Abstract
Aims
Pulmonary arterial hypertension (PAH) occurs more frequently in women than men. Oestrogen and the oestrogen-metabolising enzyme cytochrome P450 1B1 (CYP1B1) play a role in the development of PAH. Anorectic drugs such as dexfenfluramine (Dfen) have been associated with the development of PAH. Dfen mediates PAH via a serotonergic mechanism and we have shown serotonin to up-regulate expression of CYP1B1 in human pulmonary artery smooth muscle cells (PASMCs). Thus here we assess the role of CYP1B1 in the development of Dfen-induced PAH.
Methods and results
Dfen (5 mg kg−1 day−1 PO for 28 days) increased right ventricular pressure and pulmonary vascular remodelling in female mice only. Mice dosed with Dfen showed increased whole lung expression of CYP1B1 and Dfen-induced PAH was ablated in CYP1B1−/− mice. In line with this, Dfen up-regulated expression of CYP1B1 in PASMCs from PAH patients (PAH-PASMCs) and Dfen-mediated proliferation of PAH-PASMCs was ablated by pharmacological inhibition of CYP1B1. Dfen increased expression of tryptophan hydroxylase 1 (Tph1; the rate-limiting enzyme in the synthesis of serotonin) in PAH-PASMCs and both Dfen-induced proliferation and Dfen-induced up-regulation of CYP1B1 were ablated by inhibition of Tph1. 17β-Oestradiol increased expression of both Tph1 and CYP1B1 in PAH-PASMCs, and Dfen and 17β-oestradiol had synergistic effects on proliferation of PAH-PASMCs. Finally, ovariectomy protected against Dfen-induced PAH in female mice.
Conclusion
CYP1B1 is critical in the development of Dfen-induced PAH in mice in vivo and proliferation of PAH-PASMCs in vitro. CYP1B1 may provide a novel therapeutic target for PAH.
Keywords: Oestrogen, Dexfenfluramine, Pulmonary arterial hypertension, CYP1B1, Tryptophan hydroxylase
1. Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease of the pulmonary vasculature resulting in right heart failure and death. In its idiopathic and familial forms, PAH occurs more frequently in females than in males.1–3 Recent evidence suggests a role for oestrogen and the oestrogen-metabolising enzyme cytochrome P450 1B1 (CYP1B1) in the development of PAH. 17β-Oestradiol (the predominant circulating oestrogen) up-regulates components of the serotonin signalling system and mediates proliferation of human pulmonary artery smooth muscle cells (hPASMCs).4,5 In line with this, female gender is permissive in the development of PAH in mice which over-express the serotonin transporter (SERT+ mice) or S100A4/Mts1 (which functions downstream of serotonin).4,5 The PAH phenotype in female SERT+ mice is reversed by ovariectomy but restored following 17β-oestradiol replacement.5
CYP1B1 is a P450 enzyme expressed in the lung catalysing the conversion of oestrogens predominantly to 4-hydroxyoestrogens, but also to 2-hydroxy and 16-hydroxyoestrogens.6,7 CYP1B1 gene polymorphisms and dysregulated CYP1B1 expression have been associated with the risk of developing lung and other cancers,8,9 primary congenital glaucoma,10 and systemic hypertension.11 Multiple lines of evidence from both human and mouse show a role for CYP1B1 in the development of PAH. CYP1B1 gene expression is dysregulated in B-lymphocytes cultured from female PAH patients harbouring a bone morphogenetic protein receptor type II (BMPRII) mutation.12 Interestingly, cytoskeletal defects are observed in pulmonary artery endothelial cells (PAECs) derived from BMPR2 mutated, pulmonary hypertensive mice.13 We have recently shown that CYP1B1 expression is increased in the pulmonary vasculature from patients with PAH. In mice, genetic or pharmacological inhibition of CYP1B1 can protect against experimental PAH.14 Interestingly, serotonin can increase expression of CYP1B1 in hPASMCs.15
The anorectic agents aminorex and dexfenfluramine have been associated with the development of PAH since the 1960s and 1980s, respectively.16,17 Both aminorex and fenfluramine are serotonin transporter (SERT) substrates and increase extracellular concentrations of serotonin,18 leading to the hypothesis that these drugs mediate PAH via a serotonergic mechanism. In support of this hypothesis, we have shown that female mice deficient in Tph1 are protected against Dfen-induced PAH.19 Identification of the precise mechanism by which Dfen mediates PAH is essential to identify (i) other drugs which may be risk factors for development of the disease and (ii) cohorts of patients who have an increased risk of developing PAH in response to these drugs. Recently increased incidence of PAH has been noted in patients taking the drugs metamphetamine20 and benfluorex,21 both of which are structurally similar to and share pharmacological properties with Dfen. In the current study, we assess the role of CYP1B1 in the development of Dfen-induced PAH both in vivo and in vitro.
2. Methods
2.1. Ethical information
The investigation conforms with the UK Animal procedures act, 1986, with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication, 8th Edition, 2011). Experimental procedures using hPASMCs conform to the principles outlined in the Declaration of Helsinki. Ethical approval was also granted by the University Ethics Committee.
2.2. CYP1B1−/− mice
CYP1B1−/− mice were generously provided to us by the National Cancer Institute, Bethesda, MD, USA. The mice were generated on a C57BL/6 background as previously described.22 Age-matched C57BL/6 mice were studied as controls.
2.3. Dfen administration
C57BL/6 mice and CYP1B1−/− mice (10–12 weeks old) were administered Dfen (Sigma, Poole, UK) at a dose of 5 mg kg−1 day−1 by oral gavage, for 28 days. The vehicle was distilled H2O.
2.4. Bilateral ovariectomy
Female C57BL/6 mice were bilaterally ovariectomized at 8–10 weeks of age. The 28-day Dfen dosing regime commenced 2 weeks post-surgery. To determine the success of the ovariectomy procedure, the uterus was removed and weighed at necroscopy. Inhalation anaesthesia was used throughout the procedure (1–3% isofluorane supplemented with oxygen). Depth of anaesthesia was assessed by loss of pain and corneal reflexes.
2.5. Assessment of PAH
2.5.1. In vivo haemodynamic measurements
Mice were anaesthetised as described above. Pressure measurements were conducted and analysed as described previously.23 Briefly, right ventricular pressure was measured via a 25-gauge needle advanced into the right ventricle using a transdiaphragmatic approach. Systemic arterial pressure was obtained via a microcannula inserted into the carotid artery. Mice were euthanized by cervical dislocation and heart and lungs removed for subsequent analysis as described below. Ten to 12 mice per group were studied.
2.5.2. Lung histology
Sagittal lung sections were stained with Elastica-Van Gieson and microscopically assessed for muscularization of small pulmonary arteries (<80 μm external diameter) in a blinded fashion, as described previously.19 Remodelled arteries were confirmed by the presence of a double elastic laminae. Lung sections from four to six mice from each group were studied.
2.5.3. Measurement of right ventricular hypertrophy
Right ventricular hypertrophy (RVH) was assessed by measuring the weight of the RV free wall and expressing this as a ratio of the weight of the left ventricle together with the septum (LV + S).
2.6. Cells
Three distal PASMC lines (each from an individual PAH patient) were used in this study. Cells were explanted immediately following pneumonectomy, and were provided to us by Prof NW Morrell, University of Cambridge, UK. Clinical data from the three PAH patients are shown in Table 1. PAECs were obtained from Promocell (Germany).
Table 1.
Clinical details and classification of the PAH patients from whom the three PAH-PASMC lines were derived
| Patient | Age | Gender | Classification | Pulmonary artery pressure (mmHg) (systolic/mean/diastolic) |
|---|---|---|---|---|
| 1 | 41 | F | IPAH | 90/58/40 |
| 2 | 34 | M | FPAH | 74/38/24 |
| 3 | 24 | F | IPAH | 83/60/30 |
2.7. Proliferation assays
PAH-PASMCs (passages 3–6) were grown to 40% confluency prior to 24 h growth arrest in phenol-red free 0.2% foetal bovine serum (FBS) DMEM. PAH-PASMCs were then exposed to Dfen, norfenfluramine, or 17β-oestradiol for 72 h. For antagonist studies, the Tph inhibitor para-chlorophenylalanine (PCPA; 10 µmol/L) or the CYP1B1 inhibitor 2,3′,4,5′-tetramethoxystilbene (TMS; 1 µmol/L) was added 30 min prior to the addition of Dfen. As quiescent PASMCs do not readily proliferate, all experiments were performed in the presence of either 2.5% FBS or 10 ng/mL platelet-derived growth factor (PDGF). DMEM and agonists/antagonists were replaced every 48 h. For the final 24 h, 0.2 µCi [3H] thymidine was added to each well. Cells were then lysed as described previously19 and [3H] thymidine incorporation measured. Data are expressed as fold change compared with control. All experiments were conducted at least in triplicate from a minimum of two PAH-PASMC lines. Thymidine is incorporated into replicating chromosomal DNA during mitosis and measurement of [3H] thymidine incorporation is an extremely accurate method of measuring human PASMC proliferation. Indeed, we have previously reported high correlation with alternative proliferation assays in this cell type.5
2.8. RNA preparation and qRT-PCR
PAH-PASMCs were grown to 80% confluency in 12-well plates and quiesced in phenol-red free DMEM for 24 h before treatment with Dfen (0.3–3 μmol/L) or 17β-oestradiol (0.1–1 nmol/L) for 4 h. All experiments were conducted at least in triplicate from a minimum of two PAH-PASMC lines. Total RNA was isolated using miRNeasy Mini Kit (Qiagen) and reverse-transcribed using Taqman reverse transcription kit (Applied Biosystems) according to the instructions of the manufacturer. Quantitative real-time PCR was performed using Universal master mix II with Assays on Demand gene expression probes (system and probes from Applied Biosystems) for Tph1 (assay ID: Hs00188220_m1) and CYP1B1 (assay ID: Hs02382916_s1) using the comparative delta-CT method with β2-microglobulin as the endogenous control. qRT-PCR for CYP1B1 was also performed on whole lung isolated from Dfen-dosed mice (n = 6 per group) using the gene expression probe Mm00487229_m1.
2.9. Western blot analysis
Cells were grown to 80% confluency in six-well plates and quiesced in phenol-red free DMEM for 24 h before treatment with Dfen (0.3–3 μmol/L) or 17β-oestradiol (0.1–1 nmol/L) for 4 h. Cell lysates were then prepared for immunoblots as described previously.19 Primary antibodies used were rabbit anti-Tph1 (1:500, Abcam, UK), rabbit anti-CYP1B1 (1:1000, Abcam, UK), and mouse anti-alpha tubulin (1:5000; Abcam UK). Densitometrical analysis was performed using the TotalLab TL100 software. PAH-PASMC experiments were performed in triplicate from a minimum of two PAH-PASMC lines. PAEC experiments were performed in triplicate.
2.10. Serotonin ELISA assay
Serotonin concentration in conditioned media derived from PASMCs exposed to Dfen for 24 h was quantified by ELISA analysis (Genway Biotech, USA). Experiments were carried out in duplicate from each of the three PAH-PASMC lines.
2.11. Statistical analysis
Statistical comparisons were made by one-way analysis of variance with Dunnett's post-test, two-way analysis of variance followed by Bonferroni’s post-test, or Student’s t-test as appropriate. Data are expressed as mean ± SEM.
3. Results
3.1. Dfen-induced PAH is observed only in female mice
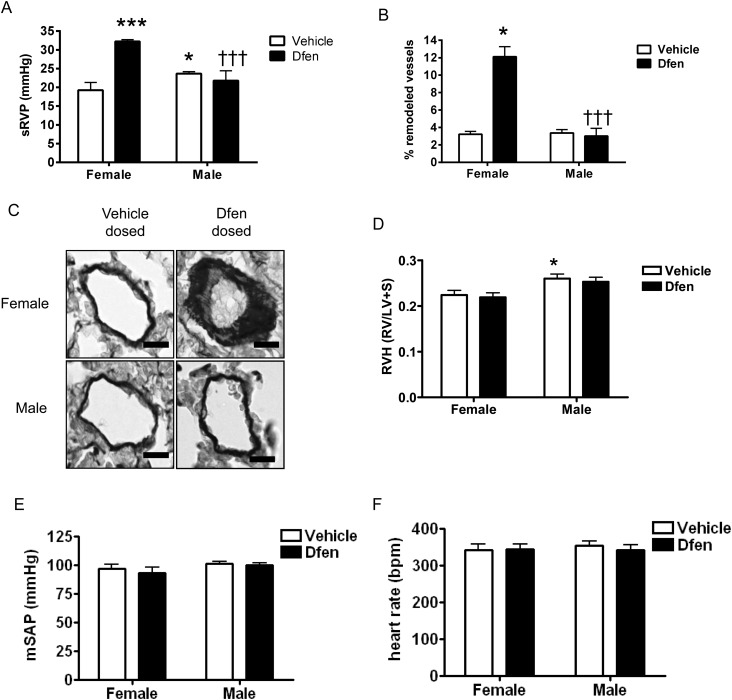
We have previously shown female gender bias in mouse models of disease where the serotonin signalling system is enhanced.4,5 Hence, we examined the development of Dfen-induced PAH in both male and female mice and observed that Dfen-induced PAH only occurred in female mice. In female mice, there was an increase in systolic RVP (sRVP; n = 6–10) and pulmonary vascular remodelling (n = 4) following Dfen administration (Figure 1A–C). RVH was significantly higher in vehicle-dosed male mice than in vehicle-dosed female mice; however, Dfen ingestion had no further effects on RVH (Figure 1D, n = 8–10). Mean systemic arterial pressure (mSAP, n = 7–10) and heart rate (n = 10–12) were unaffected by gender or Dfen administration (Figure 1E and F).
Figure 1.
The effects of Dfen on mediation of PAH are specific to female mice. Dfen-induced increases in (A) sRVP (n = 6, 6, 10, 6, respectively) and (B) pulmonary vascular remodelling (n = 4) were specific to female mice. (C) Representative images of resistance pulmonary arteries (stained with Elastica-Van Gieson) showing increased remodelling in female Dfen-dosed mice only. Male mice show a modest increase in (A) sRVP and (D) RVH (n = 10, 9, 8, 8, respectively) compared with female mice. Dfen administration has no further effects on RVH. (E) mSAP (n = 7, 8, 7, 10, respectively) and (F) heart rate (n = 10, 10, 12, 10, respectively) are unchanged between any of the groups studied. Data are expressed as mean ± SEM. Data were analysed by two-way ANOVA followed by Bonferroni’s post-test. *P < 0.05, ***P < 0.001 vs. female vehicle-dosed mice †††P < 0.001 vs. female Dfen dosed mice. Scale bars represent 20 µm.
3.2. CYP1B1 influences the development of Dfen-induced PAH in vivo
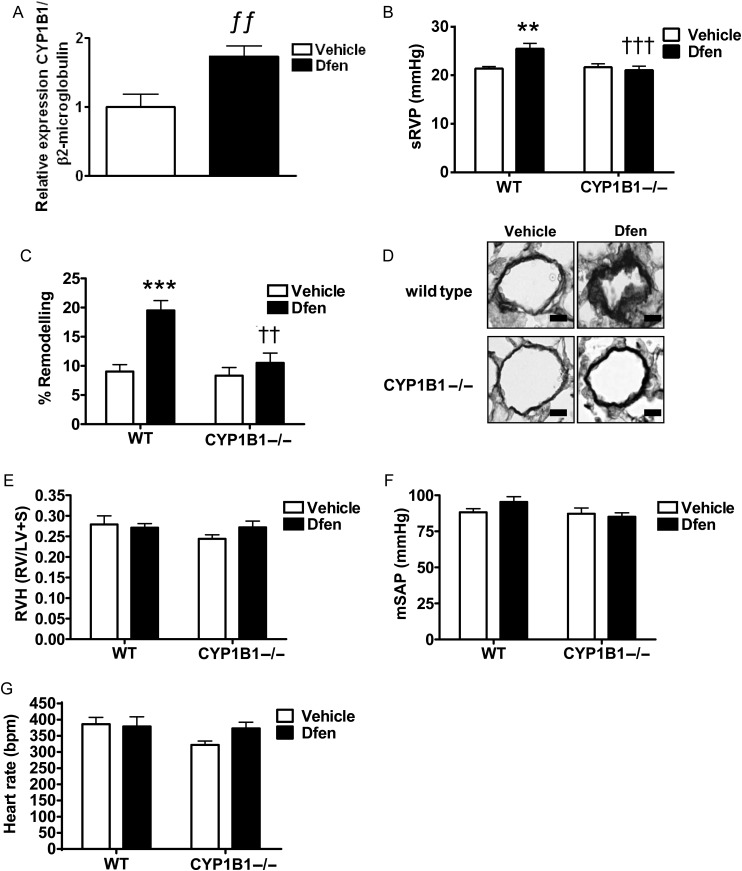
Serotonin and 17β-oestradiol can up-regulate the oestrogen-metabolizing enzyme CYP1B115 which has been shown to play a role in the development of PAH.14 Therefore, we assessed the role of CYP1B1 on development of Dfen-induced PAH in female mice. We observed increased expression of CYP1B1 in the lungs of female Dfen-dosed mice by qRT-PCR (Figure 2A, n = 6). Moreover, female CYP1B1−/− mice were protected against Dfen-induced increases in sRVP (Figure 2B, n = 9–12) and pulmonary vascular remodelling (Figure 2C and D, n = 4–5). RVH (Figure 2E, n = 8–12), mSAP (Figure 2F, n = 7–11), and heart rate (Figure 2G, n = 8–12) were unaffected by Dfen administration or genetic ablation of CYP1B1.
Figure 2.
CYP1B1 is critical for Dfen-induced PAH. (A) CYP1B1 expression was increased in Dfen-dosed mice as assessed by qRT-PCR (n = 6). (B) Dfen induced increases in sRVP (n = 9, 9, 9, 12, respectively) and (C) pulmonary vascular remodelling (n = 4, 4, 4, 5, respectively) were ablated in female CYP1B1−/− mice. (D) Representative images of resistance pulmonary arteries (stained with Elastica-Van Gieson) showing Dfen-induced remodelling is ablated in female CYP1B1−/− mice. (E) RVH (n = 8, 10, 9, 12, respectively), (F) mSAP (n = 7, 11, 8, 11), and (G) heart rate (n = 8, 11, 8, 12, respectively) were unchanged by Dfen administration or genetic ablation of CYP1B1. Data are expressed as mean ± SEM. Data were analysed by two-way ANOVA followed by Bonferroni’s post-test. ƒƒP < 0.01 vs. vehicle, **P < 0.01, ***P < 0.001 vs. female wild-type vehicle-dosed mice, ††P < 0.01, †††P < 0.001 vs. female wild-type Dfen-dosed mice. Scale bars represent 20 µm.
3.3. CYP1B1 is critical for Dfen-induced proliferation of PAH-PASMCs in vitro
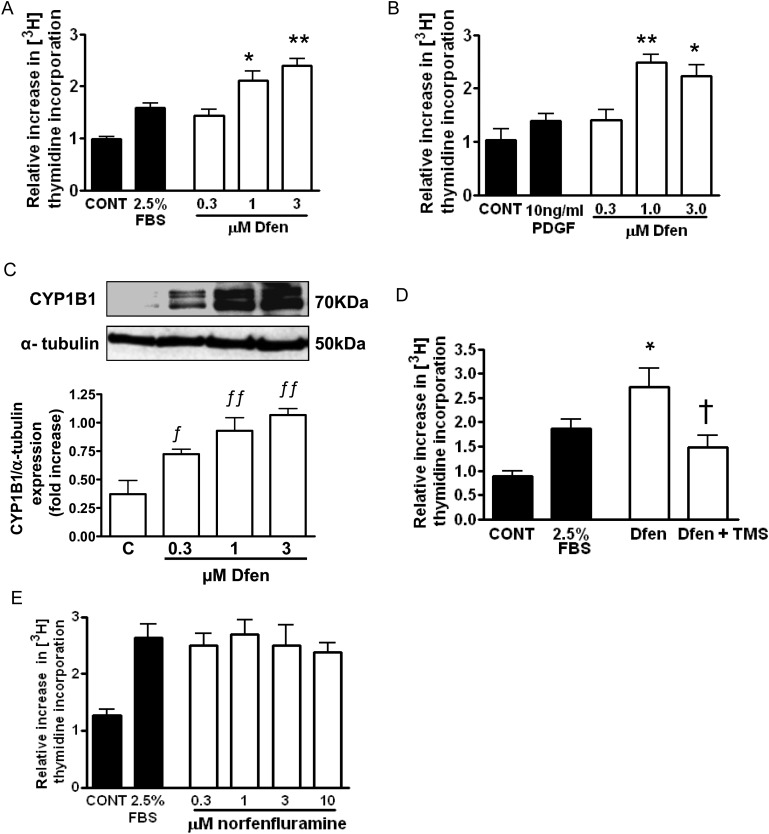
Having shown a critical role for CYP1B1 in the development of Dfen-induced PAH in vivo, we assessed the direct effects of Dfen on proliferation of PAH-PASMCs in vitro and the role of CYP1B1 in these effects. Dfen stimulated proliferation of PAH-PASMCs in the presence of 2.5% serum (Figure 3A, n = 3) or 10 ng/mL PDGF (Figure 3B, n = 3). All subsequent proliferation experiments were carried out in the presence of 2.5% serum. Dfen-induced proliferation was associated with an increase in protein expression of CYP1B1 (Figure 3C, n = 3) and was inhibited by the selective CYP1B1 inhibitor TMS (Figure 3D, n = 3). As the active Dfen metabolite norfenfluramine has also been implicated in the development of PAH,24 we assessed the effects of norfenfluramine on proliferation of PAH-PASMCs. Norfenfluramine did not mediate proliferation of PAH-PASMCs (Figure 3E, n = 3).
Figure 3.
CYP1B1 is critical for Dfen-induced proliferation of PAH-PASMCs. (A and B) Dfen-mediated proliferation of PAH-PASMCs in the presence of both FBS (n = 3) or PDGF (n = 3). (C) Dfen up-regulates expression of CYP1B1 in PAH-PASMCs as assessed by western blot (n = 3). (D) Dfen-mediated proliferation is inhibited in the presence of the selective CYP1B1 inhibitor TMS (1 µM) (n = 3). (E) Norfenfluramine had no effect on proliferation of PAH-PASMCs (n = 3). Data are expressed as mean ± SEM. Data were analysed by one-way ANOVA followed by Dunnett's post-test. *P < 0.05, **P < 0.01 vs. 2.5% serum or 10 ng/mL PDGF, ƒP < 0.05, ƒƒP < 0.01 vs. control †P < 0.05 vs. Dfen.
3.4. CYP1B1 functions downstream of serotonin in Dfen-induced proliferation of PAH-PASMCs
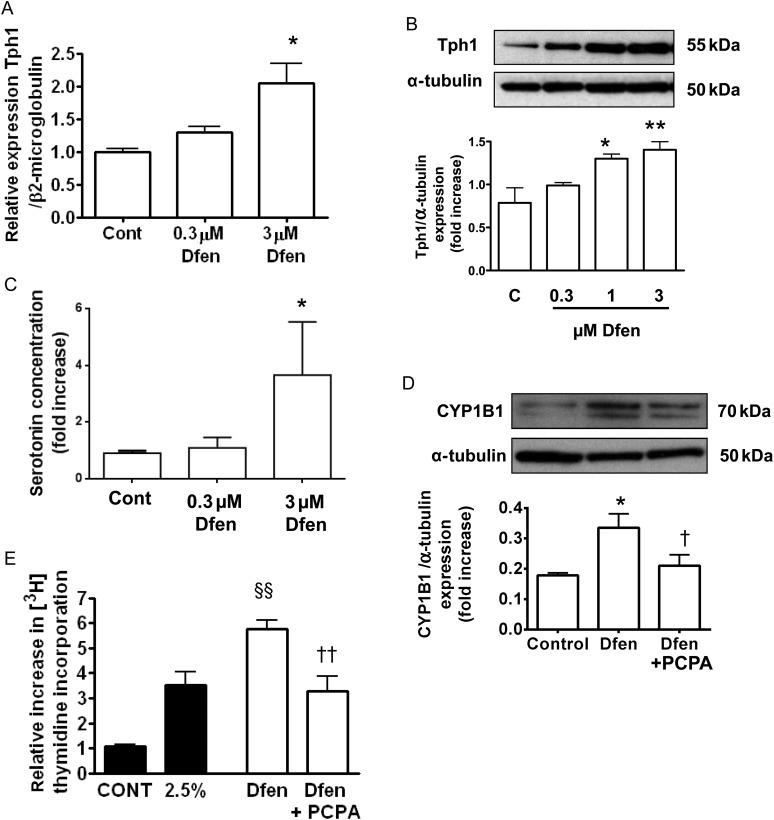
As we have previously shown that Dfen-induced PAH is dependent on peripheral serotonin in vivo,19 we investigated the role of serotonin in the effects of Dfen on PAH-PASMCs in vitro. First, we show that Dfen can up-regulate both mRNA (Figure 4A, n = 3) and protein (Figure 4B, n = 3) expression of Tph1 in PAH-PASMCs. In addition, serotonin concentration was increased in conditioned media derived from cells treated with Dfen (Figure 4C, n = 3). The Tph1 inhibitor PCPA inhibited both Dfen-induced up-regulation of CYP1B1 (Figure 4D, n = 3) and Dfen-induced proliferation of PAH-PASMCs (Figure 4E, n = 4). As endothelial cells play an important role in the development of PAH, we also assessed whether Dfen could up-regulate CYP1B1 or Tph1 in endothelial cells. Dfen had no effects on CYP1B1 expression in PAECs (Supplementary material online, Figure S1A). Dfen mediated a trend (although not statistically significant) towards increased Tph1 expression in endothelial cells (Supplementary material online, Figure S1B).
Figure 4.
CYP1B1 functions downstream of Tph1 in Dfen-induced proliferation of PAH-PASMCs. Dfen up-regulates (A) mRNA (n = 3) and (B) protein (n = 3) expression of Tph1. (C) There is an increased concentration of serotonin in conditioned media derived from cells treated with Dfen (n = 3). (D) Dfen-induced up-regulation of CYP1B1 (n = 3) and (E) Dfen-induced proliferation (n = 4) are inhibited in the presence of the Tph1 inhibitor PCPA (10 µM). Data are expressed as mean ± SEM. Data were analysed by one-way ANOVA followed by Dunnett's post-test. *P < 0.05, **P < 0.01 vs. control, §§P < 0.01 vs. 2.5% serum, †P < 0.05, ††P < 0.01 vs. Dfen.
3.5. 17β-Oestradiol plays a role in the development of Dfen-induced PAH
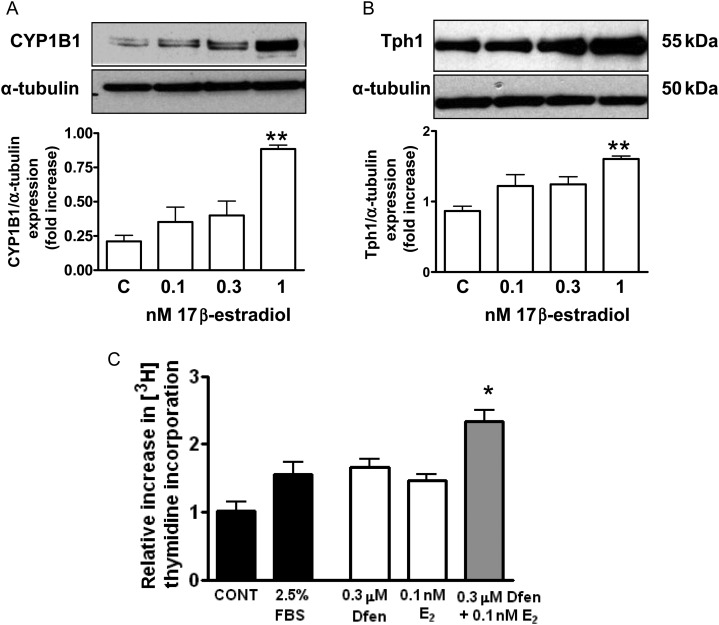
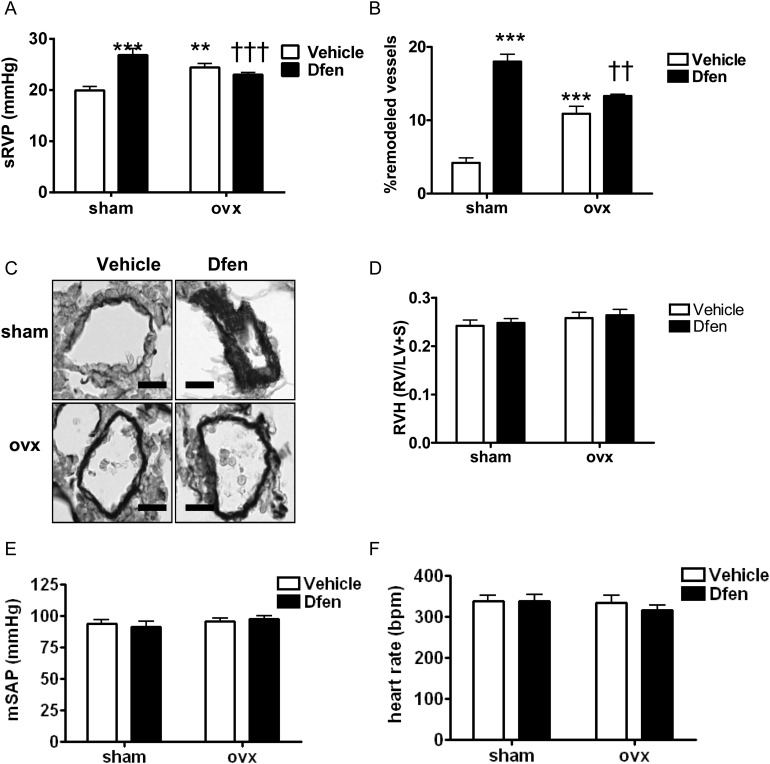
Finally, we wished to investigate the role of female sex hormones on the development of Dfen-induced PAH. We have previously shown that 17β-oestradiol can up-regulate expression of both CYP1B1 and Tph1 in human PASMCs from non-diseased controls.5,15 Now we confirm that CYP1B1 (Figure 5A, n = 3) and Tph1 (Figure 5B, n = 3) are up-regulated by 17β-oestradiol in PAH-PASMCs. In line with this, Dfen and 17β-oestradiol have synergistic effects on the proliferation of PAH-PASMCs (Figure 5C, n = 3). 17β-Oestradiol had no effects on CYP1B1 or Tph1 expression in PAECs (Supplementary material online, Figure S2). To confirm these findings in vivo, we assessed whether Dfen-induced PAH was ablated in ovariectomized mice. Success of the ovariectomy procedure was confirmed by a marked reduction of uterus weight (sham-operated mice 64.1 ± 4.3 mg cf ovariectomized mice 7.4 ± 0.4 mg, n = 24 per group, P < 0.001). Indeed, we show that Dfen-induced increases in sRVP (Figure 6A, n = 9–11) and pulmonary vascular remodelling (Figure 6B and C, n = 4) are reduced in ovariectomized mice. As described previously,5 ovariectomy increased sRVP and pulmonary vascular remodelling in vehicle-dosed mice (Figure 6A and B). There were no changes in RVH (n = 12), mSAP (n = 7–10), or heart rate (n = 9–12) in any of the groups studied (Figure 6D–F).
Figure 5.
17β-Oestradiol and Dfen have synergistic effects on PAH-PASMC proliferation. 17β-Oestradiol mediates an increase in (A) CYP1B1 (n = 3) and (B) Tph1 (n = 3) expression in PAH-PASMCs. (C) Dfen and 17β-oestradiol have synergistic effects on proliferation of PAH-PASMCs (n = 3). Data are expressed as mean ± SEM. Data were analysed by one-way ANOVA followed by Dunnett's post-test. *P < 0.05 vs. 0.3 µM Dfen P < 0.01 vs. 0.1 nM 17β-estradiol, **P < 0.01 vs. control.
Figure 6.
Female sex hormones are critical for Dfen-induced PAH. Dfen-induced increases in (A) sRVP (n = 11, 11, 9, 9, respectively) and (B) pulmonary vascular remodelling (n = 4) were inhibited in ovariectomized mice. Ovariectomized mice show a modest increase in sRVP (A) and pulmonary vascular remodelling (B) compared with sham-operated controls. (C) Representative images of resistance pulmonary arteries stained with Elastica-Van Gieson. Dfen administration or ovariectomy had no effects on (D) RVH (n = 12), (E) mSAP (n = 10, 7, 9, 9, respectively), and (F) heart rate (n = 11, 12, 9, 10, respectively). Data are expressed as mean ± SEM. Data were analysed by two-way ANOVA followed by Bonferroni’s post-test. **P < 0.001, ***P < 0.001 vs. female vehicle-dosed mice ††P < 0.01, †††P < 0.001 vs. female Dfen-dosed mice. Scale bars represent 20 µm.
4. Discussion
Here, we show a critical role for female sex hormones and the oestrogen-metabolizing enzyme CYP1B1 in the development of Dfen-induced PAH in mice. In order to translate our findings to human disease, we assessed the effects of Dfen in PASMCs derived from PAH patients. In these cells, we show that Dfen can increase CYP1B1 expression and Dfen-induced proliferation is dependent on CYP1B1 activity. We verify the role of serotonin in Dfen-mediated PAH by showing that Dfen can act directly on PAH-PASMCs to increase expression of Tph1, and pharmacological inhibition of Tph1 can inhibit both Dfen-induced proliferation and Dfen-induced up-regulation of CYP1B1. Further, we show that 17β-oestradiol can increase Tph1 and CYP1B1 expression in PAH-PASMCs and in line with this, Dfen and 17β-oestradiol have synergistic effects on proliferation of PAH-PASMCs.
We have recently described a pivotal role for CYP1B1 in the development of experimental PAH and in human PAH14 and the data shown here provide the first evidence in support of a role for CYP1B1 in a model of anorexigen-induced PAH. CYP1B1 catalyses the formation of metabolites including 2,4 and 16α-hydroxyoestrogens from 17β-estradiol.6,7 16α-Hydroxyoestrone (but not two or four hydroxyoestrogen) mediates proliferation of hPASMCs, an effect which is exaggerated in cells from PAH patients. In line with this, administration of 16α-hydroxyoestrone leads to the development of PAH in mice.14 Although CYP1B1 can mediate formation of 2-hydroxyoestrogens, these are predominantly formed by the CYP1A1/2 enzymes.6,7,14 2-Hydroxyestradiol is further converted to 2-methoxyestradiol via catechol O-methyltransferase. 2-Methoxyestradiol has been shown to be protective in the development of PAH in rodent models and also to have anti-proliferative effects on cells.25 Thus, the effects of CYP1B1 on the development of PAH may be due to a shift in the balance of oestrogen conversion to favour the pro-proliferative metabolites. In line with this, a decreased 2 hydroxyoestrogen:16α-hydroxyoestrogen ratio has been associated with PAH penetrance in female BMPRII mutation carriers.26
We have previously shown that Dfen-induced PAH in vivo is dependent on the activity of Tph1 (the Tph isoform responsible for synthesis of serotonin in the periphery).19 However, the serotonin hypothesis of PAH is still debated,27 firstly because fenfluramine-induced elevations in whole blood serotonin levels are below those necessary to induce cardiovascular side effects and secondly because compensatory genetic changes in Tph1−/− mice may be involved in the protection of these mice from Dfen-induced PAH. We have hypothesized that Dfen mediates its effects directly on the cells of the pulmonary vasculature,28,29 which would explain why Dfen can mediate PAH via a serotonergic mechanism without raising whole blood serotonin to toxic levels. In the current study, we clarify this hypothesis. First, we detected Tph1 protein in PAH-PASMCs and have shown that Dfen increases expression of Tph1 protein in these cells. Further, Dfen-induced activation of CYP1B1 and Dfen-induced proliferation of PAH-PASMCs are abolished with the Tph inhibitor PCPA, confirming the importance of de novo pulmonary arterial serotonin synthesis in response to Dfen. Dfen has previously been reported to have direct effects on pulmonary arteries, including inhibition of potassium channels,30 increased intracellular calcium,31 vasoconstriction,32 and mitogenic effects.33 However, the role of Tph1 in mediation of these effects remains to be assessed. The major metabolite of Dfen, norfenfluramine, is an agonist at the 5-HT2A and 5-HT2B receptors,34 both of which have been implicated in experimental PAH.35,36 In addition, norfenfluramine has been shown to cause more severe pulmonary vasoconstriction than Dfen in rat pulmonary arteries.24 We did not observe any effects of norfenfluramine on PAH-PASMC proliferation. Thus, while it is likely that norfenfluramine contributes to Dfen-induced PAH by mediating vasoconstriction, our results do not support a role for norfenfluramine in proliferation of PAH-PASMCs. Our results suggest that Dfen, which is a SERT substrate,34 enters the cell via SERT and increases de novo synthesis of serotonin via induction of Tph1. Serotonin can then up-regulate expression of CYP1B1.
17β-Oestradiol circulates at concentrations of 0.1–1 nmol/L.37 Here, we show that physiological concentrations of 17β-oestradiol stimulated both Tph1 and CYP1B1 expression in PAH-PASMCs. Both Dfen and 17β-oestradiol mediate proliferation of hPASMCs and these effects are synergistic at 0.3 μmol/L and 0.1 nmol/L, respectively, which are within therapeutic (Dfen) and physiological (17β-oestradiol) concentration ranges. This may indeed explain why Dfen-induced PAH was not observed in the male mice where circulating 17β-oestradiol is very low. Consistent with the data we present here, clinical studies have demonstrated a female gender bias towards Dfen-induced PAH in humans. In one study, a much larger proportion (40.9%) of female primary pulmonary hypertensive patients had used anorexigenic drugs than male primary pulmonary hypertensive patients (10.3%).16 In addition, in different patient populations, the ratio of women to men with anorexigenic-induced PAH has been reported to be 8:1, 30:1, and 34:1.38–40 It has always been assumed that the gender imbalance in the development of Dfen-induced PAH was due to a higher proportion of females using appetite suppressant drugs. However, the current study suggests a female pre-disposition to PAH in response to Dfen exposure. One limitation of our model, however, is that in the human condition, males develop Dfen-induced PAH whereas male mice do not. In addition, not all women who took Dfen developed the disease. It is of interest that BMPRII mutations have been associated with the development of fenfluramine-induced PAH,39 and polymorphisms in the gene encoding CYP1B1 increase disease penetrance in BMPRII mutation carriers by four-fold.26 Thus, assessment of CYP1B1 polymorphisms in patients with Dfen-induced PAH would be informative.
Interestingly, male C57BL/6 mice showed slightly higher sRVP and RVH than female mice and ovariectomy increased sRVP and pulmonary vascular remodelling in vehicle-dosed wild-type mice. This suggests that in the absence of activation of the serotonin system, female sex hormones may actually be protective. Indeed, oestrogen has recently been shown to be protective against hypoxic-induced PAH in male rats,41 and progesterone can protect against monocrotaline-induced PAH.42 As discussed above, as well as the formation of pro-proliferative metabolites, oestrogen metabolism can also lead to the formation of anti-proliferative metabolites. Interestingly, serotonin has been shown to up-regulate CYP1B1 in hPASMCs and in the current study we have shown Dfen to activate CYP1B1 via a serotonergic mechanism. In line with this, CYP1B1 expression is enhanced in female Dfen-dosed mice and in female mice over-expressing the serotonin transporter.15 Thus, our results suggest that enhanced serotonin signalling may lead to activation of CYP1B1, which can disrupt the balance of oestrogen metabolites to favour pro-proliferative forms.
In the present study, female mice developed increased RVP following Dfen-administration; however, RVH remained unaffected. We have previously shown a dissociation between RVP and RVH in the SERT+ mouse, mice over-expressing S100A4/Mts1, and mice dosed with Dfen.4,19,43 We are not alone in observing this phenomenon as other studies have also demonstrated elevated RVP in the absence of RVH in mice.44,45 For example, mice that express BMPR2R899X in smooth muscle or molecular loss of BMPR2 signalling in smooth muscle demonstrate elevated RVP with no RVH.44 Interestingly, it has recently been shown that in mice, testosterone may mediate RVH in response to load stress, while having no significant effects on pulmonary haemodynamics.46 Thus it is possible that sex hormones may influence RVH independently of pulmonary haemodynamics. In line with this, we show in the current study that male mice at baseline have increased RVH compared with female mice.
In conclusion, we have shown that Dfen up-regulates CYP1B1 via a serotonergic mechanism in PAH-PASMCs and CYP1B1 is critical for the development of Dfen-induced PAH in mice, and Dfen-induced proliferation of PAH-PASMCs. Our results suggest that females may have increased pre-disposition to anorexigen-induced PAH, and that further investigation of CYP1B1 as a therapeutic target for anorexigen-induced PAH may be warranted.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Medical Research Council (G0801171), the British Heart Foundation (RG/11/7/28916), and a Capacity Building Award in Integrative Mammalian Biology funded by the Biotechnology and Biological Sciences Research Council, British Pharmacological Society, Knowledge Transfer Network, Medical Research Council and Scottish Funding Council (BB/E527071/1).
Supplementary Material
Acknowledgements
We acknowledge Prof N. Morrell (University of Cambridge) for kindly supplying us with PAH-PASMCs.
Conflict of interest: none declared.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. doi:10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 2.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–109. doi: 10.1183/09031936.00092306. doi:10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 3.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J. 2007;30:1103–1110. doi: 10.1183/09031936.00042107. doi:10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 4.Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, et al. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. Respir Res. 2011;12:159. doi: 10.1186/1465-9921-12-159. doi:10.1186/1465-9921-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, MacLean MR. The serotonin transporter, gender, and 17beta oestradiol in the development of pulmonary arterial hypertension. Cardiovasc Res. 2011;90:373–382. doi: 10.1093/cvr/cvq408. doi:10.1093/cvr/cvq408. [DOI] [PubMed] [Google Scholar]
- 6.Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 2000;60:3440–3444. [PubMed] [Google Scholar]
- 7.Badawi AF, Cavalieri EL, Rogan EG. Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism. 2001;50:1001–1003. doi: 10.1053/meta.2001.25592. doi:10.1053/meta.2001.25592. [DOI] [PubMed] [Google Scholar]
- 8.Murray GI, Taylor MC, McFadyen MC, McKay JA, Greenlee WF, Burke MD, et al. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–3031. [PubMed] [Google Scholar]
- 9.Laroche-Clary A, Le Morvan V, Yamori T, Robert J. Cytochrome P450 1B1 gene polymorphisms as predictors of anticancer drug activity: studies with in vitro models. Mol Cancer Ther. 2010;9:3315–3321. doi: 10.1158/1535-7163.MCT-10-0673. doi:10.1158/1535-7163.MCT-10-0673. [DOI] [PubMed] [Google Scholar]
- 10.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997;6:641–647. doi: 10.1093/hmg/6.4.641. doi:10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- 11.Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, Fang XR, et al. Cytochrome P450 1B1 contributes to angiotensin II-induced hypertension and associated pathophysiology. Hypertension. 2010;56:667–674. doi: 10.1161/HYPERTENSIONAHA.110.154518. doi:10.1161/HYPERTENSIONAHA.110.154518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, et al. Gene expression in BMPR2 mutation carriers with and without evidence of Pulmonary Arterial Hypertension suggests pathways relevant to disease penetrance. BMC Med Genomics. 2008;1:45. doi: 10.1186/1755-8794-1-45. doi:10.1186/1755-8794-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, et al. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2012;302:L474–L484. doi: 10.1152/ajplung.00202.2011. doi:10.1152/ajplung.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, et al. Activity of the estrogen metabolising enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. doi:10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 15.White K, Loughlin L, Maqbool Z, Nilsen M, McClure J, Dempsie Y, et al. Serotonin transporter, sex, and hypoxia: microarray analysis in the pulmonary arteries of mice identifies genes with relevance to human PAH. Physiol Genomics. 2011;43:417–437. doi: 10.1152/physiolgenomics.00249.2010. doi:10.1152/physiolgenomics.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. doi:10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MS, Lane DA. Aminorex, dexfenfluramine, and primary pulmonary hypertension. J Clin Epidemiol. 1998;51:361–364. doi: 10.1016/s0895-4356(97)00289-8. doi:10.1016/S0895-4356(97)00289-8. [DOI] [PubMed] [Google Scholar]
- 18.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates - implications for primary pulmonary hypertension. Circulation. 1999;100:869–875. doi: 10.1161/01.cir.100.8.869. doi:10.1161/01.CIR.100.8.869. [DOI] [PubMed] [Google Scholar]
- 19.Dempsie Y, Morecroft I, Welsh DJ, Macritchie NA, Herold N, Loughlin L, et al. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation. 2008;117:2928–2937. doi: 10.1161/CIRCULATIONAHA.108.767558. doi:10.1161/CIRCULATIONAHA.108.767558. [DOI] [PubMed] [Google Scholar]
- 20.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. doi:10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 21.Savale L, Chaumais MC, Cottin V, Bergot E, Frachon I, Prevot G, et al. Pulmonary hypertension associated with benfluorex exposure. Eur Respir J. 2012;40:1164–1172. doi: 10.1183/09031936.00188611. doi:10.1183/09031936.00188611. [DOI] [PubMed] [Google Scholar]
- 22.Buters JT, Doehmer J, Gonzalez FJ. Cytochrome P450-null mice. Drug Metab Rev. 1999;31:437–447. doi: 10.1081/dmr-100101929. doi:10.1081/DMR-100101929. [DOI] [PubMed] [Google Scholar]
- 23.Morecroft I, Pang L, Baranowska M, Nilsen M, Loughlin L, Dempsie Y, et al. In vivo effects of a combined 5-HT1B receptor/SERT antagonist in experimental pulmonary hypertension. Cardiovasc Res. 2010;85:593–603. doi: 10.1093/cvr/cvp306. doi:10.1093/cvr/cvp306. [DOI] [PubMed] [Google Scholar]
- 24.Hong ZG, Olschewski A, Reeve HL, Nelson DP, Hong FX, Weir EK. Nordexfenfluramine causes more severe pulmonary vasoconstriction than dexfenfluramine. Am J Physiol Lung Cell Mol Physiol. 2004;286:L531–L538. doi: 10.1152/ajplung.00247.2003. doi:10.1152/ajplung.00247.2003. [DOI] [PubMed] [Google Scholar]
- 25.Tofovic SP, Zhang XC, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vasc Pharmacol. 2006;45:358–367. doi: 10.1016/j.vph.2006.05.007. doi:10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, et al. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. doi:10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman RB, Cadet JL, Dersch CM, McCoy MT, Lehrmann E, Becker KG, et al. Altered gene expression in pulmonary tissue of tryptophan hydroxylase-1 knockout mice: implications for pulmonary arterial hypertension. PLoS ONE. 2011;6:e17735. doi: 10.1371/journal.pone.0017735. doi:10.1371/journal.pone.0017735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br J Pharmacol. 2008;155:455–462. doi: 10.1038/bjp.2008.241. doi:10.1038/bjp.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLean MR. Pulmonary hypertension and the serotonin hypothesis: where are we now? Int J Clin Pract Suppl. 2007;156:27–31. doi: 10.1111/j.1742-1241.2007.01497.x. doi:10.1111/j.1742-1241.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 30.Weir EK, Reeve HL, Huang JMC, Michelakis E, Nelson DP, Hampl V, et al. Anorexic agents aminorex, fenfluramine, and dexfenfluramine inhibit potassium current in rat pulmonary vascular smooth muscle and cause pulmonary vasoconstriction. Circulation. 1996;94:2216–2220. doi: 10.1161/01.cir.94.9.2216. doi:10.1161/01.CIR.94.9.2216. [DOI] [PubMed] [Google Scholar]
- 31.Reeve HL, Archer SL, Soper M, Weir EK. Dexfenfluramine increases pulmonary smooth muscle intracellular Ca2+ independent of membrane potential. Am J Physiol Lung Cell Mol Physiol. 1999;277:L662–L666. doi: 10.1152/ajplung.1999.277.3.L662. [DOI] [PubMed] [Google Scholar]
- 32.Higenbottam T, Marriott H, Cremona G, Laude E, Bee D. The acute effects of dexfenfluramine on human and porcine pulmonary vascular tone and resistance. Chest. 1999;116:921–930. doi: 10.1378/chest.116.4.921. doi:10.1378/chest.116.4.921. [DOI] [PubMed] [Google Scholar]
- 33.Lee SL, Wang WW, Fanburg BL. Dexfenfluramine as a mitogen signal via the formation of superoxide anion. FASEB J. 2001;15:1324–1325. doi: 10.1096/fj.00-0431fje. [DOI] [PubMed] [Google Scholar]
- 34.Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. doi:10.1016/S0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 35.Launay JM, Herve P, Peoc'h K, Tournois C, Callebert J, Nebigil CG, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. doi: 10.1038/nm764. doi:10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- 36.Hironaka E, Hongo M, Sakai A, Mawatari E, Terasawa F, Okumura N, et al. Serotonin receptor antagonist inhibits monocrotaline-induced pulmonary hypertension and prolongs survival in rats. Cardiovasc Res. 2003;60:692–699. doi: 10.1016/j.cardiores.2003.09.023. doi:10.1016/j.cardiores.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Rosselli M, Imthurm B, Macas E, Keller PJ, Dubey RK. Circulating nitrite/nitrate levels increase with follicular development: indirect evidence for estradiol-mediated NO release. Biochem Biophys Res Commun. 1994;202:1543–1552. doi: 10.1006/bbrc.1994.2107. doi:10.1006/bbrc.1994.2107. [DOI] [PubMed] [Google Scholar]
- 38.Archer SL, Djaballah K, Humbert M, Weir KE, Fartoukh M, Dall'ava-Santucci J, et al. Nitric oxide deficiency in fenfluramine- and dexfenfluramine-induced pulmonary hypertension. Am J Respir Crit Care Med. 1998;158:1061–1067. doi: 10.1164/ajrccm.158.4.9802113. [DOI] [PubMed] [Google Scholar]
- 39.Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518–523. doi: 10.1183/09031936.02.01762002. doi:10.1183/09031936.02.01762002. [DOI] [PubMed] [Google Scholar]
- 40.Simonneau G, Fartoukh M, Sitbon O, Humbert M, Jagot JL, Herve P. Primary pulmonary hypertension associated with the use of fenfluramine derivatives. Chest. 1998;114(3 suppl):195S–199S. doi: 10.1378/chest.114.3_supplement.195s. doi:10.1378/chest.114.3_Supplement.195S. [DOI] [PubMed] [Google Scholar]
- 41.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, et al. 17beta-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med. 2012;185:965–980. doi: 10.1164/rccm.201107-1293OC. doi:10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tofovic PS, Zhang X, Petrusevska G. Progesterone inhibits vascular remodeling and attenuates monocrotaline-induced pulmonary hypertension in estrogen-deficient rats. Prilozi. 2009;30:25–44. [PubMed] [Google Scholar]
- 43.MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen SB, Sheward J, et al. Overexpression of the 5-hydroxytryptamine transporter gene—effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–2155. doi: 10.1161/01.CIR.0000127375.56172.92. doi:10.1161/01.CIR.0000127375.56172.92. [DOI] [PubMed] [Google Scholar]
- 44.Tada Y, Majka S, Carr M, Harral J, Crona D, Kuriyama T, et al. Molecular effects of loss of BMPR2 signaling in smooth muscle in a transgenic mouse model of PAH. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1556–L1563. doi: 10.1152/ajplung.00305.2006. doi:10.1152/ajplung.00305.2006. [DOI] [PubMed] [Google Scholar]
- 45.Weng M, Raher MJ, Leyton P, Combs TP, Scherer PE, Bloch KD. Adiponectin decreases pulmonary arterial remodeling in murine models of pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:340–347. doi: 10.1165/rcmb.2010-0316OC. doi:10.1165/rcmb.2010-0316OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hemnes AR, Maynard KB, Champion HC, Gleaves L, Penner N, West J. Testosterone negatively regulates right ventricular load stress responses in mice. Pulm Circ. 2012;2:352–358. doi: 10.4103/2045-8932.101647. doi:10.4103/2045-8932.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.