Abstract
Aims
Aberrant vascular smooth muscle cell (VSMC) proliferation and migration contribute significantly to the development of vascular pathologies, such as atherosclerosis and restenosis. MicroRNAs have recently emerged as critical modulators in cellular processes and the purpose of this study is to identify novel miRNA regulators implicated in human aortic VSMC proliferation and migration.
Methods and results
To identify miRNAs that are differentially expressed in human VSMCs, we performed miRNA microarray analysis in human aortic smooth muscle cells (SMCs) at different time points after platelet-derived growth factor (PDGF) stimulation. Here, we identified microRNA-638 (miR-638) as a transcript that was one of the most significantly down-regulated in human VSMCs after PDGF stimulation. Furthermore, we confirmed, by Quantitative RT–PCR, that miR-638 is highly expressed in human VSMCs, and its expression is markedly down-regulated in a dose- and time-dependent manner upon PDGF treatment. Consistent with a critical role in SMC proliferation, we found that miR-638 expression was significantly up-regulated in human VSMCs cultured in differentiation medium, a condition that inhibits SMC proliferation. Furthermore, we identified the orphan nuclear receptor NOR1 as a downstream target gene product of miR-638 and down-regulation of NOR1 is critical for miR-638-mediated inhibitory effects on PDGF-induced cyclin D1 expression, cell proliferation, and migration in human aortic SMCs.
Conclusion
These results indicate that miR-638 is a key molecule in regulating human VSMC proliferation and migration by targeting the NOR1/cyclin D pathway and suggest that specific modulation of miR-638 in human VSMCs may represent an attractive approach for the treatment of proliferative vascular diseases.
Keywords: miR-638, Vascular smooth muscle cell, Proliferation, Migration, Orphan Nuclear Receptor NOR1
1. Introduction
Cardiovascular diseases remain a major cause of death worldwide. Aberrant vascular smooth muscle cell (VSMC) proliferation and migration, as induced by vascular injury, have been shown to play a critical role in the pathogenesis of cardiovascular diseases, including atherosclerosis, vein graft failure, post-angioplasty restenosis, and pulmonary artery hypertension (PAH).1,2 VSMCs are highly specialized cells committed to regulate blood vessel tone and consequently blood distribution and pressure.3 Unlike many terminally differentiated cells, VSMCs can undergo phenotypic switch between quiescent contractile and proliferative synthetic phenotypes in response to changes in the local environment.4,5 Under normal circumstances, smooth muscle cells (SMCs) are maintained in a quiescent and non-migratory state. However, their proliferation and migration are markedly increased in response to various growth factors and cytokines, such as platelet-derived growth factor-BB (PDGF-BB), fibroblast growth factor, insulin-like growth factor-1, tumour necrosis factor-alpha (TNF-a), and interleukin-1.6,7 PDGF-BB, which is released primarily by vascular endothelial cells and platelets at the sites of vascular injury, has been identified as one of the most potent stimulants for the VSMC proliferation and migration, through its modulation of several transcription factors and key molecular signalling pathways.6,8 Indeed, an increased expression of signalling proteins in the PDGF pathway has been demonstrated in several cardiovascular disorders, including atherosclerosis, restenosis, and PAH.9 Accordingly, inhibition of the PDGF signalling pathway by using a PDGF receptor kinase inhibitor imatinib has been shown to effectively reduce the atherosclerotic lesion formation and PAH development, further highlighting a critical role of the PDGF pathway in proliferative vascular diseases.9–11 However, the exact mechanisms by which PDGF modulates VSMC proliferation and migration are not completely understood. Thus, the identification of novel molecular mechanisms, particularly, novel inhibitors controlling the PGDF-BB-dependent VSMC proliferation and migration, is of considerable scientific and therapeutic interest.
MicroRNAs (miRNAs) are a class of endogenous and small (20–25 nt) non-coding RNAs that negatively regulate gene expression through binding to the 3′ untranslated region (UTR) of mRNA transcripts to induce mRNA degradation or translational inhibition of target mRNAs.12 Thus far, >1000 human miRNAs have been identified and they are thought to regulate up to 60% of gene transcripts, hence, having broad implications in the regulation of a wide range of biological activities, including cell growth, apoptosis, and differentiation. Recently, multiple lines of evidence suggest that miRNAs play pivotal roles in the control of VSMC function and the response of the vascular injury through targeting transcriptional factors or key signalling molecules in SMC proliferation and migration.13,14 Indeed, a group of miRNAs, including miR-143/145, miR-221/222, miR-24, miR-26a, miR-1, miR-146a, and miR-21, has been identified to modulate VSMC differentiation, phenotypic switch, and neointimal formation following vascular injury.15–22 Among these, miR-221/222, miR-21, miR-24, and miR-1 were found to be up-regulated in response to either vascular injury or PDGF treatment and exert pro-proliferative effects on VSMCs.17–19,21 miR-143/145 is a highly enriched miRNA in VSMCs that has been extensively studied in the field of VSMC biology. The expression of miR-143/145 is markedly down-regulated in response to both vascular injury and PDGF treatment,15,16,23 and restoration of its expression attenuates the neointimal growth in the rat carotid artery after balloon injury.15 However, other studies in mice with either single deletion or the double deletion of miR-143/145 did not increase neointimal formation after carotid artery ligation,24 suggesting species-specific differences in miRNA expression are important in the regulation of VSMC biology as well as the development of vascular diseases. Furthermore, deletion of Dicer, the rate-limiting enzyme in miRNA synthesis, resulted in an impaired SMC contractility and proliferation, further indicating essential roles of miRNAs in VSMC biology.25
Thus far, our knowledge of role of miRNAs in the regulation of VSMC function was largely based on studies using rodent models of vascular injury, and those that specifically regulate human VSMC function are less explored. Therefore, in this study, we performed miRNA microarray analysis to identify the miRNAs involved in regulating human aortic SMC proliferation. We, for the first time, demonstrated that miR-638, which is abundantly expressed in human aortic SMCs, was significantly down-regulated in proliferative VSMCs. Furthermore, we found that miR-638 inhibits human VSMC proliferation and migration via directly targeting orphan nuclear receptor NOR1, which is a critical regulator implicated in proliferative vascular diseases.26
2. Methods
2.1. Cell culture
Human aortic SMCs were purchased from Lonza and cultured as previously described.27
2.2. Microarray analysis of miRNA expression
RNAs obtained from human aortic SMCs treated with PDGF-BB (20 ng/mL) at 0, 3, 6, and 24 h were subjected to the MicroRNA Microarray Service (LC Sciences, Houston, TX, USA).28
2.3. PCR analysis, RNA analysis by quantitative RT–PCR
PCR analysis for miR-638 was performed using the primers (forward: GAG AGG ATC CTG CCG CAG ATC GCT G; reverse: GAG TAA GCT TCA GGG AGT CCT CTG CC). miRNAs and mRNAs were isolated with the miRNeasy Mini Kit (Qiagen) and the RNeasy kit (Qiagen). Quantitative RT–PCR (qRT–PCR) for miRNA was performed using the miRCURY LNA™ Universal cDNA Synthesis and SYBR® Green Master Mix Kit (Exqion) as described.29 qRT–PCR for NOR1, Nurr1, and Nur77 were performed using the HotStart-IT® SYBR® Green qPCR Master Mix with UDG (2X) (USB Corporation). Primer sequences for human NOR1, Nurr1, Nur77, and 18S are presented in the Supplementary material online, Table 1.
2.4. Oligonucleotide transfection
miR-638 overexpression and miR-638 knockdown in cultured VSMCs were performed as described in the Supplementary material online, Materials and Methods.
2.5. Luciferase reporter assay
The SwitchGear GoClone reporter containing 3′-UTR of NR4A3 variant1 (3150 bp) was cotransfected with either vehicle control, miR-638 mimic, anti-miR-638 or a non-targeting control miRNA into HEK293 cells following the procedure provided by Switchgear Genomics. Forty-eight hours after transfection, luciferase activity was determined.
2.6. Construction of adenovirus
The adenovirus expressing miR-638 (Ad-miR-638) was made using RAPAd® Universal Adenoviral Expression System (Cell Biolabs, Inc.). Adenoviruses harbouring wild-type NOR1 (Ad-NOR1) and GFP (Ad-GFP) were made using AdMax (Microbix) as previously described.30
2.7. Western blot
Western blot analysis was performed using proliferating cell nuclear antigen (PCNA), Cyclin D1, α-tubulin, NOR1, and GAPDH antibodies as previously described.30
2.8. VSMC migration and proliferation and assays
Cell migration was performed by the SMC scratch wound assay as previously described.31 VSMC proliferation was determined by cell counting and MTT assay using the Vybrant® MTT Cell Proliferation Assay Kit (Invitrogen™).32
2.9. Cell cycle analysis by flow cytometry
Cells were fixed with 70% ethanol at 4°C overnight, treated with RNaseA in PBS, stained with propidium iodide (Sigma, St Louis, MO, USA), and subjected to flow cytometric DNA content analysis using FACSCalibur (Beckman Coulter, Co., USA). The percentages of cells in G1, S, and G2/M phases were analysed.19
2.10. Immunofluorescent detection of miRNA-638 in human aortic SMCs
miR-638 expression and localization was performed using the Immunofluorescence method as described.15
2.11. Statistics
All data were expressed as mean ± standard deviation. Data were analysed for statistical significance by Student's t-test or ANOVA using the SPSS software (version 18.0). A P-value <0.05 is considered statistically significant.
3. Results
3.1. miRNA expression in PDGF-stimulated human aortic SMCs
To identify the miRNAs important in the regulation of human VSMC proliferation, we performed miRNA microarray in human aortic SMCs at 0, 3, 6, and 24 h after PDGF-BB stimulation. Overall, among the 470-arrayed miRNAs, 25 miRNAs were found to be differentially expressed in human aortic SMCs during at least two time points after PDGF-BB stimulation. Seventeen miRNAs were up-regulated and eight miRNAs were down-regulated in response to PDGF stimulation (Table 1). In agreement with previously published studies,17,18,20 miR-221/222 and miR-24 were found to be significantly up-regulated in response to PDGF treatment. Among the eight miRNAs that were down-regulated by PDGF-BB stimulation, miR-638 was found to be highly enriched in human SMCs, and its expression was markedly down-regulated, in a time-dependent manner, in response to PDGF-BB stimulation (Table 1). Thus, these findings suggest that miR-638 may be a critical regulator for VSMC proliferation.
Table 1.
miRNA expression in human aortic smooth muscle cell after PDGF-BB treatment
| Fold change |
Signal intensity | ||||
|---|---|---|---|---|---|
| Hour | 0 | 3 | 6 | 24 | Baseline |
| Down-regulated | |||||
| hsa-miR-663 | 1 | 0.62 | 0.23 | 0.15 | 5552 |
| hsa-miR-638 | 1 | 0.60 | 0.40 | 0.19 | 35 390 |
| hsa-miR-612 | 1 | — | 0.07 | 0.20 | 664 |
| hsa-miR-103 | 1 | 0.66 | — | 0.64 | 5408 |
| hsa-miR-107 | 1 | 0.70 | — | 0.65 | 4937 |
| hsa-miR-191 | 1 | 0.73 | 1.3 | 0.74 | 7213 |
| hsa-miR-181a | 1 | 0.81 | — | 0.74 | 5564 |
| hsa-miR-214 | 1 | 0.78 | — | 0.74 | 16 249 |
| Up-regulated | |||||
| hsa-miR-368 | 1 | 1.68 | — | 4.32 | 782 |
| hsa-miR-376a | 1 | 2.43 | — | 3.84 | 262 |
| hsa-miR-132 | 1 | 2.33 | — | 2.10 | 668 |
| hsa-miR-29b | 1 | 1.99 | — | 2.25 | 535 |
| hsa-miR-30b | 1 | 1.52 | — | 1.95 | 1962 |
| hsa-miR-98 | 1 | 1.52 | — | 1.91 | 3321 |
| hsa-miR-23b | 1 | 1.18 | — | 1.37 | 27 680 |
| hsa-miR-15a | 1 | 1.87 | — | 1.88 | 724 |
| hsa-miR-146b | 1 | 1.99 | — | 1.79 | 1544 |
| hsa-miR-27a | 1 | 1.23 | — | 1.43 | 14 681 |
| hsa-miR-222 | 1 | — | 1.60 | 1.43 | 11 173 |
| hsa-miR-23a | 1 | 1.16 | 1.10 | 1.40 | 28 461 |
| hsa-miR-221 | 1 | — | 1.23 | 1.25 | 11 195 |
| hsa-miR-320 | 1 | 1.68 | 1.25 | 5901 | |
| hsa-miR-125b | 1 | 1.09 | 1.09 | 1.19 | 31 358 |
| hsa-miR-199a | 1 | — | 1.09 | 1.16 | 2375 |
| hsa-miR-24 | 1 | — | 1.14 | 1.15 | 16 035 |
3.2. miR-638 is down-regulated in proliferating VSMCs
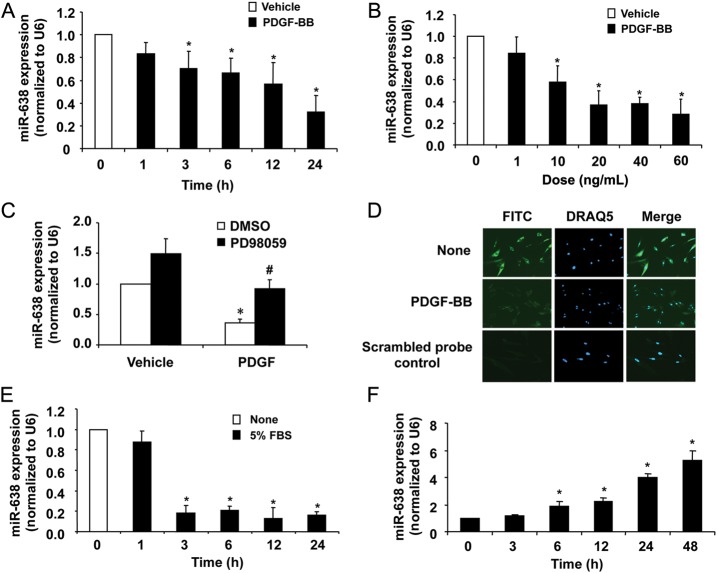
To further confirm the expression of miR-638 in proliferating VSMCs, qRT–PCR was performed in human aortic SMCs stimulated by either PDGF-BB (20 ng/mL) or 5% serum at different time points. As shown in Figure 1A, miR-638 was substantially down-regulated in response to PDGF-BB treatment. In fact, 24 h after PDGF-BB stimulation, miR-638 expression was down-regulated by ∼70%, which is consistent with changes seen in our microarray data. In addition, PDGF-BB stimulation significantly down-regulated the miR-638 expression in a dose-dependent manner, with an IC50 of ∼10 ng/mL (Figure 1B). PD98059, a selective MAPK/ERK1/2 inhibitor, prevented PDGF-induced down-regulation of miR-638 (Figure 1C), while inhibition of phosphatidylinositide 3-kinase with LY294002 had no effect on miR-638 expression, suggesting the MAPK/ERK1/2 pathway is involved in the regulation of miR-638 by PDGF-BB. Immunofluorescent staining indicated that miR-638 is primarily expressed in the cytoplasm of quiescent SMCs and down-regulated in proliferating cells (Figure 1D). To further test whether down-regulation of miR-638 was specific to PDGF-BB, human aortic SMCs were treated with 5% FBS. Similarly, as shown in Figure 1E, miR-638 was sharply down-regulated in response to FBS, with a maximal down-regulation of ∼80% at 3 h. In contrast, miR-638 was markedly up-regulated (Figure 1F), when VSMCs were cultured in smooth muscle differentiation medium, a condition that has been shown to inhibit SMC growth.33,34 In pulmonary artery SMCs, miR-638 expression was also down-regulated by PDGF-BB stimulation (see Supplementary material online, Figure 1). Taken together, these findings indicate an inverse relationship between miR-638 expression and VSMC proliferation.
Figure 1.
miR-638 is down-regulated in proliferating VSMCs. Human aortic VSMCs seeded in 6-well plates were starved with 0.5% FBS for 48 h, and then stimulated with either PDGF-BB (20 ng/mL) or 5% FBS. (A) PDGF-BB caused a time-dependent decrease in miR-638 expression in human aortic VSMCs, as demonstrated by qRT–PCR (n = 4). *P < 0.05 compared with 0 h group. (B) PDGF-BB caused a dose-dependent decrease in miR-638 expression in human aortic VSMCs at 24 h after treatment, as demonstrated by qRT–PCR (n = 4). *P < 0.05 compared with that in VSMCs without PDGF-BB. (C) Starved SMCs were pre-treated with either vehicle or PD98059 (50 μM) and then stimulated with PDGF-BB (20 ng/mL) for 24 h. The expression of miR-638 was determined by qRT–PCR (n = 4). (D) Immunofluorescent detection of microRNA-638 (green colour) in human aortic smooth muscle cells, blue colour is the cell nuclear staining by DAPI. (E) Serum (5%) caused a time-dependent decrease in miR-638 expression in human aortic VSMCs, as demonstrated by qRT–PCR (n = 4). *P < 0.05 compared with that without 5% FBS treatment. (F) qRT–PCR results showed that miR-638 was significantly up-regulated in SMC differentiation medium-treated human aortic SMCs for the indicated times (n = 4). *P < 0.05 compared with 0 h group.
3.3. miR-638 inhibits VSMC proliferation
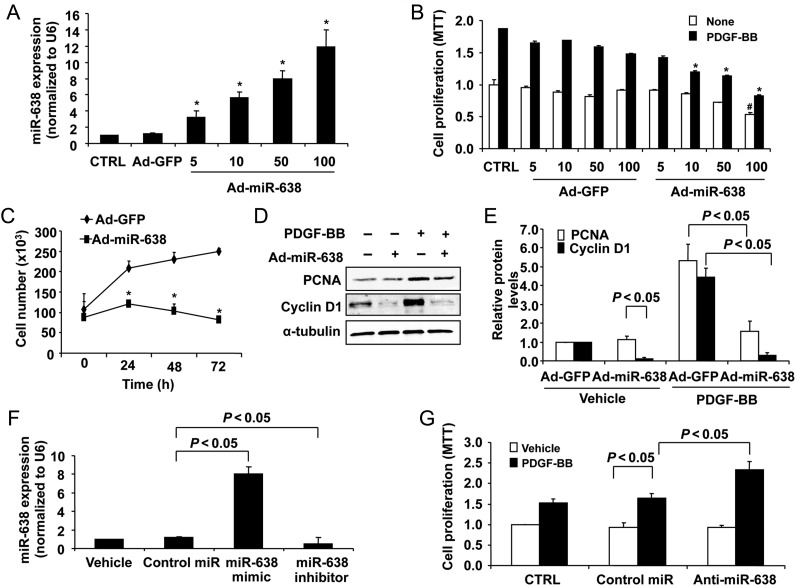
To examine the functional role of miR-638 in VSMCs, we sought to determine whether restoration of miR-638 could affect PDGF-BB-induced SMC proliferation. To this end, we constructed an adenovirus vector expressing miR-638 and showed that the transduction of human aortic SMCs with increasing multiplicity of infections (MOIs) of Ad-miR-638 significantly increased its expression in SMCs (Figure 2A). Furthermore, we showed that PDGF-BB-induced SMC proliferation, as determined by both MTT assays and cell counting, was effectively inhibited through a range of 10–100 MOIs (Figure 2B and C). The effect of miR-638 on VSMC proliferation was further confirmed by the expression of a well-known cell proliferation marker, PCNA. As shown in Figure 2D and E, PDGF-BB stimulation significantly increased the expression of PCNA, which was substantially attenuated in Ad-miR-638 transduced cells. Interestingly, loss of function studies showed that down-regulation of miR-638 by anti-miR-638 had no effect on basal SMC proliferation, but significantly enhanced the low dose PDGF-BB (2 ng/mL)-induced SMC growth (Figure 2F and G).
Figure 2.
Role of miR-638 in VSMC proliferation. (A) Human aortic SMCs were transduced with Ad-miR-638 or Ad-GFP at the indicated MOI. Seventy-two hours after transduction, total RNA was extracted and detected by qRT–PCR. Ad-miR-638 increased miR-638 levels in a dose-dependent manner. *P < 0.05 compared with control (CTRL) (n = 4). (B) Human aortic SMCs were transduced with Ad-miR-638 (100 MOI) or Ad-GFP (100 MOI). Starved VSMCs were stimulated with or without PDGF-BB (20 ng/mL) for 24 h. Ad-miR-638 significantly decreased PDGF-BB-induced human aortic VSMCs proliferation, determined by MTT proliferation assay (n = 6). *P < 0.05 compared with CTRL in base group. #P < 0.05 compared with CTRL with PDGF-BB for 24 h. (C) Ad-miR-638 reduced PDGF-BB-induced VSMC proliferation in vitro as measured by cell counts (n = 6). *P < 0.05 compared with Ad-GFP/PDGF-BB at 24, 48, and 72 h. (D) Serum-deprived SMCs transduced with Ad-miR-638 (100 MOI) or Ad-GFP (100 MOI) were stimulated with PDGF-BB (20 ng/mL) for 24 h and analysed for PCNA and CyclinD1 protein levels by western blot. (E) Densitometric analysis of CyclinD1 and PCNA protein levels as determined by western blot (n = 4). (F) Human aortic VSMCs were transfected with control miR mimics, miR-638 mimics, or miR-638 inhibitors, respectively, and subjected to qRT–PCR analysis of the miR-638 level (n = 4). (G) Anti-miR-638 (100 nM) significantly increased human aortic VSMCs proliferation stimulated by PDGF-BB (2 ng/mL), determined by the MTT proliferation assay (n = 6).
Cyclin D1 is a critical regulator for VSMC migration and proliferation.5,35 As determined by western blot analysis, overexpression of miR-638 decreased both basal and PDGF-BB-induced cyclin D1 expression in human aortic VSMCs (Figure 2D and E). Accordingly, flow cytometry analysis revealed that miR-638 significantly decreased PDGF-BB-induced cell numbers in the S phase (Table 2). Together, these results suggest that miR-638 inhibits PDGF-BB-induced VSMC proliferation by attenuating the cell cycle progression from the G0/G1 phase into the S phase.
Table 2.
Effect of miR-638 on cell cycle distribution as analysed by flow cytometry
| PDGF-BB | − | − | + | + |
|---|---|---|---|---|
| Cell cycle | Ad-GFP | Ad-miR-638 | Ad-GFP | Ad-miR-638 |
| G0/G1 | 66.3 ± 1.5% | 72.8 ± 2.6% | 65.3 ± 0.6% | 73.7 ± 0.7% |
| S | 18.5 ± 1.4% | 14.0 ± 1.3%* | 29.1 ± 1.7%* | 20.8 ± 0.8%** |
| G2/M | 15.2 ± 1.5% | 13.2 ± 2.1% | 5.6 ± 1.6% | 5.5 ± 1.4% |
*P < 0.05 vs. Ad-GFP without PDGF-BB treatment.
**P < 0.05 vs. Ad-GFP with PDGF-BB treatment.
3.4. miR-638 is implicated in VSMC migration
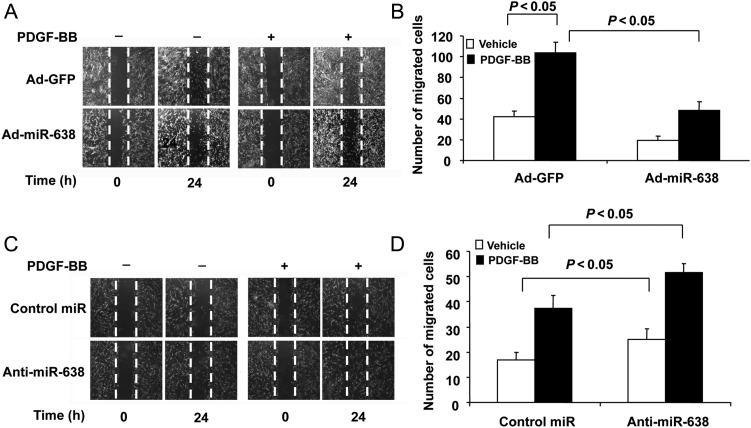
VSMC migration is also thought to contribute significantly to the development of cardiovascular diseases, including atherosclerosis and post-angioplasty restenosis.1,2 To determine whether miR-638 is also involved in VSMC migration, gain- and loss-of-function studies were performed. As shown in Figure 3A and B, restoration of miR-638 effectively inhibited both basal and PDGF-BB-induced SMC migration, as determined by scratch wound assays. Moreover, down-regulation of miR-638 by anti-miR-638 markedly enhanced both basal and PDGF-BB-induced VSMC migration (Figure 3C and D). Together, these data suggested that miR-638 regulates not only VSMC proliferation, but also cell migration.
Figure 3.
The role of miR-638 in VSMC migration. (A) Smooth muscle cell scratch wound assay. After transduction with Ad-miR-638 (100 MOI) or Ad-GFP (100 MOI) for 12 h, serum-deprived SMCs were subjected to injury by scraping and then stimulated with or without PDGF-BB (20 ng/mL) for 24 h. Migrated cells were quantitated using Image J software program and the result was shown in (B). (C) After transfected with anti-miR-638 (100 nM) or negative control oligos (100 nM), SMCs were subjected to injury by scraping and then stimulated with or without PDGF-BB (20ng/mL) for 24 h. Migrated cells were quantitated using Image J software program, and the result was shown in (D). The data are means + standard deviation of the number of migrated cells from three independent experiments.
3.5. NOR1 is a direct target of miR-638
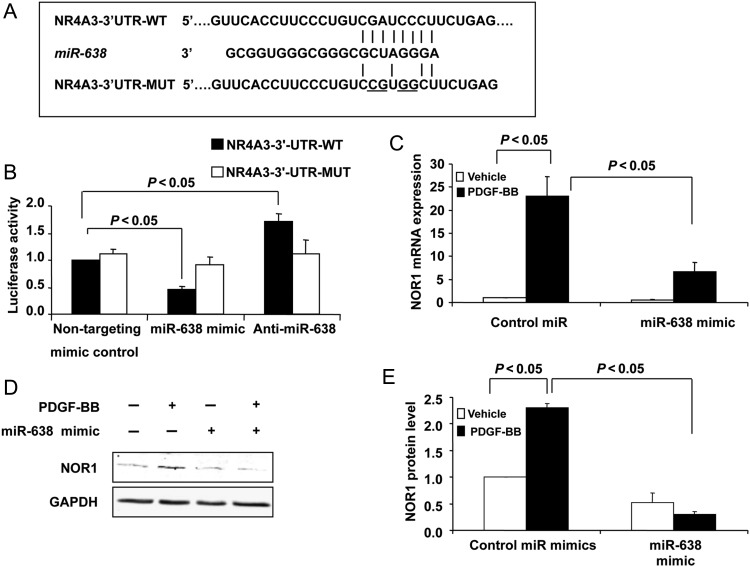
NOR1 was a potential miR-638 target according to its mRNA 3′-UTR complementary to miR-638 by searching for Targetscan database. Indeed, NOR1, together with Nur77 and Nurr1, constitute the orphan nuclear receptor NR4A family and play critical roles in VSMCs.36,37 As shown in Figure 4A, human NOR1 mRNA has a potential miR-638 binding site in its 3′-UTR. To determine whether miR-638 directly binds to the 3′-UTR sequence of human NOR1 mRNA and affects its expression, the 3′-UTR sequence of NOR1 containing the putative binding site of miR-638 was cloned into pLightSwitch_3′-UTR RenSP-luciferase reporter vector. The constructed vector was then cotransfected with either the miR-638 plasmid or non-targeting control plasmid into HEK293 cells. As shown in Figure 4B, the luciferase activity was inhibited by the transfection of cells with the miR-638 plasmid, but not by the non-targeting control plasmid, whereas transfection of anti-miR-638 increased the luciferase activity. Furthermore, the mutation of the miR-638 binding site in the 3′-UTR of NOR1 caused a complete loss of inhibitory effects of miR-638 on the luciferase activity (Figure 4B). To further verify that NOR1 is a functional target gene of miR-638 in human VSMCs, we transfected VSMCs with either a miRNA negative control or a miR-638 mimic (30 nmol) and the levels of NOR1 expression were determined by both qRT–PCR (Figure 4C) and western blot (Figure 4D and E). Consistent with previously reported studies,37 PDGF-BB significantly increased both NOR1 mRNA and protein levels in VSMCs, while miR-638 overexpression markedly inhibited both basal and PDGF-induced expression of NOR1 expression (Figure 4C–E). Importantly, the expression of two other NR4A members, Nur77 and Nurr1, was not significantly affected in human VSMCs after PDGF stimulation (data not shown). Collectively, these results suggested that miR-638 selectively binds to the 3′-UTR of human NOR1 mRNA and inhibits its expression in human VSMCs.
Figure 4.
Identification of NOR1 as a target of miR-638 in human VSMCs. (A) Schematic of the miR-638 putative binding site in human NOR1 3′-UTR and alignment of wild-type miR-638 and mutated NOR1 3′ UTR binding site of miR-638. The four mutated nucleotides are underlined. (B) HEK293 cells were cotransfected with the luciferase reporter carrying WT-NOR1 3′-UTR or mutated NOR1 3′-UTR, together with plasmid bearing miR-638 or non-targeting control or anti-miR-638. Forty-eight hours after transfection, renilla luciferase activities were measured. (C), VSMCs were transfected with miR-638 mimic or control miR mimic for 24 h and then serum-deprived for 48 h, NOR1 mRNA was measured by qRT–PCR in human aortic VSMCs 3 h after stimulation with or without PDGF-BB (20 ng/mL). (D) Effects of miR-638 overexpression on NOR1 protein expression 6 h after PDGF stimulation. (E) Densitometric analysis of NOR1 protein levels as determined by western blot.
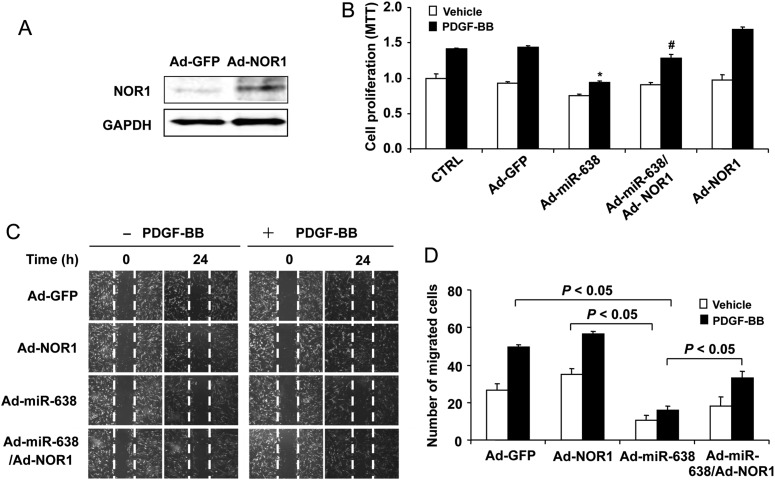
To further substantiate the functional significance of NOR1 in the miR-638-induced action on VSMCs, we determined whether NOR1 expression was able to partially rescue the inhibitory effects of miR-638 on VSMC proliferation and migration in response to PDGF-BB. As shown in Figure 5A, transduction of the cells with Ad-NOR1 significantly increased the expression of NOR1 in human VSMCs. In line with previously published data,36,37 overexpression of NOR1 markedly augmented the PDGF-BB stimulated both SMC proliferation, as determined by MTT assays (Figure 5B), and migration, as determined by scratch wound assays (Figure 5C and D). In addition, the inhibitory effects of miR-638 on PDGF-BB-induced SMC proliferation and migration were partially rescued. Taken together, these results demonstrated that miR-638 inhibits VSMC proliferation and migration, at least in part, via directly targeting the orphan nuclear receptor NOR1.
Figure 5.
Role of NOR1 in miR-638-mediated inhibitory effects on VSMC proliferation and migration. (A) NOR1 expression was determined by western blot analysis in VSMCs 48 h after transduction with Ad-NOR1 (100 MOI). (B) Human aortic VSMCs were transduced with either Ad-GFP, Ad-miR-638 or Ad-miR-638 plus Ad-GFP with a total MOI of 200. Serum-deprived VSMCs were stimulated with or without PDGF-BB (20 ng/mL) for 24 h, and cell proliferation was measured by the MTT assay (n = 6). *P < 0.05 compared with Ad-GFP with PDGF-BB. #P < 0.05 compared with Ad-miR-638 plus PDGF-BB. (C) VSMCs were transduced with adenoviruses as indicated earlier, 48 h after transduction, VSMCs were starved and cell migration was measured after PDGF stimulation for 24 h by the scratch wound assay. Migrated cells were quantitated and the results were shown in (D). The data are means + standard deviation of the number of migrated cells from three independent experiments.
4. Discussion
Despite recent progress in our understanding of the role of miRNAs in VSMC biology,14 the molecular mechanism(s) underlying SMC proliferation, migration, and proliferative vascular disease still remains elusive. This study identified miR-638 as a novel regulator for human VSMC proliferation and migration. We demonstrated that miR-638 is significantly down-regulated in human proliferative VSMCs and restoration of miR-638 markedly inhibited the PDGF-BB-induced SMC proliferation by arresting the cells in the S phase. In addition, miR-638 also suppressed the PDGF-BB-induced SMC migration. Furthermore, down-regulation of miR-638 by anti-miR-638 significantly accelerated the VSMC proliferation and migration in response to PDGF-BB stimulation. Thus, our study provides cognate evidence implicating miR-638 as a novel key regulator in human VSMC biology.
miR-638 is expressed in human and non-human primates. Recent studies have revealed that miR-638 was down-regulated in human gastric cancer, colorectal cancer, and acute leukaemia, suggesting that it may be associated with cancer cell proliferation and tumour invasion.38–40 In addition, miR-638 has been recently implicated in the pathogenesis of lupus nephritis 41 and may function as a predictor of early virological response to interferon treatment in chronic hepatitis B patients.42 However, the role of miR-638 in VSMCs and proliferative vascular diseases remains completely unknown. In the present study, we found that miR-638 is highly enriched in human aortic SMCs and its expression is nearly two to three-fold higher than that of miR-221/222 and miR-24, which have been previously shown to play critical roles in regulating VSMC phenotype and growth.17,18,20 In contrast to miR-221/222 and miR-24, which are modestly up-regulated by PDGF-BB stimulation in our study, miR-638 is substantially down-regulated at all tested time points upon PDGF-BB treatment. miR143/145, which was previously found to be down-regulated in the rodent models of vascular injury as well as in rat VSMCs after PDGF treatment,15 did not emerge as a differentially expressed miRNA in human aortic VSMCs in this study. This discrepancy suggests that the role of miRNAs in regulating VSMC function is species specific and their responses to the external stimuli are likely different.
Mechanistically, our study identified NOR1 as a direct target of miR-638 in human VSMCs. NOR1 belongs to the nuclear receptor 4A (NR4A) family and the members of this family, including Nur77, Nurr1, and NOR1, play essential roles in VSMC biology and vascular diseases.26,43 In cultured SMCs, NOR1 expression is induced by multiple stimuli, such as serum, PDGF-BB, LDL, and thrombin.36,37,44 In addition, NOR1 has been shown to be highly expressed in human atherosclerotic lesions, as well as in porcine balloon-injured vessels.36,37 PDGF-induced NOR1 expression mainly occurs at the transcriptional levels, via activating the transcriptional factor CREB and subsequently increasing its binding to the CREB-response elements located in the promoter region of NOR1.37 Thus far, the regulation of NOR1 expression by miRNAs has not yet been described. In this study, we demonstrated that miR-638 directly binds to the 3′-UTR of human NOR1 mRNA and down-regulates its expression. Furthermore, overexpression of miR-638 in human VSMCs markedly inhibited both basal and PDGF-BB-induced NOR1 expression; therefore, implicating miR-638 as a novel post-transcriptional regulator involved in the regulation of NOR1 in VSMCs. Interestingly, miR-145 is predicted to target the site located at position 849–855 of NOR-1 3′-UTR, whether miR-638 and miR-145 have synergistic effects in regulating VSMC function warrants further investigation.
Recently, NOR1 has been shown to exert pro-proliferative in VSMCs.36,37 Knockdown of NOR1 expression with anti-sense oligonucleotides in cultured SMCs has been shown to reduce SMC proliferation and migration.36,37,45 In NOR1 knockout SMCs, PDGF-induced proliferation and migration is almost completely inhibited, which coincides with strong reduction in cyclin D1 and D2 expression.45 Indeed, sequence analysis of the cyclin D1 promoter identified a functional NGFI-B response element in human (−1061 bp) and in mouse (−2197 bp),37 which may contribute to the regulation of cyclin D1 by NOR1 in VSMCs. Consistent with these studies, we demonstrated that both NOR1 and cyclin D1 expression are substantially inhibited by miR-638. Accordingly, PDGF-induced SMC proliferation and migration were also inhibited by miR-638, further highlighting the essential roles of the NOR1/cyclin D1 pathway in VSMCs. Importantly, similar to the studies performed in cultured SMCs, vascular lesion formation in response to wire injury is reduced in NOR1 knockout mice.45 Since miR-638 exists only in human and non-human primates, and its targeting sequence in the 3′-UTR of human NOR1 mRNA is not conserved among different species, further testing its functional significance by using small animal models of vascular injury is not possible. Nevertheless, it is necessary to investigate the clinical relevance of miR-638 in human proliferative vascular diseases, such as atherosclerosis and restenosis.
In summary, the present study identified miR-638 as a novel regulator in human VSMCs by targeting, at least in part, the NOR1/cyclin D pathway. miR-638 expression was substantially down-regulated in proliferative human VSMCs and restoration of its expression markedly inhibited both SMC proliferation and migration in response to PDGF stimulation. Interestingly, we also found that overexpression of miR-638 markedly increased the expression of VSMC differentiation markers, such as SM22α, SM α-actin, and calponin (see Supplementary material online, Figure 2), suggesting that miR-638 may also play a critical role in SMC differentiation. In this regard, our study provides significant novel insight into the molecular mechanisms associated with VSMC proliferation and migration, and suggests a potential therapeutic target for preventing and treating human vascular diseases, such as atherosclerosis and restenosis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This research was supported by the U.S. National Institutes of Health (R01HL103869) and the Chinese Natural Science Foundation (no. 381170114) to J.S.
Supplementary Material
References
- 1.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. doi:10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv. 2011;4:104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. doi:10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. doi:10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 4.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J. 2007;15:100–108. doi: 10.1007/BF03085963. doi:10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Zhang HN, Chen HZ, Gao P, Zhu LH, Li HL, et al. Sirt1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res. 2011;108:1180–1189. doi: 10.1161/CIRCRESAHA.110.237875. doi:10.1161/CIRCRESAHA.110.237875. [DOI] [PubMed] [Google Scholar]
- 6.Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med. 2006;16:25–28. doi: 10.1016/j.tcm.2005.11.003. doi:10.1016/j.tcm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res. 2000;86:125–130. doi: 10.1161/01.res.86.2.125. doi:10.1161/01.RES.86.2.125. [DOI] [PubMed] [Google Scholar]
- 8.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. doi:10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. doi:10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getachew R, Ballinger ML, Burch ML, Reid JJ, Khachigian LM, Wight TN, et al. PDGF beta-receptor kinase activity and erk1/2 mediate glycosaminoglycan elongation on biglycan and increases binding to LDL. Endocrinology. 2010;151:4356–4367. doi: 10.1210/en.2010-0027. doi:10.1210/en.2010-0027. [DOI] [PubMed] [Google Scholar]
- 11.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest. 2005;115:2691–2694. doi: 10.1172/JCI26593. doi:10.1172/JCI26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata A. Functions of microRNAs in cardiovascular biology and disease. Annu Rev Physiol. 2013;75:69–93. doi: 10.1146/annurev-physiol-030212-183737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang H, Hata A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol. 2012;19:224–231. doi: 10.1097/MOH.0b013e3283523e57. doi:10.1097/MOH.0b013e3283523e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald RA, Hata A, MacLean MR, Morrell NW, Baker AH. MicroRNA and vascular remodeling in acute vascular injury and pulmonary vascular remodeling. Cardiovasc Res. 2012;93:594–604. doi: 10.1093/cvr/cvr299. doi:10.1093/cvr/cvr299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. doi:10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. MiR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. doi:10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. doi:10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, et al. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. doi:10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 20.Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, et al. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 2010;29:559–573. doi: 10.1038/emboj.2009.370. doi:10.1038/emboj.2009.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. doi:10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 22.Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, et al. MiR-146a and krüppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. Embo Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. doi:10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. doi:10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. doi:10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol. 2010;306:1118–1126. doi: 10.1161/ATVBAHA.109.200873. doi:10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonta PI, Pols TW, de Vries CJ. NR4A nuclear receptors in atherosclerosis and vein-graft disease. Trends Cardiovasc Med. 2007;17:105–111. doi: 10.1016/j.tcm.2007.02.001. doi:10.1016/j.tcm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol. 2011;226:1035–1043. doi: 10.1002/jcp.22422. doi:10.1002/jcp.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762–H1769. doi: 10.1152/ajpheart.00829.2010. doi:10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HJ, Coffinier C, Choe Y, Beigneux AP, Davies BS, Yang SH, et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc Natl Acad Sci U S A. 2012;109:E423–E431. doi: 10.1073/pnas.1111780109. doi:10.1073/pnas.1111780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor nur77 suppresses endothelial cell activation through induction of ikappabalpha expression. Circ Res. 2009;104:742–749. doi: 10.1161/CIRCRESAHA.108.192286. doi:10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 31.Lim HJ, Kang DH, Lim JM, Kang DM, Seong JK, Kang SW, et al. Function of Ahnak protein in aortic smooth muscle cell migration through Rac activation. Cardiovasc Res. 2013;97:302–310. doi: 10.1093/cvr/cvs311. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Cheng Y, Liu X, Yang J, Munoz D, Zhang C. Unexpected pro-injury effect of propofol on vascular smooth muscle cells with increased oxidative stress. Crit Care Med. 2011;39:738–745. doi: 10.1097/CCM.0b013e318206bd86. doi:10.1097/CCM.0b013e318206bd86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellot JJ, Jr, Favreau LV, Karnovsky MJ, Rosenberg RD. Inhibition of vascular smooth muscle cell growth by endothelial cell-derived heparin. Possible role of a platelet endoglycosidase. J Biol Chem. 1982;257:11 256–11 260. [PubMed] [Google Scholar]
- 34.Reilly CF, Fritze LM, Rosenberg RD. Heparin inhibition of smooth muscle cell proliferation: a cellular site of action. J Cell Physiol. 1986;257:11–19. doi: 10.1002/jcp.1041290103. doi:10.1002/jcp.1041290103. [DOI] [PubMed] [Google Scholar]
- 35.Karpurapu M, Wang D, Van Quyen D, Kim TK, Kundumani-Sridharan V, Pulusani S, et al. Cyclin d1 is a bona fide target gene of nfatc1 and is sufficient in the mediation of injury-induced vascular wall remodeling. J Biol Chem. 2010;285:3510–3523. doi: 10.1074/jbc.M109.063727. doi:10.1074/jbc.M109.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez-González J, Rius J, Castelló A, Cases-Langhoff C, Badimon L. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ Res. 2003;92:96–103. doi: 10.1161/01.es.0000050921.53008.47. doi:10.1161/01.ES.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- 37.Nomiyama T, Nakamachi T, Gizard F, Heywood EB, Jones KL, Ohkura N, et al. The NR4A orphan nuclear receptor nor1 is induced by platelet-derived growth factor and mediates vascular smooth muscle cell proliferation. J Biol Chem. 2006;281:33 467–33 476. doi: 10.1074/jbc.M603436200. doi:10.1074/jbc.M603436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Wang Q, Liu C, Duan H, Zeng X, Zhang B, et al. Aberrant expression of miR-638 contributes to benzo(a)pyrene-induced human cell transformation. Toxicol Sci. 2012;125:382–391. doi: 10.1093/toxsci/kfr299. doi:10.1093/toxsci/kfr299. [DOI] [PubMed] [Google Scholar]
- 39.Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, et al. MicroRNA profiling of human gastric cancer. Mol Med Report. 2009;2:963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 40.Kahlert C, Klupp F, Brand K, Lasitschka F, Diederichs S, Kirchberg J, et al. Invasion front-specific expression and prognostic significance of microRNA in colorectal liver metastases. Cancer Sci. 2011;102:1799–1807. doi: 10.1111/j.1349-7006.2011.02023.x. doi:10.1111/j.1349-7006.2011.02023.x. [DOI] [PubMed] [Google Scholar]
- 41.Lu J, Kwan BC, Lai FM, Tam LS, Li EK, Chow KM, et al. Glomerular and tubulointerstitial miR-638, miR-198 and miR-146a expression in lupus nephritis. Nephrology (Carlton) 2012;17:346–351. doi: 10.1111/j.1440-1797.2012.01573.x. doi:10.1111/j.1440-1797.2012.01573.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Wang T, Wakita T, Yang W. Systematic identification of microRNA and messenger RNA profiles in hepatitis C virus-infected human hepatoma cells. Virology. 2010;398:57–67. doi: 10.1016/j.virol.2009.11.036. doi:10.1016/j.virol.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 43.Arkenbout EK, de Waard V, van Bragt M, van Achterberg TA, Grimbergen JM, Pichon B, et al. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation. 2002;106:1530–1535. doi: 10.1161/01.cir.0000028811.03056.bf. doi:10.1161/01.CIR.0000028811.03056.BF. [DOI] [PubMed] [Google Scholar]
- 44.Martorell L, Martinez-Gonzalez J, Crespo J, Calvayrac O, Badimon L. Neuron-derived orphan receptor-1 (NOR-1) is induced by thrombin and mediates vascular endothelial cell growth. J Thromb Haemost. 2007;5:1766–1773. doi: 10.1111/j.1538-7836.2007.02627.x. doi:10.1111/j.1538-7836.2007.02627.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Howatt DA, Gizard F, Nomiyama T, Findeisen HM, Heywood EB, et al. Deficiency of the NR4A orphan nuclear receptor nor1 decreases monocyte adhesion and atherosclerosis. Circ Res. 2010;107:501–511. doi: 10.1161/CIRCRESAHA.110.222083. doi:10.1161/CIRCRESAHA.110.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.