., 2008; Pickert et al., 2009). The role of endogenous IL-22 in promoting intestinal healing has been recently confirmed in mice deficient for IL-22BP, who displayed enhanced tissue regeneration in response to mechanical intestinal injury (Huber et al., 2012). Most recently, the ability of IL-22 to mediate epithelial healing has been extended from intestinal to cutaneous wound healing. In experimental full-thickness wounding, IL-22-deficient mice indeed exhibited defective skin repair (McGee et al., 2013).
Altogether, thedata indicate the inherent potential of endogenously produced IL-22 to serve protective functions in a diverse array of disease conditions involving deleterious insults at tissues of epithelial origin. As already alluded to, those pathogenic processes are linked to release of DAMPs from dying cells that perpetuate and amplify the inflammatory state of the affected organ. Specifically, DAMPs such as extracellular DNA, high-mobility group box-1, heat shock proteins, hyaluronan or uric acid may significantly contribute to diverse manifestations of pathogenic inflammation ranging from acetaminophen (paracetamol)-induced liver injury to ventilator-induced lung injury (Imaeda et al., 2009; Maher, 2009; Kuipers et al., 2011) and asthma bronchiale (Shim et al., 2012). IL-22 could have the potential to interfere with the injury-driven vicious cycle, present in these conditions.
Interestingly, recent studies also indicate that endogenous IL-22 not only provides essential protection during pathophysiological processes, but may play a pivotal role in preserving intestinal homeostasis under steady-state conditions. Specifically, it has been demonstrated that innate lymphoid cells/lymphoid tissue inducer-like cells located in the intestinal mucosa are able to produce ample amounts of IL-22. Either commensal microbes (Sanos et al., 2009) or ligands of the aryl hydrocarbon receptor have been implicated in this process (Lee et al., 2012). Neutralization experiments revealed that this homeostatic IL-22 activity at the intestinal host/environment interface serves pivotal antibacterial functions that avoid potentially hazardous systemic bacterial translocation (Sonnenberg et al., 2012).
Tissue-protective therapy by IL-22 administration in rodent disease
Various preclinical studies have been conducted in recent years that emphasize the broad therapeutic potential of recombinant IL-22 in liver, pancreatic, intestinal and lung pathophysiology associated with epithelial injury (Table 1). Although produced as an endogenous protective factor in most of such conditions, the data, on the whole, indicate that the modulatory potential of the IL-22/STAT3 axis is not saturated by endogenous IL-22, which consequently raises expectations for use of this cytokine in novel therapeutic strategies. Effects of IL-22 on liver pathology are particularly well characterized. Specifically, administration of recombinant IL-22 significantly alleviates murine hepatic injury in response to concanavalin A (Radaeva et al., 2004), alcohol (Ki et al., 2010; Xing et al., 2011b), LPS plus d-galactosamine (Xing et al., 2011a), acetaminophen (Scheiermann et al., 2013) or ischaemia-reperfusion (Chestovich et al., 2012). Overexpression of IL-22 by in vivo cDNA delivery likewise attenuates experimental concanavalin A-, carbon tetrachloride- or Fas-mediated liver damage (Pan et al., 2004). Protective properties of recombinant IL-22 also extended to murine high fat diet-induced steatosis. In this model, amelioration of hepatic disease by IL-22 correlates with rapid down-regulation of lipogenesis-related genes (Yang et al., 2010). In addition, using IL-22 transgenic mice, in vivo adenoviral gene provision or application of recombinant cytokine, an anti-fibrotic role of IL-22 in the liver compartment was recently demonstrated. Notably, hepatic stellate cells are regarded to be the crucial target of IL-22 in this pathophysiological context (Kong et al., 2012; Meng et al., 2012).
Table 1.
Tissue-protective therapy by provision of IL-22, in rodent disease models
| Organ | IL-22 treatment | Observed effect | Reference |
|---|---|---|---|
| Lung | Single inhalation of recombinant IL-22 (10 μg·kg−1) | Attenuation of ventilator-induced lung injury | Hoegl et al., 2011 |
| Lung | 3 intranasal doses of recombinant IL-22 (1 μg per mouse on days 14, 15, and 16 during antigen challenge) | Protection from lung inflammation in the OVA model | Besnard et al., 2011 |
| Lung | 2 intranasal doses of recombinant IL-22 (0.1 μg per mouse, 48 and 2 h before OVA challenge) | Amelioration of allergic airway inflammation in the OVA model | Takahashi et al., 2011 |
| Lung | Single intranasal dose of recombinant IL-22 (0.1, 1 or 10 μg per mouse, 1 h before OVA challenge) | Amelioration of allergic airway inflammation in the OVA model | Taube et al., 2011 |
| Lung | Intratracheal treatment with recombinant IL-22 (100 pg and 100 ng per mouse, 3×/week for 4 weeks) | Protection from lung fibrosis in the Bacillus subtilis model | Simonian et al., 2010 |
| Liver | Single i.v. dose of recombinant IL-22 (3.5 μg per mouse) | Attenuation of acetaminophen (paracetamol)-induced liver damage | Scheiermann et al., 2013 |
| Liver | IL-22 transgenic mice; administration of Ad-IL-22 | Anti-fibrotic role of IL-22 in the liver | Kong et al., 2012 |
| Liver | Single i.v. dose of recombinant IL-22 (5 μg per mouse) | Protection from hepatic ischaemia-reperfusion injury | Chestovich et al., 2012 |
| Liver | Daily doses of recombinant IL-22 (0.5 μg per mouse) for 7 days | Anti-fibrotic role of IL-22 in the liver | Meng et al., 2012 |
| Liver | Single i.v. dose of recombinant IL-22 (0.5 μg·g−1) | Protection from hepatic injury induced by LPS plus D-galactosamine | Xing et al., 2011a |
| Liver | Daily i.v. dose of recombinant IL-22 (0.5 mg·kg−1) | Protection from liver injury after acute alcohol intoxication | Xing et al., 2011b |
| Liver | IL-22 transgenic mice | Enhanced tumour formation; protection from ConA-induced liver damage; accelerated liver regeneration after partial hepatectomy | Park et al., 2011 |
| Liver | Single i.p. dose of recombinant IL-22 (1 μg·g−1) | Protection from liver injury after chronic alcohol feeding | Ki et al., 2010 |
| Liver | Daily i.p. doses of recombinant IL-22 (300 μg·kg−1) for 36 days; in ob/ob mice daily s.c. doses of recombinant IL-22 (500 μg·kg−1) for 14–40 days | Protection from high fat diet-induced steatosis | Yang et al., 2010 |
| Liver | In vivo IL-22 cDNA delivery | Protection from ConA-, carbon tetrachloride-, and Fas-induced liver damage | Pan et al., 2004 |
| Liver | Single i.v. dose of recombinant IL-22 (0.25 μg·g−1) | Protection from ConA-induced liver damage | Radaeva et al., 2004 |
| Intestinal tract, liver, spleen | Repeated i.p. doses of recombinant IL-22 (25 μg per mouse, every other day starting on day 0) | Protection from systemic translocation of commensal intestinal bacteria in the context of impaired innate lymphoid cell function | Sonnenberg et al., 2012 |
| Intestinal tract | Repeated i.p. doses of recombinant IL-22-Fc fusion protein (0.05 mg per mouse) on days 0, 3, and 6 | Protection from severe DSS-induced colitis | Cox et al., 2012 |
| Intestinal tract | In vivo delivery of an IL-22-expressing plasmid | Protection from severe infection by Citrobacter rodentium | Qiu et al., 2012 |
| Intestinal tract | In vivo delivery of an IL-22-expressing plasmid | Protection from severe infection by Citrobacter rodentium | Tumanov et al., 2011 |
| Intestinal tract | In vivo delivery of an IL-22-expressing plasmid | Protection from Th2-mediated colitis in the TCRα-KO model | Sugimoto et al., 2008 |
| Pancreas | In vivo delivery of adenovirus-IL-22; single i.p. dose of recombinant IL-22 (1 μg·g−1); IL-22 transgenic mice | Attenuation of cerulein-induced pancreatitis | Feng et al., 2012 |
| Pancreas | Single i.p. dose of recombinant IL-22 (200 ng per mouse) 24 h after onset of pancreatitis | Protection from pancreatitis induced by either cerulein or choline-deficient diet supplemented with DL-ethionine | Xue et al., 2012 |
ConA, concanavalin A; DSS, dextran sulfate sodium; OVA, ovalbumin; TCRα; T-cell receptor α-chain.
Several studies testify to the benefit of IL-22 application in disease models relating to tissues other than the liver. Specifically, provision of IL-22 as recombinant protein attenuated pancreatitis induced by a cerulein- or choline-deficient diet supplemented with DL-ethionine (Feng et al., 2012; Xue et al., 2012), probably by up-regulation of anti-apoptotic Bcl-2 and/or Bcl-XL and by modulating acinar cell autophagy (Feng et al., 2012). With regard to pulmonary insults, recombinant IL-22 once more displays the capability to ameliorate disease in the context of most diverse pathogenic entities. Local application of recombinant IL-22 was protective in murine models of allergic airway inflammation (Besnard et al., 2011; Takahashi et al., 2011; Taube et al., 2011) and lung fibrosis. In the latter pathogenic condition, IL-22 clearly reduced pulmonary collagen deposition, a hallmark of established disease (Simonian et al., 2010). Moreover, IL-22 attenuated damage in rat baro-/biotrauma initiated by ventilator-induced lung injury. Notably, in that study, recombinant rat IL-22 was applied by inhalation (Hoegl et al., 2011). In accord with the corresponding data on IL-22 blockade in intestinal disease, provision of IL-22 by in vivo delivery of IL-22-expressing plasmids ameliorated murine disease in severe infection by Citrobacter rodentium (Tumanov et al., 2011; Qiu et al., 2012) and in Th2-driven colitis observed in T-cell receptor α-chain knockout mice (Sugimoto et al., 2008). Moreover, a recombinant IL-22-Fc fusion protein was protective in experimental colitis provoked by dextran sulphate sodium-induced epithelial injury (Cox et al., 2012). Finally, administration of IL-22 protected mice from systemic translocation of intestinal commensal bacteria in the context of an impaired innate lymphoid cell function (Sonnenberg et al., 2012). Those latter data indicate the potential of IL-22 administration as a prophylactic strategy to strengthen the intestinal barrier in patients at risk for developing microbial translocation, among others HIV-infected patients (Brenchley et al., 2006). In fact, mucosal IL-22 production is impaired upon HIV infection and recombinant IL-22 can counteract gut epithelial damage induced by the virus in an in vitro model ( Kim et al., 2012).
Restrictions of IL-22 usage for tissue-protective therapy
Besides activation of the hepatic acute phase response, administration of recombinant IL-22 to healthy mice, either of BALB/c or C57Bl/6 background, in doses of up to 8 μg per animal did not evoke signs of acute systemic immunoactivation as determined by analysis of serum IL-1β, TNF-α and IL-6 (Wolk et al., 2004; Scheiermann et al., 2013). Moreover, IL-22 treatment of mice undergoing fulminant endotoxaemia failed to reduce production of these inflammatory cytokines. This observation would exclude the possibility of rapid immunosuppression by IL-22 (Scheiermann et al., 2013), a regulatory function supposed to be characteristic for the related anti-inflammatory cytokine IL-10 (Ouyang et al., 2011). Data concur with the notion that IL-22, in contrast to the related cytokines IL-10 and IL-6, does not directly affect leukocyte biology. Taken together, current preclinical observations would suggest the up-regulation of IL-22 bioactivity, for example, by administration of recombinant protein, as a promising, acutely well-tolerated and innovative pharmacological approach targeting pathogenic processes driven by epithelial injury and/or fibrosis. However, there are specific drawbacks that may rule out the long-term application of IL-22.
One particular concern is the inherent capacity of IL-22 to amplify specific aspects of ongoing autoimmune inflammation. This latent pro-inflammatory property of IL-22 is actually considered to contribute to the pathogenesis of rheumatoid arthritis and psoriasis (Pan et al., 2013), two prototypic chronic inflammatory diseases. Not only is production of IL-22 increased in rheumatoid arthritis and psoriasis patients, but it also correlates with disease severity (Boniface et al., 2007; Leipe et al., 2011; Nakajima et al., 2011). Notably, IL-22-knockout mice exhibit striking attenuation of collagen-induced arthritis (Geboes et al., 2009). Synovial fibroblasts in the arthritic joint are regarded as crucial cellular targets of IL-22, which, probably via STAT3, drives proliferation of this cell type (Ikeuchi et al., 2005). Thus, the IL-22/STAT3 pathway may contribute to the transformed-like character of synovial fibroblasts, which has been proposed to drive disease progression in rheumatoid arthritis. (Muller-Ladner et al., 1995; Aidinis et al., 2003). Besides that, activation of synovial fibroblasts by IL-22 up-regulates expression of the chemokine CCL2 (Ikeuchi et al., 2005) and of receptor activator of NF-κB ligand (KW Kim et al., 2012), which serve pro-inflammatory and joint destructive functions, respectively. Reduction of IL-22 bioactivity likewise reduces severity in experimental psoriasis (Van Belle et al., 2012), an observation that also agrees with psoriasis-like symptoms evolving in IL-22 transgenic mice (Wolk et al., 2009; Park et al., 2011). Keratinocytes are obvious targets of IL-22 in psoriasis. IL-22 modulated differentiation of keratinocytes and mediated keratinocyte expression of key inflammatory parameters, among others CXCL5, IL-20 and matrix metalloproteinases-1 and -3 (Boniface et al., 2005; Nograles et al., 2008; Sabat and Wolk, 2011). As already alluded to, under the influence of type I IFN, IL-22 significantly activates the pro-inflammatory transcription factor STAT1 (Bachmann et al., 2013), which obviously should amplify its pro-inflammatory potential (Paludan, 2000). Notably, there is up-regulation of IFN-β in the inflamed synovium of patients with rheumatoid arthritis (van Holten et al., 2005). Altogether, it is tempting to speculate that reduction of IL-22 biological activity by neutralizing antibodies, IL-22BP or antagonistic IL-22 muteins (Niv-Spector et al., 2012) would reduce signs of inflammation and disease severity in psoriasis and rheumatoid arthritis patients. The development of antagonistic IL-22 muteins (Niv-Spector et al., 2012) is particularly promising, for instance, in psoriasis. These artificial analogues of IL-22, generated by introduction of mutations, are able to bind to IL-22 receptor chain-1 with high affinity, but because they do not also recruit the IL-10 receptor chain-2, are unable to initiate IL-22 signal transduction. Because IL-20 and IL-24, at least partly, signal likewise via the IL-22 receptor chain-1 (Commins et al., 2008), those IL-22 muteins should inhibit not only IL-22, but also the biological activity of IL-20 and IL-24. Notably, along with IL-22, IL-20 and IL-24 may play a key role in the pathogenesis of psoriasis (Sa et al., 2007; Sabat and Wolk, 2011; Wang et al., 2012b).
It should also be mentioned at this point that IL-22 may aggravate tissue inflammation in some types of viral infections. Specifically, IL-22 has been shown to serve pathogenic functions in experimental hepatitis B virus infection (Y Zhang et al., 2011b) and murine West Nile virus encephalitis (P Wang et al., 2011). In both viral infections, IL-22 increases expression of local chemokines such as CXCL1, CXCL9 and CXCL10, which, via recruitment of leukocytes into liver or CNS, may potentiate collateral tissue damage and associated pathogenic inflammation. Notably, these observations contrast with the role of IL-22 in influenza A virus infection where tissue protection at the lung epithelium is the predominant effect (Kumar et al., 2013; Paget et al., 2012). On the whole, it must be concluded that in some pathogenic conditions, the latent pro-inflammatory potential of IL-22 conflicts with its tissue protective potential. The actual outcome, in such conditions, of IL-22 treatment might then be determined by context-specific parameters.
Further reservation against provision of IL-22 over the longer term as a viable pharmacological strategy lies in the pronounced and well established pro-tumourigenic role of STAT3. A variety of studies have demonstrated overt activation of STAT3 in a range of human solid tumours (Yu et al., 2009; Jarnicki et al., 2010; Johnston and Grandis, 2011). Likewise, enhanced levels of IL-22 are detectable in human cancer, including non-small cell lung (Zhang et al., 2008) and hepatocellular (Jiang et al., 2011) carcinoma as well as gastric cancer (Zhuang et al., 2012). The functional relevance of IL-22 in this context has been adequately demonstrated in experimental hepatocellular carcinoma, where IL–22-knockout mice display decreased (Jiang et al., 2011) and IL-22 transgenic mice enhanced tumour formation in the diethyl-nitrosamine model of liver carcinogenesis (Park et al., 2011). A most recent study furthermore clearly demonstrates that IL-22 promotes experimental tumourigenesis in the colon (Huber et al., 2012). Taken together, the bulk of experimental and clinical data strongly suggest a pathogenic role of the IL-22/STAT3 axis in carcinogenesis.
Conclusion
To develop novel therapeutic strategies for the treatment of diseases driven by epithelial injury is a vital challenge of current pharmacological and translational research. Preclinical data suggest augmentation of IL-22 bioactivity, for example, by administration of the recombinant protein, as a promising and entirely novel therapeutic approach for the treatment of diverse pathogenic conditions ranging from toxic liver injury and ventilator-induced lung injury to severe infections at host/environment interfaces. It can be assumed that IL-22 is characterized by a rather short half-life in circulation. Therefore, PEGylation or ligation of IL-22 to an Fc fragment will probably be necessary for efficient use of the cytokine in clinical practice (Jazayeri and Carroll, 2008; Veronese and Mero, 2008). In fact, a phase I clinical trial aiming to assess the safety profile of an IL-22-like biopharmaceutical agent (F-652) in healthy humans has recently been initiated [Generon (Shanghai) Corporation Ltd.] 12 years after introduction of the cytokine as IL–10-related T–cell-derived factor. However, potential applications of IL-22 in clinical therapy must be carefully selected (Figure 2). Whereas IL-22 appears highly suitable for tissue-protective therapy in acute disease, chronic application of the cytokine may pose a threat through its inherent potential to promote carcinogenesis and specific aspects of autoimmune inflammation.
Figure 2.
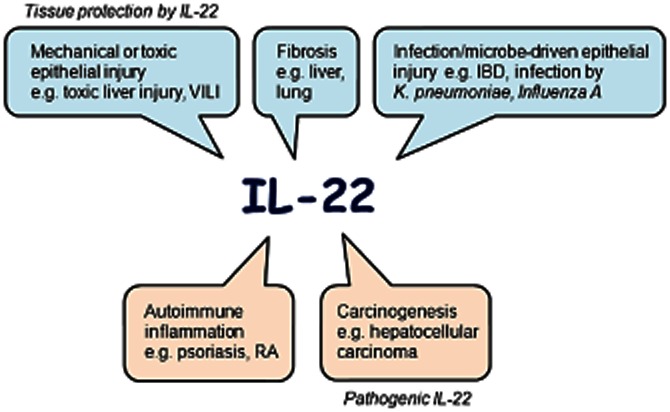
Summary scheme and contraposition of the protective and pathogenic properties of IL-22, expressed in a range of conditions. IBD, inflammatory bowel disease; RA, rheumatoid arthritis; VILI, ventilator-induced lung injury.
Conflicts of interest
There are no conflicts of interest to declare.
Note added in proof
Most recently Sarkar et al. (2013) documented the capability of systemically applied recombinant IL-22 to ameliorate collagen-induced arthritis in mice. Data, at first sight, do not concur with reduced disease severity observed in the same model using IL-22 deficient mice (Geboes et al., 2009) and may indicate the exciting possibility that therapeutically administered IL-22 can serve different functions as compared with the endogenously produced cytokine.
Glossary
- DAMPs
danger-associated molecular patterns
- IL-22BP
IL-22 binding protein
References
- Aggarwal S, Xie MH, Maruoka M, Foster J, Gurney AL. Acinar cells of the pancreas are a target of interleukin-22. J Interferon Cytokine Res. 2001;21:1047–1053. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- Aidinis V, Plows D, Haralambous S, Armaka M, Papadopoulos P, Kanaki MZ, et al. Functional analysis of an arthritogenic synovial fibroblast. Arthritis Res Ther. 2003;5:R140–R157. doi: 10.1186/ar749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med (Berl) 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M, Horn K, Rudloff I, Goren I, Holdener M, Christen U, et al. Early production of IL-22 but not IL-17 by peripheral blood mononuclear cells exposed to live Borrelia burgdorferi: the role of monocytes and interleukin-1. PLoS Pathog. 2010;6:e1001144. doi: 10.1371/journal.ppat.1001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M, Ulziibat S, Härdle L, Pfeilschifter J, Mühl H. IFNα converts IL-22 into a cytokine efficiently activating STAT1 and its downstream targets. Biochem Pharmacol. 2013;85:396–403. doi: 10.1016/j.bcp.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–1163. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
- Bingold TM, Ziesche E, Scheller B, Sadik CD, Franck K, Just L, et al. Interleukin-22 detected in patients with abdominal sepsis. Shock. 2010;34:337–340. doi: 10.1097/SHK.0b013e3181dc07b1. [DOI] [PubMed] [Google Scholar]
- Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30:196–203. doi: 10.1007/s10875-010-9368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, et al. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–G838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- Brand S, Dambacher J, Beigel F, Zitzmann K, Heeg MH, Weiss TS, et al. IL-22-mediated liver cell regeneration is abrogated by SOCS-1/3 overexpression in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1019–G1028. doi: 10.1152/ajpgi.00239.2006. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Chestovich PJ, Uchida Y, Chang W, Ajalat M, Lassman C, Sabat R, et al. Interleukin-22: implications for liver ischemia-reperfusion injury. Transplantation. 2012;93:485–492. doi: 10.1097/TP.0b013e3182449136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121:1108–1111. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, Diehl L, et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5:99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- Curd LM, Favors SE, Gregg RK. Pro-tumour activity of interleukin-22 in HPAFII human pancreatic cancer cells. Clin Exp Immunol. 2012;168:192–199. doi: 10.1111/j.1365-2249.2012.04570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- Dumoutier L, Van Roost E, Colau D, Renauld JC. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci U S A. 2000;97:10144–10149. doi: 10.1073/pnas.170291697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoutier L, Lejeune D, Colau D, Renauld JC. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J Immunol. 2001;166:7090–7095. doi: 10.4049/jimmunol.166.12.7090. [DOI] [PubMed] [Google Scholar]
- Eddens T, Kolls JK. Host defenses against bacterial lower respiratory tract infection. Curr Opin Immunol. 2012;24:424–430. doi: 10.1016/j.coi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, et al. IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol. 2011;41:1894–1901. doi: 10.1002/eji.201041197. [DOI] [PubMed] [Google Scholar]
- Feng D, Park O, Radaeva S, Wang H, Yin S, Kong X, et al. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int J Biol Sci. 2012;8:249–257. doi: 10.7150/ijbs.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Sekikawa A, Tanaka H, Fujimori Y, Katake Y, Fujii S, et al. DMBT1 is a novel gene induced by IL-22 in ulcerative colitis. Inflamm Bowel Dis. 2011;17:1177–1188. doi: 10.1002/ibd.21473. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(Suppl. 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60:390–395. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- Goh FG, Midwood KS. Intrinsic danger: activation of toll-like receptors in rheumatoid arthritis. Rheumatology (Oxford) 2012;51:7–23. doi: 10.1093/rheumatology/ker257. [DOI] [PubMed] [Google Scholar]
- Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, et al. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:369–376. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- van Holten J, Smeets TJ, Blankert P, Tak PP. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Ann Rheum Dis. 2005;64:1780–1782. doi: 10.1136/ard.2005.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–1046. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnicki A, Putoczki T, Ernst M. Stat3: linking inflammation to epithelial cancer – more than a ‘gut’ feeling? Cell Div. 2010;5:14. doi: 10.1186/1747-1028-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri JA, Carroll GJ. Fc-based cytokines : prospects for engineering superior therapeutics. BioDrugs. 2008;22:11–26. doi: 10.2165/00063030-200822010-00002. [DOI] [PubMed] [Google Scholar]
- Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–909. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Seigel B, Bengsch B, Fleming VM, Billerbeck E, Simmons R, et al. CD161(+)CD4(+) T cells are enriched in the liver during chronic hepatitis and associated with co-secretion of IL-22 and IFN-gamma. Front Immunol. 2012;3:346. doi: 10.3389/fimmu.2012.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapessidou P, Poulin L, Dumoutier L, Goldman M, Renauld JC, Braun MY. Interleukin-22 deficiency accelerates the rejection of full major histocompatibility complex-disparate heart allografts. Transplant Proc. 2008;40:1593–1597. doi: 10.1016/j.transproceed.2008.03.151. [DOI] [PubMed] [Google Scholar]
- Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64:1015–1023. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, et al. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol. 2001;166:7096–7103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- Koyasu S, Moro K. Role of innate lymphocytes in infection and inflammation. Front Immunol. 2012;3:101. doi: 10.3389/fimmu.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger B, Rudloff I, Bachmann M, Brunner F, Kapper L, Filmann N, et al. Interleukin-22 predicts severity and death in advanced liver cirrhosis: a prospective cohort study. BMC Med. 2012;10:102. doi: 10.1186/1741-7015-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers MT, van der Poll T, Schultz MJ, Wieland CW. Bench-to-bedside review: damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care. 2011;15:235. doi: 10.1186/cc10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013;6:69–82. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and ndependent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, Nigg AP, et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1453–1457. doi: 10.1136/ard.2011.152074. [DOI] [PubMed] [Google Scholar]
- Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- Lukens JR, Gross JM, Kanneganti TD. IL-1 family cytokines trigger sterile inflammatory disease. Front Immunol. 2012;3:315. doi: 10.3389/fimmu.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee HM, Schmidt BA, Booth CJ, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol. 2013;133:1321–1329. doi: 10.1038/jid.2012.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JJ. DAMPs ramp up drug toxicity. J Clin Invest. 2009;119:246–249. doi: 10.1172/JCI38178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijnissen RJ, Koenders MI, Smeets RL, Stappers MH, Nickerson-Nutter C, Joosten LA, et al. Increased expression of interleukin-22 by synovial Th17 cells during late stages of murine experimental arthritis is controlled by interleukin-1 and enhances bone degradation. Arthritis Rheum. 2011;63:2939–2948. doi: 10.1002/art.30469. [DOI] [PubMed] [Google Scholar]
- Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G, et al. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis (Edinb) 2011;91:587–593. doi: 10.1016/j.tube.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. doi: 10.1053/j.gastro.2012.05.049. e761–e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60:38–42. doi: 10.1016/j.cyto.2012.06.316. [DOI] [PubMed] [Google Scholar]
- Mühl H. Pro-inflammatory signaling by IL-10 and IL-22: bad habit stirred up by interferons? Front Immunol. 2013;4:18. doi: 10.3389/fimmu.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühl H, Chang JH, Huwiler A, Bosmann M, Paulukat J, Ninic R, et al. Nitric oxide augments release of chemokines from monocytic U937 cells: modulation by anti-inflammatory pathways. Free Radic Biol Med. 2000;29:969–980. doi: 10.1016/s0891-5849(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Mühl H, Bachmann M, Pfeilschifter J. Inducible NO synthase and antibacterial host defence in times of Th17/Th22/T22 immunity. Cell Microbiol. 2011;13:340–348. doi: 10.1111/j.1462-5822.2010.01559.x. [DOI] [PubMed] [Google Scholar]
- Muller-Ladner U, Kriegsmann J, Gay RE, Gay S. Oncogenes in rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21:675–690. [PubMed] [Google Scholar]
- Nagalakshmi ML, Rascle A, Zurawski S, Menon S, de Waal Malefyt R. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int Immunopharmacol. 2004;4:679–691. doi: 10.1016/j.intimp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Nakajima K, Tarutani M, Morishige R, Sano S. Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch Dermatol Res. 2011;303:451–455. doi: 10.1007/s00403-011-1159-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv-Spector L, Shpilman M, Levi-Bober M, Katz M, Varol C, Elinav E, et al. Preparation and characterization of mouse IL-22 and its four single-amino-acid muteins that act as IL-22 receptor-1 antagonists. Protein Eng Des Sel. 2012;25:397–404. doi: 10.1093/protein/gzs030. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287:8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan SR. Synergistic action of pro-inflammatory agents: cellular and molecular aspects. J Leukoc Biol. 2000;67:18–25. doi: 10.1002/jlb.67.1.18. [DOI] [PubMed] [Google Scholar]
- Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43–49. [PubMed] [Google Scholar]
- Pan HF, Li XP, Zheng SG, Ye DQ. Emerging role of interleukin-22 in autoimmune diseases. Cytokine Growth Factor Rev. 2013;24:51–57. doi: 10.1016/j.cytogfr.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology. 2011;54:252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol. 2012;3:287. doi: 10.3389/fimmu.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298:G74–G80. doi: 10.1152/ajpgi.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- Sabat R, Wolk K. Research in practice: IL-22 and IL-20: significance for epithelial homeostasis and psoriasis pathogenesis. J Dtsch Dermatol Ges. 2011;9:518–523. doi: 10.1111/j.1610-0387.2011.07611.x. [DOI] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Zhou X, Justa S, Bommireddy SR. Interleukin-22 reduces the severity of collagen-induced arthritis in association with increased levels of interleukin-10. Arthritis Rheum. 2013;65:960–971. doi: 10.1002/art.37849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann P, Bachmann M, Goren I, Zwissler B, Pfeilschifter J, Mühl H. Application of Interleukin-22 mediates protection in experimental acetaminophen-induced acute liver injury. Am J Pathol. 2013;182:1107–1113. doi: 10.1016/j.ajpath.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, et al. Linking genetic susceptibility to Crohn's disease with Th17 cell function: IL-22 serum levels are increased in Crohn's disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–212. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- Sestito R, Madonna S, Scarponi C, Cianfarani F, Failla CM, Cavani A, et al. STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J. 2011;25:916–927. doi: 10.1096/fj.10-172288. [DOI] [PubMed] [Google Scholar]
- Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim EJ, Chun E, Lee HS, Bang BR, Kim TW, Cho SH, et al. The role of high-mobility group box-1 (HMGB1) in the pathogenesis of asthma. Clin Exp Allergy. 2012;42:958–965. doi: 10.1111/j.1365-2222.2012.03998.x. [DOI] [PubMed] [Google Scholar]
- Shioya M, Andoh A, Kakinoki S, Nishida A, Fujiyama Y. Interleukin 22 receptor 1 expression in pancreas islets. Pancreas. 2008;36:197–199. doi: 10.1097/MPA.0b013e3181594258. [DOI] [PubMed] [Google Scholar]
- Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gamma-delta T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207:2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010a;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010b;207:1293–1305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, et al. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol. 2011;128:1067–1076. doi: 10.1016/j.jaci.2011.06.018. e1061–1066. [DOI] [PubMed] [Google Scholar]
- Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, et al. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS ONE. 2011;6:e21799. doi: 10.1371/journal.pone.0021799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe. 2011;10:44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188:462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315–329. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- Wang F, Smith N, Maier L, Xia W, Hammerberg C, Chubb H, et al. Etanercept suppresses regenerative hyperplasia in psoriasis by acutely downregulating epidermal expression of interleukin (IL)-19, IL-20 and IL-24. Br J Dermatol. 2012a;167:92–102. doi: 10.1111/j.1365-2133.2012.10961.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–550. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Bai F, Zenewicz LA, Dai J, Gate D, Cheng G, et al. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS ONE. 2012b;7:e44153. doi: 10.1371/journal.pone.0044153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–1323. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol. 2007;178:5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- Wortzel I, Seger R. The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- Xing WW, Zou MJ, Liu S, Xu T, Gao J, Wang JX, et al. Hepatoprotective effects of IL-22 on fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide in mice. Cytokine. 2011a;56:174–179. doi: 10.1016/j.cyto.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Xing WW, Zou MJ, Liu S, Xu T, Wang JX, Xu DG. Interleukin-22 protects against acute alcohol-induced hepatotoxicity in mice. Biosci Biotechnol Biochem. 2011b;75:1290–1294. doi: 10.1271/bbb.110061. [DOI] [PubMed] [Google Scholar]
- Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, et al. A soluble class II cytokine receptor, IL-22RA2, is anaturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A. 2001;98:9511–9516. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Nguyen DT, Habtezion A. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143:1670–1680. doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339–347. doi: 10.1016/j.jhep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chen Y, Wei H, Zheng C, Sun R, Zhang J, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–6439. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dang E, Shi X, Jin L, Feng Z, Hu L, et al. The pro-inflammatory cytokine IL-22 up-regulates keratin 17 expression in keratinocytes via STAT3 and ERK1/2. PLoS ONE. 2012;7:e40797. doi: 10.1371/journal.pone.0040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011a;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cobleigh MA, Lian JQ, Huang CX, Booth CJ, Bai XF, et al. A proinflammatory role for interleukin-22 in the immune response to hepatitis B virus. Gastroenterology. 2011b;141:1897–1906. doi: 10.1053/j.gastro.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Guo G, et al. Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol Immunother. 2012;61:1965–1975. doi: 10.1007/s00262-012-1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziesche E, Bachmann M, Kleinert H, Pfeilschifter J, Mühl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282:16006–16015. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]


