Summary
The polymeric immunoglobulin receptor (pIgR) mediates transcytosis of dimeric immunoglobulin A (dIgA) and its release into mucosal secretions. The present study reveals the complexity of the trafficking of pIgR to the apical plasma membrane in epithelial cells with exocrine secretory functions; in rabbit lacrimal gland acinar cells (LGACs), trafficking of pIgR involves both the transcytotic pathway and one arm of the regulated secretory pathway. By specifically tracking pIgR endocytosed from the basolateral membrane, we show here that the Rab11a-regulated transcytotic pathway mediates the basal-to-apical transport of pIgR, and that pIgR sorted into the transcytotic pathway does not access the regulated secretory pathway. However, previous work in LGACs expanded in the present study has shown that some pIgR is localized to Rab3D-enriched mature secretory vesicles (SVs). Myosin Vb and myosin Vc motors modulate release of proteins from the Rab11a-regulated transcytotic pathway and the Rab3D-enriched secretory pathway in LGACs, respectively. Confocal fluorescence microscopy and biochemical assays showed that inhibition of myosin Vb and myosin Vc activity by overexpression of their dominant-negative mutants each significantly but differentially impaired aspects of apically targeted pIgR trafficking and secretory component release, suggesting that these motors function to regulate pIgR trafficking in both the transcytotic and exocytotic pathways. Intriguingly, a second mature SV population enriched in Rab27b was devoid of pIgR cargo, suggesting the specialization of Rab3D-enriched mature SVs to carry a particular subset of cargo proteins from the trans-Golgi network to the apical plasma membrane.
Key words: Transcytosis, Secretion, Polymeric immunoglobulin receptor, Rab11a, Rab3D, Rab27b, Myosin V
Introduction
The polymeric immunoglobulin receptor (pIgR) is expressed in multiple polarized cells, including hepatocytes, intestinal epithelial cells, and acinar cells of the mammary, salivary and lacrimal glands (LGs) (Kaetzel, 2005; Evans et al., 2008; Kimura et al., 2008). pIgR mediates the transcellular transport of immunoglobulins A and M (Rojas and Apodaca, 2002), playing an important role in mucosal immune defense. In pIgR-transfected Madin–Darby canine kidney (MDCK) cells, newly synthesized pIgR traffics from the trans-Golgi network to the basolateral plasma membrane (BLM), where it binds to its ligand dIgA (Apodaca et al., 1994; Mostov et al., 1995; Song et al., 1994). With or without dIgA, pIgR is endocytosed from the BLM and transported through a series of endosomal compartments to the apical plasma membrane (APM) (Apodaca et al., 1994; Mostov et al., 1995; Su et al., 2010). At the APM, the pIgR extracellular domain is proteolytically cleaved and released as either secretory component (SC) bound to dIgA (sIgA) or free SC, both of which perform protective functions (Rojas and Apodaca, 2002; Bonner et al., 2007).
Although the transfected MDCK system provided valuable insights regarding transcytosis of pIgR, these cells do not express other apically targeted secretory/exocytotic pathways characteristic of the glandular epithelial cells responsible for significant physiological secretion of pIgR in vivo. Investigation of pIgR trafficking in these models reveals additional complexity. For instance, lacrimal gland acinar cells (LGACs) are epithelial cells specialized for production, packaging and regulated exocytosis of diverse tear proteins which protect the vulnerable ocular surface. LGACs use both the regulated secretory pathway and the transcytotic pathway to transport proteins into tear fluid (Evans et al., 2008; Chiang et al., 2011; Xu et al., 2011).
Multiple Rab proteins regulate protein secretion (Hutagalung and Novick, 2011), and two of them are constituents of regulated secretory vesicles (SVs) in LGACs: Rab3D and Rab27b (Marchelletta et al., 2008; Chiang et al., 2011; Chiang et al., 2012). Our previous study showed that a cohort of pIgR and free SC is localized to Rab3D-enriched SVs, and the kinetics of SC release following exposure of LGACs to the agonist carbachol (CCh) suggested a ‘burst’ effect consistent with rapid mobilization and release from this pool of SVs (Evans et al., 2008). Thus in LGACs, pIgR is present in the regulated secretory pathway and can be cleaved intravesicularly to create a pool of SC available for rapid release by the discharge of SVs. On the other hand, Rab27b labels a pool of SVs that package and secrete a heterologously expressed protein, syncollin (Chiang et al., 2011). Despite the proximity of Rab27b- and Rab3D-enriched mature SVs in the subapical region of LGACs, the relationships between these populations of SVs are not well characterized and are a focus of the current study within the context of pIgR trafficking. Another participant in the regulated secretory pathway in LGACs is the actin-based motor, myosin Vc (Marchelletta et al., 2008; Chiang et al., 2011).
Apart from the regulated secretory pathway, we have characterized a transcytotic pathway in LGACs for mediating transcytosis of pIgR and dIgA from the BLM to the APM (Xu et al., 2011). Key molecular regulators of the transcytotic pathway in LGACs are Rab11a, the small GTPase involved in regulating membrane recycling and transcytosis in other cells (Lapierre et al., 2001; Volpicelli et al., 2002; Ducharme et al., 2007; Wang et al., 2008), and myosin Vb (Wilcke et al., 2000; Lapierre et al., 2001; Volpicelli et al., 2002).
Utilizing strategies which selectively impair transcytotic versus exocytotic trafficking in LGACs, we show that the transcytotic pathway mediates basal-to-apical transport of pIgR, and that the Rab3D-enriched mature SVs contain a separate pool of pIgR, presumably acquired from the biosynthetic pathway, that appears inaccessible to pIgR trafficked from the BLM. Moreover, Rab27b-enriched mature SVs appear devoid of pIgR and physically distinct from the Rab3D-enriched mature SVs. A model is proposed in which the three apically directed trafficking pathways characterized here are distinct with respect to their acquisition of cargo, their regulation by Rabs, and their regulation by the myosin V class of molecular motors.
Results
Expression of fluorescent pIgR constructs in primary LGACs
Adenovirus (Ad) constructs are capable of transducing rabbit primary LGACs at efficiencies >80% (Jerdeva et al., 2005; Evans et al., 2008; Chiang et al., 2011; Xu et al., 2011). We constructed Ad encoding human pIgR fused with enhanced green fluorescent protein (EGFP) at the C′ terminus of the cytoplasmic domain of the hpIgR (hpIgR-EGFP) (Fig. 1A). Co-transduction of cells with Ad hpIgR-EGFP and Adeno-X Tet-On® followed by doxycycline treatment induced expression of hpIgR-EGFP as verified by western blotting: hpIgR-EGFP was recognized by both anti-human SC (hSC) and anti-GFP antibodies (Fig. 1B). hpIgR-EGFP migrates as a doublet, a common feature of pIgR expressed in other cells due to different levels of glycosylation (Kühn et al., 1983; Deitcher et al., 1986; Asano et al., 1998; Ogura, 2005). The anti-hSC antibody specificity for hpIgR-EGFP and not endogenous rabbit pIgR is further shown in supplementary material Fig. S1A,B. hpIgR-EGFP was mostly localized to the subapical cytoplasm beneath the APM, as well as to the BLM, comparable to endogenous pIgR in non-transduced cells (Fig. 1C,D), suggesting that EGFP did not interfere with the appropriate localization of hpIgR-EGFP. In addition, we have previously noted that endogenous pIgR is extensively colocalized with exogenous EGFP-Rab11a (Xu et al., 2011); conversely, hpIgR-EGFP showed substantial colocalization with endogenous Rab11 (Fig. 1E).
Fig. 1.
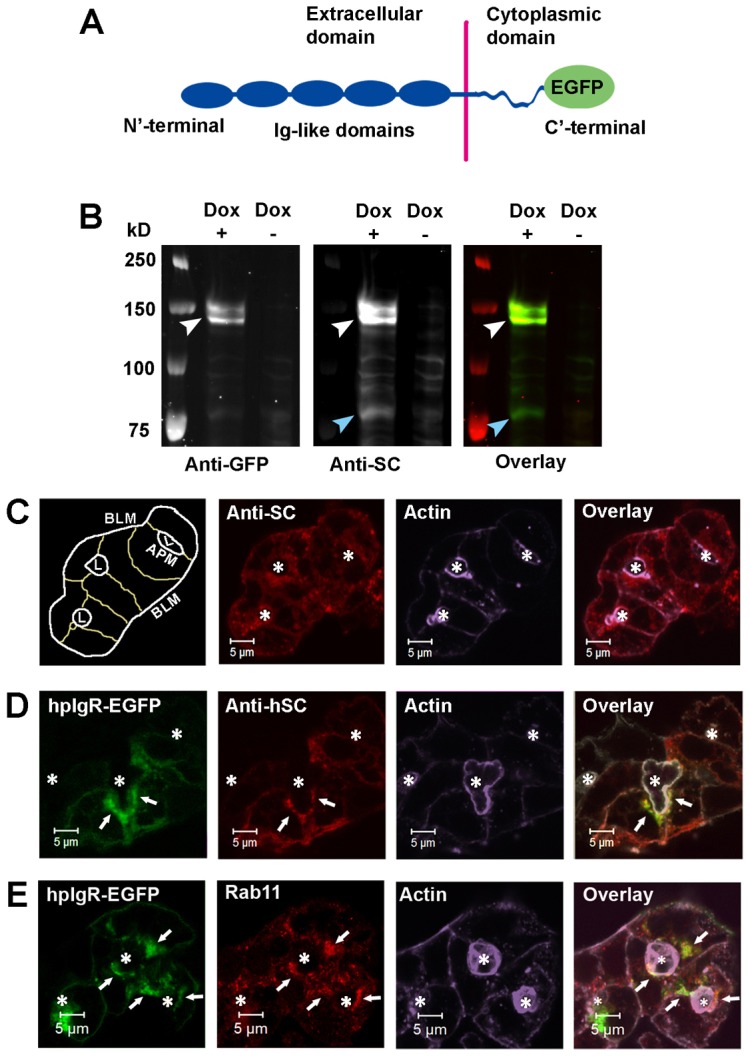
Characterization of the hpIgR construct. (A) Schematic of the hpIgR-EGFP construct showing EGFP fusion to the C′-terminal cytoplasmic domain of hpIgR. (B) LGACs were co-transduced with Ad hpIgR-EGFP and Adeno-X Tet-On®. After induction with 0.1 µg/ml doxycycline (+) or not (−) overnight, cells were lysed with non-denaturing lysis buffer. Lysate was pre-cleared as in the Materials and Methods, and analyzed by western blotting using primary goat anti-hSC or mouse anti-GFP antibodies, as well as secondary IRDye®800-conjugated donkey anti-goat or IRDye®700-conjugated goat anti-mouse antibodies, respectively. White arrowhead, hpIgR-EGFP; blue arrowhead, SC. (C,D) Non-transduced LGACs (C) or LGACs expressing hpIgR-EGFP (D) were fixed, permeabilized, and labeled with primary goat anti-hSC antibody, as well as secondary Alexa Fluor®-568-conjugated donkey anti-goat antibody and Alexa Fluor®-647-conjugated phalloidin. (E) LGACs expressing hpIgR-EGFP were fixed, permeabilized, and labeled with primary mouse anti-Rab11 antibody, secondary Alexa Fluor®-568-conjugated goat anti-mouse antibody and Alexa Fluor®-647-conjugated phalloidin. White arrows, colocalization between red and green; *, lumena. Scale bars: 5 µm.
Trafficking of pIgR in the transcytotic pathway
Previous work suggested that Rab11a regulates transcytosis of pIgR and dIgA in LGACs (Xu et al., 2011). To further investigate this, we specifically labeled the pool of hpIgR-EGFP at the BLM with its natural ligand, dIgA, or with anti-hSC antibody to track the basolaterally endocytosed hpIgR-EGFP. Since virtually all apical pIgR has the SC region cleaved, the anti-SC antibody does not bind apical pIgR; also since the antibody is added to live acini cooled to 4°C to impair endocytosis, it should be accessible only to plasma membrane and not intracellular hpIgR-EGFP.
Labeling the endogenous rabbit pIgR pool is theoretically achievable through use of rabbit dIgA. Although we have generated rabbit dIgA (Xu et al., 2011), the complex formed by fluorophore-conjugated rabbit dIgA and endogenous rabbit pIgR does not generate resolvable fluorescent signals in imaging experiments (data not shown). However, confocal fluorescence microscopy showed that rabbit dIgA can bind to hpIgR-EGFP: LGACs overexpressing hpIgR-EGFP were co-incubated at 4°C with rhodamine-conjugated dIgA and an anti-hSC antibody that does not react with endogenous rabbit SC (supplementary material Fig. S1C). hpIgR-EGFP on the BLM was clearly labeled by anti-hSC antibody and rhodamine-conjugated dIgA, whereas hpIgR-EGFP inside LGACs was not labeled because the antibodies could only access the cell surface (Fig. 2A). The label was exclusively basolateral at 0 minutes (prior to cell warming and internalization of receptor), and the immunofluorescence signal on the APM was negligible. After warming to 37°C to allow endocytosis and trafficking of the single cohort of basolateral hpIgR-EGFP, time-course experiments clearly showed that the anti-hSC–hpIgR-EGFP complex colocalized with dIgA–hpIgR-EGFP complex at all time points (Fig. 2A). This result suggests that the hpIgR-EGFP labeled by anti-hSC antibody is endocytosed and trafficked together with the dIgA–hpIgR-EGFP complex. This novel approach was thus able to resolve the trafficking of basolaterally endocytosed pIgR from the total pIgR pool, allowing us to investigate the interrelationship between pIgR present in the transcytotic pathway and the regulated secretory pathway.
Fig. 2.
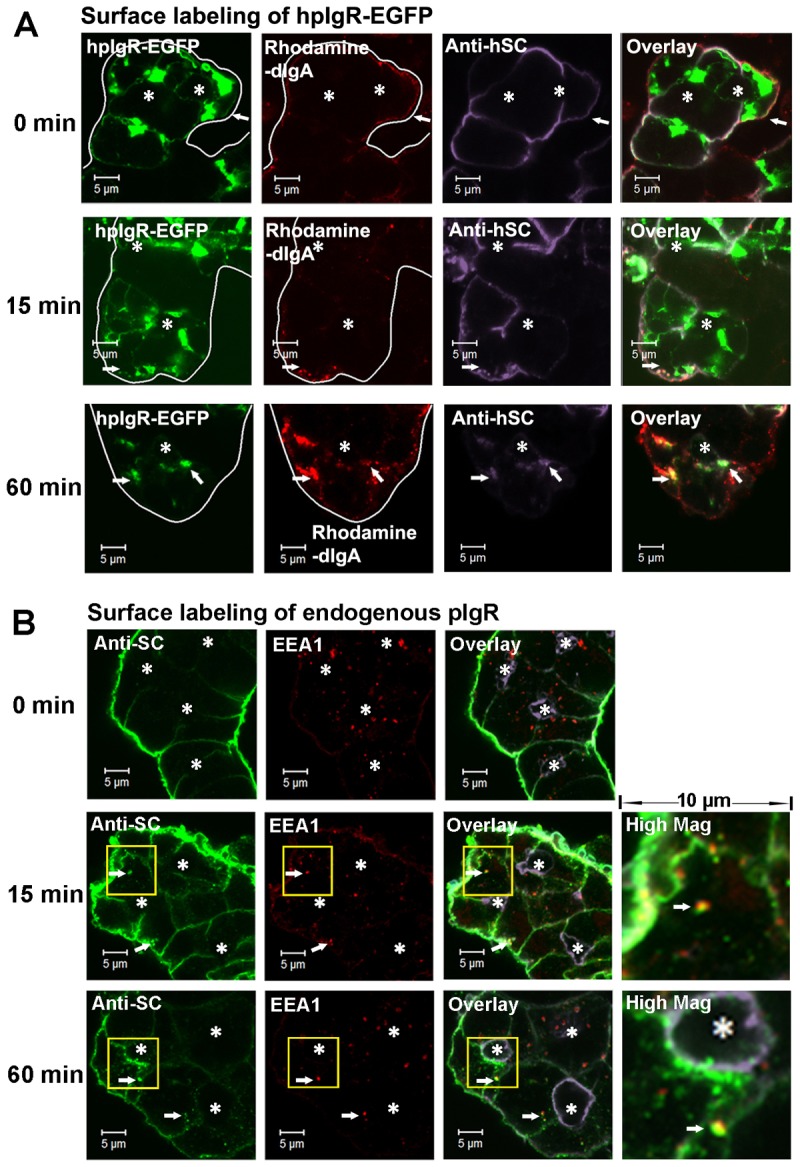
Post-endocytotic trafficking of basolateral hpIgR-EGFP in LGACs. (A) LGACs expressing hpIgR-EGFP were co-incubated with 100 µg/ml rhodamine-conjugated rabbit dIgA and 4 µg/ml primary mouse anti-hSC antibody for 1 hour at 4°C. Subsequently, LGACs were rinsed and incubated at 37°C, fixed at the time points indicated, and labeled with secondary Alexa Fluor®-633-conjugated goat anti-mouse antibody. Cellular outlines and positions of lumena were obtained by differential interference contrast imaging. White arrows, colocalization between red, green and purple. (B) Non-transduced LGACs were incubated with sheep anti-rabbit SC serum for 1 hour at 4°C, rinsed and incubated at 37°C. Cells were fixed at the time points indicated, permeabilized, and labeled with primary mouse anti-EEA1 antibody, secondary Alexa Fluor®-488-conjugated donkey anti-sheep and Alexa Fluor®-568-conjugated goat anti-mouse antibodies, and Alexa-Fluor®-647-conjugated phalloidin. Actin is displayed in purple in the overlay and high magnification images. White arrows, colocalization between red and green; *, lumena. Scale bars: 5 µm.
Because hpIgR-EGFP occupies one fluorescence filter channel for confocal microscopy, labeling endogenous rabbit pIgR would enable tracking of the post-endocytotic trafficking of pIgR with additional labels. We labeled endogenous rabbit pIgR on the BLM with sheep antiserum raised against the SC domain of rabbit pIgR. In order to verify the specificity of anti-rabbit SC antibody in sheep serum for binding pIgR on the BLM, SC purified from rabbit bile was used as the competitor for antibody binding. Escalating concentrations of rabbit SC significantly reduced the indirect fluorescent signal on the BLM (supplementary material Fig. S2A), indicating that sheep anti-rabbit SC antibodies bind to pIgR with high specificity. When pre-immune sheep serum was used as control, no endocytosed cargo with immunofluorescence was observed (supplementary material Fig. S2B); together, this observation plus the competition experiment suggest that Fc receptor does not non-specifically bind or endocytose anti-SC antibodies. Therefore, labeling basolateral pIgR with anti-SC antibodies provides a reliable method for tracking the post-endocytotic trafficking of endogenous pIgR.
After warming to 37°C, basolaterally endocytosed pIgR accumulated in early endosomes labeled by EEA1 by 15 minutes; at 30 minutes (not shown) and 60 minutes, a subset of pIgR still remained in the early endosomes (Fig. 2B). However, at 30 minutes, endocytosed pIgR was also present in the subapical compartment labeled by EGFP-Rab11a (Fig. 3A), and continued to accumulate in this compartment by 60 minutes (Fig. 3A). These observations indicate that endocytosed pIgR is eventually sorted from early endosomes into Rab11a-enriched subapical vesicles involved in the transcytotic pathway.
Fig. 3.
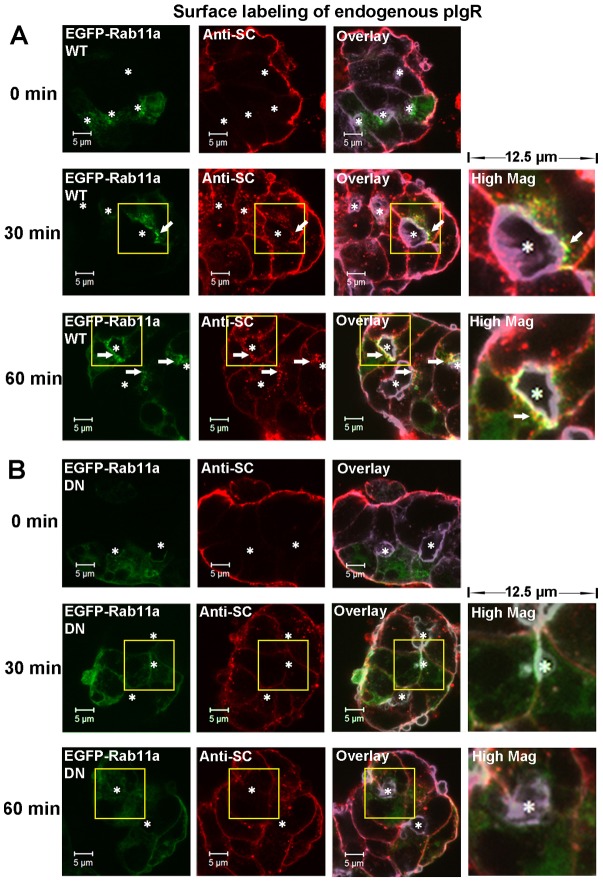
Rab11a regulates the basal-to-apical transcytosis of pIgR. LGACs expressing EGFP-Rab11a WT (A) or EGFP-Rab11a DN (B) were incubated with sheep anti-rabbit SC antiserum for 1 hour at 4°C, rinsed and incubated at 37°C. Cells were fixed at the time points indicated, permeabilized, and labeled with secondary Alexa Fluor®-568-conjugated donkey anti-sheep antibody and Alexa Fluor®-647-conjugated phalloidin. Actin is displayed in purple in the overlay and high magnification images. White arrows, colocalization between red and green; *, lumena. Scale bars: 5 µm.
In order to further probe the role of Rab11a in the transcytosis of pIgR, we expressed EGFP-Rab11a DN (dominant negative) in LGACs using an Ad construct. EGFP-Rab11a DN significantly reduced the amount of basolaterally endocytosed pIgR in the subapical region (Fig. 3B), indicating the inhibition of basal-to-apical transport of endocytosed pIgR. These observations indicate that the basal-to-apical transport of pIgR requires Rab11a functionality.
Trafficking of pIgR in the regulated secretory pathway
Rab3D is a well-known marker of SVs in LGACs (Evans et al., 2008) and other secretory cells (Ohnishi et al., 1996; Tian et al., 2010). Confocal microscopy of immunofluorescence showed that endogenous Rab3D was also partially colocalized with γ-adaptin (supplementary material Fig. S3A), a marker of late-Golgi or TGN trafficking (Wong and Brodsky, 1992; Chiang et al., 2012). This suggests that the Rab3D-enriched membrane compartment originates from the biosynthetic compartment in LGACs. In this study, we constructed an Ad vector encoding rabbit Rab3D linked to mCherry fluorescent protein on the N′-terminus (mCherry-Rab3D) (Fig. 4A). mCherry-Rab3D expressed in LGACs showed similar subapical distribution to endogenous Rab3D (Fig. 4B) (Evans et al., 2008), but the morphology of the membrane compartment labeled by mCherry-Rab3D seemed to be slightly altered by the overexpression of mCherry-Rab3D. Morphological changes in membrane compartments caused by overexpression of other Rab proteins have been described previously (Bucci et al., 1992; Wilson et al., 1994; Wilcke et al., 2000; Mesa et al., 2001; Hölttä-Vuori et al., 2002). Our previous work showed a direct interaction between Rab3D and the cytoplasmic domain of pIgR (Evans et al., 2008). Co-immunoprecipitation experiments likewise demonstrated a direct interaction between mCherry-Rab3D and hpIgR-EGFP (Fig. 4C,D), suggesting that mCherry-Rab3D is functionally comparable with endogenous Rab3D. This result is also consistent with the extensive colocalization of subapical hpIgR-EGFP with mCherry-Rab3D (Fig. 5A).
Fig. 4.
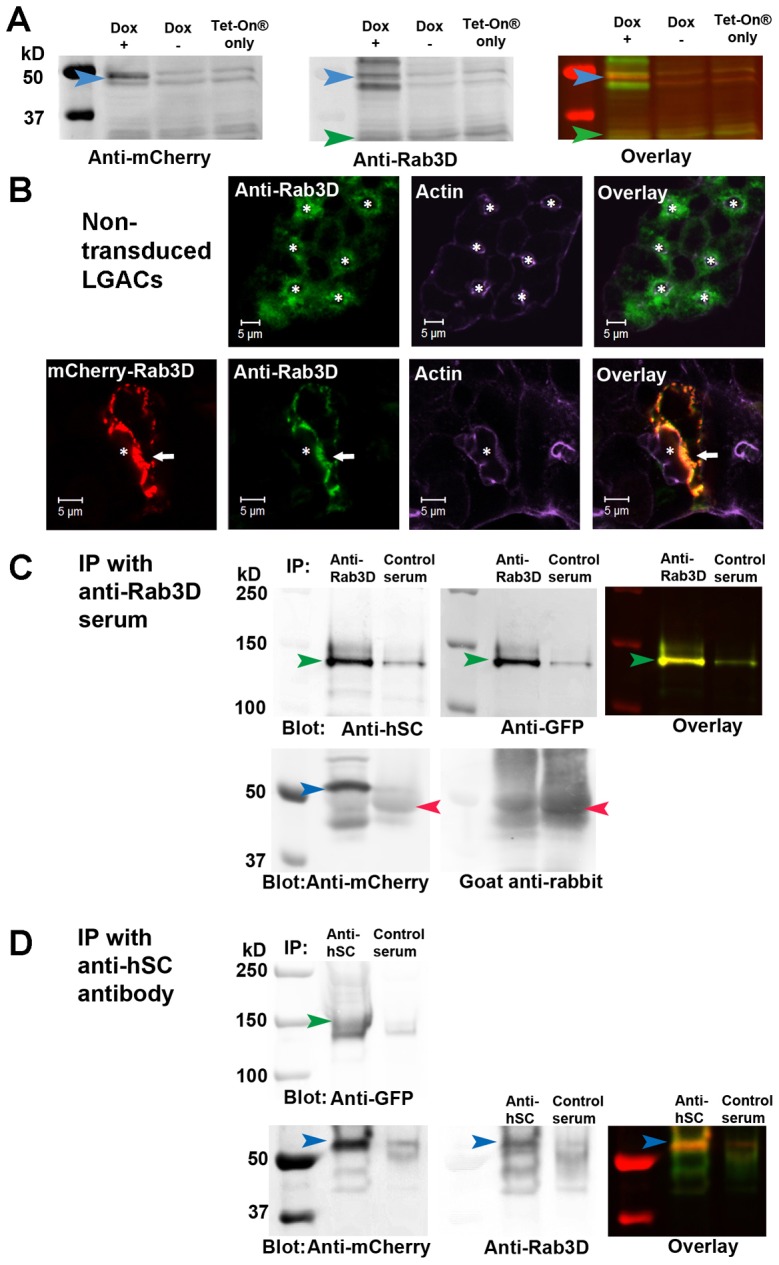
Characterization of mCherry-Rab3D expressed in LGACs. (A) LGACs co-transduced with Ad mCherry-Rab3D and Adeno-X Tet-On® were cultured overnight with 0.1 µg/ml doxycycline (Dox +) or not (Dox −); LGACs transduced with Adeno-X Tet-On® alone were cultured overnight with 0.1 µg/ml doxycycline (Tet-On® only). All LGACs were lysed with non-denaturing lysis buffer. Lysate was pre-cleared as in the Materials and Methods, and analyzed by western blotting using primary anti-Rab3D rabbit antiserum and mouse anti-mCherry monoclonal antibody, as well as secondary IRDye®800-conjugated goat anti-rabbit (green in the overlay image) and IRDye®700-conjugated goat anti-mouse (red in the overlay image) antibodies. Blue arrowhead, mCherry-Rab3D; green arrowhead, endogenous Rab3D. The expression level of mCherry-Rab3D was 5-fold higher than endogenous Rab3D. (B) LGACs expressing mCherry-Rab3D and non-transduced LGACs were fixed, permeabilized and labeled with primary rabbit anti-Rab3D serum, secondary Alexa Fluor®-488-conjugated donkey anti-rabbit antibody and Alexa Fluor®-647-conjugated phalloidin. White arrow, colocalization between red and green; *, lumena. Scale bars: 5 µm. (C,D) Immunoprecipitation (IP) was performed on lysates of LGACs co-expressing mCherry-Rab3D and hpIgR-EGFP as described in Materials and Methods. Rabbit anti-Rab3D serum was used to immunoprecipitate mCherry-Rab3D, and pre-immune rabbit serum was used as a control (C). Goat anti-hSC antibody was used to immunoprecipitate pIgR-EGFP, and pre-immune goat serum was used as control (D). The immunoprecipitated mCherry-Rab3D and hpIgR-EGFP were detected by western blotting, using mouse anti-mCherry, mouse anti-GFP and goat anti-hSC primary antibodies, as well as IRDye®700-conjugated goat anti-mouse (red in overlay), IRDye®800-conjugated donkey anti-goat or goat anti-rabbit (green in overlay) secondary antibodies. This image is representative for western blotting results from three preparations. Green arrowhead, hpIgR-EGFP; blue arrowhead, mCherry-Rab3D; red arrowhead, rabbit immunoglobulin heavy chain.
Fig. 5.
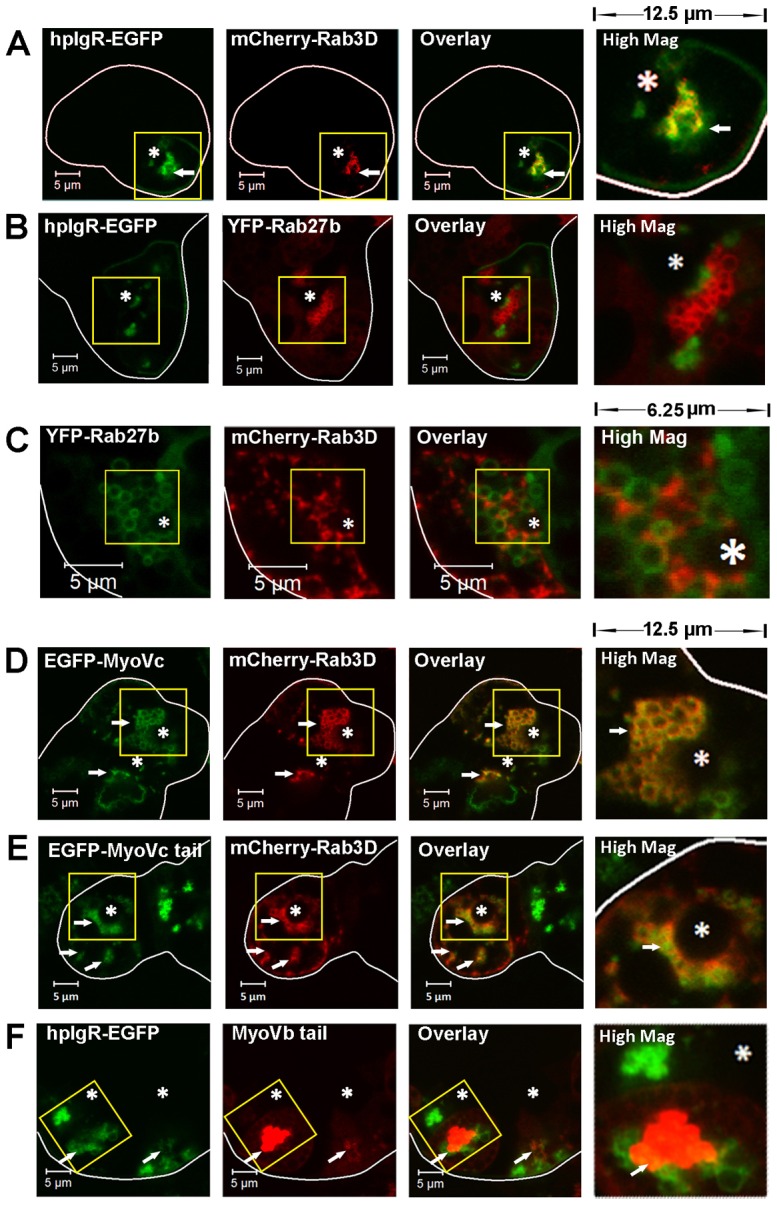
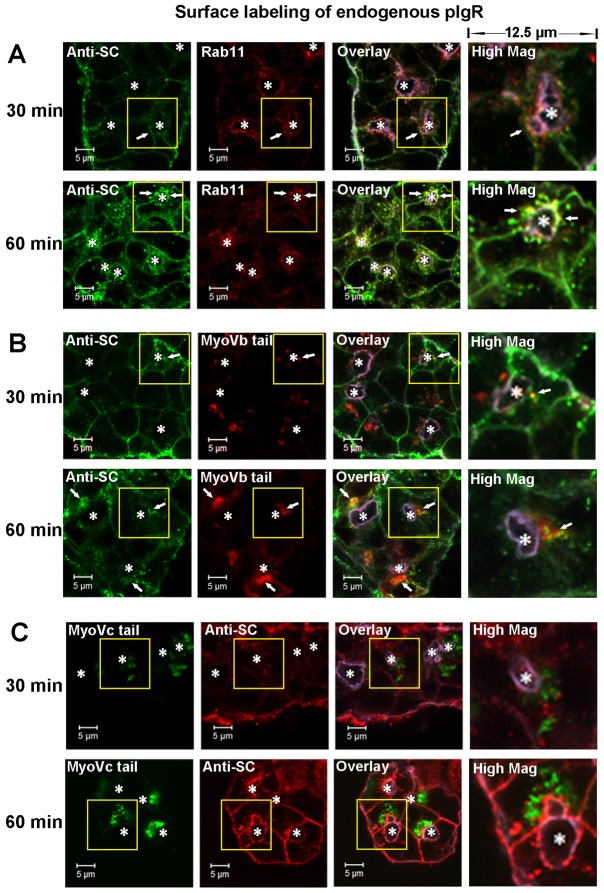
The distribution of hpIgR-EGFP in the regulated secretory pathway and the transcytotic pathway. Live LGACs co-expressing fluorescent fusion proteins were imaged by confocal fluorescence microscopy to show localization patterns of various markers. Cellular outlines were obtained by differential interference contrast imaging. (A) hpIgR-EGFP colocalized with mCherry-Rab3D. (B) The hpIgR-EGFP-enriched compartment was distinct from YFP-Rab27b-enriched SVs. (C) YFP-Rab27b-enriched SVs were distinct from mCherry-Rab3D-enriched SVs. (D) EGFP-myosin Vc was enriched in the mCherry-Rab3D-enriched SVs. (E) EGFP-myosin Vc tail colocalized with the membrane compartment labeled by mCherry-Rab3D. (F) hpIgR-EGFP partially colocalized with mCherry-myosin Vb tail. The high magnification image displays a region of 12.5×12.5 µm (A,B,D,E,F) or 6.25×6.25 µm (C) from the yellow boxed image to the left. White arrows, colocalization between red and green; *, lumena. Scale bars: 5 µm.
Interestingly, hpIgR-EGFP did not colocalize extensively with yellow fluorescent protein (YFP)-Rab27b (Fig. 5B), another marker of mature SVs in LGACs (Chiang et al., 2011) and other secretory cells (Chen et al., 2004; Imai et al., 2004). Moreover, mCherry-Rab3D and YFP-Rab27b labeled two different types of vesicles that did not extensively colocalize (Fig. 5C). Syncollin has been identified as a marker of the regulated secretory pathway in LGACs (Jerdeva et al., 2005) and other secretory cells (Wäsle et al., 2004; Wäsle et al., 2005), and syncollin-GFP expressed in LGACs is present in YFP-Rab27b-enriched SVs (Chiang et al., 2011). Confocal fluorescence microscopy showed that syncollin-GFP was not enriched in mCherry-Rab3D-labeled SVs (supplementary material Movies 1, 2). Moreover, treatment with 100 mM CCh substantially accelerated the release of syncollin-GFP-enriched vesicles at the APM compared with the resting stage, which occurred with different dynamics than the modest changes of mCherry-Rab3D-enriched SV movements (Movies 1, 2). These observations suggest that Rab3D and Rab27b label two partially distinct subsets of mature SVs in LGACs. In addition, pIgR is associated with Rab3D, but not Rab27b, while syncollin-GFP is associated with Rab27b, but not Rab3D.
Myosin Vc is associated with Rab3D-enriched mature SVs
Our previous study showed the association of the myosin Vc motor protein with the Rab3D-enriched SVs in LGACs (Marchelletta et al., 2008). Here, live cell imaging showed the co-localization of EGFP-full-length-myosin Vc with mCherry-Rab3D (Fig. 5D). Moreover, the overexpression of EGFP-full-length-myosin Vc altered the morphology of overexpressed mCherry-Rab3D-labeled compartments compared to those in LGACs without overexpressed myosin Vc (Fig. 5 A,C,D). Specifically, in LGACs co-expressing the EGFP-full-length-myosin Vc and mCherry-Rab3D, mCherry-Rab3D was more uniformly distributed around the SVs, and was completely colocalized with myosin Vc. These observations suggest a functional interaction between myosin Vc and Rab3D-enriched secretory pathway; further, myosin Vc may be essential for either the recruitment of Rab3D to vesicles or the formation or stabilization of vesicles capable of recruiting Rab3D. We propose that the saturation of available endogenous myosin Vc motor by overexpression of mCherry-Rab3D caused the morphological differences in the mCherry-Rab3D-enriched compartment compared to the endogenous Rab3D-enriched compartment in non-transduced LGACs. However, overexpression of EGFP-full-length-myosin Vc can restore the balance between mCherry-Rab3D and available myosin Vc, making the morphology of mCherry-Rab3D-enriched compartment similar to the endogenous Rab3D-enriched compartment.
The myosin Vc tail domain fused to EGFP on the N′-terminus (EGFP-myosin Vc tail) competes with endogenous myosin Vc for the vesicle binding, but does not have motor function, thus it acts as the DN mutant of myosin Vc (Rodriguez and Cheney, 2002). Comparably to EGFP-full-length-myosin Vc, EGFP-myosin Vc tail was also highly colocalized with mCherry-Rab3D (Fig. 5E), and elicited more association of overexpressed mCherry-Rab3D with SVs.
EGFP-myosin Vc tail was also extensively colocalized with YFP-Rab27b on large SVs (supplementary material Fig. S4A), consistent with the observed colocalization described in previous studies (Jacobs et al., 2009; Chiang et al., 2012). These observations indicate that myosin Vc participates in the trafficking of both Rab3D-enriched and the Rab27b-enriched membrane compartments, but that the cargo of these compartments are largely distinct from each other.
pIgR that enters the transcytotic pathway remains distinct from the regulated secretory pathway
Though both the Rab11a-regulated transcytotic pathway and the Rab3D-regulated secretory pathway participate in pIgR trafficking (Evans et al., 2008; Xu et al., 2011), whether these two pathways are independent or exchange cargo has not been studied. Therefore, we interrogated whether these two pathways exchange pIgR as their cargo.
To study the effect of motor proteins on the trafficking of pIgR, we used an Ad construct that encodes a truncated mutant of myosin Vb fused with mCherry fluorescent protein on the N′-terminus (mCherry-myosin Vb tail). Similar to EGFP-myosin Vc tail, mCherry-myosin Vb tail associates with vesicle cargo but does not have the motor function, acting as a DN inhibitor of myosin Vb. Our previous study suggested that the motility of Rab11a-enriched vesicles utilized myosin Vb (Xu et al., 2011), and also showed the presence of endogenous pIgR in the membrane compartment labeled by EGFP-myosin Vc tail (Marchelletta et al., 2008). Here, confocal fluorescence microscopy of live LGACs showed that a subset of hpIgR-EGFP was also localized to the membrane compartment labeled by mCherry-myosin Vb tail (Fig. 5F).
mCherry-myosin Vb tail did not colocalize with either EGFP-myosin Vc tail (supplementary material Fig. S4B) or Rab3D (supplementary material Fig. S4C), markers of the regulated secretory pathway. These data indicate that myosin Vb and Vc motors are associated distinctly and independently with the transcytotic pathway and the regulated secretory pathway, respectively. Based on these observations, we hypothesized that pIgR is distributed into the transcytotic pathway and the Rab3D-enriched secretory pathway, and that overexpression of mCherry-myosin Vb tail traps the pIgR sorted into the transcytotic pathway, but does not interfere with trafficking of the pIgR in the Rab3D-regulated secretory pathway. On the other hand, overexpression of EGFP-myosin Vc tail traps the pIgR sorted into the regulated secretory pathway, but does not interfere with trafficking of the pIgR in the transcytotic pathway.
To test our hypothesis, we tracked the cohort of basolaterally endocytosed pIgR labeled by anti-SC antibody. In LGACs expressing either mCherry-myosin Vb tail or EGFP-myosin Vc tail, endocytosis of pIgR from the BLM was not visibly affected (data not shown). However, basolaterally endocytosed pIgR in LGACs expressing mCherry-myosin Vb tail accumulated in the membrane compartment labeled by mCherry-myosin Vb tail (Fig. 6A,B) and did not show the same extent of subapical distribution as in non-transduced LGACs. In contrast, EGFP-myosin Vc tail did not appear to alter the basal-to-apical transport of endocytosed pIgR: basolaterally endocytosed pIgR accumulated subapically, but not in the membrane compartment labeled by EGFP-myosin Vc tail (Fig. 6C). This indicated that basolaterally endocytosed pIgR is not sorted into the regulated secretory pathway to any significant extent, and the transcytotic and the regulated secretory pathways are distinctively regulated by different myosin motor proteins.
Fig. 6.
pIgR endocytosed from the basolateral membrane enters the transcytotic pathway but does not intersect with the regulated secretory pathway. (A) Non-transduced LGACs were incubated with sheep anti-rabbit SC antiserum for 1 hour at 4°C, rinsed and incubated at 37°C. Cells were fixed at the time points indicated, permeabilized, and labeled with primary mouse anti-Rab11 antibody, secondary Alexa Fluor®-488-conjugated donkey anti-sheep and Alexa Fluor®-568-conjugated goat anti-mouse antibodies, and Alexa Fluor® 647-conjugated phalloidin. LGACs expressing mCherry-myosin Vb tail (B) or EGFP-myosin Vc tail (C) were incubated with sheep anti-rabbit SC antiserum for 1 hour at 4°C, rinsed and incubated at 37°C. Cells were fixed at the time points indicated, permeabilized, and labeled with secondary Alexa Fluor®-488-conjugated donkey anti-sheep antibody (B) or Alexa Fluor®-568-conjugated donkey anti-sheep antibody (C), and Alexa Fluor®-647-conjugated phalloidin. Actin is displayed in purple in overlay and high magnification images. White arrows, colocalization between red and green; *, lumena. Scale bars: 5 µm.
The transcytotic and the regulated secretory pathways distinctively mediate apically targeted trafficking of pIgR
We distinguished the roles of the transcytotic pathway and the regulated secretory pathway in the trafficking of pIgR by quantifying the cleaved SC in the culture supernatant. Apically released proteins, including SC, can be recovered from culture supernatant since the supernatant is contiguous with that within the lumena formed in reconstituted acinar cultures (Jerdeva et al., 2005; Chiang et al., 2011). Therefore, hSC cleaved from hpIgR-EGFP and released into the culture supernatant was quantified by western blotting using an anti-hSC antibody that does not cross-react with endogenous rabbit SC or pIgR (supplementary material Fig. S1A,B).
As a cholinergic agonist, CCh stimulates the release of SC into the culture supernatant by LGACs (Evans et al., 2008). We investigated the contribution of the transcytotic and the regulated secretory pathways to the acute-phase release of hSC upon CCh stimulation.
We transduced LGACs for the co-expression of hpIgR-EGFP with mCherry-myosin Vb tail, EGFP-myosin Vc tail, or LifeAct–TagRFP. rAVCMVLifeAct–TagRFP, which expresses RFP-tagged LifeAct that labels F-actin but does not interfere with actin dynamics (Riedl et al., 2008; Riedl et al., 2010), was used as a control for co-transduction for the myosin tail domain constructs. The control construct known not to alter the intracellular trafficking of pIgR provided the baseline for determining the change of LGAC secretion due to real alteration of intracellular trafficking rather than the process of Ad transduction itself. mCherry-myosin Vb tail and EGFP-myosin Vc tail were expressed at comparable levels in transduced LGACs (supplementary material Fig. S4D).
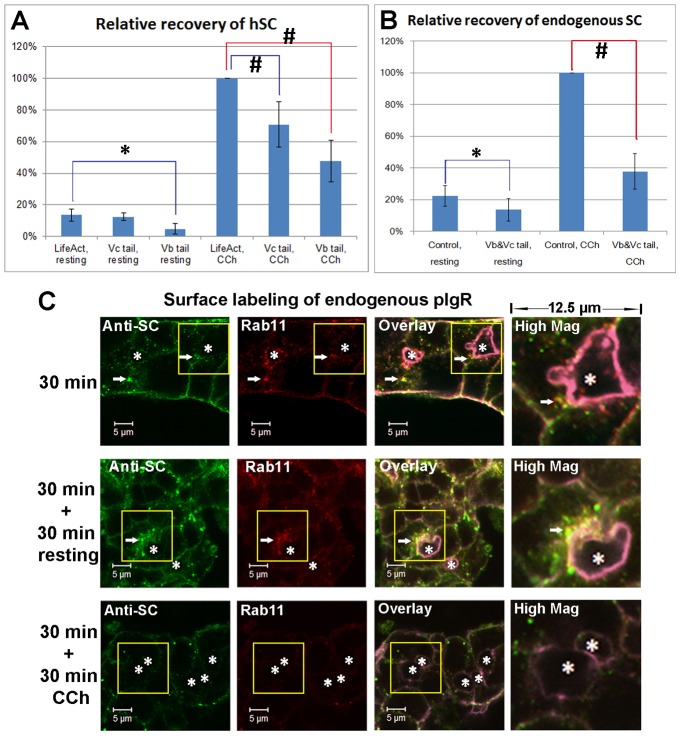
Either at the resting stage or with 30-minute CCh treatment, the LGACs overexpressing mCherry-myosin Vb tail released significantly less hSC than the control (Fig. 7A), indicating that the mCherry-myosin Vb tail inhibits constitutive transcytosis and CCh-stimulated transcytosis. However, LGACs overexpressing mCherry-myosin Vb tail still released significantly more hSC under CCh treatment than at the resting stage (Fig. 7A), suggesting that either the myosin Vc-regulated secretory pathway was not inhibited by overexpressed mCherry-myosin Vb tail and could still be stimulated by CCh, or CCh stimulation could overcome the inhibitory effects of the myosin Vb tail.
Fig. 7.
CCh accelerates the SC release on the apical membrane of LGAC acini. (A) Quantification of hSC released into culture supernatant by LGACs co-transduced with Ad hpIgR-EGFP, Adeno-X Tet-On® and rAVCMVLifeAct–TagRFP, Ad EGFP-myosin Vc tail or Ad mCherry-myosin Vb tail were cultured overnight with doxycycline. LifeAct-TagRFP is a control for EGFP myosin-Vc tail or mCherry-myosin-Vb tail transduction. (B) Quantification of endogenous rabbit SC released into culture supernatant. LGACs were co-transduced with rAVCMVLifeAct–TagRFP and Ad syncollin-GFP (control combination), or with Ad EGFP-myosin Vc tail and Ad mCherry-myosin Vb tail (experimental treatment combination). For both A and B, the net release of hSC or endogenous rabbit SC into the culture supernatant during a 30-minute treatment was quantified by western blotting, as described in Materials and Methods. n = 5; #P<0.05, one-sample Wilcoxon signed rank test; *P<0.05, Student's t-test. (C) Non-transduced LGACs were incubated with sheep anti-rabbit SC antiserum for 1 hour at 4°C, rinsed and incubated at 37°C for 30 minutes. Cells were then treated with or without 100 µM CCh for an additional 30 minutes. Cells were fixed at the time points indicated, permeabilized, and labeled with primary mouse anti-Rab11 antibody, secondary Alexa Fluor®-488-conjugated donkey anti-sheep and Alexa Fluor®-568 goat anti-mouse antibodies, and Alexa Fluor®-647-conjugated phalloidin. Actin labeled with Alexa Fluor®-647-conjugated phalloidin is displayed in purple in overlay and high magnification images. White arrows, colocalization; *, lumena. Scale bars: 5 µm.
With CCh treatment, LGACs overexpressing EGFP-myosin Vc tail released significantly less hSC than the control, indicating the inhibition of SC release from the regulated secretory pathway. However, LGACs overexpressing EGFP-myosin Vc tail also released significantly more hSC under CCh treatment than at the resting stage (Fig. 7A), suggesting that either the transcytotic pathway was not inhibited by overexpressed EGFP-myosin Vc tail and could be stimulated by CCh, or CCh stimulation could overcome the inhibitory effects of the myosin Vc tail. We were unable to probe the simultaneous effects of myosin Vb and Vc tail expression on hSC release, since this would necessitate use of four adenoviral constructs which affects normal cell viability/morphology. Co-expression of mCherry-myosin Vb tail and EGFP-myosin Vc tail significantly reduced but did not completely inhibit the release of endogenous SC in LGACs relative to those co-transduced with rAVCMVLifeAct–TagRFP and Ad-syncollin-GFP (neither with effects on trafficking) (Fig. 7B), suggesting that a minimal amount of SC might still be released via alternative mechanisms that do not depend on myosin Vb or Vc motors.
The conclusions above are also consistent with the confocal fluorescence microscopy analysis of basolaterally endocytosed pIgR. At the 30-minute time point, endocytosed pIgR labeled by anti-SC antibody accumulated in the subapical region beneath the lumen; when additional 30-minute incubation at the resting stage was applied, endocytosed pIgR continued to accumulate in the subapical region (Fig. 7C). However, when an additional 30-minute CCh treatment was applied, most of the endocytosed pIgR in the subapical region was depleted (Fig. 7C). This observation suggested that CCh possibly accelerates the terminal steps of the transcytotic pathway.
On the other hand, CCh treatment was not able to deplete the endocytosed pIgR trapped in the membrane compartment labeled by mCherry-myosin Vb tail (Fig. 8A). This indicated that the CCh-induced accelerated release of SC via the transcytotic pathway is dependent on the myosin Vb motor, and suggested that the additional SC released under CCh stimulation by LGACs expressing mCherry-myosin Vb tail was likely secreted via the regulated secretory pathway. However, the overexpression of EGFP-myosin Vc tail did not inhibit the depletion of endocytosed pIgR in the subapical region upon CCh treatment (Fig. 8B), further indicating that the transcytotic pathway is not regulated by myosin Vc. At the resting stage, a subset of endogenous pIgR was enriched in the membrane compartment labeled by EGFP-myosin Vc tail, and 30-minute CCh treatment was not able to induce the release of endogenous pIgR from this compartment (Fig. 8C). This finding is again consistent with the inhibition of the regulated secretory pathway and accounts for the overall reduction of hSC released into the culture supernatant under CCh treatment (Fig. 7A). These results indicate that, although the transcytotic and secretory pathways are associated with different myosin motors and are regulated by different Rab proteins, CCh accelerates pIgR trafficking and SC release through both of these exocytotic pathways.
Fig. 8.
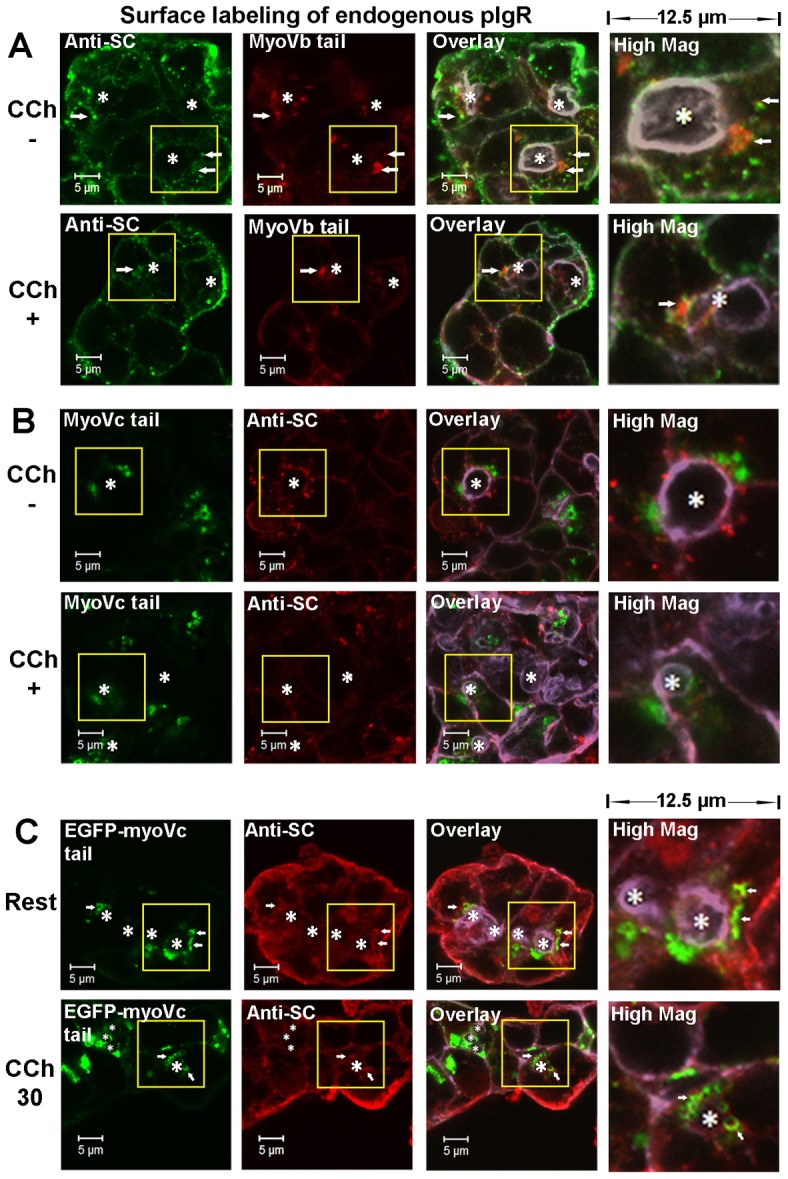
Apically targeted trafficking of pIgR is regulated by the transcytotic pathway and the regulated secretory pathway distinctively. LGACs expressing mCherry-myosin Vb tail (A) or EGFP-myosin Vc tail (B) were incubated with sheep anti-rabbit SC antiserum for 1 hour at 4°C, rinsed and incubated at 37°C for 30 minutes. Cells were then treated with or without 100 µM CCh for an additional 30 minutes. Cells were fixed, permeabilized, and labeled with secondary Alexa Fluor®-488-conjugated donkey anti-sheep antibody (A) or Alexa Fluor®-568-conjugated donkey anti-sheep antibody (B), and Alexa Fluor®-647-conjugated phalloidin. (C) LGACs expressing EGFP-myosin Vc tail were treated with 100 µM CCh or not for 30 minutes. Cells were fixed, permeabilized and labeled with primary sheep anti-SC serum, secondary Alexa Fluor®-568-conjugated donkey anti-sheep antibody and Alexa-Fluor®-647-conjugated phalloidin. Actin is displayed in purple in overlay and high magnification images. White arrows, colocalization between red and green; *, lumena. Scale bars: 5 µm.
Discussion
We have used rabbit LGACs to characterize the trafficking of pIgR and secretion of SC through tandem apically directed regulated exocytosis and transcytosis. By tracking cargoes separately along the regulated secretory and transcytotic pathways, we demonstrated three pathways for apically directed secretion in LGACs: the transcytotic pathway and two regulated secretory pathways. Moreover, we show that apically directed pIgR in the transcytotic pathway was sorted independently of pIgR in the regulated secretory pathway, i.e. the pathways did not appear to intersect. In addition, we showed that the pIgR appeared to occupy only one of two identifiable regulated SV populations.
With respect to pIgR transcytosis, we relied upon the ability to transduce LGACs with a novel Ad hpIgR-EGFP construct that recapitulates our previous findings with endogenous pIgR. In particular, basolaterally endocytosed pIgR accumulated in subapical Rab11a-enriched vesicles, and this leg of the transcytotic pathway was dependent upon functional Rab11a. Moreover, basolaterally endocytosed pIgR did not enter the regulated secretory compartment, although its transcytosis was stimulated by CCh.
While the Rab11a-regulated transcytotic pathway is responsible for the basal-to-apical transport of basolaterally endocytosed pIgR, the Rab3D-regulated secretory pathway associated with myosin Vc contains a subset of pIgR that likely originates directly from the biosynthetic pathway. This finding is consistent with previous studies suggesting the participation of both transcytotic and secretory pathways in the trafficking of pIgR and secretion of SC by LGACs (Evans et al., 2008; Xu et al., 2011).
We also further characterized the regulated secretions in LGACs with respect to the Rab3D- and Rab27b-enriched compartments. Rab3D and Rab27b are both present in pancreatic (Ohnishi et al., 1996; Valentijn et al., 1996; Chen et al., 2004) and parotid (Raffaniello et al., 1999; Nguyen et al., 2003; Imai et al., 2004; Imai et al., 2009) acinar cells and LGACs (Evans et al., 2008; Chiang et al., 2011). In rat parotid acinar cells, Rab27b regulates the release of amylase (Imai et al., 2004), and Rab3D is localized to the zymogen granule membrane (Raffaniello et al., 1999). Interestingly, salivary glands of Sjögren's syndrome patients typically show lower levels of Rab3D associated with loss of cell polarity and secretory dysfunction (Bahamondes et al., 2011), providing clinically relevant evidence for a role of Rab3D in acinar cell secretion. However, previous studies did not characterize the interrelation between the membrane compartments labeled by Rab3D and Rab27b in the same cell. In this study, mCherry-Rab3D was capable of interacting with pIgR and SVs comparably to the endogenous Rab3D protein. Its use, in parallel with YFP-Rab27b, showed that Rab3D and Rab27b label two largely distinct pools of SVs in LGACs: Rab3D regulates the apical release of pIgR, but not syncollin, while Rab27b regulates the apical release of syncollin, but not pIgR. Although our work is reliant on overexpression studies, analogously, in pancreatic acinar cells, VAMP 2-positive zymogen granules mediate the majority of constitutive secretion, whereas VAMP 8-positive zymogen granules are more largely involved in Ca2+-stimulated secretion (Weng et al., 2007). Thus, Rab and SNARE proteins may in coordination sequester specific secretory cargo proteins into distinct populations of SVs.
Other work has also recognized that LGACs display SVs of significantly different composition. EM analysis reveals serous and mucous granules with clearly distinct content (Edman et al., 2010) but the nature of the molecular machinery that resides on each type of granule has not been identified. Our recent analysis of SV composition in Rab27a/b-deficient mouse LGs by EM shows that mouse LGs still contain significant numbers of SVs (Chiang et al., 2011). Moreover, comparable analysis of the exocrine pancreas in Rab3D knockout mice revealed a robust array of unusually large SV (Riedel et al., 2002), and this phenomenon is recapitulated in LG of Rab3D knockout mice (data not shown). There is also evidence that regulation of secretion by Rab3D may be more complex. In the AR42J cell line derived from rat pancreatic tumor, Rab3D may regulate the level of amylase but is not involved in exocytosis induced by cholecystokinin (Limi et al., 2012). In addition, Rab3D has been shown to regulate secretory granule maturation in neuroendocrine PC12 cells by regulating the removal of cargo from immature secretory granules (Kogel and Gerdes, 2010).
Finally, the LG is innervated by both sympathetic and parasympathetic nervous systems, and LGACs respond both to adrenergic and cholinergic stimulation through distinct pathways (Hodges et al., 1992). Diversification of secretory pathways for sequestration of different cargo and coupling of distinct pathways to different stimuli offers the potential to customize physiological responses.
This work distinguishes the function of two myosin V motors in parallel in the same cell: myosin Vb with Rab11a-enriched vesicles, and myosin Vc with Rab3D- and Rab27b-enriched vesicles. Myosin Vb and Vc did not show functional overlap, as overexpression of mCherry-myosin Vb did not alter the distribution of Rab3D.
On the basis of these observations, we propose a model for the trafficking of pIgR in LGACs (Fig. 9). A subset of newly synthesized pIgR is transported to the BLM. Bound to dIgA or not, basolateral pIgR is rapidly endocytosed and transported into early endosomes. Thereafter, they are sorted into Rab11a-enriched vesicles, which are transported on microtubules (MTs) towards the subapical region, where they acquire the myosin Vb motor and are released constitutively in a process that can also be accelerated by cholinergic secretagogue stimulation.
Fig. 9.
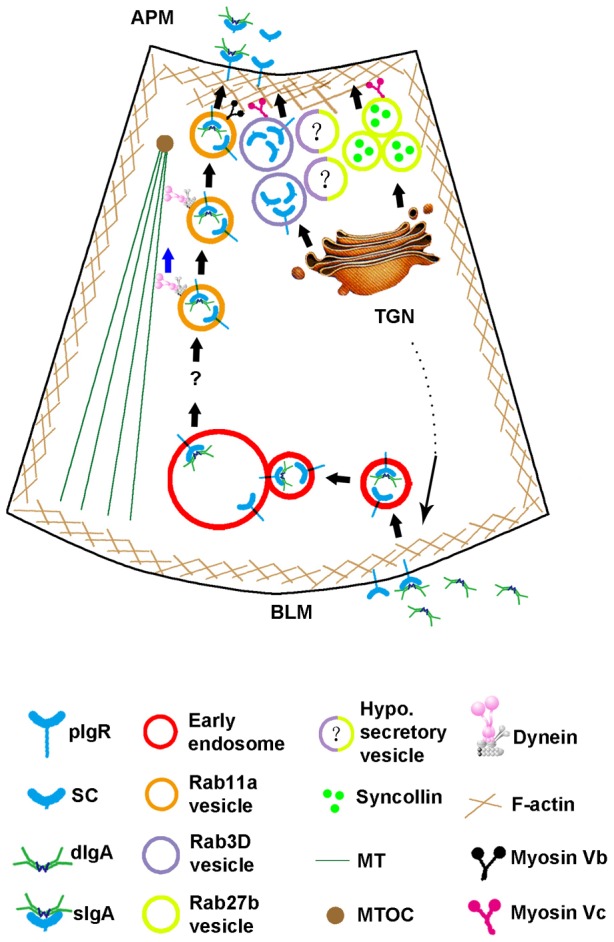
Working model for pIgR trafficking in LGACs.
Another subset of newly synthesized pIgR is directly sorted into Rab3D-enriched SVs. Myosin Vc is required for the apically targeted trafficking of SC in Rab3D-enriched vesicles. Although Rab27b-enriched SVs also recruit myosin Vc, they are clearly distinct from Rab3D-enriched SVs as evidenced by their ability to fuse and release syncollin-GFP without any apparent association or recruitment of mCherry-Rab3D. Additional vesicle populations labeled by both Rab27b and Rab3D are hypothetically shown that may carry distinct cargo proteins and/or represent vesicles at a different maturational or regulatory states. Evidence from other systems is consistent with the selective secretion of cargo proteins regulated by specific Rabs. For example, in anterior neurulation in Xenopus, Rab3D regulates specifically the secretion of the morphogen Noggin, but not that of Chordin or Wnts (Kim and Han, 2011).
The mechanism that distinguishes basolaterally endocytosed pIgR from that sorted to the secretory pathway requires further study. However, one possibility is that the phosphorylation of serine 726 and/or serine 664 on the cytoplasmic domain of pIgR that provides the crucial signals for internalization and transcytosis (Casanova et al., 1990; Okamoto et al., 1994), respectively, in MDCK cells, may also serve as a sorting signal for entry and trafficking through the transcytotic pathway in LGACs, thereby differentiating endocytosed pIgR from newly synthesized pIgR. On the other hand, non-phosphorylated pIgR or pIgR phosphorylated only on serine 726 may be trafficked into the secretory pathway via its interaction with the AP-1 clathrin adaptor at the TGN (Orzech et al., 1999). Moreover, the direct binding between pIgR and Rab3D shown previously (Evans et al., 2008) and in this study may explain the sequestration of a subset of newly synthesized pIgR by Rab3D into SVs or the recruitment of Rab3D onto nascent SVs by pIgR.
Our model suggests physiological functions for different trafficking populations of pIgR: sequestration of apically targeted cargoes into different membrane compartments enables the exocrine cells to release these cargoes separately under the control of different sets of regulators, and therefore provide different responses under physiological conditions. The transcytotic pathway may provide a constitutive mechanism for transporting dIgA from the serum, and for release of sIgA into tears. This pathway may be essential for maintaining normal or baseline functions of the ocular mucosal immune system. However, the regulated secretory pathway may provide important reinforcement of mucosal innate immunity under conditions of ocular surface challenge associated with physiological reflex and triggering of tear flow through regulated secretion by LGACs. Free SC provides protective functions against certain pathogens (Giugliano et al., 1995; Hammerschmidt et al., 1997; Dallas and Rolfe, 1998; de Araújo and Giugliano, 2001; de Oliveira et al., 2001; Perrier et al., 2006). The rapid release of SC may immediately increase the SC concentration in tears, to saturate and neutralize pathogens, principal sources of eye irritation. LGACs may in fact deliberately sequester SC in a specialized population of Rab3D-enriched SVs that may be mobilized by specific stimuli just for this purpose. Given the responsiveness of the LGAC to multiple sympathetic and parasympathetic agonists, it is possible that ocular surface irritations of different origins may translate to specific neural signals resulting in release of specific SV populations with contents customized to address the particular pathological challenge. The advent of more sophisticated tools for investigation of the spectrum of tear proteins released should enable further explorations of each individual vesicle population to the health of the ocular surface, and the link between each population to the nature of the physiological stimulus (e.g. acetylcholine, epinephrine and norepinephrine).
In summary, we show here that the Rab11a-regulated transcytotic pathway mediates the basal-to-apical transcytosis of pIgR in LGACs, and that pIgR and its cleaved SC domain appear to remain segregated in Rab3D-enriched SVs (lacking Rab27b). In addition, exocytosis of SC from pIgR enriched in these vesicle pools can be stimulated with cholinergic agonists. Finally, different pools of SVs with different cargoes and regulatory effectors have been characterized together in a dynamic fashion.
Materials and Methods
Materials
CCh was purchased from Sigma-Aldrich (St. Louis, MO, USA). Protease inhibitor cocktail for preparation of lysates is described in a previous study (Vilalta et al., 1998). The Prolong® anti-fade Kit, Alexa Fluor®-conjugated secondary antibodies and phalloidin were from Invitrogen (Carlsbad, CA, USA). Goat polyclonal anti-hSC antibody was from R&D systems (Minneapolis, MN, USA). Mouse monoclonal anti-hSC antibody was from NeoMarkers (Fremont, CA, USA). Mouse anti-GFP antibody, pre-immune rabbit serum, sheep serum and mouse IgG, as well as Protein A/G-conjugated agarose resin, were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-mCherry monoclonal antibody was from MBL (Woburn, MA, USA). Rabbit anti-Rab3D serum was raised as described (Evans et al., 2008). Sheep anti-rabbit SC serum was prepared by Capralogics (Hardwick, MA, USA) using SC from rabbit gall bladder bile as antigen. Rabbit dIgA was generated as described previously (Xu et al., 2011). IRDye®-conjugated secondary antibodies were from Rockland (Gilbertsville, PA, USA). Blocking buffer was from Li-COR Biosciences (Lincoln, NB, USA). Doxycycline was obtained from Clontech (Mountain View, CA, USA). Peter's Complete Medium (PCM) was prepared according to methods in our previous study (Xu et al., 2011). All animal experiments were performed according to approved guidelines.
Preparation of primary LGACs
Preparation of primary LGACs from LGs from female New Zealand white rabbits was as described previously (Xu et al., 2011).
Production and purification of Ad constructs
For constructing Ad hpIgR-EGFP, the open reading frame for the human pIgR precursor cDNA sequence was PCR amplified from the cloning vector (GenBank Entry: BC110494.2) with sense primer, 5′- TACTGCTAGCTCAACGGGAGAGAAGGAAGTGG-3′, and anti-sense primer, 5′-TAGACTCGAGATAGGCTTCCTGGGGGCCGTC-3′. The PCR product was linked into the pCR® II-TOPO vector from Invitrogen (Carlsbad, CA, USA). The pCR® II-TOPO hpIgR was digested with NheI and XhoI restriction enzymes, and the 2381 bp fragment encoding hpIgR was inserted into pEGFP-N1 vector.
The pEGFP-N1 hpIgR was digested with NheI and NotI restriction enzymes to generate a 3172 bp fragment encoding hpIgR-EGFP, which was subcloned into the pTRE-Shuttle2 vector and further subcloned into the Adeno-X System 1 viral DNA vector (supplementary material Table S1, pIgR-EGFP sequence). The recombinant viral DNA vector linearized with PacI was used to transfect HEK293 cells for packaging virus, in accordance with the manufacturer's protocol.
For constructing Ad mCherry-Rab3D, mCherry with a 12-aa linker of 3 G-G-S-G sequence repeats was fused to the N′-terminal of rabbit Rab3D (supplementary material Table S1, mCherry-Rab3D sequence) by full-gene synthesis subcontracted to GenScript (Piscataway, NJ, USA). The cloning vector containing the mCherry-Rab3D sequences was sent to Vector Biolabs (Philadelphia, PA, USA) for customized construction of the Ad encoding this protein in the Ad5 (dE1/E3) backbone.
Ad hpIgR-EGFP and Ad mCherry-Rab3D require co-transduction with Adeno-X Tet-On® and doxycycline induction for protein expression. Doxycycline-inducible Ad EGFP-Rab11a WT (wild type) and Ad EGFP-Rab11a DN were also described in our previous study (Xu et al., 2011) and derived from the kind gifts of Dr James Goldenring (Vanderbilt University).
Ad mCherry-myosin Vb tail and Ad EGFP-myosin Vc tail were used in our previous studies (Marchelletta et al., 2008; Xu et al., 2011) and were the kind gifts of Dr Lixin Zhu (SUNY, Buffalo) and Richard Cheney (University of North Carolina), respectively. Ad YFP-Rab27b and Ad syncollin-GFP used in our previous studies (Jerdeva et al., 2005; Chiang et al., 2011) were the kind gifts of Dr Serhan Karvar (University of Southern California) and Dr Christopher Rhodes (University of Chicago), respectively. rAVCMVLifeAct–TagRFP was from Ibidi GmbH (Martinsried, Germany). All Ad vectors were amplified in HEK293 cells according to established protocols (Wang et al., 2003).
Ad transduction
LGACs were transduced as described in our previous study (Xu et al., 2011). The Adeno-X Tet-On® encodes a regulatory protein that enables the expression of the protein of interest only when it binds doxycycline in the culture medium. Doxycycline was added at 0.1 µg/ml (Ad hpIgR-EGFP, Ad mCherry-Rab3D) or 1 µg/ml (Ad EGFP-Rab11a WT, Ad EGFP-Rab11a DN) after removal of virus and replacement of culture medium. Ad mCherry-myosin Vb tail, Ad EGFP-myosin Vc tail, Ad YFP-Rab27b and rAVCMVLifeAct–TagRFP were used alone at an MOI of 5 for 2 hours of incubation at 37°C for transduction. However, when these constructs were used for co-transduction with doxycyline-inducible constructs, the Adeno-X Tet-On® and doxycycline were also applied. After virus transduction, LGACs were cultured 16–18 hours before analysis.
Controls for Ad transduction were tailored to the individual experiment. For direct comparison of the effects of EGFP-Rab11a DN, EGFP-Rab11a WT was utilized. For the evaluation of the impact of myosin Vb and/or Vc-tail constructs on SC release, Ad transduction controls included use of equivalent titers of constructs encoding small proteins with no known effects on intracellular trafficking such as syncollin-GFP and LifeAct–Tag-RFP
Confocal fluorescence microscopy
Analysis of fluorescent live and fixed LGACs utilized a Zeiss LSM 510 Meta NLO equipped with Argon, red HeNe and green HeNe lasers as described in our previous study (Xu et al., 2011). Procedures for fixation and permeabilization of LGACs for indirect immunofluorescence are also as previously published (Xu et al., 2011).
Uptake of antibody-labeled pIgR
LGACs were incubated at 4°C for 1 hour with Rhodamine-conjugated rabbit dIgA (100 µg/ml) and/or sheep anti-SC serum (1∶20) diluted in binding medium (PCM containing 20 mM HEPES, 2 mM CaCl2, 2 mM MgCl2 and 3% m/v BSA), or goat anti-hSC antibody diluted in binding medium (2 µg/well). After extensive washing with PCM, LGACs were incubated at 37°C for uptake and trafficking of antibody-labeled pIgR. LGACs were fixed and labeled at 0-minute, 15-minute, 30-minute or 60-minute time points.
Co-immunoprecipitation
LGACs co-expressing hpIgR-EGFP and mCherry-Rab3D were collected on day 3 of culture by centrifugation. LGACs were lysed in non-denaturing lysis buffer (20 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, pH 8) with protease inhibitors by 30-minute agitation at 4°C. 1 ml lysis buffer was used for 2×107 cells/sample. Lysate was centrifuged at 10,000×g for 10 minutes and post-nuclear supernatant was pre-cleared with 40 µl (50% v/v) CL2B Sepharose beads twice. 20 µl (50% v/v) protein A/G agarose resin beads, 10 µl rabbit anti-Rab3D serum or goat anti-hSC antibody was added into the supernatant, and pre-immune rabbit or goat serum was used as controls. After end-to-end rotation at 4°C overnight in the mixture, beads were extensively washed with lysis buffer and mixed with SDS-PAGE loading buffer at room temperature. The sample was analyzed by western blotting using mouse anti-mCherry, mouse anti-GFP and goat anti-hSC primary antibodies, as well as IRDye®-conjugated secondary antibodies and imaged with Odyssey® 3 software.
Quantification of hSC or endogenous rabbit SC released by LGACs
LGACs grown on Matrigel™-coated 12-well plates were co-transduced on day 2. On day 3, the old medium was aspirated and 500 µl fresh PCM was added into each well. LGACs were incubated for 30 minutes before treatments, and the secretion into culture supernatant during this period is quantified as ‘background release’. LGACs were then treated with 100 µM CCh or not (resting stage) for 30 minutes, and the culture supernatant was harvested for quantification of secretion. Cell pellets dissolved with 0.5 M NaOH were quantified using BCA protein assay to normalize secretion to cellular protein. Harvested culture supernatant was concentrated ∼10 times with Vivaspin-500 concentrators (Littleton, MA, USA) and analyzed by western blotting, using goat anti-hSC primary antibody and IRDye®800-conjugated donkey anti-goat secondary antibody for probing hSC; or sheep anti-SC serum and Alexa Fluor®680-conjugated donkey anti-sheep secondary antibody for probing endogenous SC. Fluorescent signals on membranes were scanned using an Odyssey® Imaging System from LI-COR and quantified with the Odyssey® 3 software. The amount of hSC or endogenous SC in treatment medium minus background release indicates the net release under each condition, and is presented as relative recovery normalized to hSC or endogenous SC release of control LGACs with CCh treatment.
Statistics
For quantification of hSC or endogenous SC, five repetitions were performed, each from a separate preparation. A one-sample Wilcoxon signed rank test was applied to compare different treatment groups with the control. Student's t-test based on unequal variances was applied to compare hSC or endogenous SC release at the resting stage. For immunofluorescence and western blots, a minimum of three repetitions was performed with each repetition from a separate preparation.
Supplementary Material
Footnotes
Author contributions
S.X. and L.M. performed most of the experiments. E.E. guided the design and analysis of experiments involving uptake of antibody-labeled pIgR. S.X., C.T.O. and S.F.H.-A. designed the experiments, analyzed the data and wrote the manuscript.
Funding
This work was supported by the National Institutes of Health [grant numbers EY011386 to S.F.H.-A., EY016985 to S.F.H.-A. and C.T.O.]. Additional support to S.F.H.-A. was from the National Institutes of Health [grant numbers 5UL1RR031986, P30DK048522, EY017293]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.122242/-/DC1
References
- Apodaca G., Katz L. A., Mostov K. E. (1994). Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J. Cell Biol. 125, 67–86 10.1083/jcb.125.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M., Saito M., Fujita H., Wada M., Kobayashi K., Vaerman J. P., Moro I. (1998). Molecular maturation and functional expression of mouse polymeric immunoglobulin receptor. J. Immunol. Methods 214, 131–139 10.1016/S0022-1759(98)00052-0 [DOI] [PubMed] [Google Scholar]
- Bahamondes V., Albornoz A., Aguilera S., Alliende C., Molina C., Castro I., Urzúa U., Quest A. F., Barrera M. J., González S. et al. (2011). Changes in Rab3D expression and distribution in the acini of Sjögren's syndrome patients are associated with loss of cell polarity and secretory dysfunction. Arthritis Rheum. 63, 3126–3135 10.1002/art.30500 [DOI] [PubMed] [Google Scholar]
- Bonner A., Perrier C., Corthésy B., Perkins S. J. (2007). Solution structure of human secretory component and implications for biological function. J. Biol. Chem. 282, 16969–16980 10.1074/jbc.M701281200 [DOI] [PubMed] [Google Scholar]
- Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. (1992). The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70, 715–728 10.1016/0092-8674(92)90306-W [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. (1990). Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science 248, 742–745 10.1126/science.2110383 [DOI] [PubMed] [Google Scholar]
- Chen X., Li C., Izumi T., Ernst S. A., Andrews P. C., Williams J. A. (2004). Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem. Biophys. Res. Commun. 323, 1157–1162 10.1016/j.bbrc.2004.08.212 [DOI] [PubMed] [Google Scholar]
- Chiang L., Ngo J., Schechter J. E., Karvar S., Tolmachova T., Seabra M. C., Hume A. N., Hamm-Alvarez S. F. (2011). Rab27b regulates exocytosis of secretory vesicles in acinar epithelial cells from the lacrimal gland. Am. J. Physiol. Cell Physiol. 301, C507–C521 10.1152/ajpcell.00355.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L., Karvar S., Hamm-Alvarez S. F. (2012). Direct imaging of RAB27B-enriched secretory vesicle biogenesis in lacrimal acinar cells reveals origins on a nascent vesicle budding site. PLoS ONE 7, e31789 10.1371/journal.pone.0031789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas S. D., Rolfe R. D. (1998). Binding of Clostridium difficile toxin A to human milk secretory component. J. Med. Microbiol. 47, 879–888 10.1099/00222615-47-10-879 [DOI] [PubMed] [Google Scholar]
- de Araújo A. N., Giugliano L. G. (2001). Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells. BMC Microbiol. 1, 25 10.1186/1471-2180-1-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira I. R., de Araújo A. N., Bao S. N., Giugliano L. G. (2001). Binding of lactoferrin and free secretory component to enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 203, 29–33 [DOI] [PubMed] [Google Scholar]
- Deitcher D. L., Neutra M. R., Mostov K. E. (1986). Functional expression of the polymeric immunoglobulin receptor from cloned cDNA in fibroblasts. J. Cell Biol. 102, 911–919 10.1083/jcb.102.3.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme N. A., Williams J. A., Oztan A., Apodaca G., Lapierre L. A., Goldenring J. R. (2007). Rab11-FIP2 regulates differentiable steps in transcytosis. Am. J. Physiol. Cell Physiol. 293, C1059–C1072 10.1152/ajpcell.00078.2007 [DOI] [PubMed] [Google Scholar]
- Edman M., Marchelletta R. R., Hamm-Alvarez S. F. (2010). Ocular surface: lacrimal gland overview. Encyclopedia of the Eye, Vol. 2 Besharse J C, Dana R, Dartt D A, ed522–527Amsterdam: Elsevier/Academic Press [Google Scholar]
- Evans E., Zhang W., Jerdeva G., Chen C. Y., Chen X., Hamm-Alvarez S. F., Okamoto C. T. (2008). Direct interaction between Rab3D and the polymeric immunoglobulin receptor and trafficking through regulated secretory vesicles in lacrimal gland acinar cells. Am. J. Physiol. Cell Physiol. 294, C662–C674 10.1152/ajpcell.00623.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano L. G., Ribeiro S. T., Vainstein M. H., Ulhoa C. J. (1995). Free secretory component and lactoferrin of human milk inhibit the adhesion of enterotoxigenic Escherichia coli. J. Med. Microbiol. 42, 3–9 10.1099/00222615-42-1-3 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt S., Talay S. R., Brandtzaeg P., Chhatwal G. S. (1997). SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 25, 1113–1124 10.1046/j.1365-2958.1997.5391899.x [DOI] [PubMed] [Google Scholar]
- Hodges R. R., Dicker D. M., Rose P. E., Dartt D. A. (1992). Alpha 1-adrenergic and cholinergic agonists use separate signal transduction pathways in lacrimal gland. Am. J. Physiol. 262, G1087–G1096 [DOI] [PubMed] [Google Scholar]
- Hölttä-Vuori M., Tanhuanpää K., Möbius W., Somerharju P., Ikonen E. (2002). Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol. Biol. Cell 13, 3107–3122 10.1091/mbc.E02-01-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung A. H., Novick P. J. (2011). Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A., Yoshie S., Nashida T., Shimomura H., Fukuda M. (2004). The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J. Cell Sci. 117, 1945–1953 10.1242/jcs.01048 [DOI] [PubMed] [Google Scholar]
- Imai A., Yoshie S., Nashida T., Fukuda M., Shimomura H. (2009). Redistribution of small GTP-binding protein, Rab27B, in rat parotid acinar cells after stimulation with isoproterenol. Eur. J. Oral Sci. 117, 224–230 10.1111/j.1600-0722.2009.00618.x [DOI] [PubMed] [Google Scholar]
- Jacobs D. T., Weigert R., Grode K. D., Donaldson J. G., Cheney R. E. (2009). Myosin Vc is a molecular motor that functions in secretory granule trafficking. Mol. Biol. Cell 20, 4471–4488 10.1091/mbc.E08-08-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerdeva G. V., Yarber F. A., Trousdale M. D., Rhodes C. J., Okamoto C. T., Dartt D. A., Hamm-Alvarez S. F. (2005). Dominant-negative PKC-epsilon impairs apical actin remodeling in parallel with inhibition of carbachol-stimulated secretion in rabbit lacrimal acini. Am. J. Physiol. Cell Physiol. 289, C1052–C1068 10.1152/ajpcell.00546.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel C. S. (2005). The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 206, 83–99 10.1111/j.0105-2896.2005.00278.x [DOI] [PubMed] [Google Scholar]
- Kim H., Han J. K. (2011). Rab3D is required for Xenopus anterior neurulation by regulating Noggin secretion. Dev Dyn. 240, 1430–1439 10.1002/dvdy.22643 [DOI] [PubMed] [Google Scholar]
- Kimura F., Aizawa K., Tanabe K., Shimizu K., Kon M., Lee H., Akimoto T., Akama T., Kono I. (2008). A rat model of saliva secretory immunoglobulin: a suppression caused by intense exercise. Scand. J. Med. Sci. Sports 18, 367–372 10.1111/j.1600-0838.2007.00642.x [DOI] [PubMed] [Google Scholar]
- Kögel T., Gerdes H. H. (2010). Roles of myosin Va and Rab3D in membrane remodeling of immature secretory granules. Cell. Mol. Neurobiol. 30, 1303–1308 10.1007/s10571-010-9597-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., Kocher H. P., Hanly W. C., Cook L., Jaton J. C., Kraehenbuhl J. P. (1983). Structural and genetic heterogeneity of the receptor mediating translocation of immunoglobulin A dimer antibodies across epithelia in the rabbit. J. Biol. Chem. 258, 6653–6659 [PubMed] [Google Scholar]
- Lapierre L. A., Kumar R., Hales C. M., Navarre J., Bhartur S. G., Burnette J. O., Provance D. W. J., Jr, Mercer J. A., Bähler M., Goldenring J. R. (2001). Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limi S., Ojakian G., Raffaniello R. (2012). Rab3D regulates amylase levels, not agonist-induced amylase release, in AR42J cells. Cell. Mol. Biol. Lett. 17, 258–273 10.2478/s11658-012-0008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchelletta R. R., Jacobs D. T., Schechter J. E., Cheney R. E., Hamm-Alvarez S. F. (2008). The class V myosin motor, myosin 5c, localizes to mature secretory vesicles and facilitates exocytosis in lacrimal acini. Am. J. Physiol. Cell Physiol. 295, C13–C28 10.1152/ajpcell.00330.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa R., Salomón C., Roggero M., Stahl P. D., Mayorga L. S. (2001). Rab22a affects the morphology and function of the endocytic pathway. J. Cell Sci. 114, 4041–4049 [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Altschuler Y., Chapin S. J., Enrich C., Low S. H., Luton F., Richman-Eisenstat J., Singer K. L., Tang K., Weimbs T. (1995). Regulation of protein traffic in polarized epithelial cells: the polymeric immunoglobulin receptor model. Cold Spring Harb. Symp. Quant. Biol. 60, 775–781 10.1101/SQB.1995.060.01.083 [DOI] [PubMed] [Google Scholar]
- Nguyen D., Jones A., Ojakian G. K., Raffaniello R. D. (2003). Rab3D redistribution and function in rat parotid acini. J. Cell. Physiol. 197, 400–408 10.1002/jcp.10373 [DOI] [PubMed] [Google Scholar]
- Ogura Y. (2005). Transient expression of polymeric immunoglobulin receptor in human adenocarcinoma cell line HT-29. J. Oral Sci. 47, 15–20 10.2334/josnusd.47.15 [DOI] [PubMed] [Google Scholar]
- Ohnishi H., Ernst S. A., Wys N., McNiven M., Williams J. A. (1996). Rab3D localizes to zymogen granules in rat pancreatic acini and other exocrine glands. Am. J. Physiol. 271, G531–G538 [DOI] [PubMed] [Google Scholar]
- Okamoto C. T., Song W., Bomsel M., Mostov K. E. (1994). Rapid internalization of the polymeric immunoglobulin receptor requires phosphorylated serine 726. J. Biol. Chem. 269, 15676–15682 [PubMed] [Google Scholar]
- Orzech E., Schlessinger K., Weiss A., Okamoto C. T., Aroeti B. (1999). Interactions of the AP-1 Golgi adaptor with the polymeric immunoglobulin receptor and their possible role in mediating brefeldin A-sensitive basolateral targeting from the trans-Golgi network. J. Biol. Chem. 274, 2201–2215 10.1074/jbc.274.4.2201 [DOI] [PubMed] [Google Scholar]
- Perrier C., Sprenger N., Corthésy B. (2006). Glycans on secretory component participate in innate protection against mucosal pathogens. J. Biol. Chem. 281, 14280–14287 10.1074/jbc.M512958200 [DOI] [PubMed] [Google Scholar]
- Raffaniello R. D., Lin J., Schwimmer R., Ojakian G. K. (1999). Expression and localization of Rab3D in rat parotid gland. Biochim. Biophys. Acta 1450, 352–363 10.1016/S0167-4889(99)00052-X [DOI] [PubMed] [Google Scholar]
- Riedel D., Antonin W., Fernandez-Chacon R., Alvarez de Toledo G., Jo T., Geppert M., Valentijn J. A., Valentijn K., Jamieson J. D., Südhof T. C. et al. (2002). Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol. Cell. Biol. 22, 6487–6497 10.1128/MCB.22.18.6487-6497.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J., Crevenna A. H., Kessenbrock K., Yu J. H., Neukirchen D., Bista M., Bradke F., Jenne D., Holak T. A., Werb Z. et al. (2008). Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 10.1038/nmeth.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J., Flynn K. C., Raducanu A., Gärtner F., Beck G., Bösl M., Bradke F., Massberg S., Aszodi A., Sixt M. et al. (2010). Lifeact mice for studying F-actin dynamics. Nat. Methods 7, 168–169 10.1038/nmeth0310-168 [DOI] [PubMed] [Google Scholar]
- Rodriguez O. C., Cheney R. E. (2002). Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J. Cell Sci. 115, 991–1004 [DOI] [PubMed] [Google Scholar]
- Rojas R., Apodaca G. (2002). Immunoglobulin transport across polarized epithelial cells. Nat. Rev. Mol. Cell Biol. 3, 944–955 10.1038/nrm972 [DOI] [PubMed] [Google Scholar]
- Song W., Apodaca G., Mostov K. (1994). Transcytosis of the polymeric immunoglobulin receptor is regulated in multiple intracellular compartments. J. Biol. Chem. 269, 29474–29480 [PubMed] [Google Scholar]
- Su T., Bryant D. M., Luton F., Vergés M., Ulrich S. M., Hansen K. C., Datta A., Eastburn D. J., Burlingame A. L., Shokat K. M. et al. (2010). A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat. Cell Biol. 12, 1143–1153 10.1038/ncb2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Jin R. U., Bredemeyer A. J., Oates E. J., Błazewska K. M., McKenna C. E., Mills J. C. (2010). RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol. Cell. Biol. 30, 1269–1284 10.1128/MCB.01328-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn J. A., Sengupta D., Gumkowski F. D., Tang L. H., Konieczko E. M., Jamieson J. D. (1996). Rab3D localizes to secretory granules in rat pancreatic acinar cells. Eur. J. Cell Biol. 70, 33–41 [PubMed] [Google Scholar]
- Vilalta P. M., Zhang L., Hamm-Alvarez S. F. (1998). A novel taxol-induced vimentin phosphorylation and stabilization revealed by studies on stable microtubules and vimentin intermediate filaments. J. Cell Sci. 111, 1841–1852 [DOI] [PubMed] [Google Scholar]
- Volpicelli L. A., Lah J. J., Fang G., Goldenring J. R., Levey A. I. (2002). Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 22, 9776–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jerdeva G., Yarber F. A., da Costa S. R., Xie J., Qian L., Rose C. M., Mazurek C., Kasahara N., Mircheff A. K. et al. (2003). Cytoplasmic dynein participates in apically targeted stimulated secretory traffic in primary rabbit lacrimal acinar epithelial cells. J. Cell Sci. 116, 2051–2065 10.1242/jcs.00398 [DOI] [PubMed] [Google Scholar]
- Wang Z., Edwards J. G., Riley N., Provance D. W. J., Jr, Karcher R., Li X. D., Davison I. G., Ikebe M., Mercer J. A., Kauer J. A. et al. (2008). Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135, 535–548 10.1016/j.cell.2008.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäsle B., Hays L. B., Rhodes C. J., Edwardson J. M. (2004). Syncollin inhibits regulated corticotropin secretion from AtT-20 cells through a reduction in the secretory vesicle population. Biochem. J. 380, 897–905 10.1042/BJ20031726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wäsle B., Turvey M., Larina O., Thorn P., Skepper J., Morton A. J., Edwardson J. M. (2005). Syncollin is required for efficient zymogen granule exocytosis. Biochem. J. 385, 721–727 10.1042/BJ20041064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng N., Thomas D. D., Groblewski G. E. (2007). Pancreatic acinar cells express vesicle-associated membrane protein 2- and 8-specific populations of zymogen granules with distinct and overlapping roles in secretion. J. Biol. Chem. 282, 9635–9645 10.1074/jbc.M611108200 [DOI] [PubMed] [Google Scholar]
- Wilcke M., Johannes L., Galli T., Mayau V., Goud B., Salamero J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J. Cell Biol. 151, 1207–1220 10.1083/jcb.151.6.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Nuoffer C., Meinkoth J. L., McCaffery M., Feramisco J. R., Balch W. E., Farquhar M. G. (1994). A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J. Cell Biol. 125, 557–571 10.1083/jcb.125.3.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. H., Brodsky F. M. (1992). 100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties. J. Cell Biol. 117, 1171–1179 10.1083/jcb.117.6.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Edman M., Kothawala M. S., Sun G., Chiang L., Mircheff A., Zhu L., Okamoto C., Hamm-Alvarez S. (2011). A Rab11a-enriched subapical membrane compartment regulates a cytoskeleton-dependent transcytoticpathway in secretory epithelial cells of the lacrimal gland. J. Cell Sci. 124, 3503–3514 10.1242/jcs.088906 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.