Abstract
Background and Purpose
It is well recognized that vasopressin modulates the neurogenic control of the circulation. Here, we report the central mechanisms by which vasopressin modulates cardiovascular response to stress induced by immobilization.
Experimental Approach
Experiments were performed in conscious male Wistar rats equipped with radiotelemetric device for continuous measurement of haemodynamic parameters: systolic and diastolic BP and heart rate (HR). The functioning of the spontaneous baro-receptor reflex (BRR) was evaluated using the sequence method and the following parameters were evaluated: BRR sensitivity (BRS) and BRR effectiveness index (BEI).
Key Results
Under baseline physiological conditions intracerebroventricular injection of 100 and 500 ng of selective non-peptide V1a or V1b or V2 receptor antagonist did not modify BP, HR and BRR. Rats exposed to 15 min long stress by immobilization exhibited increase of BP, HR, reduction of BRS and no change in BEI. Pretreatment of rats with V1a receptor antagonist did not modulate the BP, HR, BRS and BEI response to stress. Pretreatment of rats with V1b receptor and V2 receptor antagonist, at both doses, prevented BRR desensitization and tachycardia, but failed to modulate stress-induced hypertension.
Conclusions and Implications
Vasopressin by the stimulation of central V1b- and V2-like receptors mediates stress-induced tachycardia and BRR desensitization. If these mechanisms are involved, BRR desensitization in heart failure and hypertension associated with poor outcome, they could be considered as novel targets for cardiovascular drug development.
Keywords: V1a receptor antagonist, V1b receptor antagonist, V2 receptor antagonist, stress, baro-receptor reflex
Introduction
The baro-receptor reflex (BRR) is the major corrector of arterial BP. Its malfunction has been associated with increased BP variability and the poor outcome of cardiovascular disease such as hypertension and congestive heart failure (Mortara et al., 1997; Narkiewicz and Grassi, 2008). Many neurohumoural factors have been found to regulate the function of the BRR, and vasopressin is among the key ones.
The major sources of vasopressin are the paraventricular nucleus (PVN) and the supraoptic nucleus of the hypothalamus. The magnocellular part of the PVN that projects to the neurohypophysis releases vasopressin in the circulation where it acts as an antidiuretic hormone and vasoconstrictor agent in most vascular beds (Burbach et al., 2001; Murphy et al., 2012). Peripherally released vasopressin also sensitizes the BBR via the area postrema, a circumventricular organ devoid of blood–brain barrier, to decrease cardiac output and limit the increase in BP associated with the augmentation of the peripheral resistance (Hasser and Bishop, 1990). Vasopressin also acts in the brain as neurotransmitter/modulator in cardiovascular control. Neurons from the parvocellular part of the PVN project to the nucleus of the solitary tract (NTS), where BRR afferents terminate, the rostral part of the ventrolateral medulla (RVLM) and the intermediolateral column of the spinal cord (IML) to modulate tonic sympathetic outflow to the heart, blood vessels and the kidneys (Pyner, 2009; Chen and Toney, 2010).
We hypothesized that centrally released vasopressin might be involved in the modulation of the BRR modulation under physiological conditions, baseline and/or stress. A number of studies indicate that vasopressin receptors are widely distributed in the brain neuronal network involved in cardiovascular control (Brinton et al., 1984; Ostrowski et al., 1994; Hernando et al., 2001; Vargas et al., 2009; Sato et al., 2011). Because vasopressin produces its effects by the stimulation of three distinct G–protein-linked membrane receptors, we injected selective and non-peptide V1a, V1b, V2 vasopressin antagonists in the lateral ventricle of freely moving adult male Wistar rats both under baseline conditions and during exposure to stress, when vasopressin release is enhanced (Carrasco and Van de Kar, 2003). The major strengths of this study are the use of highly selective non-peptide vasopressin V1a, V1b and V2 receptor antagonists, without agonist properties, as well as the sequence methodology for the evaluation of the spontaneous BRR that circumvents the use of vasoactive drugs, that by itself interfere with BRR functioning.
Methods
All experimental procedures in this study conformed to European Communities Council Directive of November 24, 1986 (86/609/EEC). The experimental protocol was approved by the School of Medicine, University of Belgrade, ethics review board (approval reference number 6R/2010).
Animals
Experiments were performed in male, 12-week-old Wistar rats bred at a local animal facility, weighing 310–360 g. Rats were housed individually in a controlled environment: 12 h/12 h light–dark cycle, temperature 21°C ± 2 and humidity 65% ± 9, with access to standard food pellets (0.2 % sodium content, Veterinarski zavod Subotica, Serbia) and tap water ad libitum. The number of rats in each protocol was calculated statistically taking into account intra-group variability, using software ‘Power Sample Size Calculation’ available at: http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/PowerSampleSize, for power of 90% and type I error probability of 0.05. At the end of the experiment, the rats were euthanized by an overdose of thiopentone sodium (150 mg, i.p.).
Surgery
Rats underwent two surgical procedures at 10 days intervals. Under combined ketamine (100 mg·kg−1, i.m.) and xylazine (10 mg·kg−1, i.m.) anaesthesia, a 3 cm long medial abdominal incision was made and the intestine retracted to expose the abdominal aorta. The tip of the catheter of the radio telemetric probe (TA11-PA C40, DSI, Transoma Medical, St. Paul, MN, USA) was inserted into the aorta using a 21 G needle. The inserted catheter was fixed with 3 M Vetbond® and tissue cellulose patch (DSI, Transoma Medical). The transmitter was attached to the anterior abdominal wall and the wound was closed by suture. To prevent bacterial infection, neomycin and bacitracin were sprayed topically, and the rats were treated with gentamicin (25 mg·kg−1 i.m.) 3 days previous, and on the day of surgery. To reduce pain, rats received metamizole (200 mg·kg−1·day−1, i.m.) on the day of surgery and for the next 2 days. Each rat was housed in a Plexiglas cage (30 × 30 × 30 cm) and left to recover fully for 10 days prior to the second surgery.
A second surgery was performed under the same anaesthesia. Each rat's head was mounted in the stereotaxic frame and the skin was incised 1 mm to expose the skull. The stereotaxic coordinates were derived from Paxinos and Watson (2005). The guide cannula (G22) protruded in the lateral ventricle 4 mm beneath the skull and was positioned anterior-posterior = 1.08 mm (from bregma) and lateral = 1.5 mm (from midline), and fixed with dental cement. The skin above was sutured and sprayed with antibiotics (neomycin and bacytracin), and the guide was plugged with a stainless-steel pin. In the post-operative period, rats were treated with gentamicin (25 mg·kg−1 i.m.) and metamizole (200 mg·kg−1·day−1, i.m.) 1 day before and 3 days after surgery. Five days elapsed before rats were exposed to experimentation.
Pilot experiments
To define the selective dose of vasopressin antagonists, we used previous experience (Milutinović et al., 2006). The cardiovascular effect of vasopressin administered in a dose of 50 ng·5 μL–1 (i.c.v.) to conscious rats was assessed without and with pretreatment of rats (n = 6) with 100 ng and 500 ng of V1a or V1b or V2 receptor antagonists at 2 days intervals (wash-out period).
Experimental design
All experiments started at 10:00 h in a quiet surrounding under controlled environmental conditions, following 60 min long baseline recordings. Cardiovascular parameters and arterial BRR were evaluated under baseline physiological conditions and during exposure to stress induced by 15 min long immobilization period under opaque Plexiglas restrainer (6 cm in width by 4 cm in height). A group of rats (n = 6) was treated with 5 μL of vehicle (i.c.v.), 100 ng/5 μL (i.c.v.) and 500 ng/5 μL (i.c.v.) of vasopressin antagonist (V1a or V1b or V2 receptor antagonist) at 2 h intervals. A period of 2 days elapsed between different drug administrations. Another group of six rats was injected with three consecutive injections of vehicle 5 μL (i.c.v.) of 0.9% NaCl or 5% dimethyl sulfoxide (DMSO) or 10% DMSO respecting the same timeline for drugs. This experimental group was introduced to eliminate effects of volume or vehicle on cardiovascular parameters. Each injection of drug or vehicle was followed by 30 min long recording periods of arterial BP.
In the stress protocol, 36 rats were randomized in six experimental groups: V1a-100, V1a-500, V1b-100, V1b-500, V2-100 and V2-500. Rats were first recorded 15 min for baseline BP, then a vehicle or drug was injected i.c.v. followed by 15 min long recordings of BP. This was followed by 15 min long immobilizations during which BP was also recorded.
Cardiovascular signal processing and analysis
Arterial BP was digitalized at 1000 Hz in Dataquest A.R.T. 4.0 software, (DSI, Transoma Medical). Systolic BP (SBP) and diastolic BP (DBP) and pulse interval (PI) or its inverse, heart rate (HR), were derived from the arterial pulse pressure as maximum, minimum and inter-beat interval of the pulse pressure wave, respectively. Mean BP (MBP) was calculated as the integral of the arterial pulse pressure wave. For each registration period mean value of SBP, MBP, DBP, HR and PI was calculated, and again averaged ± SEM for the whole experimental group (shown in tables and graphs).
Evaluation of the spontaneous BRR by the method of sequences
The method is explained in detail elsewhere (Bajić et al., 2010). Briefly, a spontaneous BRR sequence is a stream of consecutively increasing/decreasing SBP samples, followed by a stream of increasing/decreasing PI interval samples delayed by three, four or five beats in respect to SBP. A threshold for sequence length was set to four beats (Lončar-Turukalo et al., 2011). The following BRR features were evaluated
BRR sensitivity (BRS, ms·mmHg–1) assessed as a linear regression coefficient averaged over all identified sequences (pulse interval = BRS·SBP + const, where fitting of the curve is done in a least square sense);
BRR effectiveness index (BEI) calculated as the ratio of number of sequences versus number of SBP ramps.
Drugs
Nonpeptide and selective V1a (SR49059), V1b (SSR149415) and V2 (SR121463) antagonists were kindly donated by Dr. Claudine Serradeil-Le Gal from Exploratory Research Department of Sanofy-Synthélabo Recherche (Toulouse, France). [Arg8]-vasopressin acetate was purchased from Sigma-Aldrich (Munich, Germany) and neomycin bacitracin spray from Galenika (Belgrade, Serbia). Ketamine, xylazine and thiopental sodium injections were purchased from Marlo Farma (Belgrade, Serbia). Metamizol sodium and gentamicin injections were purchased from Hemofarm (Vrsac, Serbia).
Vasopressin [(Arg8)-vasopressin acetate] and the V2 antagonist (SR121463) were dissolved in pyrogen-free saline while the V1a antagonist (SR49059) and V1b antagonist (SSR149415) were dissolved in 10% (vol/vol) and 5% (vol/vol) DMSO, respectively.
Statistics
Cardiovascular parameters are presented as mean ± SEM. Differences between experimental protocols were analysed by two-way anova for repeated measures followed by post hoc Bonferroni test using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was considered at P < 0.05.
Results
Pilot studies
Vasopressin injected at a dose of 50 ng (i.c.v.) induced a consistent and significant increase in BP that lasted up to 20 min: SBP increased by 17.5 mmHg ± 4.3, P < 0.01, and DBP by 8 mmHg ± 2.2, P < 0.05. Vasopressin did not affect HR significantly. The hypertensive effect of vasopressin was inhibited by pretreatment of rats by100 ng (i.c.v.) of the V1a antagonist (SBP increased only by 1.6 mmHg ± 5.7, P > 0.05, and DBP by 1 mmHg ± 2.3, P > 0.05), and by 500 ng (i.c.v.) of the V1a antagonist (SBP increased by 2.5 mmHg ± 8.3, P > 0.05, and DBP by 1.8 mmHg ± 2.9 P > 0.05). V1b antagonist pretreatment by 100 ng (i.c.v.) did not prevent the significant rise of SBP induced by vasopressin (21 mmHg ± 5.7, P < 0.05) while DBP did not change significantly (9.8 mmHg ± 4.6). V1b antagonist pretreatment in a dose of 500 ng (i.c.v.) followed by 50 ng (i.c.v) of vasopressin decreased SBP by 0.5 mmHg ± 3.7 (P > 0.05) and DBP increased by 3.8 mmHg ± 3.4 (P > 0.05). Rats pretreated with 100 ng (i.c.v.) and 500 ng (i.c.v.) of the V2 antagonist exhibited hypertension following vasopressin injection (50 ng, i.c.v.). SBP and DBP increased, respectively by 19.9 mmHg ± 3.8 (P < 0.05), and by 9.2 mmHg ± 3.1 (P < 0.05) for 100 ng of V2 antagonist pretreatment and 19.9 mmHg ± 3.3 (P < 0.01), and 7.8 mmHg ± 1.1 (P < 0.05) for 500 ng of V2 antagonist pretreatment. Based on these studies, we used both 100 and 500 ng doses to ascertain functional separation between the effects of V1a, V1b and V2 receptor antagonists.
Effects of non-peptide vasopressin antagonists on BP, HR and BRR under baseline physiological conditions
Table 1 shows that under baseline physiological conditions, V1a, V1b and V2 antagonists applied i.c.v. in a dose of 100 and 500 ng did not change the mean values of SBP, MBP, DBP and HR and did not modulate BRS (Table 1). BEI was also unaffected by drug treatment (Figure 1).
Table 1.
Effects of V1a, V1b and V2 receptor antagonist on SBP, MBP, DBP, HR and BRS under baseline physiological conditions
| I.C.V. | SBP (mmHg) | MBP (mmHg) | DBP (mmHg) | HR (bpm) | BRS (ms·mmHg−1) |
|---|---|---|---|---|---|
| 10% DMSO (5 μL) | 120 ± 6 | 93 ± 4 | 80 ± 2 | 377 ± 19 | 1.4 ± 0.2 |
| V1a (100 ng·5 μL−1) | 123 ± 5 | 94 ± 3 | 79 ± 3 | 386 ± 22 | 1.3 ± 0.3 |
| V1a (500 ng·5 μL−1) | 122 ± 4 | 94 ± 4 | 80 ± 2 | 390 ± 20 | 1.2 ± 0.2 |
| 5% DMSO (5 μL) | 126 ± 3 | 96 ± 4 | 81 ± 3 | 373 ± 18 | 1.1 ± 0.2 |
| V1b (100 ng·5 μL−1) | 122 ± 4 | 95 ± 4 | 81 ± 4 | 371 ± 19 | 1.4 ± 0.4 |
| V1b (500 ng·5 μL−1) | 121 ± 3 | 93 ± 5 | 79 ± 2 | 371 ± 22 | 1.4 ± 0.2 |
| 0.9% NaCl (5 μL) | 123 ± 3 | 96 ± 3 | 83 ± 3 | 398 ± 17 | 1.2 ± 0.1 |
| V2 (100 ng·5 μL−1) | 124 ± 6 | 96 ± 4 | 82 ± 3 | 394 ± 23 | 1.1 ± 0.2 |
| V2 (500 ng·5 μL−1) | 125 ± 3 | 96 ± 3 | 82 ± 6 | 383 ± 17 | 1.1 ± 0.2 |
Values are mean ± SEM of six experiments. In this and the following tables, V1a, V1a-receptor antagonist; V1b, V1b-receptor antagonist; V2, V2-receptor antagonist.
Figure 1.
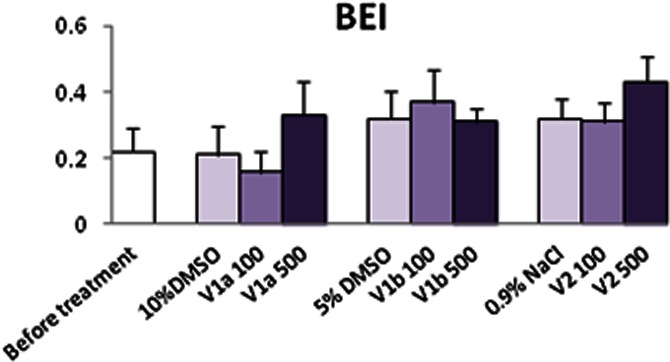
Effects of vasopressin V1a, V1b and V2 receptor antagonist on BEI under baseline physiological conditions. Values are mean ± SEM of six experiments.
Effects of immobilization on BP, HR and BRR
Exposure of rats to 15 min long immobilizations increased SBP, MBP, DBP and HR (Tables 4). This was associated with a decrease in BRS (Figure 2), while BEI remained unchanged (Figure 3).
Table 4.
Effects of stress on SBP, MBP, DBP, HR and BRS in rats pretreated with V2-receptor antagonist or vehicle (0.9% NaCl)
| I.C.V. | SBP (mmHg) | MBP (mmHg) | DBP (mmHg) | HR (bpm) | BRS (ms·mmHg−1) |
|---|---|---|---|---|---|
| BASELINE 0.9% NaCl (5 μL) | 123 ± 4 | 96 ± 4 | 83 ± 4 | 392 ± 16 | 1.3 ± 0.2 |
| STRESS 0.9% NaCl (5 μL) | +22 ± 4** | +23 ± 4** | +26 ± 5** | +58 ± 12* | 0.59 ± 0.2 * |
| BASELINE V2 (100 ng·5 μL−1) | 125 ± 3 | 100 ± 4 | 87 ± 5 | 392 ± 17 | 1.1 ± 0.1 |
| STRESS V2 (100 ng·5 μL−1) | +16 ± 7** | +14 ± 4** | +13 ± 3** | +24 ± 23 | 1.3 ± 0.7 |
| BASELINE V2 (500 ng·5 μL−1) | 124 ± 1 | 98 ± 4 | 85 ± 1 | 380 ± 5 | 1.3 ± 0.2 |
| STRESS V2 (500 ng·5 μL−1) | +16 ± 4** | +18 ± 2** | +21 ± 3** | +26 ± 17 | 1.0 ± 0.3 |
Values are mean ± SEM of six experiments. V2 antagonist pretreatment, at both doses, reduces stress-induced HR increase and prevents BRR desensitization. *P < 0.05, **P < 0.01 versus corresponding baseline.
Figure 2.
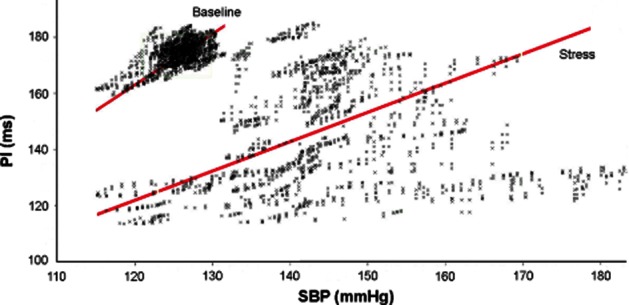
Effects of stress by immobilization on the BRS in one conscious rat. Note a decrease of BRS by stress (BRS, line slope).
Figure 3.
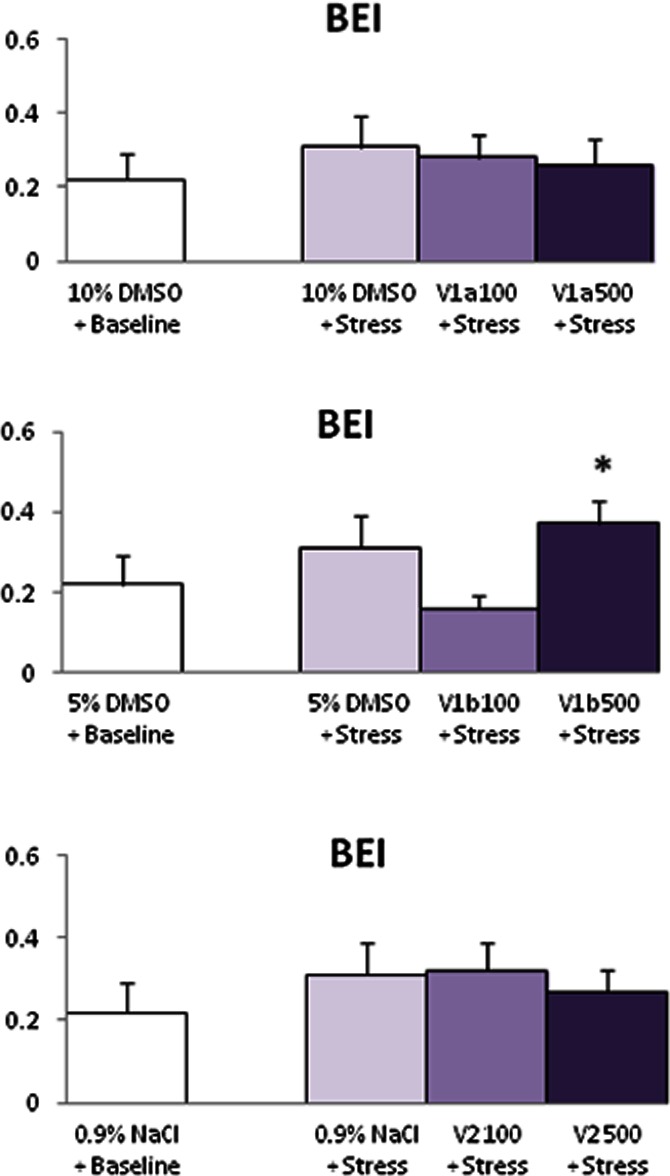
Effects of stress on the BRR effectiveness index under V1a or V1b or V2 receptor blockade. Note that exposure of rats to stress by immobilization did not modify BEI without or with treatment, except for 500 ng of V1b antagonist. For reasons of readability only one baseline value is shown. Values are mean ± SEM for six experiments. *P < 0.05, **P < 0.01 versus baseline.
Effects of immobilization on BP, HR and BRR under vasopressin receptor blockade
The V1a antagonist applied i.c.v. to conscious rats in a dose of 100 and 500 ng did not modify the effect of stress on SBP, MBP, DBP, HR, BRS (Table 2) and BEI (Figure 3, upper panel).
Table 2.
Effects of stress on SBP, MBP, DBP, HR and BRS in rats pretreated with V1a-receptor antagonist or vehicle (10% DMSO)
| I.C.V. | SBP (mmHg) | MBP (mmHg) | DBP (mmHg) | HR (bpm) | BRS (ms·mmHg−1) |
|---|---|---|---|---|---|
| BASELINE 10% DMSO (5 μL) | 123 ± 4 | 96 ± 4 | 83 ± 4 | 390 ± 16 | 1.4 ± 0.2 |
| STRESS 10% DMSO (5 μL) | +20 ± 4** | +22 ± 4** | +23 ± 5** | +55 ± 12* | 0.66 ± 0.2 * |
| BASELINE V1a (100 ng·5 μL−1) | 120 ± 4 | 96 ± 3 | 84 ± 3 | 370 ± 10 | 1.4 ± 0.3 |
| STRESS V1a (100 ng·5 μL−1) | +15 ± 3** | +16 ± 3** | +16 ± 3** | +59 ± 19* | 0.75 ± 0.1 * |
| BASELINE V1a (500 ng·5 μL−1) | 120 ± 5 | 95 ± 4 | 82 ± 3 | 395 ± 13 | 1.8 ± 0.3 |
| STRESS V1a (500 ng·5 μL−1) | +12 ± 1* | +10 ± 5* | +9 ± 2* | 50 ± 22 * | 1.1 ± 0.4* |
Values are mean ± SEM of six experiments. Stress increases SBP, MBP, DBP, HR and decreases BRS. Note that V1a antagonist pretreatment does not modify the stress response. *P < 0.05, **P < 0.01 versus corresponding baseline.
Rats pretreated with 100 and 500 ng (i.c.v.) of the V1b antagonist and exposed to immobilization exhibited comparable increase of SBP, MBP and DBP with non-treated rats (Table 3). However, V1b antagonist at both doses blunted the HR response to stress, prevented BRS desensitization (Table 3), and only at 500 ng increased BEI (Figure 3, middle panel).
Table 3.
Effects of stress on SBP, MBP, DBP, HR and BRS in rats pretreated with V1b receptor antagonist or vehicle (5% DMSO)
| I.C.V. | SBP (mmHg) | MBP (mmHg) | DBP (mmHg) | HR (bpm) | BRS (ms·mmHg−1) |
|---|---|---|---|---|---|
| BASELINE 5% DMSO (5 μL) | 123 ± 4 | 96 ± 4 | 83 ± 4 | 390 ± 16 | 1.4 ± 0.2 |
| STRESS 5% DMSO (5 μL) | +21 ± 4** | +22 ± 4** | +24 ± 5** | +55 ± 12* | 0.65 ± 0.2 * |
| BASELINE V1b (100 ng·5 μL−1) | 123 ± 3 | 95 ± 4 | 81 ± 5 | 400 ± 17 | 1.2 ± 0.1 |
| STRESS V1b (100 ng·5 μL−1) | +19 ± 2** | +10 ± 1* | +9 ± 2* | +17 ± 10 | 1.3 ± 0.3 |
| BASELINE V1b (500 ng·5 μL−1) | 121 ± 7 | 98 ± 5 | 87 ± 4 | 382 ± 8 | 1.3 ± 0.2 |
| STRESS V1b (500 ng·5 μL−1) | +17 ± 6** | +10 ± 3* | +6 ± 2 | +26 ± 20 | 1.2 ± 0.3 |
Values are mean ± SEM of six experiments. V1b antagonist, at both doses, decreases stress-induced tachycardia and BRR desensitization. *P < 0.05, **P < 0.01 versus corresponding baseline.
V2 antagonist pretreated rats exposed to stress exhibited a comparable increase of SBP, MBP and DBP to non-treated rats, at both doses. However, the stress-induced tachycardia and BRS desensitization were inhibited (Table 4) while BEI was not modified by treatment (Figure 3, lower panel).
Discussion and conclusions
The present study in conscious rats shows that, under basal physiological conditions, central vasopressin does not modulate BP, HR and baro-receptor control of the circulation, whereas during exposure to stress induced by immobilization, vasopressin mediates HR increase and BRR desensitization by the stimulation of central V1b- and V2-like receptors.
Our experiments also show that, under basal physiological conditions, centrally acting vasopressin does not participate in BP maintenance. This finding is in line with those of Imai and co-workers (1990), who reported that i.c.v. injected desmopressin, a selective V2 agonist or i.c.v. injected selective V1 antagonist do not affect BP and HR of rats under basal physiological conditions. They also found that BP and HR of rats with hereditary diabetes insipidus (DI) under basal conditions are similar to Long-Evans rats, a parent rat strain without DI. However, it was recently reported that V1a receptor knockout mice exhibit lower BP levels in comparison to wild-type controls and that this hypotension is due to the lack of peripheral V1a receptors resulting in attenuation of RAS functioning and BRS (Fujiwara et al., 2012). In DI rats, both the decrease in BRS (Imai et al., 1983) and no change in BRS (Gardiner and Bennett, 1988) have been reported. Nonetheless, electrical stimulation of PVN (Ciriello and Caralesu, 1980) as well as microinjection of vasopressin in NTS (Matsuguchi et al., 1982; Michelini and Bonagamba, 1988; Kubo and Kihara, 1990; Hegarty and Felder, 1995) have been found to produce baro-reflex desensitization and tachycardia. On the other hand, injection of vasopressin into the area postrema (Hasser and Bishop, 1990) [note that the area postrema can be reached by drugs injected via both sides of the blood–brain barrier] was found to enhance BRS (Unger et al., 1986; Brizzee and Walker, 1990). Therefore, we cannot entirely rule out a possibility that i.c.v. injected drugs in our experiments may have acted at multiple sites to produce concomitantly opposing effects on the BRR.
In the present experiments, immobilization of rats induced a typical haemodynamic defence with instantaneous BP and HR increase, and a reduction of BRS, as part of a complex neuroendocrine response to stress. Injection of vasopressin antagonists previous to exposure to stress modified the cardiovascular response. Particularly, the V1b receptor antagonist prevented the stress-induced HR increase and BRS desensitization. An interesting finding is that a V2 receptor antagonist also efficiently prevented BRR desensitization. Although this study cannot pinpoint the exact brain structure where vasopressin V1b and V2 receptors are stimulated during stress, the injection site of antagonists in the lateral cerebral ventricle of the rat points towards adjacent brain structures bed nucleus of the stria terminalis (BNST), PVN and periaqueductal grey matter (PAG), all of which are well known to play an important role in cardiovascular regulation and related neuroendocrine functions (Loewy and McKellar, 1980). This view is further supported by the morphological finding of vasopressin receptors in BNST, PVN and PAG of the rat brain (Brinton et al., 1984; Ostrowski et al., 1994; Hernando et al., 2001; Yang et al., 2006; 2007; Vargas et al., 2009).
V1b receptors found in BNST have been found to contribute crucially to the emotional response to stress (Griebel et al., 2002), and strong emotions can increase BP and HR. BNST is abundantly interconnected with the hypothalamus, PAG and brainstem vagal nuclear complex (Berk and Finkelstein, 1981; Ter Horst and Luiten, 1986; Thompson et al., 1996) and they act collectively to mediate integrated fear response (Walker et al., 2003). Microinjections of the V1b antagonist in the BNST were found to exert anxiolytic and antidepressant effects (Griebel et al., 2002). We reported previously that centrally applied V1b antagonist reduces sympatho-respiratory response of rats exposed to stress and shortens the recovery of BP and HR (Milutinović et al., 2006; Stojičić et al., 2006; 2008). In the present experiments, V1b antagonist was most effective in reducing HR increase induced by immobilization and prevented BRR desensitization. Our findings complement those of Roper and co-workers (2010) who demonstrate that V1b knock-out mice exhibit reduced blood ACTH and corticosterone response to stress.
Another possible site of action of V1b receptor antagonist in our experiments is within the PVN, a major integrative site of neuro-endocrine and behavioural response to stress. Morphological studies indicate abundance of V1a and V1b vasopressin and oxytocin receptors on somata and dendrites of magnocellular neurons in the PVN (Ostrowski et al., 1994; Hernando et al., 2001). The role of these receptors is not yet elucidated. It has been suggested that they could have a role in the paracrine and autocrine regulation of magnocellular neurons (Tobin et al., 2012). Using specific antisense oligomers directed against oxytocin mRNA in the PVN of rats, evidence was provided that oxytocin-containing neurons that project to the brainstem are critical for the HR response to shaker stress (Morris et al., 1995). Microdialysis studies confirmed that, during exposure to stress, there is an increase in oxytocin content in the PVN (Morris et al., 1995; Nishioka et al., 1998). Callahan and co-workers have shown that electrolytic (Callahan et al. 1989) and chemical (Callahan et al. 1992) lesions of PVN, as well as central administration of only oxytocin antagonist, prevents stress-induced tachycardia. They also showed that DI rats that lack vasopressin, but not oxytocin, exhibit normal HR response to foot-shock. Their results altogether suggest that PVN-derived oxytocin, not vasopressin, mediates stress-induced tachycardia. This is in line with present report that V1a antagonist failed to prevent stress-induced tachycardia. It also does not support a role of V1b receptors in PVN in stress-induced BRR desenzitization.
The effects of V2 receptor antagonism are intriguing. Pharmacological experimentation supports the existence of V2 receptors in adult rat brain (Imai et al., 1990; Sampey et al., 1999; Yang et al., 2006; 2007). Recent studies using V2 antagonists and small interfering RNA gene knock-down technology suggest that V2 receptors in PVN and PAG might be involved in anti-nociception (Yang et al., 2006) and nociception (Yang et al., 2007), respectively. Morphological studies indicate that PVN has abundant connections with the defence area in the PAG (Swanson and Kuypers, 1980; Geerling et al., 2010) and that PAG neuronal projections to the medulla are involved in stress-induced tachycardia and BRR inhibition (Dean and Coote, 1986; Farkas et al., 1998; De Menezes et al., 2009; Netzer et al., 2011). Therefore, it is possible that V2-like receptors in PAG are involved in the stress-induced BRR desensitization, in our experiments.
It can be concluded that, in conscious rats, central vasopressin V1b and V2 receptors mediate stress-induced BRR desensitization enabling concurrent BP and HR increase. If V1b and V2-like central receptors are involved in BRR desensitization in heart failure and hypertension associated with poor outcome, our findings could open new perspectives in the treatment of these neurocardiogenic deregulations.
Acknowledgments
This work was supported by grant III41013 of the Ministry of Education, Science and Technology (RS) and an International Junior Research Grant of the Physiological Society (UK).
Glossary
- BEI
baro-receptor reflex effectiveness index
- BNST
bed nucleus of the stria terminalis
- BRR
baro-receptor reflex
- BRS
baro-receptor reflex sensitivity
- DBP
diastolic BP
- HR
heart rate
- IML
intermediolateral column of the spinal cord
- MBP
mean BP
- NTS
nucleus of the solitary tract
- PAG
periaqueductal grey matter
- PI
pulse interval
- PVN
paraventricular nucleus
- RVLM
rostoventrolateral medulla
- SBP
systolic BP
Conflict of interest
The authors state no conflict of interest.
References
- Bajić D, Lončar-Turukalo T, Stojičić S, Šarenac O, Bojić T, Murphy D, et al. Temporal analysis of the spontaneous baroreceptor reflex during mild emotional stress in the rat. Stress. 2010;13:142–154. doi: 10.3109/10253890903089842. [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. Afferent projections to the preoptic area and hypothalamic regions in the rat brain. Neuroscience. 1981;6:1601–1624. doi: 10.1016/0306-4522(81)90227-x. [DOI] [PubMed] [Google Scholar]
- Brinton RE, Gee KW, Wamsley JK, Davis TP, Yamamura HI. Regional distribution of putative vasopressin receptors in the rat brain and pituitary by quantitative autoradiography. Proc Natl Acad Sci U S A. 1984;81:7248–7252. doi: 10.1073/pnas.81.22.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzee BL, Walker BR. Vasopressinergic augmentation of cardiac baroreceptor reflex in conscious rats. Am J Physiol. 1990;258(4 Pt 2):R860–R868. doi: 10.1152/ajpregu.1990.258.4.R860. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- Callahan MF, Kirby RF, Cunningham JT, Eskridge-Sloop SL, Johnson AK, McCarty R, et al. Central oxytocin systems may mediate a cardiovascular response to acute stress in rats. Am J Physiol. 1989;256(5 Pt 2):H1369–H1377. doi: 10.1152/ajpheart.1989.256.5.H1369. [DOI] [PubMed] [Google Scholar]
- Callahan MF, Thore CR, Sundberg DK, Gruber KA, O'Steen K, Morris M. Excitotoxin paraventricular nucleus lesions: stress and endocrine reactivity and oxytocin mRNA levels. Brain Res. 1992;597:8–15. doi: 10.1016/0006-8993(92)91499-5. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol. 2010;103:4–15. doi: 10.1152/jn.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, Caralesu FR. Role of paraventricular and supraoptic nuclei in central cardiovascular regulation in the cat. Am J Physiol. 1980;239:R137–R142. doi: 10.1152/ajpregu.1980.239.1.R137. [DOI] [PubMed] [Google Scholar]
- De Menezes RC, Zaretsky DV, Fontes MA, DiMicco JA. Cardiovascular and thermal responses evoked from the periaqueductal grey require neuronal activity in the hypothalamus. J Physiol. 2009;587:1201–1215. doi: 10.1113/jphysiol.2008.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Coote JH. Discharge patterns in postganglionic neurons to skeletal muscle and kidney during activation of the hypothalamic and midbrain defense areas in the cat. Brain Res. 1986;377:271–278. doi: 10.1016/0006-8993(86)90868-1. [DOI] [PubMed] [Google Scholar]
- Farkas E, Jansen AS, Loewy AD. Periaqueductal grey matter input to cardiac-related sympathetic premotor neurons. Brain Res. 1998;792:179–192. doi: 10.1016/s0006-8993(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Tanoue A, Tsujimoto G, Koshimizu TA. The roles of V1a vasopressin receptors in blood pressure homeostasis: a review of studies on V1a receptor knockout mice. Clin Exp Nephrol. 2012;16:30–34. doi: 10.1007/s10157-011-0497-y. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Bennett T. Cardiac baroreflex sensitivities in conscious, unrestrained, Long-Evans and Brattleboro rats. J Auton Nerv Syst. 1988;23:213–219. doi: 10.1016/0165-1838(88)90096-3. [DOI] [PubMed] [Google Scholar]
- Geerling JC, Shin J-W, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasser EM, Bishop VS. Reflex effect of vasopressin after blockade of V1 receptors in the area postrema. Circ Res. 1990;67:265–271. doi: 10.1161/01.res.67.2.265. [DOI] [PubMed] [Google Scholar]
- Hegarty AA, Felder RB. Antagonism of vasopressin V1 receptors in NTS attenuates baroreflex control of renal nerve activity. Am J Physiol. 1995;269:H1080–H1086. doi: 10.1152/ajpheart.1995.269.3.H1080. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JPH. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- Imai Y, Nolan PL, Johnston CI. Restoration of suppressed baroreflex sensitivity in rats with hereditary diabetes insipidus (Brattleboro rats) by arginine-vasopressin and DDAVP. Circ Res. 1983;53:140–149. doi: 10.1161/01.res.53.2.140. [DOI] [PubMed] [Google Scholar]
- Imai Y, Abe K, Sasaki S, Minami N, Munakata M, Sakuma H, et al. Cardiovascular depression and stabilization by central vasopressin in rats. Hypertension. 1990;15:291–300. doi: 10.1161/01.hyp.15.3.291. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Modulation of the aortic baroreceptor reflex by neuropeptide Y, neurotensin and vasopressin microinjected into the nucleus tractus solitarii of the rat. Naunyn Scmiedebergs Arch Pharmacol. 1990;342:182–188. doi: 10.1007/BF00166962. [DOI] [PubMed] [Google Scholar]
- Loewy AD, McKellar S. The neuroanatomical basis of central cardiovascular control. Fed Proc. 1980;39:2495–2503. [PubMed] [Google Scholar]
- Lončar-Turukalo L, Bajić D, Japundžić-Žigon N. Temporal sequence parameters in isodistributional surrogate data: model and exact expressions. IEEE Trans Biomed Eng. 2011;58:16–24. doi: 10.1109/TBME.2010.2083661. [DOI] [PubMed] [Google Scholar]
- Matsuguchi H, Sharabi FM, Gordon FJ, Johnson AK, Schmid PG. Blood pressure and heart rate responses to microinjection of vasopressin into the nucleus tractus solitaries region of the rat. Neuropharamacology. 1982;21:687–693. doi: 10.1016/0028-3908(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Bonagamba LG. Baroreceptor reflex modulation by vasopressin microinjected into the nucleus tractus solitarii of conscious rats. Hypertension. 1988;11(2 Pt 2):I75–I79. doi: 10.1161/01.hyp.11.2_pt_2.i75. [DOI] [PubMed] [Google Scholar]
- Milutinović S, Murphy D, Japundžić-Žigon N. The role of central vasopressin receptors in the modulation of autonomic cardiovascular controls: a spectral analysis study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1579–R1591. doi: 10.1152/ajpregu.00764.2005. [DOI] [PubMed] [Google Scholar]
- Morris M, Callahan MF, Li P, Lucion AB. Central oxytocin mediates stress-induced tachycardia. J Neuroendocrinol. 1995;7:455–459. doi: 10.1111/j.1365-2826.1995.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, et al. Arterial baroreflex modulation of heart rate in chronic heart failure. Clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- Murphy D, Konopacka A, Hindmarch C, Paton JFR, Sweedler JV, Gillette MU, et al. The hypothalamic-Neurohypophyseal System: from genome to physiology. J Neuroendocrinol. 2012;24:539–553. doi: 10.1111/j.1365-2826.2011.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Grassi G. Impaired baroreflex sensitivity as a potential marker of cardiovascular risk in hypertension. J Hypertens. 2008;26:1303–1304. doi: 10.1097/HJH.0b013e328305e1a5. [DOI] [PubMed] [Google Scholar]
- Netzer F, Bernard J-F, Verberne AJM, Harmon M, Camus F, Benoliel J-J, et al. Brain circuits mediating baroreflex bradycardia inhibition in rats: an anatomical and functional link between the cuneiform nucleus and the periaqueductal grey. J Physiol. 2011;589(Pt 8):2079–2091. doi: 10.1113/jphysiol.2010.203737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:56–60. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Lolait SJ, Young WS., 3rd Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: implications for cardiovascular regulation. J Chem Neuroanat. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Roper JA, Craighead M, O'Carroll A-M, Lolait SJ. Attenuated stress response to acute restraint and forced swimming stress in arginine vasopressin V1b receptor subtype (Avpr1b) receptor knockout mice and wild-type mice treated with a novel Avpr1b receptor antagonist. J Neuroendocrinol. 2010;22:1173–1180. doi: 10.1111/j.1365-2826.2010.02070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey DB, Burrell LM, Widdop RE. Vasopressin V2 receptor enhances gain of baroreflex in conscious spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 1999;45:R872–R879. doi: 10.1152/ajpregu.1999.276.3.R872. [DOI] [PubMed] [Google Scholar]
- Sato K, Numata T, Saito T, Ueta Y, Okada Y. V2 receptor mediated autocrine role of somatodendritic release of AVP in rat vasopressin neurons under hypo-osmotic conditions. Sci Signal. 2011;4:ra5. doi: 10.1126/scisignal.2001279. [DOI] [PubMed] [Google Scholar]
- Stojičić S, Milutinović S, Šarenac O, Živković S, Japundžić-Žigon N. Central vasopressin V(1a) and V(1b) receptors modulate the cardiovascular response to air-jet stress in conscious rats. Biomed Tech (Berl) 2006;51:268–271. doi: 10.1515/BMT.2006.053. [DOI] [PubMed] [Google Scholar]
- Stojičić S, Milutinović-Smiljanić S, Šarenac O, Milosavljević S, Paton JFR, Murphy D, et al. Blockade of central vasopressin receptors reduces the cardiovascular response to acute stress in freely moving rats. Neuropharmacology. 2008;54:824–836. doi: 10.1016/j.neuropharm.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Luiten PGM. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Cannteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tobin V, Leng G, Ludwig M. The involvement of actin, calcium channels and exocytosis proteins in somato-dendritic oxytocin and vasopressin release. Front Physiol. 2012;3:261. doi: 10.3389/fphys.2012.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T, Rohmeiss P, Demmert G, Ganten D, Lang RE, Luft FC. Differential modulation of the baroreceptor reflex by brain and plasma vasopressin. Hypertension. 1986;8(6 Pt 2):II157–II162. doi: 10.1161/01.hyp.8.6_pt_2.ii157. [DOI] [PubMed] [Google Scholar]
- Vargas KJ, Sarmiento JM, Ehrenfeld P, Anazco CC, Villanueva CI, Carmona PL, et al. Postnatal expression of V2 vasopressin receptor splice variants in the rat cerebellum. Differentiation. 2009;77:377–385. doi: 10.1016/j.diff.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdale in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen J-M, Song C-Y, Liu W-Y, Wang G, Wang C-H, et al. Through the central V2, not V1 receptors influencing the endogenous opiate peptide system, arginine vasopressin, not oxytocin in the hypothalamic paraventricular nucleus involves in the antinociception in the rat. Brain Res. 2006;1069:127–138. doi: 10.1016/j.brainres.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang Y, Xu HT, Chen JM, Liu WJ, Lin BC. Arginine vasopressin induces periaqueductal gray release of enkephalin and endorphin relating to pain modulation in the rat. Regul Pept. 2007;142:29–36. doi: 10.1016/j.regpep.2007.01.006. [DOI] [PubMed] [Google Scholar]


