Abstract
N–3 long-chain polyunsaturated fatty acids (n–3 LC-PUFAs), in particular α-linolenic acid (18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) are receiving much attention because of their presumed beneficial health effects. To explain these, a variety of mechanisms have been proposed, but their interactions with the endocannabinoid system have received relatively little attention so far. However, it has already been shown some time ago that consumption of n–3 LC-PUFAs not only affects the synthesis of prototypic endocannabinoids like anandamide but also stimulates the formation of specific n–3 LC-PUFA-derived conjugates with ethanolamine, dopamine, serotonin or other amines. Some of these fatty amides show overlapping biological activities with those of typical endocannabinoids, whereas others possess distinct and sometimes largely unknown receptor affinities and other properties. The ethanolamine and dopamine conjugates of DHA have been the most investigated thus far. These mediators may provide promising new leads to the field of inflammatory and neurological disorders and for other pharmacological applications, including their use as carrier molecules for neurotransmitters to target the brain. Furthermore, combinations of n–3 LC-PUFA-derived fatty acid amides, their precursors and FAAH inhibitors offer possibilities to optimise their effects in health and disease.
Linked Articles
This article is part of a themed section on Cannabinoids. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2013.169.issue-4 & http://dx.doi.org/10.1111/bph.2012.167.issue-8
Keywords: endocannabinoid system; n-3; PUFA, DHA, EPA; fish oil
Introduction
Conjugates of fatty acids with ethanolamine, amino acids or monoamine neurotransmitters occur widely in nature (Di Marzo et al., 2007; Farrell and Merkler, 2008; Connor et al., 2010; Ezzili et al., 2010). Chemically, they are categorized as fatty acid amides and further divided into subclasses, including the N-acyl ethanolamines (NAEs) and N-acyl amines (Lipid Maps class FA08; http://www.lipidmaps.org). The best studied representative to date is anandamide (N-arachidonoyl ethanolamine, AEA), a prototypic endocannabinoid well-known for its pleiotropic effects ranging from energy homeostasis to immune functioning (Di Marzo et al., 2007). The biological and pharmacological properties of fatty (acid) amides do not follow their chemical classification and have shown to be very diverse. Anandamide (Figure 1) is a known ligand for both the cannabinoid type-1 (CB1) and CB2 receptors (receptor nomenclature follows Alexander et al., 2011) and belongs to the NAE subclass. However, several other NAEs, such as N-palmitoyl ethanolamine (PEA), N-oleoyl ethanolamine (OEA) and N-stearoyl ethanolamine (SEA), show different receptor preferences, including affinity for GPR55, GPR18, GPR119, TRPV1 (transient receptor potential channel type V1) or PPARα, while often showing less or no affinity for CB1 or CB2 receptors (Alexander and Kendall, 2007; Di Marzo et al., 2007; Farrell and Merkler, 2008; Hansen and Diep, 2009). The N-acyl amine subclass contains more than 80 different conjugates of long-chain fatty acids with amino acids (lipoamino acids; elmiric acids) or neurotransmitters (Burstein and Zurier, 2009; Connor et al., 2010; Tan et al., 2010).
Figure 1.
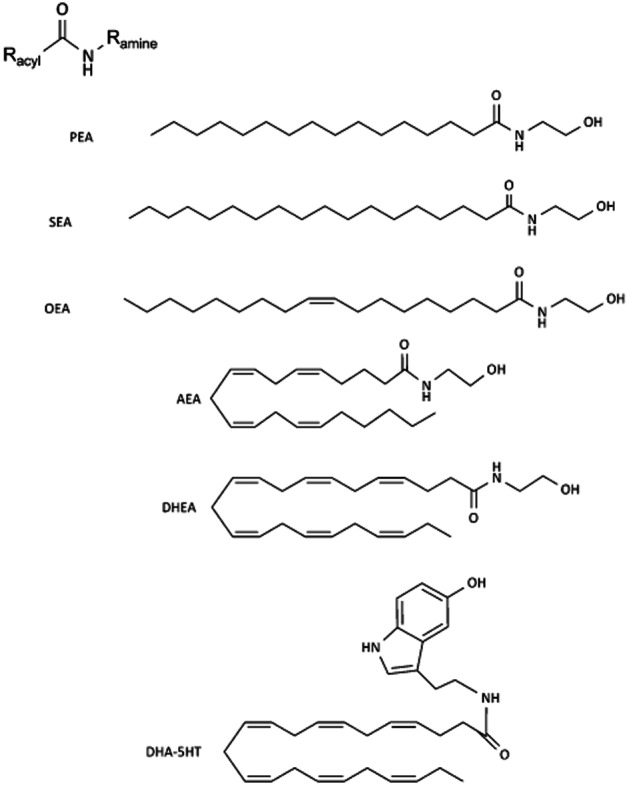
General chemical structure of fatty acid amides and some examples of conjugates of dietary relevant fatty acids with ethanolamine or serotonin.
For many of these molecules, relatively little is known so far about their biological significance or pharmacological potential. In many cases, only in vitro data are available, often obtained from testing single compounds. However, in vivo, fatty acid amides are known to occur in fluctuating mixtures of structurally related molecules with pleiotropic and tissue specific activities. With regard to the fatty acid moiety, the majority of studies so far have focused on conjugates of the most abundant fatty acids in higher animals, in particular those of arachidonic acid (20:4n-6), palmitic acid (16:0), oleic acid (18:1n-9) and stearic acid (18:0). Compared with these, much less is known on the biology and pharmacology of fatty amides of long-chain polyunsaturated (n–3) fatty acids (n–3 LC-PUFAs), including those of the dietary most relevant α-linolenic acid (18:3n-3), eicosapentaenoic acid (EPA; 20:5n-3) and docosahexaenoic acid (DHA; 22:6n-3) (see Figure 2).
Figure 2.
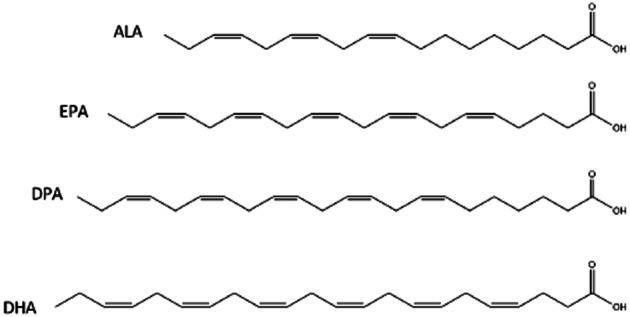
Chemical structure of four major natural n-3 LC-PUFAs: ALA (18:3n-3), EPA (20:5n-3), DPA (22:5n-3) and DHA (22:6n-3).
Because of their (alleged) positive effects in health and disease, n–3 LC-PUFAs are of much interest both from a nutritional and a pharmacological perspective. As will be described below, several mechanisms have been proposed to explain these effects, but a possible involvement of fatty acid amides has not yet received much attention. During the last few years, new data have become available, suggesting that the formation and effects of fatty acid amides derived from n–3 LC-PUFAs may be more important than previously assumed. For example, although the existence of N-docosahexaenoyl ethanolamine (DHEA) in bovine brain was already reported in 1997 by the group of Raphael Mechoulam (Sheskin et al., 1997), only few studies have further investigated its physiological role or pharmacological effects. However, evidence is increasing that several amine conjugates of n–3 acids possess an interesting spectrum of activities. Furthermore, at least some of them, including DHEA, are present in brain and gut tissue in concentrations similar to or even higher than those of AEA (Berger et al., 2001; Wood et al., 2010; Balvers et al., 2012a).
The aim of the present review is to summarize and discuss these findings from a physiological and pharmacological perspective. The focus is primarily on DHEA, the most studied representative of this group, but other conjugates of DHA, EPA and α-linolenic acid with ethanolamine, dopamine, serotonin or other amines will also be given attention.
Long-chain n–3 PUFAs – presence, health effects and metabolic pathways
Polyunsaturated fatty acids contain more than one double bond in the aliphatic chain. The natural PUFAs are often categorized into two groups: the n–6 (or ω-6) and the n–3 (or ω-3) fatty acids, based on the position of the first double bond starting from the methyl (omega, ω) position. Mammals do not have enzymes to insert a double bond in the n–6 or n–3 position, and a lack of linoleic acid (18:2n-6) or α-linolenic acid (ALA, 18:3n-3) in the diet can give rise to symptoms of deficiency in humans (Hansen and Artmann, 2008; De Caterina, 2011). Significant amounts of ALA are found in a number of green plants, nuts, flaxseed (linseed) and some vegetable oils, including soybean and rapeseed oils (Calder, 2011). Via elongation of the acyl chain and insertion of extra double bonds ALA can be converted to EPA (20:5n-3) via the intermediate stearidonic acid (18:4n-3). EPA can be further metabolized to docosapentaenoic acid (22:5n-3; DPA) and finally to DHA (22:6n-3). However, endogenous conversion of ALA to EPA and DHA is very limited in humans, in particular in adults (Brenna et al., 2009). Details on these pathways are described in a number of recent reviews (Russo, 2009; Calder, 2011; De Caterina, 2011).
EPA, DPA and DHA are particularly found in ‘oily’ fish (herring, salmon, mackerel), in certain algae and in ‘krill oil’. One oily fish meal can provide between 1.5 and 3.5 g of these n–3 LC-PUFAs (Russo, 2009; Calder, 2011). Consumption of n–3 LC-PUFAs has been associated with a variety of positive health effects (Parker et al., 2006; Carlson, 2009; Bazan et al., 2011; Calder, 2011). However, for most of these presumed effects, the evidence in humans is far from conclusive, in particular when considering the DHA/EPA intake obtained from the commonly recommended one to two servings of oily fish per week. Benefits in humans seem to be most consistent for mortality from coronary heart disease and sudden cardiac death (Riediger et al., 2009; de Roos et al., 2009; De Caterina, 2011; Mozaffarian and Wu, 2011). At the same time, recent meta-analyses on the relation between consumption of fish and/or n–3 LC-PUFAs and the incidence of diabetes type 2, for example, did not provide clear evidence for such associations (Wallin et al., 2012; Xun and He, 2012). In rodent studies, n–3 LC-PUFAs exhibit immune-modulating, anti-inflammatory and cellular protective properties (Calder, 2009; 2011). However, doses used in these studies are often rather high, which complicates extrapolation to humans. Effects on inflammatory markers have been reported from human studies as well, although doses are sometimes high compared with those commonly obtained from the diet (see Tur et al., 2012). The immune-modulating and/or anti-inflammatory effects have been explained from different mechanisms (see Figure 3), and it seems conceivable that these will at least partly be acting in parallel (Calder, 2011).
Figure 3.
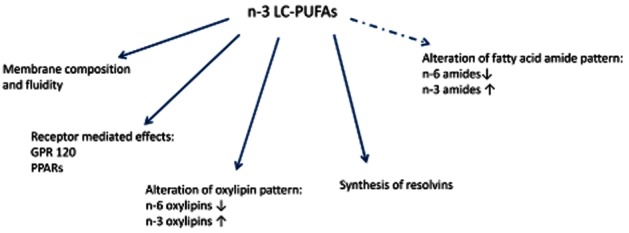
Schematic representation of different mechanisms via which n–3 LC-PUFAs can influence biological processes. Dotted arrows depict the modulation of the endocannabinoid system as described in this review.
Dietary n–3 LC-PUFAs induce shifts in endocannabinoid patterns
The endocannabinoid system controls food intake and energy balance through a number of central and peripheral mechanisms (see Maccarrone et al., 2010b). Vice versa, both the absolute and relative endocannabinoid tissue concentrations are determined by feeding status and dietary intake patterns. A number of studies in rodents and humans have shown that increasing the relative proportion of n–3 LC-PUFAs in the diet can lead to a decrease in the formation of the ‘prototypic’ endocannabinoids anandamide (AEA) and 2-AG (Batetta et al., 2009; Banni and Di Marzo, 2010; Maccarrone et al., 2010b). In the past, this was sometimes interpreted as an overall reduction of activity of the endocannabinoid system. For example, in the study of Watanabe et al. (2003), the conclusion was drawn that modulation of dietary n–3 PUFA status might provide a way to modify physiological and pathological events mediated by 2-AG through cannabinoid receptors in the CNS. However, although lower anandamide and 2-AG levels after fish oil diets have indeed been shown in many studies, these changes are a direct consequence from a shift in n–3/n–6 balance of membrane lipids. This results in compensatory increases in n–3 LC-PUFA-derived acyl conjugates. As will be discussed below, some of these molecules also show affinity for CB1 or CB2 receptors or share other activities with, for example, anandamide. Therefore, it seems important to pay attention to a broader spectrum of fatty amides and if possible include other classes of fatty acid metabolites, such as the oxygenated lipid species formed by COXs or lipoxygenases (Balvers et al., 2012a).
Formation and turnover of n–3 LC-PUFA-derived fatty amides
Given the high proportion of its precursor DHA in phospholipids of brain synapses and retina, it is not surprising that DHEA was first discovered in brain tissue and retina (Sugiura et al., 1996; Sheskin et al., 1997; Bisogno et al., 1999).
In 2001, Berger et al. demonstrated that brain levels of the ethanolamine conjugates of DHA and EPA, DHEA and EPEA (N-eicosapentaenoyl ethanolamine) in piglets were modulated by the amount of n–3 LC-PUFAs in the feed (Berger et al., 2001). Since then several other studies in different species have confirmed an increased formation of DHEA and EPEA in various tissues after administering fish oil or individual n–3 LC-PUFAs (see Maccarrone et al., 2010b). A more recent example is the study of Artmann et al. (2008) who showed that a fish-oil rich diet given to rats increased jenunal levels of DHEA and EPEA, while at the same time decreasing levels of AEA, OEA and PEA. Specifically for brain, Wood et al. (2010) showed that 2 weeks of fish oil supplementation in mice caused a shift in NAE (and also glycerol-ester) patterns in favour of DHEA and EPEA at the expense of their arachidonoyl and oleoyl homologues. Remarkably, these studies also showed that even with control diets not enriched with n–3 LC-PUFAs, brain concentrations of DHEA were higher than those of AEA and of the same order of magnitude as AEA in other tissues like ileum and liver. Tissue levels of EPEA appear to be low compared with those of DHEA but increase with fish oil diets and after LPS treatment, in particular in the gut (Artmann et al., 2008; Balvers et al., 2012a). Using deuterated (d5) DHA and EPA, we showed that differentiated 3T3-L1 adipocytes are able to synthesize DHEA and EPEA from their precursors (Balvers et al., 2010). Recently, human breast and prostate cancer cell lines as well as hippocampal neuron cultures were also shown to perform these conversions (Brown et al., 2011; Kim et al., 2011a,b).
Pilot studies with human volunteers in our own lab showed that daily intake of fish oil food supplements (480 mg EPA plus 360 mg DHA per day) doubled plasma DHEA levels in 3 weeks. All these findings are consistent with the concept that the local relative availability of fatty acid precursors, which in turn is modulated by dietary intake of lipids, determines the pattern of amide conjugates formed. The same holds true for the local availability of amines. For example, we showed that serotonin conjugates with fatty acids, including those of DHA and EPA, are formed by gut tissue, where most of the body's serotonin resides (Verhoeckx et al., 2011). As expected, intestinal levels of DHA-serotonin and EPA-serotonin were higher in mice fed a fish oil rich diet. In addition to the ethanolamines EPEA and DHEA, several n–3 LC-PUFA-derived fatty amides have so far been identified with different amines, in organisms ranging from Homo sapiens to Hydra. Examples taken from different studies are given in Table 1.
Table 1.
General literature overview of n–3 LC-PUFA-derived fatty amides identified so far in different organisms and tissues and brief indication of their bioactivity and receptor affinity (if known)
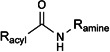 | |||
|---|---|---|---|
| Racyl | Ramine | Selected references | Presence shown in species (P) Receptor affinity studies (R) Bioactivity data available (B) |
| DHA (22:6n-3) | Ethanolamine | (Sheskin et al., 1997; Berger et al., 2001; Artmann et al., 2008; Balvers et al., 2010; Wood et al., 2010; Brown et al., 2011; Meijerink et al., 2011; Rapoport et al., 2011; Tsuboi et al., 2011) | P, R, B (see text) |
| Dopamine | (Shashoua and Hesse, 1996; Bisogno et al., 2000; Bezuglov et al., 2001; Bobrov et al., 2006; Ostroumova et al., 2010; Dang et al., 2011; Sakharova et al., 2012) | P: rodent brain Fresh water hydra; see further text | |
| B: Hydra tissue development, mouse embryo development; uptake in mouse brain; antipyretic, analgesic, cataleptic in rats; FAAH inhibition; AEA uptake; inhibition of NO and cytokines; antioxidant and neuroprotective in rats, anti-Parkinson (see also text) | |||
| Serotonin | (Verhoeckx et al., 2011) | P: pig, mouse | |
| B: FAAH inhibition; GLP-1 release | |||
| Glutamic acid and glutamine | (Tan et al., 2010) | P: bovine brain | |
| GABA | (Tan et al., 2010) | P: bovine brain | |
| Phenylalanine | (Tan et al., 2010) | P: bovine brain | |
| Histidine | (Tan et al., 2010) | P: bovine brain | |
| EPA (20:5n-3) | Ethanolamine | (Berger et al., 2001; Artmann et al., 2008; Balvers et al., 2010; 2012a, b; Wood et al., 2010) | P,R,B, (see text) |
| Dopamine | (Bisogno et al., 2000; Bezuglov et al., 2001) | R: CB1 receptors | |
| B: antipyretic, analgesic, cataleptic in rats; FAAH inhibition; AEA uptake (see also text) | |||
| Serotonin | (Verhoeckx et al., 2011) | P: pig, mouse | |
| B: FAAH inhibition; GLP-1 release (see also text) | |||
| DPA (22:5n-3) | Ethanolamine | (Berger et al., 2001) | P: pig brain |
| Dopamine | (Bisogno et al., 2000; Bezuglov et al., 2001) | R: CB1 receptors B: antipyretic, analgesic, cataleptic in rats | |
| ALA (18:3n-3) | Ethanolamine | (Sheskin et al., 1997; Movahed et al., 2005; Meijerink et al., 2011) | R: CB1 receptors; no affinity; activates TRVP1 receptors |
| P: rat mesenteric arteries B: No effect on NO and CCL2 release in RAW264.7 cells | |||
| Serotonin | (Ortar et al., 2007) | R: TRVP1 receptors | |
| B: FAAH inhibition | |||
| Dopamine | (Bisogno et al., 2000) | R: CB1, CB2 receptors | |
| B: FAAH inhibition; AEA uptake (see text) | |||
It is conceivable that n–3 LC-PUFA derived fatty amides will be formed and broken down via pathways similar to those described for other amides. Depending on the structure, synthesis can take place via different routes. An extensive review of these falls outside the scope of this paper, and readers are referred to several excellent recent reviews on this topic (Di Marzo et al., 2007; Bisogno, 2008; Muccioli, 2010; Ueda et al., 2010a). Briefly, according to the most studied transacylation–PDE pathway, NAEs are formed from glycerophospholipids via N-acylphosphatidyl ethanolamine (NAPE), by sequential catalysis involving Ca2+-dependent N-acyltransferase and NAPE-hydrolyzing PLD. The biosynthetic NAPE precursor of DHEA has indeed been found in bovine retina (Bisogno et al., 1999) and in rat brain (Sugiura et al., 1996). Other pathways involve different enzymes including PLA2. The biosynthesis of conjugates with simple amino acids does not follow the phospholipid pathways (Bradshaw et al., 2009). The breakdown of n–3 derived fatty acid conjugates is likely to follow routes similar to those described for other fatty acid amides. The primary NAE-degrading enzyme is fatty acid amide hydrolase (FAAH, now also known as FAAH-1), localized on the endoplasmatic reticulum (Bisogno, 2008). A second FAAH enzyme, now called FAAH-2, was found in humans, located on cytoplasmic lipid droplets (Wei et al., 2006; Bisogno, 2008). Apparently, rodents do not possess FAAH-2. Both enzymes show distinct but overlapping substrate specificity and tissue distribution. To reach their sites of catabolism within the cell, NEAs are bound to different proteins including fatty acid binding proteins 5 and 7, heat shock protein 70, albumin and the FAAH-like AEA transporter protein (Kaczocha et al., 2009; Fowler, 2012). Intracellular trafficking of NAEs is also important to reach the intracellular receptors (Maccarrone et al., 2010a; Kaczocha et al., 2012). DHEA inhibited the hydrolysis of [14C]AEA although to a lesser extent than AEA itself (Bisogno et al., 1999). This suggests that FAAH recognizes DHEA, but that it is a worse substrate than AEA. From their studies in the (human) LNCaP prostate cancer cell line, Brown et al. (2010) also obtained further evidence that FAAH metabolizes both EPEA and DHEA. Recently, a third NAE hydrolyzing enzyme, NAE-hydrolyzing acid amidase (NAAA), has been identified (Ueda et al., 2010b). Next to hydrolysis, NAEs are substrates for oxidative enzymes including COXs, lipoxygenases (LOXs) and cytochrome P450 enzymes, yielding a range of prostamides (prostaglandin-amides) and hydroperoxy derivatives (Vandevoorde and Lambert, 2007; Woodward et al., 2008: Dainese et al., 2012 #3478; Rouzer and Marnett, 2011). A number of COX metabolites and LOX metabolites of NAEs have shown to possess biological activities (Vandevoorde and Lambert, 2007; Rouzer and Marnett, 2011). DHEA was oxidised by LOX in human PBMCs and mouse brain homogenates leading to the formation of several oxygenated molecules including, including 17-hydroxy-DHEA, 10,17-dihydroxy-DHEA and 15-hydroxy-16(17)-epoxy-DHEA (15-HEDPEA) (Yang et al. (2011). These authors also showed that some of these oxygenated metabolites possess biological activity, including effects on inflammatory processes, and may play important organ protecting roles.
Activities of DHEA and EPEA
Anti-inflammatory properties
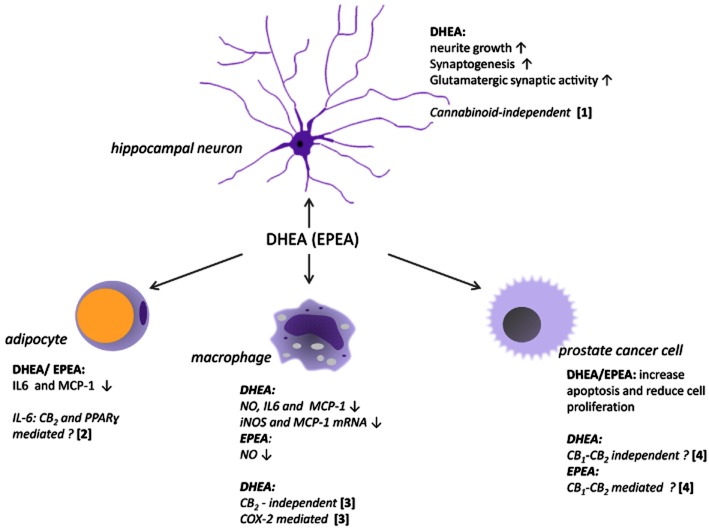
Several fatty amides, including (but not limited to) those binding to cannabinoid receptors, show anti-inflammatory or general immune modulating properties (Burstein and Zurier, 2009; Pandey et al., 2009). Comparing a number of NAEs for their ability to inhibit NO release from stimulated RAW264.7 macrophages, we showed that DHEA was the most potent of the compound series tested, inducing a dose-dependent inhibition of NO release (Meijerink et al., 2011). EPEA and DEA were also able to inhibit NO release, whereas anandamide and LNEA were inactive in this assay. Interestingly, the precursor of DHEA, DHA showed a much smaller effect. In the same cell line, DHEA significantly suppressed the production of the chemokine CCL2 (MCP-1) and in LPS-stimulated mouse peritoneal macrophages it reduced CCL2, IL-6 and NO production. The inhibition took place at a transcriptional level, as gene expression of CCL2 and inducible NOS was inhibited by DHEA. In differentiated 3T3-L1 adipocytes DHEA and EPEA reduced LPS-induced production of CCL2 and IL-6 (Balvers et al., 2010). Both compounds were already effective at concentrations as low as 1 nM. Interestingly and similar to what has been shown for anandamide during inflammation, DHEA and EPEA tissue levels were found to increase after an inflammatory stimulus in mice fed fish oil (Balvers et al., 2012b). This could indicate that these compounds have a role as endogenous anti-inflammatory mediators.
Other biological effects of DHEA and EPEA
Brown et al. (2010) suggested that DHEA and EPEA may possess potential anti-carcinogenic properties as the compounds displayed anti-proliferative and cell growth inhibitory effects in LNCaP and PC3 prostate cancer cells. NAEs had greater anti-proliferative potency than their parent compounds DHA and EPA. The inhibition resulted from an increased apoptosis and changes in cell cycle arrest. However, no consistent pattern was observed as specific effects exerted by the compounds differed between both n–3 NAEs and both cell lines. Hence, the mechanisms behind the anti-carcinogenic effects are still unclear. Increasing evidence suggests that DHEA and other DHA conjugates are important for brain development and the maintenance of brain functioning, and that they play roles in neuroprotection and the control of inflammation during disease or resulting from tissue damage. Like its parent compound DHA, DHEA and other conjugates, including that of dopamine are found in relatively high concentrations in brain (Sheskin et al., 1997; Berger et al., 2001; Tan et al., 2010; Wood et al., 2010). Although other several pathways are being proposed to explain the effects of DHA on brain (Bazan et al., 2011), it is conceivable that mechanisms taking place via their amine conjugates will be involved here as well. For example, the presence of DHEA (called by the authors ‘synaptamide’) was demonstrated in mouse hippocampus and shown to be a potent stimulator of neurite growth and synaptogenesis in hippocampal neurons (Kim et al., 2011a,b). Furthermore, it enhanced glutamatergic synaptic activity. Again, the bio-activities of DHEA were higher than those of the parent compound DHA. Yang et al. (2011) recently identified a series of oxygenated metabolites from DHEA in mice brain that regulated leukocyte motility. The authors conclude that these metabolites might serve as anti-inflammatory and organ-protective mediators in brain.
A remarkable activity of EPEA with possible links to endocannabinoid function was reported for Caenorhabditis elegans (Lucanic et al., 2011). This conjugate was not only present in the nematode but was also found to inhibit the typical dietary restriction induced lifespan extension (Lucanic et al., 2011). The authors concluded that EPEA might have a role in ageing and represents a signal that coordinates nutrient status.
Are DHEA and EPEA members of the endocannabinoid family?
From their structural analogy to corresponding arachidonic acid N-conjugates, it is likely that there will be a number of candidate receptors to which different n–3 derived fatty amides may show affinity, including CB1 and CB2, GPR18, 55, 92, 119, TRVP1 and PPARs (Alexander and Kendall, 2007; Di Marzo et al., 2007; de Novellis et al., 2008). However, data on this are scattered, and the overall picture is not complete. Published data consistently suggest that DHEA and EPEA are relatively weak ligands for cannabinoid receptors. Binding affinity of DHEA to CB1 receptors has been compared to anandamide in a number of studies (see Felder et al., 1993; Sheskin et al., 1997 for binding data). Low-affinity (compared with anandamide) binding of EPEA to CB1 receptors has been shown (Adams et al., 1995). More recently, Brown et al. (2010) reported values of 633 nM and 124 nM for binding of DHEA to mouse brain CB1 receptors in the absence and presence of the FAAH inhibitor PMSF, respectively. For binding of EPEA to CB1 receptors (in the presence of PMSF), slightly lower Ki values were found. The same authors also showed that DHEA and EPEA can bind to CB2 receptors, although with a slightly lower affinities compared with those for CB1 receptors. DHEA and EPEA behaved as CB1 and CB2 receptor agonists as indicated by their ability to produce a concentration related stimulation of [35S]GTPγS binding to mouse brain and CHO-hCB2 cell membranes. In both membrane preparations, DHEA displayed higher potency than EPEA. Using a commercially available human CB2 receptor preparation (membranes from Sf9 cells; PerkinElmer, the Netherlands), we also found that DHEA binds to these receptors in the nanomolar range (Ki estimated 5.7 nM), with an approximately eight-fold lower affinity compared to Win55,212-2 (unpublished data).
Role of cannabinoid and PPAR receptors in biological effects of DHEA and EPEA
Although DHEA and EPEA have been shown to bind and activate CB1 and CB2 receptors, this has not been, so far, linked to their immune-modulating activities. In LPS-stimulated peritoneal macrophages collected from CB2–/– mice, DHEA still produced a reduction of NO release, which would conflict with any involvement of the CB2 receptor (Meijerink et al., unpublished data). Similar studies in our lab with CB1 receptor antagonists indicated that these receptors did not play a role either in this effect (unpublished data). In LPS-stimulated 3T3-L1 adipocytes, inhibition of IL-6 release by DHEA or EPEA could be blocked by a combination of a PPAR-γ (GW9662) and a CB2 receptor antagonist (SR144528), while the individual antagonists showed much smaller effects (Balvers et al., 2010). However, neither the combination nor the individual antagonists reversed the inhibitory effects of DHEA or EPEA on CCL2 release. These observations are in line with the studies of Brown et al. (2010), showing that the DHEA-mediated decrease in proliferation of their prostate cancer cell lines could not be blocked by CB1 or CB2 receptor antagonists. By contrast, the anti-proliferative potency of EPEA was reduced by AM281 and AM630, selective antagonists for CB1 and CB2 receptors respectively (Brown et al., 2010). Finally, recent data on the effects of DHEA on neurite outgrowth and synaptogenesis in mice also show that these effects are apparently independent of interaction with CB receptors (Kim and Spector, 2012). Taken together, connections between biological effects of DHEA and EPEA found so far and receptor-specific interactions need to be analysed further.
Effects on COX-2
To further elucidate the underlying mechanism(s) of DHEA-exerted immune-modulatory activity, we studied its effects on different key inflammatory mediators. DHEA dose-dependently reduced levels of prostaglandins and thromboxane B2 generated by COX-2 in LPS stimulated RAW264.7 macrophages. At low concentrations, DHEA caused a less pro-inflammatory secretory oxylipin profile in the activated macrophages, whereas its parent compound DHA did not change levels of metabolites formed by COX-2 in that concentration range. The activity of NF-κB and IFN-β, both important players of the MyD88-dependent and the MyD88-independent pathway, respectively, were not affected by DHEA (Meijerink et al., unpublished data). As COX-2 protein expression was not altered these effects could be due to competition of DHEA or its oxygenated metabolites with arachidonic acid. Whether DHEA indeed acts as a substrate for COX-2, thereby generating active or non-active metabolites or whether it mainly exerts its effects by inducing a shift in pro-inflammatory mediators, remains to be investigated. Figure 4 shows a summarizing overview of the activities and putative mechanisms of DHEA and EPEA found so far.
Figure 4.
Summary of DHEA and EPEA effects on different cell types. (1): Kim and Spector (2012); (2): Balvers et al. (2010); (3): Meijerink et al. unpublished data; (4): Brown et al. (2010).
Activities and possible molecular targets of n–3 LC-PUFA-derived fatty amides other than DHEA and EPEA
Dopamine- and other N-acyl-conjugates of DHA
Apart from DHEA, conjugates of DHA have been found with serotonin, dopamine, glycine, alanine, glutamine and glutamic acid, GABA, histidine and phenylalanine (Table 1). However, in most cases, information is limited to a demonstration of their existence, and even basic molecular properties have often not yet been established. As mentioned above, we found DHA-serotonin in the gut of mice fed a fish oil-rich diet. Studies on its biological effects and role are ongoing. Unlike its EPA analogue, DHA-serotonin did not inhibit FAAH, and although it stimulated GLP-1 release in vitro, its potency was similar to that of its parent compound DHA (Verhoeckx et al., 2011). More is known about the dopamine-conjugate of DHA. Like its analogues N-oleoyl dopamine (OLDA) and N-arachidonoyl dopamine (NADA) and also the ethanolamine conjugate DHEA (described above), this compound may be of interest because of its potential properties in relation to brain function and neuroprotection, including positive effects on hypoxic–ischaemic injury or brain inflammatory processes. This is in line with the observation that the parent DHA shows marked accumulation in the CNS, where it is a major component of brain synapses and retina and known to play important developmental roles (Rapoport et al., 2011). Furthermore, DHA by itself is of great interest for its role in neuroprotection after brain hypoxia and ischaemia (Mayurasakorn et al., 2011). Vice versa, high levels of dietary n–6 fatty acids contribute to reduced levels of DHA in the developing brain and inhibit secondary neurite growth (Novak et al., 2008).
Nutritional n-3 (omega-3) deficiency also abolishes endocannabinoid-mediated neuronal functions (Lafourcade et al., 2011). Testing a series of dopamine fatty acid conjugates, Bisogno et al. (2000) reported that DHA-dopamine is a better CB1 receptor ligand than AEA tested under the same conditions. Synthesis of DHA-dopamine (and EPA-dopamine) and its further testing have also been reported by Bezuglov et al. (2001). The compound produced hypothermic, cataleptic and (some) analgesic effects as well as hypo-activity. An interesting application of DHA-dopamine was described by Shashoua and Hesse (1996) who studied the ability of different dopamine conjugates to act as carrier to increase brain dopamine content. Remarkably, and apparently in line with the tendency of DHA to accumulate in brain tissue, the DHA conjugate was the most active in increasing dopamine uptake by the brain. In addition, the conjugate depressed general locomotor activity of mice in a dose-dependent manner and suppressed the appetite of mice and rats. A similar concept was described by Yehuda (2002) who suggested that N-(α-linolenoyl) tyrosine could potentially be used as an anti-Parkinson agent. Finally, Bobrov et al. (2006) showed that DHA-dopamine exhibited antioxidant activity and produced a dose-dependent protective effect on cultured granular cells from rat cerebellum under conditions of oxidative stress. It also decelerated the development of Parkinson's disease-like symptoms in a MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model. At present, it is unclear whether this interesting application has been taken further since there appear to be no reports published since 2006.
Dopamine- and serotonin conjugates of EPA
Synthesis of EPA-dopamine has been reported by Bezuglov et al. (2001). The compound produced displacement of radioactive ligand from a CB1 receptor preparation with a Ki of the same order of magnitude as that of NADA. As observed with DHA-dopamine (described above), the EPA analogue showed hypothermic, cataleptic and analgesic effects and also produced hypoactivity in rats. The analgesic effect, as measured with the hot plate test, was greater than that of DHA-dopamine and comparable with NADA. As N-arachidonoyl serotonin is a FAAH inhibitor (Maione et al., 2007; de Novellis et al., 2008), we investigated a series of other amides, including DHA-serotonin and EPA-serotonin for this effect (Verhoeckx et al., 2011)and found that EPA-serotonin, but not DHA-serotonin, was able to inhibit FAAH. However, we also found that some of the parent fatty acids, including AA and EPA had the same effect.
N-acyl conjugates of ALA
Data on the activity of amine conjugates from α-linolenic acid (18:3n-3) in animals appear to be scarce, and in some reports, the structure seems to be confused with that of its γ-isomer, which is an n–6 fatty acid. The ethanolamine conjugate (αLNEA) was found in rat mesenteric arteries and dorsal root ganglia, and shown to activate TRVP1 receptors (Movahed et al., 2005). In our laboratory, the compound was not active in inhibiting NO and CCL2 release from LPS-activated RAW264.7 macrophages (Meijerink et al., 2011). The serotonin conjugate of ALA was synthesized and further tested by Ortar et al. (2007). This compound also showed TRVP1 activity and furthermore inhibition of FAAH. Finally, and as described in a previous section, N-(α-linolenoyl) tyrosine may have potential in Parkinson's disease (Yehuda, 2002). Although it is possible that this molecule can be formed endogenously, to our knowledge this has not been demonstrated so far.
Conclusions and future perspectives
N-acylamines of DHA and other n–3 polyunsatured fatty acids are members of a large group of endogenous mediators of which the full biological significance remains to be established. Their formation is time- and tissue-specific and modulated by various endogenous (such as energy status or inflammation) and environmental factors, including diet. From a physiological perspective, it is important to realise that these molecules occur in fluctuating mixtures of structurally related molecules with pleiotropic and tissue-specific activities. To make it even more complicated, there is a constant interplay with other biochemical routes, including the formation of eicosanoids and different intermediates (LOX, COX, CYP450 products) (Balvers et al., 2012a; 2012b). Therefore, especially when studying their physiological roles, lipidomic and multi-target approaches are needed to fully comprehend their pathways and effects.
Given these complicating factors, evidence is accumulating that DHEA, DHA-dopamine and other n–3 LC-PUFA-derived fatty amides possess several interesting properties that merit further studies in relation to for example inflammatory and neural disorders. In the brain, DHEA is present at levels comparable with those of AEA. Although its affinity for CB1 receptors is lower than that of AEA, recent studies suggest that the compound or its metabolites do play important roles in normal brain functioning and modulation of inflammatory processes. Notwithstanding the association between dietary intake of n–3 LC-PUFAs and the formation of their respective fatty acid amides, including DHEA, their role and significance in mediating the alleged health effects of fish oil remains speculative. In this respect, fatty acid amides represent a group of molecules of interest to both the pharmacological and nutritional research fields. In addition to directly administering the compounds, the ability to use DHA conjugates as carriers of neurotransmitters through the blood–brain barrier also merits further investigation. Not only for dopamine as suggested before (Shashoua and Hesse, 1996) but perhaps also for serotonin. The fact that fatty acid amides are a part of endogenous pathways could be advantageous for pharmacological applications. At the same time, this may have consequences for their metabolic stability. Therefore, in future pharmacological studies, it is recommended to pay particular attention to their pharmacokinetic properties. In addition to their administration as single compounds, combinations with FAAH inhibitors (Pillarisetti et al., 2009) and (or) their fatty acid precursors could be of interest to further increase (local) concentrations.
Glossary
- 2-AG
2-arachidonoylglycerol
- AEA
N-arachidonoyl ethanolamine (anandamide)
- ALA
α-linolenic acid (18:3n-3)
- αLNEA
α-N-linolenoyl ethanolamine (conjugate of ALA)
- CHD
coronary heart disease
- DEA
N-docosatetraenoyl ethanolamine
- DHA
docosahexaenoic acid (22:6n-3)
- DHA-5HT
N-docosahexaenoyl serotonin
- DHA-DA
N-docosahexaenoyl dopamine
- DHEA
N-docosahexaenoyl ethanolamine
- DPA
docosapentaenoic acid (22:5n-3)
- EPA
eicosapentaenoic acid (20:5n-3)
- n–3 LC-PUFA
(n–3) long-chain polyunsaturated fatty acid
- NAEs
N-acyl ethanolamines
- OEA
N-oleoyl ethanolamine
- PEA
N-palmitoyl ethanolamine
- SEA
N-stearoyl ethanolamine
- TRVP1
transient receptor potential channel type V1
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Adams IB, Ryan W, Singer M, Thomas BF, Compton DR, Razdan RK, et al. Evaluation of cannabinoid receptor binding and in vivo activities for anandamide analogs. J Pharmacol Exp Ther. 1995;273:1172–1181. [PubMed] [Google Scholar]
- Alexander SPH, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol. 2007;152:602–623. doi: 10.1038/sj.bjp.0707456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br. J. Pharmacol. (5th edn.) 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Balvers MGJ, Verhoeckx KCM, Plastina P, Wortelboer HM, Meijerink J, Witkamp RF. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochim Biophys Acta. 2010;1801:1107–1114. doi: 10.1016/j.bbalip.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Balvers MGJ, Verhoeckx KCM, Bijlsma S, Rubingh CM, Meijerink J, Wortelboer HM, et al. Fish oil and inflammatory status alter the n-3 to n-6 balance of the endocannabinoid and oxylipin metabolomes in mouse plasma and tissues. Metabolomics. 2012a;8:1130–1147. doi: 10.1007/s11306-012-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balvers MGJ, Verhoeckx KCM, Meijerink J, Bijlsma S, Rubingh CM, Wortelboer HM, et al. Time-dependent effect of in vivo inflammation on eicosanoid and endocannabinoid levels in plasma, liver, ileum and adipose tissue in C57BL/6 mice fed a fish-oil diet. Int Immunopharmacol. 2012b;13:204–214. doi: 10.1016/j.intimp.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Banni S, Di Marzo V. Effect of dietary fat on endocannabinoids and related mediators: consequences on energy homeostasis, inflammation and mood. Mol Nutr Food Res. 2010;54:82–92. doi: 10.1002/mnfr.200900516. [DOI] [PubMed] [Google Scholar]
- Batetta B, Griinari M, Carta G, Murru E, Ligresti A, Cordeddu L, et al. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J Nutr. 2009;139:1495–1501. doi: 10.3945/jn.109.104844. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci U S A. 2001;98:6402–6406. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezuglov V, Bobrov M, Gretskaya N, Gonchar A, Zinchenko G, Melck D, et al. Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg Med Chem Lett. 2001;11:447–449. doi: 10.1016/s0960-894x(00)00689-2. [DOI] [PubMed] [Google Scholar]
- Bisogno T. Endogenous cannabinoids: structure and metabolism. J Neuroendocrinol. 2008;20(Suppl. 1):1–9. doi: 10.1111/j.1365-2826.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Delton-Vandenbroucke I, Milone A, Lagarde M, Di Marzo V. Biosynthesis and inactivation of N-arachidonoylethanolamine (anandamide) and N-docosahexaenoylethanolamine in bovine retina. Arch Biochem Biophys. 1999;370:300–307. doi: 10.1006/abbi.1999.1410. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, et al. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351:817–824. [PMC free article] [PubMed] [Google Scholar]
- Bobrov MY, Lyzhin AA, Andrianova EL, Gretskaya NM, Zinchenko GN, Frumkina LE, et al. Antioxidant and neuroprotective properties of N-docosahexaenoyl dopamine. Bull Exp Biol Med. 2006;142:425–427. doi: 10.1007/s10517-006-0383-x. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Hu SSJ, Burstein S, Walker JM. Chapter 8. Novel endogenous N-acyl glycines: identification and characterization. In: Gerald L, editor. Vitamins & Hormones. Vol. 81. San Diego, CA: Elsevier-Academic Press; 2009. pp. 191–205. [DOI] [PubMed] [Google Scholar]
- Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA, et al. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis. 2010;31:1584–1591. doi: 10.1093/carcin/bgq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I, Wahle KWJ, Cascio MG, Smoum-Jaouni R, Mechoulam R, Pertwee RG, et al. Omega-3 N-acylethanolamines are endogenously synthesised from omega-3 fatty acids in different human prostate and breast cancer cell lines. Prostaglandins Leukot Essent Fatty Acids. 2011;85:305–310. doi: 10.1016/j.plefa.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Burstein S, Zurier R. Cannabinoids, endocannabinoids, and related analogs in inflammation. AAPS J. 2009;11:109–119. doi: 10.1208/s12248-009-9084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009;91:791–795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Calder PC. Fatty acids and inflammation: the cutting edge between food and pharma. Eur J Pharmacol. 2011;668(Suppl. 1):S50–S58. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- Carlson SE. Docosahexaenoic acid supplementation in pregnancy and lactation. Am J Clin Nutr. 2009;89:678S–6684. doi: 10.3945/ajcn.2008.26811E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Vandenberg RJ. N-Acyl amino acids and N-acyl neurotransmitter conjugates: neuromodulators and probes for new drug targets. Br J Pharmacol. 2010;160:1857–1871. doi: 10.1111/j.1476-5381.2010.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainese E, Sabatucci A, Angelucci CB, Barsacchi D, Chiarini M, MacCarrone M. Impact of embedded endocannabinoids and their oxygenation by lipoxygenase on membrane properties. ACS Chem Neurosci. 2012;3:386–392. doi: 10.1021/cn300016c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang HT, Kang GJ, Yoo ES, Hong J, Choi JS, Kim HS, et al. Evaluation of endogenous fatty acid amides and their synthetic analogues as potential anti-inflammatory leads. Bioorg Med Chem. 2011;19:1520–1527. doi: 10.1016/j.bmc.2010.12.046. [DOI] [PubMed] [Google Scholar]
- De Caterina R. n–3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chem Biol. 2007;14:741–756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Ezzili C, Otrubova K, Boger DL. Fatty acid amide signaling molecules. Bioorg Med Chem Lett. 2010;20:5959–5968. doi: 10.1016/j.bmcl.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell EK, Merkler DJ. Biosynthesis, degradation and pharmacological importance of the fatty acid amides. Drug Discov Today. 2008;13:558–568. doi: 10.1016/j.drudis.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. Anandamide uptake explained? Trends Pharmacol Sci. 2012;33:181–185. doi: 10.1016/j.tips.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Artmann A. Endocannabinoids and nutrition. J Neuroendocrinol. 2008;20(Suppl. 1):94–99. doi: 10.1111/j.1365-2826.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Diep TA. N-acylethanolamines, anandamide and food intake. Biochem Pharmacol. 2009;78:553–560. doi: 10.1016/j.bcp.2009.04.024. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG. Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem. 2012;287:3415–3424. doi: 10.1074/jbc.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, et al. N-docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem J. 2011b;435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-Y, Spector AA. Synaptamide, endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukot Essent Fatty Acids. 2012 doi: 10.1016/j.plefa.2012.08.002. http://dx.doi.org/10.1016/j.plefa.2012.08.002 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-Y, Spector AA, Xiong Z-M. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat. 2011a;96:114–120. doi: 10.1016/j.prostaglandins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, Matias I, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- Lucanic M, Held JM, Vantipalli MC, Klang IM, Graham JB, Gibson BW, et al. N-acylethanolamine signalling mediates the effect of diet on lifespan in Caenorhabditis elegans. Nature. 2011;473:226–229. doi: 10.1038/nature10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Dainese E, Oddi S. Intracellular trafficking of anandamide: new concepts for signaling. Trends Biochem Sci. 2010a;35:601–608. doi: 10.1016/j.tibs.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Gasperi V, Catani MV, Diep TA, Dainese E, Hansen HS, et al. The endocannabinoid system and its relevance for nutrition. Annu Rev Nutr. 2010b;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- Maione S, De Petrocellis L, De Novellis V, Moriello AS, Petrosino S, Palazzo E, et al. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br J Pharmacol. 2007;150:766–781. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayurasakorn KA, Williams JJA, Ten VSB, Deckelbaum RJAB. Docosahexaenoic acid: brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr Opin Clin Nutr Metab Care. 2011;14:158–167. doi: 10.1097/MCO.0b013e328342cba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijerink J, Plastina P, Vincken J-P, Poland M, Attya M, Balvers M, et al. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: evidence for a new link between fish oil and inflammation. Br J Nutr. 2011;105:1798–1807. doi: 10.1017/S0007114510005635. [DOI] [PubMed] [Google Scholar]
- Movahed P, Jonsson BA, Birnir B, Wingstrand JA, Jorgensen TD, Ermund A. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280:38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- Muccioli GG. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov Today. 2010;15:474–483. doi: 10.1016/j.drudis.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Novak EM, Dyer RA, Innis SM. High dietary ω-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 2008;1237:136–145. doi: 10.1016/j.brainres.2008.07.107. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Palazzo E, Rossi F, De Petrocellis L, Petrosino S, Guida F, et al. The analgesic effect of N-arachidonoyl-serotonin, a FAAH inhibitor and TRPV1 receptor antagonist, associated with changes in rostral ventromedial medulla and locus coeruleus cell activity in rats. Neuropharmacology. 2008;55:1105–1113. doi: 10.1016/j.neuropharm.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Ortar G, Cascio MG, De Petrocellis L, Morera E, Rossi F, Schiano-Moriello A, et al. New N-arachidonoylserotonin analogues with potential ‘dual’ mechanism of action against pain. J Med Chem. 2007;50:6554–6569. doi: 10.1021/jm070678q. [DOI] [PubMed] [Google Scholar]
- Ostroumova TV, Markova LN, Akimov MG, Gretskaya NM, Bezuglov VV. Docosahexaenoyl dopamine in freshwater hydra: effects on regeneration and metabolic changes. Russ J Dev Biol. 2010;41:164–167. [PubMed] [Google Scholar]
- Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. Endocannabinoids and immune regulation. Pharmacol Res. 2009;60:85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Gibson NA, Brotchie H, Heruc G, Rees A-M, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- Pillarisetti S, Alexander CW, Khanna I. Pain and beyond: fatty acid amides and fatty acid amide hydrolase inhibitors in cardiovascular and metabolic diseases. Drug Discov Today. 2009;14:1098–1111. doi: 10.1016/j.drudis.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Rapoport SI, Ramadan E, Basselin M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins Other Lipid Mediat. 2011;96:109–113. doi: 10.1016/j.prostaglandins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109:668–679. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- de Roos B, Yiannis M, Ingeborg AB. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. Br J Pharmacol. 2009;158:413–428. doi: 10.1111/j.1476-5381.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev. 2011;111:5899–5921. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol. 2009;77:937–946. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Sakharova NY, Markova LN, Smirnov AA, Vikhlyantseva EF, Fialkovskaya LA, Bezuglov VV. Effect of docosahexaenoyl dopamine on the in vitro development of early mouse embryos. Dokl Biol Sci. 2012;442:38–41. doi: 10.1134/S0012496612010115. [DOI] [PubMed] [Google Scholar]
- Shashoua VE, Hesse GW. N-docosahexaenoyl, 3 hydroxytyramine: a dopaminergic compound that penetrates the blood-brain barrier and suppresses appetite. Life Sci. 1996;58:1347–1357. doi: 10.1016/0024-3205(96)00101-4. [DOI] [PubMed] [Google Scholar]
- Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Tonegawa T, Nakane S, Yamashita A, et al. Transacylase-mediated and phosphodiesterase-mediated synthesis of N-arachidonoylethanolamine, an endogenous cannabinoid-receptor ligand, in rat brain microsomes. Eur J Biochem. 1996;240:53–62. doi: 10.1111/j.1432-1033.1996.0053h.x. [DOI] [PubMed] [Google Scholar]
- Tan B, O'Dell DK, Yu YW, Monn MF, Hughes HV, Burstein S, et al. Identification of endogenous acyl amino acids based on a targeted lipidomics approach. J Lipid Res. 2010;51:112–119. doi: 10.1194/jlr.M900198-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K, Okamoto Y, Ikematsu N, Inoue M, Shimizu Y, Uyama T, et al. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase d-dependent and -independent pathways. Biochim Biophys Acta. 2011;1811:565–577. doi: 10.1016/j.bbalip.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Tur JA, Bibiloni MM, Sureda A, Pons A. Dietary sources of omega 3 fatty acids: public health risks and benefits. Br J Nutr. 2012;107(Suppl. 2):S23–S52. doi: 10.1017/S0007114512001456. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta. 2010a;1801:1274–1285. doi: 10.1016/j.bbalip.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA) Prog Lipid Res. 2010b;49:299–315. doi: 10.1016/j.plipres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Vandevoorde S, Lambert DM. The multiple pathways of endocannabinoid metabolism: a zoom out. Chem Biodivers. 2007;4:1858–1881. doi: 10.1002/cbdv.200790156. [DOI] [PubMed] [Google Scholar]
- Verhoeckx KCM, Voortman T, Balvers MGJ, Hendriks HFJ, Mwortelboer H, Witkamp RF. Presence, formation and putative biological activities of N-acyl serotonins, a novel class of fatty-acid derived mediators, in the intestinal tract. Biochim Biophys Acta. 2011;1811:578–586. doi: 10.1016/j.bbalip.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Wallin A, Di Giuseppe D, Orsini N, Patel PS, Forouhi NG, Wolk A. Fish consumption, dietary long-chain n-3 fatty acids, and risk of type 2 diabetes. Diabetes Care. 2012;35:918–929. doi: 10.2337/dc11-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Doshi M, Hamazaki T. n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot Essent Fatty Acids. 2003;69:51–59. doi: 10.1016/s0952-3278(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Carling RWC, Cornell CL, Fliri HG, Martos JL, Pettit SN, et al. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol Ther. 2008;120:71–80. doi: 10.1016/j.pharmthera.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Xun P, He K. Fish consumption and incidence of diabetes. Diabetes Care. 2012;35:930–938. doi: 10.2337/dc11-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Fredman G, Krishnamoorthy S, Agrawal N, Irimia D, Piomelli D, et al. Decoding functional metabolomics with docosahexaenoyl ethanolamide (DHEA) identifies novel bioactive signals. J Biol Chem. 2011;286:31532–31541. doi: 10.1074/jbc.M111.237990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda S. Possible anti-Parkinson properties of N-(α-linolenoyl) tyrosine: a new molecule. Pharmacol Biochem Behav. 2002;72:7–11. doi: 10.1016/s0091-3057(01)00646-3. [DOI] [PubMed] [Google Scholar]