Abstract
Retrovirus tropism can be restricted by cellular factors such as Fv1, Ref1, and Lv1 that inhibit infection by targeting the incoming viral capsid. Here, we show that rodent cells exhibit differential sensitivity to infection by vesicular stomatitis virus G-pseudotyped lentiviruses and that differences between human immunodeficiency virus type 1 and simian immunodeficiency virus (SIVmac) infectivity are sometimes, but not always, governed by determinants in capsid-p2. In at least one case, resistance to SIVmac infection could be eliminated by saturation of target cells with noninfectious SIVmac particles. However, cross-saturation experiments and analysis of Fv1-null cells engineered to express natural or artificial Fv1 proteins revealed that lentivirus restriction in mouse cells is independent of Fv1. Overall, these findings indicate that novel restriction factors in rodents can modulate sensitivity to specific primate lentiviruses.
Human immunodeficiency virus type 1 (HIV-1) exhibits a highly restricted host cell tropism and is only capable of efficient replication in primary or immortalized human T cells and macrophages. The discovery of human gene products that are expressed in these cells and are essential for virus replication has raised the possibility that nonhuman organisms, particularly rodents, might be engineered to express these molecules, thereby rendering them able to support a productive HIV-1 replication cycle (8, 20, 26). There are several, apparently recessive, blocks to HIV-1 replication in rodent cells, some of which can be overcome by expression of human versions of the HIV-1 receptors (CD4 and CXCR4 or CCR5) and the essential Tat cofactor, cyclin T1 (4, 5, 14, 26, 37). If rodent cells are engineered to express these molecules, then the early steps of the HIV-1 life cycle proceed with reasonable efficiency (4, 14, 21, 26). Additional and as-yet-poorly characterized blocks in late steps of the virus life cycle ultimately lead to a profound defect (103- to 104-fold) in the yield of infectious virions per infected cell (4, 26). Importantly, these late-stage defects can be rescued by fusing HIV-1-infected rodent cells to uninfected human cells (4, 25), suggesting that they are the consequence of a lack of necessary factors rather than the presence of dominant inhibitors of late HIV-1 replication steps in rodents.
Retroviral tropism is also influenced by the presence of dominant inhibitory activities. One such activity is exhibited by the product of a murine gene, Fv1, which can confer substantial resistance to infection by murine leukemia virus (MLV) (15, 24, 28, 30). Fv1 acts by targeting the capsid of the incoming virus and prevents the establishment of an integrated provirus (11, 19, 23, 32). Fv1 itself encodes a protein that exhibits approximately 60% homology to the Gag proteins encoded by the HERV-L and MERV-L family of endogenous retroviruses (3), and two major allelic variants of Fv1 are present in laboratory mice (Fv1n and Fv1b) that confer resistance to B-tropic (B-MLV) and N-tropic (N-MLV) MLV strains, respectively (15, 22, 30).
The determinants of N- versus B-tropism have been mapped to a single amino acid residue within the MLV capsid (CA) protein (23). Fv1-mediated restriction of MLV infection can be overcome at high multiplicities of infection, and infection of restricting cells can give rise to multihit titration curves, implying that infection by one virus particle is facilitated by the presence of others (1, 7, 10, 13, 31, 34). This inhibition of restriction is highly specific in that unrestricted viral particles do not inhibit restriction, presumably because Fv1 does not recognize and therefore cannot be saturated by unrestricted capsids.
Recently, it has become clear that “Fv1-like” inhibitors of retroviral infection are prevalent among mammals. Indeed, human cells exhibit substantial and specific resistance to infection by N-MLV (35). Precisely the same amino acid that governs N- versus B-tropism in mice also controls MLV tropism for human cells (35). Because of this and the fact that precisely the same saturation phenomenon that was described in the context of murine Fv1-mediated restriction is also observed in human cells (36), it is probable that humans also express an inhibitor, termed Ref1, that exhibits restriction specificity similar to that of Fv1b.
Nonhuman primate cells are resistant to infection by HIV-1, and studies of viral DNA formation and those involving pseudotyped viruses indicate that the block is at an early postentry step (17, 18, 33). In fact, blocks to lentivirus infection in primate cells exhibit several of the same characteristics that are evident in the context of Fv1- and Ref1-mediated restriction of MLV (2, 9, 27). Thus, nonhuman primate cells are assumed to express an inhibitor, termed Lv1, which inhibits infection by lentiviruses.
Recent evidence suggests that Ref1 in humans and Lv1 in African green monkeys possess remarkable properties in that they restrict infection by divergent retroviruses whose capsids posses little sequence homology, yet discriminate between the almost-identical capsids encoded by N-MLV and B-MLV (16). This conclusion is based on “cross-saturation” experiments in which target cells are treated with virus-like particles (VLPs) derived from a restricted lentivirus prior to challenge with N-MLV. In human and African green monkey cells, treatment with restricted lentivirus VLPs is able to completely restore susceptibility to N-MLV infection that is ordinarily inhibited by 50- to 1,000-fold.
Susceptibility of NIH 3T3 cells to primate lentiviruses.
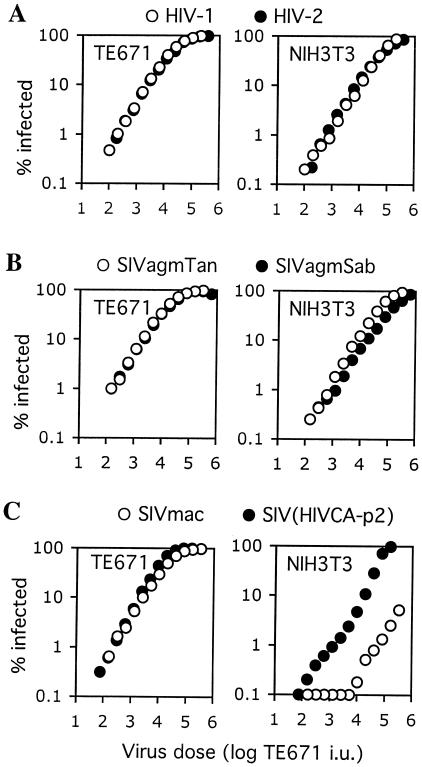
The notion that restriction factors in humans (Ref1) and African green monkeys (Lv1) are able to restrict very divergent retroviruses (16) prompted us to ask the question of whether the murine Fv1 gene product is able to recognize and restrict primate lentiviruses. Previously, we and others have shown that there is no strong postentry restriction to HIV-1 infection of murine NIH 3T3 cells, which are Fv1n/n (4, 14, 26). To extend these observations, we tested the properties of a series of vesicular stomatitis virus G (VSV-G)-pseudotyped lentivirus-green fluorescent protein (GFP) reporter viruses in NIH 3T3 cells. These were based on the genomes of HIV-1, HIV-2, and the simian immunodeficiency viruses SIVmac, SIVagmTan, and SIVagmSab, which were rendered envelope defective and in which the nef gene was replaced with a cDNA encoding GFP (details of the construction are available on request). Virus stocks, generated in 293T cells as previously described (9, 16), were first titrated on human TE671 cells to normalize infectivity. Infections were carried out using serially diluted virus stock in the presence of 5 μg of Polybrene/ml, and infected cells were enumerated by fluorescence-activated cell sorter analysis of GFP expression 48 h later, as previously described (9, 16).
As can be seen in Fig. 1, each of these viruses gave linear titration curves on this human cell line that were virtually superimposable. Because primate lentivirus Tat proteins do not function efficiently in rodent cells, we titrated each of the reporter viruses on a previously described NIH 3T3-derived cell line that was engineered to express a murine cyclin T1 protein in which a single amino acid residue is altered to confer Tat responsiveness (NIH 3T3/CycT) (4). As is shown in Fig. 1A and B, HIV-1, HIV-2, SIVagmTan, and SIVagmSab were only slightly less infectious on NIH 3T3/CycT cells than on TE671 cells (about 2.5- to 5-fold). This difference in susceptibility between the two cell lines was quite consistent, irrespective of which of these viruses was used, and was within the range of variation in sensitivity to VSV-G-pseudotyped primate lentiviruses that we have observed among human cell lines. Thus, it was not considered significant.
FIG. 1.
Comparison of primate lentivirus infectivity in TE671 and NIH 3T3 cells. Human TE671 cells and mouse NIH 3T3 cells were inoculated with equivalent doses of the indicated VSV-G pseudotyped GFP reporter viruses. The dose of each virus used was normalized to give equivalent titers in infectious units (i.u.) in human TE671 cells, which do not restrict any of the primate lentiviruses used here. The results presented are representative of several experiments done using independently prepared virus stocks.
In contrast, NIH 3T3/CycT cells appeared highly resistant to infection by the SIVmac reporter virus. In this case, SIVmac was about 100-fold less infectious on NIH 3T3/CycT cells than on TE671 cells (Fig. 1C). Moreover, at high levels of SIVmac inoculum, the titration curve exhibited nonlinearity, a characteristic that is frequently observed as a restriction factor becomes saturated with increasing incoming virus dose. Therefore, we undertook a series of experiments designed to determine whether NIH 3T3 cells express a saturable capsid targeting an inhibitor of SIVmac infection and whether this inhibitor is Fv1.
CA-p2 is an important lentivirus tropism determinant in NIH 3T3 cells.
We and others have previously demonstrated that replacement of the CA-p2 domain of SIVmac with that of HIV-1 results in a virus construct, SIV(HIV CA-p2), which behaves largely, in terms of restriction in primate cells, like HIV-1 rather than SIVmac (9, 12, 16, 29). Therefore, to determine whether the reduced susceptibility of NIH 3T3/CycT cells to SIVmac compared to that with HIV-1 was governed by determinants within the viral CA-p2 domain, we challenged them with the SIV(HIV CA-p2) reporter virus. As can be seen in Fig. 1C, SIV(HIV CA-p2) was about 20-fold more infectious than SIVmac on NIH 3T3/CycT target cells. Although the transfer of the HIV-1 CA-p2 domain to SIV conferred increased infectivity in NIH 3T3 cells, it did not completely restore the HIV-1 phenotype. SIV(HIV CA-p2) remained slightly less infectious than HIV-1 on NIH 3T3 cells, and the titration curve was not perfectly linear. At present, the reasons for this are unclear. Nonetheless, this result clearly demonstrates that the origin of the CA-p2 domain in Gag is an important determinant of primate lentivirus tropism for NIH 3T3 cells.
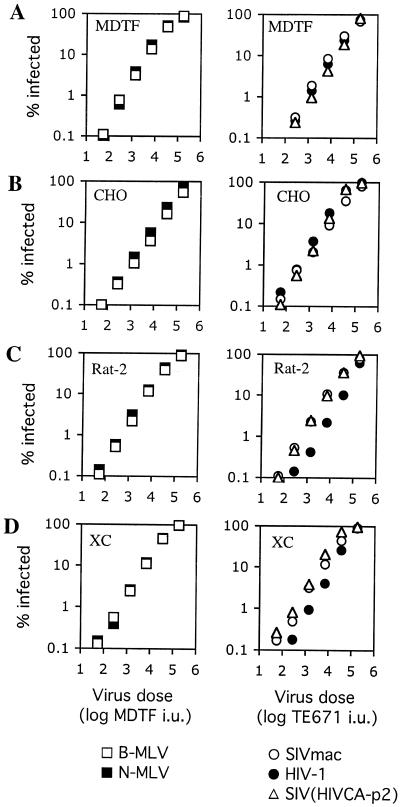
Capsid-dependent and -independent lentivirus tropism determinants in rodents.
NIH 3T3 cells carry the N-allele of Fv1, which could potentially be responsible for capsid-dependent resistance to SIVmac infection. If Fv1 is indeed responsible for restriction of SIVmac infection in NIH 3T3 cells, then no SIVmac restriction should be evident in rodent cell lines that are Fv1-null. Therefore, we next examined a panel of rodent cell lines from mouse, rat, and hamster to determine whether the specific, CA-p2-dependent resistance to SIVmac infection could be observed in the absence of an Fv1 allele. To obviate the requirement for cyclin T1 expression in the target cells, we used a vector system in which the GFP reporter gene is driven by a cytomegalovirus promoter encoded within a packageable vector genome and in which HIV-1, SIVmac, or SIV(HIV CA-p2) Gag-Pol proteins are expressed in trans. Otherwise, infections were carried out as for the full-length reporter viruses, except that infected cells were treated with 6 mM sodium butyrate for the 24 h preceding fluorescence-activated cell sorter analysis to boost the level of the GFP signal. As is shown in Fig. 2, we confirmed that, like Fv-1-null Mus dunni cells (MDTF) and in contrast to NIH 3T3 or TE671 cells, Chinese hamster ovary (CHO), White Reston rat (Rat-2), and Fisher rat (XC) cell lines did not exhibit any specific resistance to N-MLV or B-MLV. We then challenged them with HIV-1, SIVmac, or SIV(HIV CA-p2) vector stocks that were normalized for infectious titer on human TE671 cells. Neither MDTF nor CHO cells exhibited any noticeable difference in sensitivity to HIV-1, SIVmac, or SIV(HIV CA-p2) vectors (Fig. 2A and B). In contrast, both the Rat-2 and XC cell lines were less susceptible to HIV-1 than to SIVmac (Fig. 2C and D). The magnitude of the relative resistance to HIV-1 was quite small (approximately three- to fivefold) but was observed in multiple repetitions of this experiment and when using independently prepared virus stocks. Interestingly, this differential susceptibility to HIV-1 versus SIVmac was not governed by capsid, since the SIV(HIV CA-p2) virus exhibited an infectivity on rat cells that was nearly identical to that of SIVmac (Fig. 2C and D). Overall, however, none of the Fv1-null rodent cell lines displayed the strong, CA-p2-dependent resistance to SIVmac infection that was evident in Fv1n/n NIH 3T3 cells.
FIG. 2.
HIV-1, SIVmac, and SIV(HIV CA-p2) infectivity in Fv1-null rodent cells. Rodent cell lines MDTF (M. dunni) (A), CHO (B), Rat-2 (White-Weston rat) (C), and XC (Fischer rat) (D) were inoculated with increasing amounts of N-MLV, B-MLV, HIV-1, SIVmac, or chimeric SIV(HIV CA-p2) GFP vector stocks as indicated. The doses of N-MLV and B-MLV vectors are given as the titer (infectious units [i.u.]) of each virus measured on MDTF cells. The dose of HIV-1, SIVmac, and SIV(HIV CA-p2) vectors are given as the titer (i.u.) of each virus measured on TE671 cells. The results presented were obtained using single HIV-1, SIVmac, and SIV(HIV CA-p2) stocks but are representative of several experiments done using independently prepared stocks.
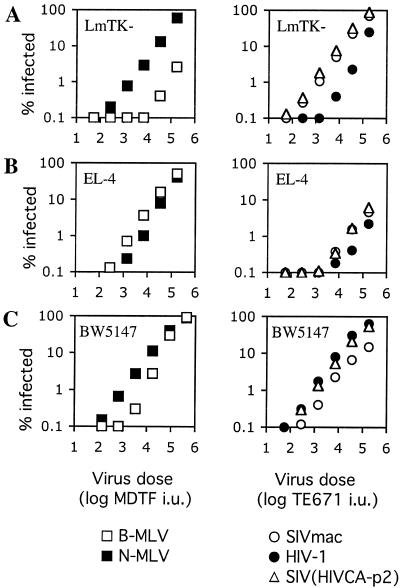
Mouse cell lines exhibit variable relative susceptibility to HIV-1 and SIVmac vectors.
We next tested additional Fv1-positive mouse cell lines for sensitivity to the HIV-1, SIVmac, and SIV(HIV CA-p2) vectors. These cells were of either fibroblast or T-cell origin and were originally derived from several laboratory mouse strains. As is shown in Fig. 3, a variety of phenotypes in terms of susceptibility to HIV-1 SIVmac, and SIV(HIV CA-p2) infection were observed. LmTK- cells (from C3H/An mice) are Fv1n/n and, like NIH 3T3 cells, are about 30-fold less susceptible to B-MLV than to N-MLV (Fig. 3A). However, in marked contrast to NIH 3T3 cells, LmTK- cells were less sensitive to HIV-1 than to SIVmac. In fact, the phenotype exhibited by LmTK- cells was somewhat similar to that exhibited by the rat cells (Fig. 2C and D), although the difference in titer between SIVmac and HIV-1 in LmTK- cells was larger (approximately 10- to 20-fold) (Fig. 3A). These cells also shared the property with rat cells of specific resistance to HIV-1 infection that was not determined by the viral capsid, since SIV(HIV CA-p2) had the same relative infectivity as SIVmac and not that of HIV-1.
FIG. 3.
Resistance of Fv1-positive murine cells to lentivirus infection. Mouse cell lines LmTK- (A), BW5147 (B), and EL-4 (C) were inoculated with increasing amounts of N-MLV, B-MLV, HIV-1, SIVmac, or chimeric SIV(HIV CA-p2) GFP vector stocks as indicated. The dose of N-MLV and B-MLV vectors are given as the titer (infectious units [i.u.]) of each virus measured on MDTF cells. The doses of HIV-1, SIVmac, and SIV(HIV CA-p2) vectors are given as the titer (infectious units [i.u.]) of each virus measured on TE671 cells. The results presented were obtained using single HIV-1, SIVmac, and SIV(HIV CA-p2) stocks but are representative of several experiments done using independently prepared stocks.
We also tested the susceptibility of two T-cell lines, EL-4 (from C57BL/6N mice) and BW5147 (from AKR/J mice), to HIV-1 and SIVmac vectors. Although EL-4 cells are Fv1b/b, only a modest difference in susceptibility to N-MLV versus B-MLV was observed, suggesting that Fv1 is either poorly expressed or is not able to restrict MLV infection in this cell line (Fig. 3B). EL-4 cells were, however, quite strongly resistant to infection by both HIV-1 and SIVmac. A modestly reduced susceptibility to HIV-1 compared to SIVmac was observed (approximately threefold) but, as was the case in the LmTK- and rat cell lines, this difference was not determined by the viral capsid, since SIVmac and SIV(HIV CA-p2) had nearly identical titers on this cell line. BW5147 cells, which are Fv1n/n, were approximately 10-fold less susceptible to B-MLV than to N-MLV at a low virus dose (Fig. 3C). In contrast to EL-4 cells, however, this T-cell line was quite sensitive to both HIV-1 and SIVmac infection. SIVmac was about threefold less infectious in BW5147 cells than was HIV-1, and this difference was primarily attributable to capsid, since SIV(HIV CA-p2) exhibited similar infectivity to HIV-1 (Fig. 3C). Overall, the rodent cell lines tested exhibited distinct relative susceptibilities to HIV-1 and SIVmac that were sometimes but not always determined by the origin of the viral capsid and did not correlate well with the presence or function of any particular Fv1 allele.
NIH 3T3 cells express a saturable inhibitor of SIVmac infection.
Because NIH 3T3 cells exhibited the strongest specific resistance to a primate lentivirus (SIVmac) that was apparently governed by determinants within the CA-p2 Gag domain, we tested whether resistance was due to the presence of a saturable inhibitor and whether this inhibitor was Fv1 that is functionally expressed therein. As is shown in Fig. 4A, treatment of NIH 3T3 cells with increasing doses of SIVmac VLPs increased infection by a fixed inoculum of SIV GFP by up to ninefold. However, the same treatment did not affect infection by either unrestricted N-MLV or Fv1n-restricted B-MLV. In a reciprocal experiment, shown in Fig. 4B, saturation of NIH 3T3 cells with restricted B-MLV-Neo, but not unrestricted N-MLV-Neo, vector particles increased susceptibility to a restricted GFP-expressing B-MLV vector by >50-fold. In contrast, the same manipulations had only marginal effects on SIVmac infection. Thus, in NIH 3T3 cells, under conditions where Fv1n is apparently saturated by incoming B-MLV particles, SIVmac remains restricted. Moreover, when sufficient SIVmac particles are incoming to saturate whatever is responsible for SIVmac restriction in NIH 3T3 cells, Fv1n-mediated restriction of B-MLV infection remains effective.
FIG. 4.
Resistance of NIH 3T3 cells to SIVmac is saturable by SIVmac but not B-MLV particles. (A) Inhibition of SIVmac-GFP restriction in NIH 3T3 cells by SIVmac VLPs. NIH 3T3 cells were inoculated with a fixed and equivalent dose (as measured on MDTF cells) of N-MLV, B-MLV, or SIVmac GFP reporter viruses, in the presence of increasing amounts of SIVmac VLPs. The VLP dose is given in nanograms of reverse transcriptase per well. (B) B-MLV particles cannot inhibit restriction to SIVmac infection in NIH 3T3 cells. NIH 3T3 cells were inoculated with a fixed and equivalent dose (as measured on Fv1-null MDTF cells) of B-MLV or SIVmac GFP reporter viruses, in the presence of increasing amounts of N-neo or B-neo virus particles. The inhibiting (neo) virus dose is given as the titer (in infectious units [i.u.]) as measured on MDTF cells.
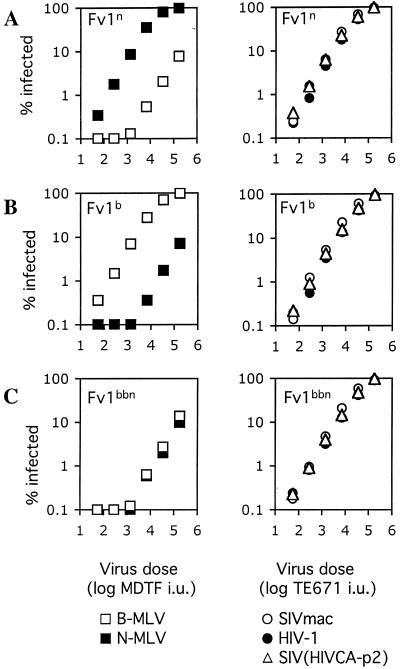
Fv1 does not confer resistance to HIV-1 or SIVmac infection.
The above data suggested that the capsid-dependent, saturable restriction of SIVmac infection that was observed in NIH 3T3 cells was mediated by a factor or factors other than Fv1. To unequivocally determine whether Fv1 was capable of conferring resistance to SIVmac or HIV-1 infection, we engineered Fv1-null cells to stably express Fv1n or Fv1b. MDTF cells were transduced with LNCX-derived retroviral vectors expressing Fv1n or Fv1b, and the G418-resistant populations were used as a pool. We also expressed an artificial chimeric form of Fv1, termed Fv1bbn, that possesses expanded specificity (6). This Fv1 protein confers resistance to both N-tropic and B-tropic MLV as well as NB-tropic MLV that is not ordinarily restricted by either parental Fv1 allele. As expected, MDTF/Fv1n cells exhibited specific, approximately 50-fold resistance to B-MLV, while MDTF/Fv1b cells exhibited the same degree of specific resistance to N-MLV (Fig. 5A and B). As was previously reported, MDTF cells expressing Fv1bbn were resistant to both B-MLV and N-MLV (Fig. 5C) (6). In contrast, MDTF cells expressing either the natural or artificial Fv1 variants did not exhibit any specific resistance to either HIV-1, SIVmac, or SIV(HIV CA-p2) and were at least as susceptible to infection by these viruses, as were control MDTF cells carrying a vector lacking Fv1 (Fig. 5 and data not shown). Thus, none of the Fv1 alleles tested could block infection by these primate lentiviruses.
FIG. 5.
Restriction of lentiviruses in murine cells is not mediated by Fv1. Fv1-null MDTF cells were engineered to stably express different alleles of Fv1, Fv1n (A), Fv1b (B), and a chimeric Fv1bbn that restricts both N- and B-MLV (C). Target cells were inoculated with increasing amounts of N-MLV, B-MLV, HIV-1, SIVmac, or chimeric SIV(HIV CA-p2) GFP vector stocks as indicated. The virus dose of N-MLV and B-MLV is given as the titer (in infectious units [i.u.]) of each virus measured on MDTF cells. The dose of HIV-1, SIVmac, and SIV(HIV CA-p2) is given as the titer (i.u.) of each virus as measured on TE671 cells.
Conclusions and implications.
The apparently broad specificity of retroviral restriction factors in cells of human (Ref1) and African green monkey (Lv1) origin, both of which are superficially Fv1b-like in that they restrict infection by N-MLV but not B-MLV (16, 35), suggested that perhaps Fv1 itself might be able to restrict infection by lentiviruses. This proved not to be the case. In general, however, we observed several unexpected phenotypes in terms of relative susceptibility to HIV-1 and SIVmac vectors among rodent cell lines. In some cell lines, we found no evidence for specific resistance to either virus. In others, variable (3- to 20-fold) relative resistance to HIV-1 compared to SIVmac was observed. In this case, the differential susceptibility to the two viruses was not due to determinants in the capsid. In addition, we found that two mouse cell lines exhibited specific resistance to SIVmac. Therein, the viral determinants of the phenotype resided predominantly within the CA-p2 domain, since SIV(HIV CA-p2) behaved more like HIV-1 and not like SIVmac. In BW5147 cells, the phenotype was subtle, but in NIH 3T3 cells the origin of CA-p2 had dramatic effects in infectivity. In addition to these specific effects on either HIV-1 or SIVmac infectivity, one cell line, EL-4, appeared to have a superimposed defect in terms of sensitivity to both HIV-1 and SIVmac infection. However, this cell line was quite efficiently infected by a B-MLV vector.
We have not determined precisely the step at which infection is attenuated in rodent cells. However, all of the viruses and vectors were pseudotyped with VSV-G, effectively precluding entry restriction as a factor. Moreover, with the exception of the data shown in Fig. 1, all of the findings described herein were obtained using vector genomes in which the GFP reporter gene was driven by a cytomegalovirus promoter. The vectors each gave robust GFP expression in all the cells tested, particularly after sodium butyrate treatment. Thus, the virus-specific restrictions occur at some point between virus entry and gene expression.
These findings should be an important consideration in the development of rodent models of lentivirus infection. Thus far, two reports have been published describing the construction of transgenic rodents that are engineered to be susceptible to HIV-1 infection (8, 20). In both cases, susceptibility to infection of transgenic cells in vitro was documented, but in neither case was robust, persistent infection established. There are several obstacles to the development of such rodent models, and postentry, preintegration restriction is likely to also be an additional important factor in selecting a mouse (or other rodent) strain for the introduction of human genes that are required to support HIV-1 replication.
The fact that cell lines exhibit differences in their relative susceptibilities to HIV-1 versus SIVmac strongly suggests that important and specific host-virus interactions occur during the postentry, preintegration phase of the lentivirus life cycle in rodent cells. Moreover, these findings further suggest that host gene products involved in these interactions may be polymorphic among laboratory mouse strains. At present, it is unclear whether some of the phenotypes documented herein reflect the action of facilitators or inhibitors of infection. However, in at least one case, namely SIVmac infection of NIH 3T3 cells, resistance appears to be due predominantly to the presence of a saturable, capsid-targeting inhibitor of infection. Assuming that these findings can be demonstrated to be consequent to the mouse strain and not to differential expression of genes in immortalized versus primary mouse cells, the application of genetic studies in mice could be a useful means by which host factors that control susceptibility to lentivirus infection might be identified.
Acknowledgments
We thank Greg Towers, Jonathan Stoye, Heinrich Gottlinger, and Kyriacos Mitrophanous for gifts of reagents. Infectious molecular clones of SIVagmSab and SIVagmTan were obtained from Beatrice Hahn through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH).
This work was supported by a grant from the NIH (RO1 AI50111). P.D.B. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Bassin, R. H., G. Duran-Troise, B. I. Gerwin, and A. Rein. 1978. Abrogation of Fv-1b restriction with murine leukemia viruses inactivated by heat or by gamma irradiation. J. Virol. 26:306-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 99:11920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 4.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieniasz, P. D., T. A. Grdina, H. P. Bogerd, and B. R. Cullen. 1998. Recruitment of a protein complex containing Tat and cyclin T1 to TAR governs the species specificity of HIV-1 Tat. EMBO J. 17:7056-7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock, M., K. N. Bishop, G. Towers, and J. P. Stoye. 2000. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J. Virol. 74:7422-7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boone, L. R., C. L. Innes, and C. K. Heitman. 1990. Abrogation of Fv-1 restriction by genome-deficient virions produced by a retrovirus packaging cell line. J. Virol. 64:3376-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning, J., J. W. Horner, M. Pettoello-Mantovani, C. Raker, S. Yurasov, R. A. DePinho, and H. Goldstein. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. USA 94:14637-14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowan, S., T. Hatziioannou, T. Cunningham, M. A. Muesing, H. G. Gottlinger, and P. D. Bieniasz. 2002. Cellular inhibitors with Fv1-like activity restrict human and simian immunodeficiency virus tropism. Proc. Natl. Acad. Sci. USA 99:11914-11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decleve, A., O. Niwa, E. Gelmann, and H. S. Kaplan. 1975. Replication kinetics of N- and B-tropic murine leukemia viruses on permissive and nonpermissive cells in vitro. Virology 65:320-332. [DOI] [PubMed] [Google Scholar]
- 11.DesGroseillers, L., and P. Jolicoeur. 1983. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J. Virol. 48:685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfman, T., and H. G. Gottlinger. 1996. The human immunodeficiency virus type 1 capsid p2 domain confers sensitivity to the cyclophilin-binding drug SDZ NIM 811. J. Virol. 70:5751-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duran-Troise, G., R. H. Bassin, A. Rein, and B. I. Gerwin. 1977. Loss of Fv-1 restriction in Balb/3T3 cells following infection with a single N tropic murine leukemia virus particle. Cell 10:479-488. [DOI] [PubMed] [Google Scholar]
- 14.Garber, M. E., P. Wei, V. N. KewalRamani, T. P. Mayall, C. H. Herrmann, A. P. Rice, D. R. Littman, and K. A. Jones. 1998. The interaction between HIV-1 Tat and human cyclin T1 requires zinc and a critical cysteine residue that is not conserved in the murine CycT1 protein. Genes Dev. 12:3512-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley, J. W., W. P. Rowe, and R. J. Huebner. 1970. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J. Virol. 5:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatziioannou, T., S. Cowan, S. P. Goff, P. D. Bieniasz, and G. Towers. 2003. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 22:385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himathongkham, S., and P. A. Luciw. 1996. Restriction of HIV-1 (subtype B) replication at the entry step in rhesus macaque cells. Virology 219:485-488. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann, W., D. Schubert, J. LaBonte, L. Munson, S. Gibson, J. Scammell, P. Ferrigno, and J. Sodroski. 1999. Species-specific, postentry barriers to primate immunodeficiency virus infection. J. Virol. 73:10020-10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolicoeur, P., and D. Baltimore. 1976. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc. Natl. Acad. Sci. USA 73:2236-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keppler, O. T., F. J. Welte, T. A. Ngo, P. S. Chin, K. S. Patton, C. L. Tsou, N. W. Abbey, M. E. Sharkey, R. M. Grant, Y. You, J. D. Scarborough, W. Ellmeier, D. R. Littman, M. Stevenson, I. F. Charo, B. G. Herndier, R. F. Speck, and M. A. Goldsmith. 2002. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195:719-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keppler, O. T., W. Yonemoto, F. J. Welte, K. S. Patton, D. Iacovides, R. E. Atchison, T. Ngo, D. L. Hirschberg, R. F. Speck, and M. A. Goldsmith. 2001. Susceptibility of rat-derived cells to replication by human immunodeficiency virus type 1. J. Virol. 75:8063-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak, C. A. 1985. Analysis of wild-derived mice for Fv-1 and Fv-2 murine leukemia virus restriction loci: a novel wild mouse Fv-1 allele responsible for lack of host range restriction. J. Virol. 55:281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozak, C. A., and A. Chakraborti. 1996. Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology 225:300-305. [DOI] [PubMed] [Google Scholar]
- 24.Lilly, F. 1967. Susceptibility to two strains of Friend leukemia virus in mice. Science 155:461-462. [DOI] [PubMed] [Google Scholar]
- 25.Mariani, R., B. A. Rasala, G. Rutter, K. Wiegers, S. M. Brandt, H. G. Krausslich, and N. R. Landau. 2001. Mouse-human heterokaryons support efficient human immunodeficiency virus type 1 assembly. J. Virol. 75:3141-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariani, R., G. Rutter, M. E. Harris, T. J. Hope, H. G. Krausslich, and N. R. Landau. 2000. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74:3859-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odaka, T., and T. Yamamoto. 1965. Inheritance of susceptibility to Friend mouse leukemia virus. 11. Spleen foci method applied to test the susceptibility of crossbred progeny between a sensitive and a resistant strain. Jpn. J. Exp. Med. 35:311-314. [PubMed] [Google Scholar]
- 29.Owens, C. M., P. C. Yang, H. Gottlinger, and J. Sodroski. 2003. Human and simian immunodeficiency virus capsid proteins are major viral determinants of early, postentry replication blocks in simian cells. J. Virol. 77:726-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pincus, T., J. W. Hartley, and W. P. Rowe. 1971. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J. Exp. Med. 133:1219-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pincus, T., J. W. Hartley, and W. P. Rowe. 1975. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology 65:333-342. [DOI] [PubMed] [Google Scholar]
- 32.Pryciak, P. M., and H. E. Varmus. 1992. Fv-1 restriction and its effects on murine leukemia virus integration in vivo and in vitro. J. Virol. 66:5959-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata, R., H. Sakai, M. Kawamura, K. Tokunaga, and A. Adachi. 1995. Early replication block of human immunodeficiency virus type 1 in monkey cells. J. Gen. Virol. 76:2723-2730. [DOI] [PubMed] [Google Scholar]
- 34.Tennant, R. W., J. A. Otten, A. Brown, W. K. Yang, and S. J. Kennel. 1979. Characterization of Fv-1 host range strains of murine retroviruses by titration and p30 protein characteristics. Virology 99:349-357. [DOI] [PubMed] [Google Scholar]
- 35.Towers, G., M. Bock, S. Martin, Y. Takeuchi, J. P. Stoye, and O. Danos. 2000. A conserved mechanism of retrovirus restriction in mammals. Proc. Natl. Acad. Sci. USA 97:12295-12299.11027299 [Google Scholar]
- 36.Towers, G., M. Collins, and Y. Takeuchi. 2002. Abrogation of Ref1 retrovirus restriction in human cells. J. Virol. 76:2548-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]