Abstract
CD8 T-cell responses are thought to be crucial for control of viremia in human immunodeficiency virus (HIV) infection but ultimately fail to control viremia in most infected persons. Studies in acute infection have demonstrated strong CD8-mediated selection pressure and evolution of mutations conferring escape from recognition, but the ability of CD8 T-cell responses that persist in late-stage infection to recognize viruses present in vivo has not been determined. Therefore, we studied 24 subjects with advanced HIV disease (median viral load = 142,000 copies/ml; median CD4 count = 71/μl) and determined HIV-1-specific CD8 T-cell responses to all expressed viral proteins using overlapping peptides by gamma interferon Elispot assay. Chronic-stage virus was sequenced to evaluate autologous sequences within Gag epitopes, and functional avidity of detected responses was determined. In these subjects, the median number of epitopic regions targeted was 13 (range, 2 to 39) and the median cumulative magnitude of CD8 T-cell responses was 5,760 spot-forming cells/106 peripheral blood mononuclear cells (range, 185 to 24,700). On average six (range, one to 8) proteins were targeted. For 89% of evaluated CD8 T-cell responses, the autologous viral sequence was predicted to be well recognized by these responses and the majority of analyzed optimal epitopes were recognized with medium to high functional avidity by the contemporary CD8 T cells. Withdrawal of antigen by highly active antiretroviral therapy led to a significant decline both in breadth (P = 0.032) and magnitude (P = 0.0098) of these CD8 T-cell responses, providing further evidence that these responses had been driven by recognition of autologous virus. These results indicate that strong, broadly directed, and high-avidity gamma-interferon-positive CD8 T-cells directed at autologous virus persist in late disease stages, and the absence of mutations within viral epitopes indicates a lack of strong selection pressure mediated by these responses. These data imply functional impairment of CD8 T-cell responses in late-stage infection that may not be reflected by gamma interferon-based screening techniques.
In 1987, the existence of circulating human immunodeficiency virus (HIV)-specific cytotoxic CD8 T cells was reported in persons with chronic HIV infection (42, 52). Since then methods to screen for virus-specific CD8 T-cell responses have improved substantially. Comprehensive screening techniques with gamma interferon-based Elispot or intracellular cytokine staining assays and overlapping peptides have allowed assessment of T-cell responses towards the entire expressed genome of HIV type 1 (HIV-1) (1, 10). When these techniques are used, the breadth and magnitude of CD8 T-cell responses can be evaluated at the single epitope level (1, 24). These studies demonstrate that HIV is highly immunogenic and that all expressed proteins are targets of CD8 T-cell responses.
There is a correlation between disease progression in HIV infection and the level of set-point viral load that subjects reach in untreated acute HIV infection after the initial high viremia (34). The finding that HIV-specific CD8 T-cell responses appear coincident with the drop in viremia suggests a role for these cells in immune containment (12, 20, 33). Further evidence is provided by the simian AIDS model, in which animals fail to contain initial viremia if their CD8 T cells are depleted at the time of infection (30, 44), whereas depletion of B cells does not affect initial control (45). Moreover, there is growing evidence of viral escape within CD8 T-cell epitopes during the acute phase of simian immunodeficiency virus or HIV infection indicative of strong immune selection pressure (3, 13, 38, 43.) Therefore, there is strong evidence for the antiviral activity of CD8 T-cell responses during acute HIV infection.
The situation in the chronic phase of infection is less clear. CD8 T-cell depletion studies indeed showed an increase in viral load in the majority of SIV-infected animals (30). In contrast, there is no clear connection between the gradual rise in viremia during chronic infection and changes in CD8 T-cell responses. The best documented association between loss of immune control and CD8 T-cell responses in chronic HIV infection is escape within the HLA B27-restricted epitope KRWIILGLNK (26, 31). On the contrary, it was shown recently that there is no correlation between the viral load and the total magnitude or the total breadth of CD8 T-cell responses (1, 10, 24). Nevertheless, a primary aim of vaccine and immunotherapy trials is to induce HIV-specific CD8 T-cell responses with the intention of achieving control of viremia.
In order to determine the significance of CD8 T-cell responses in chronic HIV infection, we studied the extent of CD8 T-cell responses in subjects with high viral loads and/or low CD4 counts—a group that is largely underrepresented in studies to date. We then sequenced the chronic-stage autologous virus to evaluate to what extent the overlapping peptides used matched the autologous virus. Finally, we determined the functional avidity of epitope-specific responses, as it has been shown in animal models that high-avidity T-cell responses may preferentially be able to control viral infections (2, 22).
MATERIALS AND METHODS
Study subjects.
Twenty-four chronically HIV-1 infected individuals were studied at the Massachusetts General Hospital (MGH) and the Lemuel Shattuck Hospital, Boston, Mass. Participants had to fulfill one of the following criteria: a viral load of >45,000 copies/ml (Roche Amplicor Assay Version 1.0) or a CD4 count that was < 350/μl or an AIDS-defining illness. The upper cutoff for viral load testing was 500,000 copies/ml (Lemuel Shattuck Hospital) and 750,000 copies/ml (MGH). All subjects had been without antiretroviral treatment for at least 3 months at the time of the first blood draw. Relevant clinical data for all study subjects are summarized in Table 1. Eight individuals started highly active antiretroviral therapy (HAART) after the first blood draw and were evaluated at a second time point 8 to 12 weeks after starting treatment. Five HIV-1 seronegative individuals were studied as control subjects.
TABLE 1.
Clinical characteristics of study subjects
| Patient code | HLA class I type
|
Viral load (copies/ml) | CD4 count (cells/μl) | Opportunistic diseasea | ||
|---|---|---|---|---|---|---|
| HLA A | HLA B | HLA Cw | ||||
| PR 1 | 2, 3 | 35, 44 | 4, 7 | 12,900 | 12 | MAC infection, AIDS dementia |
| PR 2 | 23, 68 | 7, 57 | 7 | 24,400 | 55 | HIV wasting |
| PR 3 | 1, 3 | 8, 58 | 7 | 25,362 | 329 | ⊘ |
| PR 4 | 24, 30 | 13, 40 | 6, 7 | 30,000 | 290 | ⊘ |
| PR 5 | 3, 25 | 42, 57 | 3 | 48,700 | 408 | ⊘ |
| PR 6 | 24, 68 | 7, 13 | 7, 8 | 50,600 | 488 | ⊘ |
| PR 7 | 29, 31 | 35, 40 | 2, 4 | 61,000 | 29 | ⊘ |
| PR 8 | 2, 11 | 35, 40 | 3, 4 | 96,100 | 436 | ⊘ |
| PR 9 | 2, 30 | 49, 51 | 7, 16 | 98,500 | 423 | ⊘ |
| PR 10 | 33, 68 | 15, 53 | 3, 4 | 98,800 | 28 | PCP |
| PR 11 | 1, 2 | 15, 53 | 2, 6 | 118,000 | 27 | KS |
| PR 12 | 1, 2 | 15, 44 | 3, 5 | 124,000 | 82 | PCP |
| PR 13 | 1 | 8, 57 | 6, 7 | 160,000 | 60 | ⊘ |
| PR 14 | 1, 3 | 7 | 7 | 171,000 | 277 | KS |
| PR 15 | 2, 26 | 38, 44 | 5, 12 | 200,000 | 150 | ⊘ |
| PR 16 | 1, 2 | 18, 27 | 2, 12 | 249,000 | 115 | KS |
| PR 17 | 2, 68 | 7, 44 | 7 | 269,000 | 220 | ⊘ |
| PR 18 | 2, 26 | 8, 44 | 5, 7 | 292,000 | 129 | PML |
| PR 19 | 2, 26 | 44, 45 | 5, 6 | 376,000 | 3 | MAC infection |
| PR 20 | 30, 36 | 35, 58 | 6, 7 | >500,000 | 0 | ⊘ |
| PR 21 | 29, 68 | 40, 52 | 2, 15 | >500,000 | 11 | PCP |
| PR 22 | 2, 24 | 18, 35 | 4, 7 | >500,000 | 17 | Cryptococcal meningitis |
| PR 23 | 2, 66 | 15, 41 | 14, 17 | >750,000 | 33 | Oral candidiasis |
| PR 24 | 1, 3 | 7, 57 | 6, 7 | >750,000 | 24 | PCP |
MAC, Mycobacterium avium complex; PCP, Pneumocystis carinii pneumonia; KS, Kaposi's sarcoma; PML, progressive multifocal leukoencephalopathy; ⊘, no opportunistic diseases.
HLA class I typing was performed at Dynal Biotech (Oxford, United Kingdom) by using sequence-specific primer-PCR. The HLA class I genotypes of the study population were diverse and were not selected for a certain HLA allele. The study was approved by the Institutional Review Board of MGH and the Lemuel Shattuck Hospital. All individuals gave informed consent for participation in the studies.
Peptides.
Peptides were synthesized with an automated peptide synthesizer (MBS 396; Advanced Chemtech, Louisville, Ky.) by using fluorenylmethoxycarbonyl chemistry. Four hundred and ten peptides (16 to 19 amino acids long, 10-amino-acid overlap, consensus sequence clade B 2001 [http://hiv-web.lanl.gov]) spanning all expressed HIV proteins (Gag, Nef, Rev, Tat, Vpu, Vpr, Pol, Env, and Vif) were synthesized. To detect single-epitope responses, we used a matrix containing pools of peptides; since each peptide was present in two different wells, this allowed for independent confirmation of results in a second assay (1). In addition, peptides according to the sequence of the autologous virus of subjects and peptides corresponding to the optimal epitope indicated were synthesized and tested for recognition.
CD8 T-cell avidity was investigated by using peptide titrations in an Elispot assay and was defined as peptide concentration that resulted in 50% maximum gamma interferon production (38). Experiments were done in duplicate, and values are the mean of both experiments.
Elispot assay.
HIV-1 specific CD8 T-cell responses were quantified by Elispot assay by using fresh or frozen peripheral blood mononuclear cells (PBMC) (0.5 to 1 × 105 per well) and single peptides (final concentration: 14 μg/ml), as described previously (7). The incubation period was 14 to 16 h. Gamma interferon-producing cells were counted by direct visualization and are expressed as spot-forming cells (SFC) per 106 PBMC. Negative controls were always < 30 SFC per 106 input cells. The positive controls consisted of incubation of PBMC with phytohemagglutinin. Wells were counted as positive if they were at least 50 SFC/106 PBMC and exhibited at least three times the background level. We chose an upper cutoff of 200 spots per well, i.e., 2000 SFC/106 PBMC, when adding 105 PBMC per well and 4000 SFC/106 PBMC when adding 0.5 × 105 PBMC per well. CD8 dependence for the majority of responses in Elispot assays was confirmed by intracellular cytokine staining and flow cytometry (23).
The subjects studied here have lower CD4 counts, which might lead to a higher percentage of CD8 T cells within PBMC than for controllers with normal CD4 counts. The percentage of CD8 T cells within the PBMC in fluorescence-activated cell sorter analysis in the study cohort was between 32 and 59% (normal range, 9 to 48% [MGH]), and adjusting the value to absolute CD8 counts did not change the results significantly (data not shown). Therefore, we expressed the magnitude as SFC/106 PBMC, as this is the widely used standard.
Sequencing of autologous virus with viral RNA or proviral DNA.
Viral RNA was extracted from the patients' plasma with the QIAamp RNA Viral Mini Kit (Qiagen, Valencia, Calif.), or viral DNA was extracted from PBMC by using the Puregene DNA Isolation Kit (Gentra, Minneapolis, Minn.) according to the manufacturer's protocol. Viral RNA was transcribed into cDNA by using the SuperScript First-Strand Synthesis System for reverse transcriptase PCR (Invitrogen, Carlsbad, Calif.) and the specific primer 5′-TGG TGG GGC TGT TGG CT-3′. DNA was amplified by using nested PCR and primers as described earlier (5). PCR cycling conditions were as follows: 94°C for 2 min, 35 to 40 cycles of 30 s at 94°C, 30 s at 56°C, 1.5 min at 72°C, and a final extension of 68°C for 20 min. In the nested PCR the extension time was shortened to 1 min. For five subjects (PR1, PR3, PR7, PR8, and PR18) regions were cloned: PCR fragments were gel purified (QIAquick Gel Extraction Kit; Qiagen) and were cloned by using the TOPO TA Cloning Kit (Invitrogen). Plasmid DNA was isolated by using QiaPrep Turbo Miniprep (Qiagen). PCR products or plasmid DNA were sequenced bidirectionally by the Sequencing Core Facility, MGH. Sequencher 4.1 (Gene Codes Corporation) was used to edit and align sequences. Clone data confirmed population sequence data.
Statistical analysis.
Statistical analysis was performed by using Graph Pad Prism 3.0. In order to avoid overestimation of the total breadth of the HIV-1-specific CD8 response, responses to two adjacent overlapping peptides were counted as responses to one epitopic region (1), since some T-cell epitopes can be located in the overlapping region of two adjacent peptides, resulting in responses to both overlapping peptides. Similarly, in the analysis of the total magnitude of HIV-1 specific CD8 T-cell response, we also considered only the higher response of two adjacent overlapping peptides for final calculations. While avoiding overestimation of responses, this approach can potentially underestimate the total breadth and magnitude of responses (27).
In order to take the length of each protein into consideration to calculate the immunogenicity of each protein, we calculated the relative size of each protein as amino acids of the protein divided by the amino acids of all expressed HIV proteins together. We then used a score dividing the relative contribution of each protein to the total breadth of responses by the relative size of the protein. The results are grouped as a score of >1, a score that is = 1, or a score of <1.
RESULTS
HIV-specific, gamma interferon-positive CD8 T-cell responses are strong and broadly directed in subjects with progressing HIV infection.
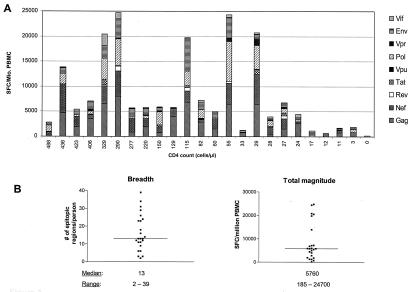
Twenty-four HIV-infected individuals who did not control viremia (Table 1) were evaluated for breadth and specificity of CD8 T-cell responses by using an gamma interferon Elispot assay and peptides spanning all expressed viral proteins. The median viral load was 142,000 copies/ml (range, 12,900 to >750,000 copies/ml); the median CD4 count was 71/μl (range, 0 to 488/μl). No subject had received HAART for at least 3 months prior to the first blood draw. Results of cumulative responses for each individual are shown in Fig. 1A, stratified by CD4 count. HIV-specific CD8-T-cell responses to at least two epitopic regions were detected in all HIV-infected subjects, and no HIV-specific CD8 T-cell responses were detected in five uninfected controls (data not shown). The weakest CD8 T-cell responses were those in subject PR 20, with a viral load of >500,000 copies/ml and a CD4 count of 0/μl. In this individual, only three CD8 T-cell responses were detected, with a total magnitude of 185 SFC/106 PBMC. However, overall there was no consistency between viral load and breadth or strength of the CD8 T-cell response. For example, subject PR 24 (viral load = >750,000 copies/ml; CD4 count = 24/μl) had a higher level of responses than did subject PR 6 (viral load = 50,600 copies/ml; CD4 count = 488/μl). The median breadth of epitopic regions for each subject was 13 (range, 2 to 39), and the median cumulative magnitude was 5,760 SFC/106 PBMC (range, 185 to 24,700 SFC/106 PBMC) (Fig. 1B). When compared to a group of 21 controllers (defined as subjects with a normal CD4 count and viral load that was < 2,000 copies/ml in the absence of antiretroviral treatment for at least 2 years), there was no significant difference in either breadth or magnitude of responses: for the breadth of responses, it was 13 versus 16 epitopic regions/person (P = 0.69), and for the magnitude of responses, it was 5,760 SFC/106 PBMC versus 9,800 SFC/106 PBMC (P = 0.22).
FIG. 1.
(A) Cumulative CD8 T-cell responses measured by gamma interferon Elispot for each HIV protein for each study subject stratified according to CD4 counts. (B) Median and range of breadth and total magnitude of epitopic regions recognized by study subjects.
All expressed HIV proteins are broadly targeted by the study cohort.
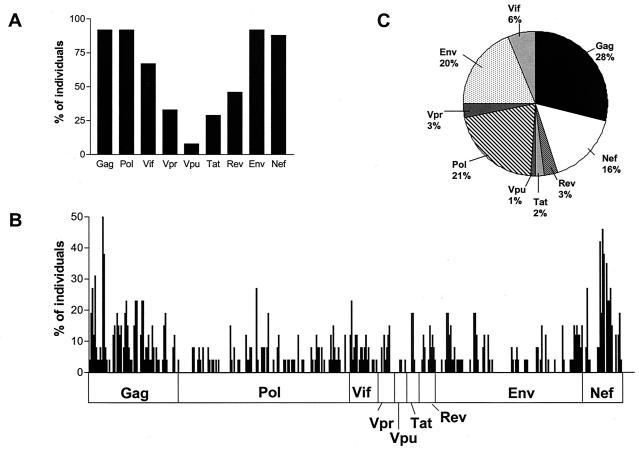
As there was no difference in breadth or magnitude of CD8 T-cell responses in these subjects from that found in cohorts who appear to contain HIV-1 replication, we were interested in determining if their responses were more narrowly focused on certain regions of the HIV genome. As shown in Fig. 2A, all nine expressed HIV proteins are targeted by this cohort. Twenty-two of the 24 (92%) subjects had at least one response towards Env, Gag, and Pol. Eighty-eight percent had a response towards Nef. Vpu was the least targeted protein, which is consistent with reports in cohorts examined at earlier stages of diseases (4). Figure 2B shows the percentage of subjects recognizing each individual peptide. Overall the responses are broadly distributed within each protein. Clusters of responses were present within Gag and Nef, again similar to findings in cohorts examined at earlier stages of disease (1). Figure 2C shows the relative contribution of individual proteins to the total breadth of HIV-specific CD8 T-cell responses of all subjects evaluated together. Gag was the most immunogenic (28%), followed by Pol (21%), Env (20%), and Nef (16%). In order to take the length of each protein into consideration, we used a score dividing the relative contribution of each protein to the total breadth of responses by the relative size of the protein for all expressed HIV proteins. Here Gag and Nef were the most immunogenic (score > 1), followed by Vpr and Vif (score = 1) and Rev, Tat, Pol, Env, and Vpu (score < 1).
FIG. 2.
(A) Percentage of individuals who recognize at least one peptide of each HIV protein. (B) Peptide recognition across the entire expressed HIV genome. Shown is the percentage of subjects who recognize each individual of the 410 overlapping peptides (listed on the x axes). (C) Relative contribution of each protein to the total breadth of the HIV-specific CD8 T-cell responses of all subjects together.
Peptide sequences match the autologous virus for most of the evaluated CD8 T-cell responses.
Viral escape by mutation of critical amino acids within epitopes is the best studied mechanism of immune evasion from CD8 T-cell responses (3, 26, 31, 38). Therefore, we next addressed whether there was a frequent mismatch between the overlapping peptides used to screen for CD8 T-cell responses and the autologous viral sequence, as nonrecognition of the autologous virus could be an explanation for the discrepancy between CD8 T-cell responses and high viral loads.
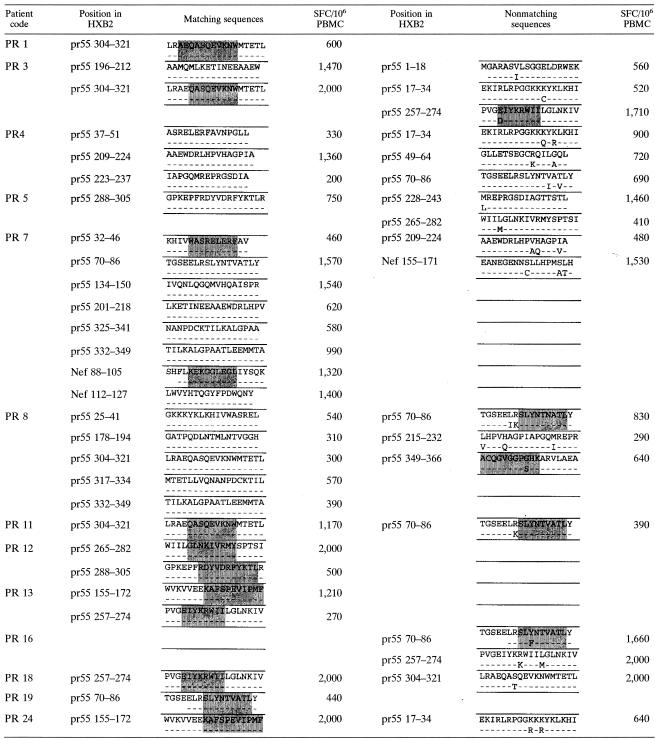
Gag was sequenced, since it was the most frequently targeted protein and the medium strong and strong responses (≥200 SFC/106 PBMC) were evaluated. In Table 2, the sequences of autologous virus are presented for 13 subjects according to the individual CD8 T-cell responses detected. The top row shows the sequence of the peptide that was used for screening (which is the consensus sequence of 2001 for clade B [http://hiv-web.lanl.gov]), and the bottom row shows the sequence of the autologous virus of the indicated subject. Confirmed optimal epitopes are shown in gray shading. For 28 out of the 46 (61%) responses, the sequence of the peptide matched exactly the sequence of the autologous virus.
TABLE 2.
Comparison of peptide sequence (top lines) and autologous sequence (bottom lines) corresponding to the CD8 T-cell responses detected in study subjectsa
Shaded in gray are confirmed, recognized optimal epitopes. Positions of the peptides within Gag or Nef are given according to the HXB2 numbering system of the Los Alamos database.
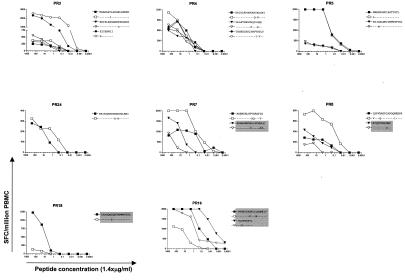
For 18 of 46 responses tested, the consensus sequence and the autologous sequence did not match. We next synthesized peptides according to the autologous sequence and compared recognition of both peptides in peptide titration assays (Table 2 and Fig. 3). For two patients (PR 10 and PR 13), the amino acid changes were outside the optimal epitope that the subjects recognized (A2 SLYNTVATL in both cases), and previous studies suggest that these changes did not alter antigen processing (15). In the cases in which the optimal epitope contained an amino acid change compared to the consensus sequence, recognition of the autologous optimal epitope was tested. For 13 of these 18, the response towards the autologous peptide was equal to or stronger than towards the consensus sequence, indicating that the autologous virus should be targeted by the established response and the autologous peptide was a better match. For 5 out of the 18 responses, the response towards the autologous peptides was weaker than towards the consensus peptide, suggesting that the amino acid change might represent escape variants.
FIG. 3.
Peptide titration curves comparing the consensus peptide and the peptide corresponding to the autologous sequence of indicated individuals. Highlighted in gray are nonmatching sequences where the CD8 T-cell response towards the autologous peptide was weaker than towards the consensus sequence clade B peptide. Peptide concentrations are shown in micrograms per milliliter.
Taken together, these data indicate that, for 41 out of 46 (89%) responses within Gag, the virus in vivo should be well recognized by the persistent CD8 T-cell response.
Functional avidity of detected responses.
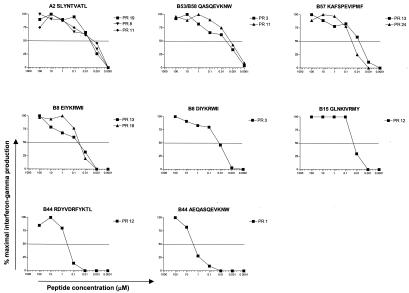
It has been reported that high-functional-avidity CD8 T-cell responses can preferentially recognize virally infected cells and therefore may represent particularly potent CTL responses (2, 22). We investigated next if the responses that persisted in the setting of high viral load were of a low functional avidity and therefore not likely to control viremia to a low level. This evaluation was done for 13 CD8 T-cell responses from a total of nine subjects for whom the optimal epitope was defined (Table 2 and Fig. 4). For six of those responses, we found a high functional avidity (1 to 10 nM). Five displayed an intermediate avidity (10 to 100 nM), and two displayed a low avidity (>100 nM) (Table 3). Of the 11 high- or intermediate-avidity responses, 8 were immunodominant responses of the corresponding subject. Interestingly, the functional avidity for the B57-restricted epitope KAFSPEVIPMF was high in subject PR 13 and intermediate in subject PR 24 (Fig. 4 and Table 3).
FIG. 4.
Peptide titration curves for confirmed optimal epitopes recognized by indicated study subjects. Peptide concentrations are presented in micromolars; CD8 T-cell responses are presented as percent maximal gamma interferon production. Functional avidity was determined as 50% maximal gamma interferon production.
TABLE 3.
Functional avidity for CD8 T-cell epitopes tested
| Functional avidity (1/2max response [nM]) (range) | CD8 T-cell epitope | HLA restric- tion | na |
|---|---|---|---|
| High: (1-10 nM) | |||
| 3.5 (1.1-5.0) | SL9 (SLYNTVATL) | A2 | 3 |
| 2.4 (1.1-4.0) | QW9 (QASQEVKNW) | B53, B58 | 2 |
| 6.5 (6.0-7.0) | KF11 (KAFSPEVIPMF) | B57 | 1 |
| Intermediate: (10-100 nM) | |||
| 32.5 (25.0-40.0) | KF11 (KAFSPEVIPMF) | B57 | 1 |
| 42 (27-67) | EI8 (EIYKRWII) | B8 | 2 |
| 13 | DI8 (DIYKRWII) | B8 | 1 |
| 18.5 (18.0-19.0) | GY9 (GLNKIVRMY) | B15 | 1 |
| Low: (>100 nM) | |||
| 430 | RL11 (RDYVDRFYKTL) | B44 | 1 |
| 1,530 (440-2,600) | AW11 (AEQASQEVKNW) | B44 | 1 |
n, number of subjects recognizing each epitope.
These results indicate that lack of sufficient immune control in progressive HIV infection occurs despite the presence of CD8 T-cell responses of high functional avidity.
CD8 T-cell responses decline when antigen is withdrawn.
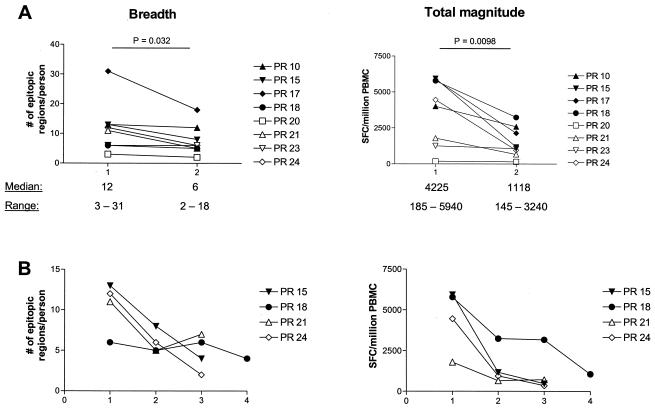
Eight of the study subjects were further evaluated following initiation of HAART. Figure 5A shows the results prior to treatment (time point 1) and 8 to 12 weeks after the initiation of HAART (time point 2). In seven out of eight subjects, the breadth of CD8 T-cell responses declined, while the total magnitude of responses decreased for all eight subjects studied. Differences were statistically significant (P = 0.032 for the breadth and P = 0.0098 for the magnitude). Four of these eight subjects remained on HAART with a suppressed viral load and were studied at additional time points after the initiation of HAART (Fig. 5B [time points 3 and 4]). This shows that there is a continuous decrease in magnitude and also in breadth of CD8 T-cell responses.
FIG. 5.
(A) Breadth and total magnitude of CD8 T-cell responses for eight subjects at two time points: time point 1 is without HAART; time point 2 is 8 to 12 weeks after initiation of HAART. Statistical analysis was done with the paired t test. (B) Longitudinal follow-up of breadth and magnitude of responses after the initiation of HAART. Time point 3 is 3 to 6 months after starting HAART, and time point 4 is at 1 year on therapy.
These results suggest that the CD8 T-cell responses are being driven by persistent exposure to antigen and provide further evidence that they recognize the viral sequence in vivo.
DISCUSSION
CD8 T-cell responses are thought to be crucial for control of viremia in HIV-1 infection (9, 12, 14, 30, 44). In the presence of a functional CD8 T-cell response, antigenic variation is an important mechanism of immune evasion caused by immune selection pressure (41). Based on the knowledge about viral escape derived largely from acute and early-stage infection with AIDS viruses, the CD8 T-cell response usually declines after escape occurs, since the antigen is essentially withdrawn (3, 13, 26, 31). We demonstrate here that strong and broadly directed, gamma interferon-positive CD8 T-cell responses exist in the presence of high viral loads and progressive disease course. Based on the sequence data presented, the predominant viral population in vivo in subjects who are failing to contain HIV-1 replication is predicted to be well recognized for 89% of evaluated CD8 T-cell responses. The fact that these CD8 T-cell responses are present at high frequencies and decline when antigen exposure is diminished by HAART supports the conclusion that they recognize the autologous virus. Therefore, these data strongly suggest a lack of strong immune selection pressure mediated by the majority of CD8 T-cell responses detected by gamma interferon secretion in the chronic progressive phase of HIV infection.
The lack of detectable immune selection pressure presented here is different from antiretroviral treatment, where drug selection pressure consistently results in viral resistance mutations in the presence of detectable viral loads. Reasons for this difference could be the availability of similar drug concentrations in all compartments, whereas there might be blind niches where CD8 T cells do not reach the virus. The efficacy of CD8 T-cell responses also depend on the type of infected cell and the way that antigen is presented. Down-regulation of major histocompatibility complex class I by Nef is one example of impaired antigen presentation and protection against cytotoxic-T-lymphocyte (CTL) killing (19, 46). In addition, CD8 T-cell responses are thought to be dependent on help by CD4 T cells (54), which are depleted in the cohort presented here. It is also possible that presentation of some of these epitopes may be affected by mutations in flanking regions. However, detailed analysis of at least one Gag A2 epitope failed to reveal this as a mechanism (15).
These data provide additional support for the hypothesis that lack of sufficient immune pressure is due to in vivo defects in CD8 T-cell function. This has been widely discussed recently, and previous studies have examined different features of CD8 T-cell function. Among them are low percentages of CD45RA+/CCR7− HIV-specific CD8 T cells (18) or high CD27 expression (8, 49) in contrast to controlled chronic infections like those with Epstein-Barr virus or cytomegalovirus. Others have described a limited proliferative capacity and low perforin expression of HIV-specific CD8 T cells in progressive HIV infection (37, 56). In addition, high-avidity CD8 T cells were shown to provide better protection against viral diseases in mouse models (2, 22). In simian immunodeficiency virus infection, it was shown that responses of high functional avidity induced escape within the first 4 weeks of infection (38). The study presented here extends the knowledge about CD8 T-cell avidity in that high-avidity CD8 T cells can be found despite uncontrolled viremia. Interestingly, the same epitope can display different levels of avidity in different subjects, a finding consistent with a recent report in primary HIV infection (17). Gag is one of the more conserved proteins of HIV-1. It has been theorized that lack of sequence variation might be due to major consequences for viral replicative capacity (51). If this—and not an impairment of CD8 T-cell function—was the reason for lack of escape in the present study, the subjects studied would be expected to have lower viral loads.
These data extend earlier studies of CD8 T-cell function by examining autologous viral sequences in relation to detectable responses in the chronic phase of HIV infection. There are limited prior reports examining escape from CTL responses in progressors, examining either a single case or one specific CTL epitope (28, 35). Similar conclusions were recently reported in a cohort of chronically infected B57+ subjects (36), limited to analysis of epitopes presented by HLA B57. As in this earlier study, our study is limited to one sequencing time point since most persons were started on HAART.
Based on the knowledge of escape in acute and early HIV infection (3, 13, 38, 43), it is quite possible that all the subjects studied here may have had viral escape towards CD8 T-cell responses that may have been generated during earlier stages of infection. This most likely includes epitopes within Gag and other HIV proteins. One reason for the relative lack of putative escape mutations in late infection might be that CTL specificities in chronic infection differ from those in acute infection. It has been shown for the A2-restricted epitope SLYNTVATL and the B57-restricted KAFSPEVIPMF—both found in the present cohort—that they arise later in the disease course (7a, 25), and a change of immunodominance has been described in chronic compared to acute viral infection in the lymphocytic choriomeningitis virus model (53). One could speculate that the responses detected in the present study evolved late in the disease course, maybe even at a time point when CD4 T cells had declined, and that they are therefore of a quality different from that of CD8 T cells in the early stages of HIV infection. On the other hand, late escape from a B27-restricted epitope was associated with disease progression (26, 31), which suggests that functional CD8 T-cell responses and strong immune selection pressure can exist in the chronic phase of infection. It was also shown that CTL clones can induce viral escape very rapidly under optimal in vitro conditions (55). But the CTL clones in that study were generated over weeks under optimal circumstances, including the presence of exogenous interleukin 2, which might restore functional defects (47).
Earlier reports have found a correlation between disease progression and CTL responses towards certain HIV proteins (32, 39, 48), but more recent studies using comprehensive screening techniques fail to confirm this (1, 11, 37). Although the subjects in the present study are more advanced in their disease course than cohorts studied before, data presented here are in accordance with the latter observation, as differences to controllers were not statistically significant.
There are limitations to the comprehensive screening technique used here. Screening with 18-amino-acid-long peptides might underestimate the real magnitude of a response substantially compared to using the optimal epitope (6). In addition, using the autologous sequence to screen for CTL responses leads to the detection of a broader response than any other sequence, even than the consensus sequence (4a). Therefore, additional CD8 T-cell responses might have been missed in this study.
Taken together, this study shows that, in the presence of viral antigen, strong and broadly directed gamma interferon-positive CD8 T-cell responses are detectable in chronic progressive HIV infection. The majority of these responses are likely to recognize the virus as indicated by the sequence data but most probably do not exert strong immune selection pressure on the virus. Low functional avidity does not explain the ineffectiveness of the CD8 T-cell responses, and they rapidly decline once the antigen is withdrawn. This has implications for the general classification of HIV-specific CD8 T-cell responses in the pathogenesis of HIV infection, because it might imply that there are different qualities of HIV-specific CD8 T-cell responses. The distinction between CD8 T cells of different quality is not possible by measuring gamma interferon production; therefore, new assays are needed to distinguish between them. If this is true, then it is significant for vaccine and immunotherapy trials. These trials aim at inducing functional CD8 T-cell responses and mainly utilize gamma interferon Elispot or intracellular cytokine assays to screen for responses (16, 21, 29, 40, 50). The data presented here suggest rethinking this approach.
Acknowledgments
We thank all study participants and the dedicated clinical research staff at the collaborating sites.
This study was supported by the National Institutes of Health (RO1 AI28568 and AI054178-01, contract no. NO1-A1-15442), the Deutsche Forschungsgemeinschaft DR424/1-1 (R.D.), the Howard Hughes Medical Institute (R.D. and B.D.W.), the Doris Duke Charitable Foundation (B.D.W.), the Partners/Fenway/Shattuck CFAR, the Merck Medical School Grant (T.M.A.), the Concerned Parents for AIDS Research (CPFA) (M.M.A.), and AmfAR (70577-31-RFT) (M.M.A.).
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander-Miller, M. A., G. R. Leggatt, and J. A. Berzofsky. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 4.Altfeld, M., M. M. Addo, R. L. Eldridge, X. G. Yu, S. Thomas, A. Khatri, D. Strick, M. N. Phillips, G. B. Cohen, S. A. Islam, S. A. Kalams, C. Brander, P. J. Goulder, E. S. Rosenberg, and B. D. Walker. 2001. Vpr is preferentially targeted by CTL during HIV-1 infection. J. Immunol. 167:2743-2752. [DOI] [PubMed] [Google Scholar]
- 4a.Altfeld, M., et al. 2003. Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences. J. Virol. 77:7330-7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altfeld, M., T. M. Allen, X. G. Yu, M. N. Johnston, D. Agrawal, B. T. Korber, D. C. Montefiori, D. H. O'Connor, B. T. Davis, P. K. Lee, E. L. Maier, J. Harlow, P. J. Goulder, C. Brander, E. S. Rosenberg, and B. D. Walker. 2002. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 420:434-439. [DOI] [PubMed] [Google Scholar]
- 6.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altfeld, M. A., A. Trocha, R. L. Eldridge, E. S. Rosenberg, M. N. Phillips, M. M. Addo, R. P. Sekaly, S. A. Kalams, S. A. Burchett, K. McIntosh, B. D. Walker, and P. J. Goulder. 2000. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J. Virol. 74:8541-8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Altfeld, M. A., et al. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS, in press. [DOI] [PubMed]
- 8.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 9.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 10.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betts, M. R., J. P. Casazza, and R. A. Koup. 2001. Monitoring HIV-specific CD8+ T cell responses by intracellular cytokine production. Immunol. Lett. 79:117-125. [DOI] [PubMed] [Google Scholar]
- 12.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 14.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr. Opin. Immunol. 11:451-459. [DOI] [PubMed] [Google Scholar]
- 15.Brander, C., O. O. Yang, N. G. Jones, Y. Lee, P. Goulder, R. P. Johnson, A. Trocha, D. Colbert, C. Hay, S. Buchbinder, C. C. Bergmann, H. J. Zweerink, S. Wolinsky, W. A. Blattner, S. A. Kalams, and B. D. Walker. 1999. Efficient processing of the immunodominant, HLA-A*0201-restricted human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitope despite multiple variations in the epitope flanking sequences. J. Virol. 73:10191-10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao, H., P. Kaleebu, D. Hom, J. Flores, D. Agrawal, N. Jones, J. Serwanga, M. Okello, C. Walker, H. Sheppard, R. El-Habib, M. Klein, E. Mbidde, P. Mugyenyi, B. Walker, J. Ellner, and R. Mugerwa. 2003. Immunogenicity of a recombinant human immunodeficiency virus (HIV)-canarypox vaccine in HIV-seronegative Ugandan volunteers: results of the HIV Network for Prevention Trials 007 Vaccine Study. J. Infect. Dis. 187:887-895. [DOI] [PubMed] [Google Scholar]
- 17.Cao, J., J. McNevin, S. Holte, L. Fink, L. Corey, and M. J. McElrath. 2003. Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. J. Virol. 77:6867-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 19.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 20.Connick, E., R. L. Schlichtemeier, M. B. Purner, K. M. Schneider, D. M. Anderson, S. MaWhinney, T. B. Campbell, D. R. Kuritzkes, J. M. Douglas, Jr., F. N. Judson, and R. T. Schooley. 2001. Relationship between human immunodeficiency virus type 1 (HIV-1)-specific memory cytotoxic T lymphocytes and virus load after recent HIV-1 seroconversion. J. Infect. Dis. 184:1465-1469. [DOI] [PubMed] [Google Scholar]
- 21.Couillin, I., F. Letourneur, P. Lefebvre, J. G. Guillet, and F. Martinon. 2001. DNA vaccination of macaques with several different Nef sequences induces multispecific T cell responses. Virology 279:136-145. [DOI] [PubMed] [Google Scholar]
- 22.Derby, M., M. Alexander-Miller, R. Tse, and J. Berzofsky. 2001. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J. Immunol. 166:1690-1697. [DOI] [PubMed] [Google Scholar]
- 23.Draenert, R., M. Altfeld, C. Brander, N. Basgoz, C. Corcoran, A. G. Wurcel, D. R. Stone, S. A. Kalams, A. Trocha, M. M. Addo, P. J. Goulder, and B. D. Walker. 2003. Comparison of overlapping peptide sets for detection of antiviral CD8 and CD4 T cell responses. J. Immunol. Methods 275:19-29. [DOI] [PubMed] [Google Scholar]
- 24.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the Gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulder, P. J., M. A. Altfeld, E. S. Rosenberg, T. Nguyen, Y. Tang, R. L. Eldridge, M. M. Addo, S. He, J. S. Mukherjee, M. N. Phillips, M. Bunce, S. A. Kalams, R. P. Sekaly, B. D. Walker, and C. Brander. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 27.Goulder, P. J., Y. Tang, S. I. Pelton, and B. D. Walker. 2000. HLA-B57-restricted cytotoxic T-lymphocyte activity in a single infected subject toward two optimal epitopes, one of which is entirely contained within the other. J. Virol. 74:5291-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay, C. M., D. J. Ruhl, N. O. Basgoz, C. C. Wilson, J. M. Billingsley, M. P. DePasquale, R. T. D'Aquila, S. M. Wolinsky, J. M. Crawford, D. C. Montefiori, and B. D. Walker. 1999. Lack of viral escape and defective in vivo activation of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes in rapidly progressive infection. J. Virol. 73:5509-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelleher, A. D., C. Long, E. C. Holmes, R. L. Allen, J. Wilson, C. Conlon, C. Workman, S. Shaunak, K. Olson, P. Goulder, C. Brander, G. Ogg, J. S. Sullivan, W. Dyer, I. Jones, A. J. McMichael, S. Rowland-Jones, and R. E. Phillips. 2001. Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J. Exp. Med. 193:375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 35.Meyerhans, A., G. Dadaglio, J. P. Vartanian, P. Langlade-Demoyen, R. Frank, B. Asjo, F. Plata, and S. Wain-Hobson. 1991. In vivo persistence of a HIV-1-encoded HLA-B27-restricted cytotoxic T lymphocyte epitope despite specific in vitro reactivity. Eur. J. Immunol. 21:2637-2640. [DOI] [PubMed] [Google Scholar]
- 36.Migueles, S. A., A. C. Laborico, H. Imamichi, W. L. Shupert, C. Royce, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, C. W. Hallahan, and M. Connors. 2003. The differential ability of HLA B(*)5701+ long-term nonprogressors and progressors to restrict human immunodeficiency virus replication is not caused by loss of recognition of autologous viral gag sequences. J. Virol. 77:6889-6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493-499. [DOI] [PubMed] [Google Scholar]
- 39.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 40.Oxenius, A., D. A. Price, H. F. Gunthard, S. J. Dawson, C. Fagard, L. Perrin, M. Fischer, R. Weber, M. Plana, F. Garcia, B. Hirschel, A. McLean, and R. E. Phillips. 2002. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc. Natl. Acad. Sci. USA 99:13747-13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips, R. E. 2002. Immunology taught by Darwin. Nat. Immunol. 3:987-989. [DOI] [PubMed] [Google Scholar]
- 42.Plata, F., B. Autran, L. P. Martins, S. Wain-Hobson, M. Raphael, C. Mayaud, M. Denis, J. M. Guillon, and P. Debre. 1987. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature 328:348-351. [DOI] [PubMed] [Google Scholar]
- 43.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 45.Schmitz, J. E., M. J. Kuroda, S. Santra, M. A. Simon, M. A. Lifton, W. Lin, R. Khunkhun, M. Piatak, J. D. Lifson, G. Grosschupff, R. S. Gelman, P. Racz, K. Tenner-Racz, K. A. Mansfield, N. L. Letvin, D. C. Montefiori, and K. A. Reimann. 2003. Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 77:2165-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 47.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 48.van Baalen, C. A., O. Pontesilli, R. C. Huisman, A. M. Geretti, M. R. Klein, F. de Wolf, F. Miedema, R. A. Gruters, and A. D. Osterhaus. 1997. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J. Gen. Virol. 78:1913-1918. [DOI] [PubMed] [Google Scholar]
- 49.van Baarle, D., S. Kostense, E. Hovenkamp, G. Ogg, N. Nanlohy, M. F. Callan, N. H. Dukers, A. J. McMichael, M. H. van Oers, and F. Miedema. 2002. Lack of Epstein-Barr virus- and HIV-specific CD27− CD8+ T cells is associated with progression to viral disease in HIV-infection. AIDS 16:2001-2011. [DOI] [PubMed] [Google Scholar]
- 50.Vogel, T. U., H. Horton, D. H. Fuller, D. K. Carter, K. Vielhuber, D. H. O'Connor, T. Shipley, J. Fuller, G. Sutter, V. Erfle, N. Wilson, L. J. Picker, and D. I. Watkins. 2002. Differences between T cell epitopes recognized after immunization and after infection. J. Immunol. 169:4511-4521. [DOI] [PubMed] [Google Scholar]
- 51.Wagner, R., B. Leschonsky, E. Harrer, C. Paulus, C. Weber, B. D. Walker, S. Buchbinder, H. Wolf, J. R. Kalden, and T. Harrer. 1999. Molecular and functional analysis of a conserved CTL epitope in HIV-1 p24 recognized from a long-term nonprogressor: constraints on immune escape associated with targeting a sequence essential for viral replication. J. Immunol. 162:3727-3734. [PubMed] [Google Scholar]
- 52.Walker, B. D., S. Chakrabarti, B. Moss, T. J. Paradis, T. Flynn, A. G. Durno, R. S. Blumberg, J. C. Kaplan, M. S. Hirsch, and R. T. Schooley. 1987. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328:345-348. [DOI] [PubMed] [Google Scholar]
- 53.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wodarz, D., and V. A. Jansen. 2001. The role of T cell help for anti-viral CTL responses. J. Theor. Biol. 211:419-432. [DOI] [PubMed] [Google Scholar]
- 55.Yang, O. O., P. T. Sarkis, A. Ali, J. D. Harlow, C. Brander, S. A. Kalams, and B. D. Walker. 2003. Determinants of HIV-1 mutational escape from cytotoxic T lymphocytes. J. Exp. Med. 197:1365-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, D., P. Shankar, Z. Xu, B. Harnisch, G. Chen, C. Lange, S. J. Lee, H. Valdez, M. M. Lederman, and J. Lieberman. 2003. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood 101:226-235. [DOI] [PubMed] [Google Scholar]