Abstract
Aims
Myxomatous mitral valve disease (MMVD) is associated with leaflet thickening, fibrosis, matrix remodelling, and leaflet prolapse. Molecular mechanisms contributing to MMVD, however, remain poorly understood. We tested the hypothesis that increased transforming growth factor-β (TGF-β) signalling and reactive oxygen species (ROS) are major contributors to pro-fibrotic gene expression in human and mouse mitral valves.
Methods and results
Using qRT–PCR, we found that increased expression of TGF-β1 in mitral valves from humans with MMVD (n = 24) was associated with increased expression of connective tissue growth factor (CTGF) and matrix metalloproteinase 2 (MMP2). Increased levels of phospho-SMAD2/3 (western blotting) and expression of SMAD-specific E3 ubiquitin-protein ligases (SMURF) 1 and 2 (qRT–PCR) suggested that TGF-β1 signalling occurred through canonical signalling cascades. Oxidative stress (dihydroethidium staining) was increased in human MMVD tissue and associated with increases in NAD(P)H oxidase catalytic subunits (Nox) 2 and 4, occurring despite increases in superoxide dismutase 1 (SOD1). In mitral valves from SOD1-deficient mice, expression of CTGF, MMP2, Nox2, and Nox4 was significantly increased, suggesting that ROS can independently activate pro-fibrotic and matrix remodelling gene expression patterns. Furthermore, treatment of mouse mitral valve interstitial cells with cell permeable antioxidants attenuated TGF-β1-induced pro-fibrotic and matrix remodelling gene expression in vitro.
Conclusion
Activation of canonical TGF-β signalling is a major contributor to fibrosis and matrix remodelling in MMVD, and is amplified by increases in oxidative stress. Treatments aimed at reducing TGF-β activation and oxidative stress in early MMVD may slow progression of MMVD.
Keywords: Cardiovascular surgery, Valves, Mitral valve, Regurgitation, Antioxidants
1. Introduction
Myxomatous mitral valve disease (MMVD) is a common cardiac condition affecting between 2 and 3% of the population.1,2 Myxomatous changes in the mitral valve include leaflet thickening, fibrosis, and matrix remodelling, ultimately contributing to prolapse and regurgitation.
In unstressed conditions, transforming growth factor-β (TGF-β) appears to tonically suppress cellular proliferation and promote valve interstitial cell-cycle arrest.3 Following injury, however, TGF-β can induce differentiation of quiescent valvular interstitial cells to a myofibroblast-like phenotype.4–6 These activated myofibroblasts express extracellular matrix proteins7 and fibrotic markers such as smooth muscle α-actin (SM α-actin)4,8 and connective tissue growth factor (CTGF).9–11
Many histological and biochemical changes in myxomatous mitral valves are consistent with TGF-β receptor activation. Previous work has shown that multiple TGF-β receptors and their ligands are increased in myxomatous mitral valves.12,13 TGF-β receptors exert many of their effects through SMAD2/3 phosphorylation14,15 (i.e. canonical signalling), which is antagonized by the intracellular E3 ubiquitin ligases SMURF1 and SMURF2.16–19 Insights into changes in endogenous inhibitors of TGF-β signalling in human MMVD, however, are limited.
Reactive oxygen species (ROS) play a critical role in the pathogenesis of numerous cardiac diseases, and are often associated with TGF-β signalling,20 matrix remodelling,21 and fibrosis.22–24 The primary enzymatic source of ROS in a variety of cardiovascular diseases is from NAD(P)H oxidases that can be activated in response to growth factors and/or cytokines such as TGF-β1. Specifically, NAD(P)H oxidase catalytic subunit 4 (Nox4)-derived ROS have been shown to play a critical role in the transduction of TGF-β1-induced differentiation of cardiac myofibroblasts.25,26 Importantly, Nox4-derived ROS may also serve as an alternative pathway activating pro-fibrotic and matrix remodelling gene expression.27–29 While previous studies have reported increases in systemic markers of oxidative stress in patients with mitral valve prolapse,30 we are not aware of studies characterizing changes in oxidative stress in mitral valve tissue from this population, nor have there been investigations examining the role of ROS in the initiation and/or amplification of transcriptional responses to pro-fibrotic stimuli in mitral valve interstitial cells.
The aims of this study were, thus, (i) to determine whether oxidative stress is increased in mitral valves from humans with MMVD, (ii) to determine whether increases in oxidative stress are associated with increases in NAD(P)H oxidase levels and canonical TGF-β signalling pathways in human MMVD, (iii) to determine whether reductions in antioxidant gene expression are independent contributors to increases in pro-fibrotic and matrix remodelling gene expression in mitral valves in vivo, and (iv) to determine whether increasing intracellular antioxidant capacity can attenuate TGF-β1 signalling in mitral valve interstitial cells in vitro.
2. Methods
2.1. Tissue
Studies were performed on 48 samples of human mitral valve tissue collected during surgery at Mayo Clinic, Rochester. The 24 controls were non-myxomatous mitral valves explanted from cardiac transplant recipients. The other 24 samples were collected from individuals with myxomatous mitral valve degeneration during mitral valve repair procedures. Patients with MMVD were identified following clinical evaluation in the Mayo Clinic Valvular Heart Disease Clinic. The diagnosis of MMVD was confirmed by clinical histopathological analysis of tissue following surgery (gross and microscopic features consistent with MMVD, i.e. evaluated by Verhoeff–Van Gieson staining). These histopathological changes were associated clinically with severe mitral regurgitation, no mitral stenosis, and a history of mitral valve prolapse with chordal rupture. Valve tissue from patients with mitral prolapse of a defined genetic origin (e.g. Marfan syndrome, Loeys–Dietz, osteogenesis imperfecta, etc.) was not used in the current study.
2.2. Animal model
Superoxide dismutase 1 (SOD1) knockout and wild-type mice littermates (n = 12 and n= 11, respectively) were aged 3 months. Animals were euthanized via intraperitoneal injection of pentobarbital sodium (0.5 mL). Samples of mitral valve tissue were harvested and stored in Trizol (Invitrogen).
2.3. Ethical considerations
For human studies, all protocols and procedures were approved by the Mayo Clinic Institutional Review Board and conformed to the principles outlined in the Declaration of Helsinki. For animal studies, all protocols and procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee and conformed to the guidelines set forth by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals.
2.4. Cell culture
Mitral valves from three C57BL/6 mice were harvested and pooled. All cells were grown in complete media (40% Dulbecco's modified Eagle's medium, 40% Ham's F-10, 20% foetal bovine serum, and antibiotics) between passages 3 and 9 and harvested at 90–100% confluence. Treatments included TGF-β1 (10 ng/mL) and polyethylene glycol-superoxide dismutase (PEG-SOD) (200 U/mL) for 24 h. Cells were harvested using lysis buffer (Invitrogen), 1% 2-mercaptoethanol.
2.5. RNA isolation and complementary DNA synthesis
Intact valve tissue was pulverized and transferred to Trizol reagent (Invitrogen). Total RNA was extracted using chloroform extraction techniques (Invitrogen). Complementary DNA (cDNA) was synthesized from total RNA using the Superscript VILO cDNA Synthesis Kit (Invitrogen).
2.6. Gene expression
Gene expression for TGFB1, CTGF, MMP2, MMP9, NOX2, NOX4, SOD1, SOD2, SOD3, CAT (Catalase), ACTA2 (SM α-actin), SMURF1, SMURF2 was measured using TaqMan Gene Expression Assay primers and Master Mix using an Applied Biosystems 96-well Thermal Cycler. GAPDH and HPRT1 were used to normalize gene expression levels.
2.7. Western blotting
Protein was isolated from pulverized tissue or from lysed cells. Gel electrophoresis was performed using Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies against p-SMAD2 (1:1000, Cell Signaling), p-SMAD3 (1:2500, Abcam), or GAPDH (1:10 000, Abcam) for 18 h at 4°C and subsequently incubated with a goat anti-rabbit/mouse HRP-conjugated secondary antibody for 1.5 h at room temperature (1:5000, Fisher Scientific). After addition of a chemiluminescent substrate (Thermo Scientific), band detection was accomplished using digital imaging techniques (Fluorochem M) and quantified using the ImageJ (NIH) or Cell Biosystems software.
2.8. Dihydroethidium staining
ROS were measured in cryosectioned myxomatous and non-myxomatous human mitral valve tissue samples frozen in OCT compound (see Detailed Methods in the Supplementary material online).
ROS were also measured in freshly excised, wild-type and CuZnSOD-deficient mouse mitral valves. Tissue was stained, wet-mounted en bloc on glass slides, and imaged using confocal microscopy (see Detailed Methods in the Supplementary material online).
2.9. Statistical methods
All data are reported as mean ± SE. Significant differences between non-myxomatous and myxomatous human valve tissue were detected using unpaired t-tests. Significant differences between littermate-matched wild-type and SOD1 knockout mice were detected using paired t-tests. For cell culture experiments examining interactions between TGF-β1 and antioxidant levels, main effects were detected using an analysis of variance, and Bonferroni-corrected paired t-tests were used for post hoc testing.
3. Results
3.1. Mitral valve function in non-myxomatous and myxomatous mitral valves
Evidence of mild, ischaemic mitral valve regurgitation was present in 58% of non-myxomatous mitral valves. In contrast, severe mitral valve regurgitation due to leaflet prolapse was present in all patients with MMVD, as evidenced by abnormal mitral regurgitant volumes (71 ± 6.3 cc) and left atrial volume indices (56 ± 3.7 cc/m2). Left ventricular function was well maintained in patients with MMVD.
3.2. TGF-β1 expression and SMAD2, 3 phosphorylation in human tissue
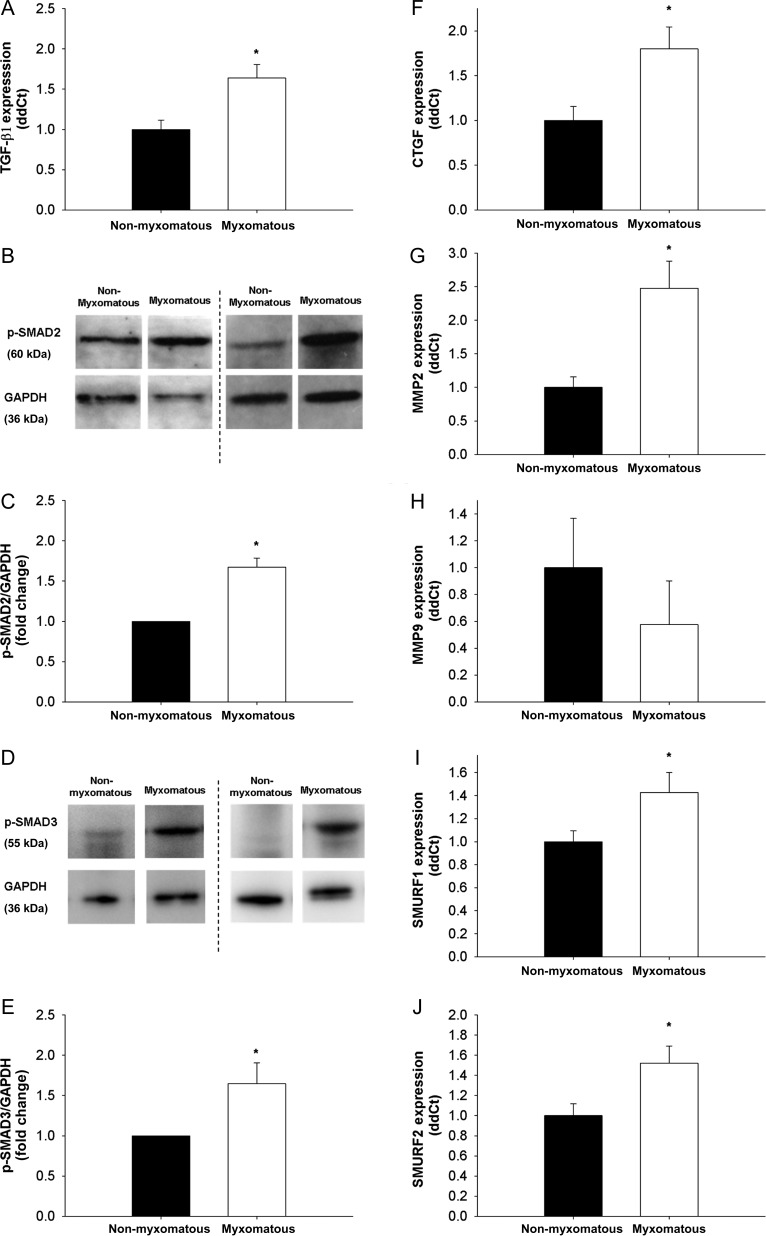
Expression of TGF-β1 was increased in human myxomatous mitral valves compared with non-myxomatous valves (Figure 1A) and was associated with increases in protein levels of p-SMAD2/3 (Figure 1B–E).
Figure 1.
TGF-β1 expression, levels of canonical TGF-β1 signalling molecules, and SMAD target gene expression in non-myxomatous and myxomatous human mitral valve tissue. (A) TGF-β1 expression is significantly increased in MMVD (qRT–PCR, n= 24 non-myxomatous valves, n= 24 myxomatous valves). (B–E) Western blots showing SMAD2/3 phosphorylation (B and D) and subsequent quantitation using densitometry (C and E). Note that SMAD2/3 phosphorylation—indicative of canonical TGF-β1 signalling—is significantly increased in MMVD (n= 5 non-myxomatous valves, n= 5 myxomatous valves). (F–H) Changes in CTGF, MMP2, and MMP9 in MMVD. Note that CTGF (F) and MMP2 (G) are markedly increased in MMVD (qRT–PCR, n= 24 non-myxomatous valves, n= 24 myxomatous valves). (I and J) Changes in expression of the intracellular E3 ubiquitin ligases SMURF1 and SMURF2, which are key negative regulators of canonical Smad signalling. Note that both SMURF1 and SMURF2 are markedly increased in human MMVD (n= 24 non-myxomatous valves, n= 24 myxomatous valves). *P < 0.05 in all figures.
3.3. Expression of TGF-β1 target genes in human tissue
CTGF was significantly increased in human myxomatous mitral valves compared with non-myxomatous mitral valves (Figure 1F). While matrix metalloproteinase 2 (MMP2) was significantly increased in patients with MMVD (Figure 1G), MMP9, however, was comparable between non-myxomatous and myxomatous valves (Figure 1H). Expression of SMURF1 and SMURF2, endogenous inhibitors of TGF-β1 signalling, was also elevated in mitral valves with MMVD (Figure 1I and J).
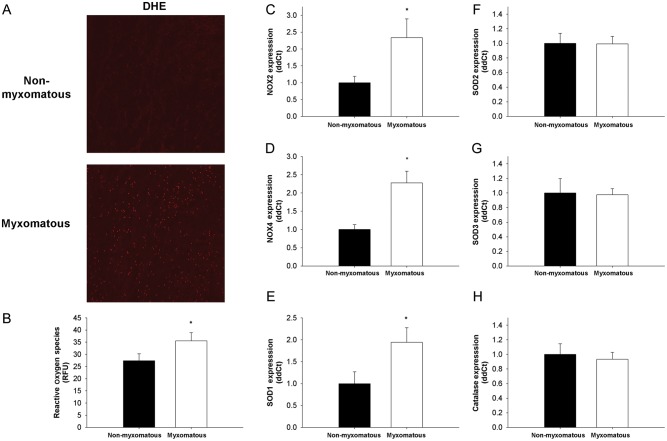
3.4. Oxidative stress in human tissue
DHE fluorescence was low in non-myxomatous human mitral valves, but was elevated in mitral valves with MMVD (Figure 2A and B).
Figure 2.
ROS and pro/antioxidant gene expression in non-myxomatous and myxomatous human mitral valves. (A and B) Micrographs and quantitation of dihydroethidium staining in non-myxomatous and myxomatous human mitral valves. Note that ROS are significantly increased in valve tissue from patients with MMVD (n= 5 non-myxomatous valves, n= 5 myxomatous valves). (C–F) Expression of pro- and antioxidant genes in non-myxomatous and myxomatous human mitral valve tissue. Expression of Nox2 (C), Nox4 (D), and SOD1 (E) is significantly increased in myxomatous tissue. (F–H) Expression of other SOD isoforms (SOD2 and SOD3) and catalase was relatively unchanged (n= 24 non-myxomatous valves, n= 24 myxomatous valves for panels (C)–(F). *P < 0.05 in all figures.
3.5. Pro-oxidant and antioxidant gene expression in human tissue
Expression of Nox2 and 4, catalytic subunits of the NAD(P)H oxidase enzymatic complex, was increased in myxomatous mitral valve tissue (Figure 2C and D). Humans with MMVD had elevated gene expression of the cytosolic antioxidant SOD1 (Figure 2E). Expression of other antioxidants such as SOD2 (mitochondria), SOD3 (extracellular), and catalase (peroxisomes) did not differ between myxomatous and non-myxomatous mitral valve tissue (Figure 2F–H).
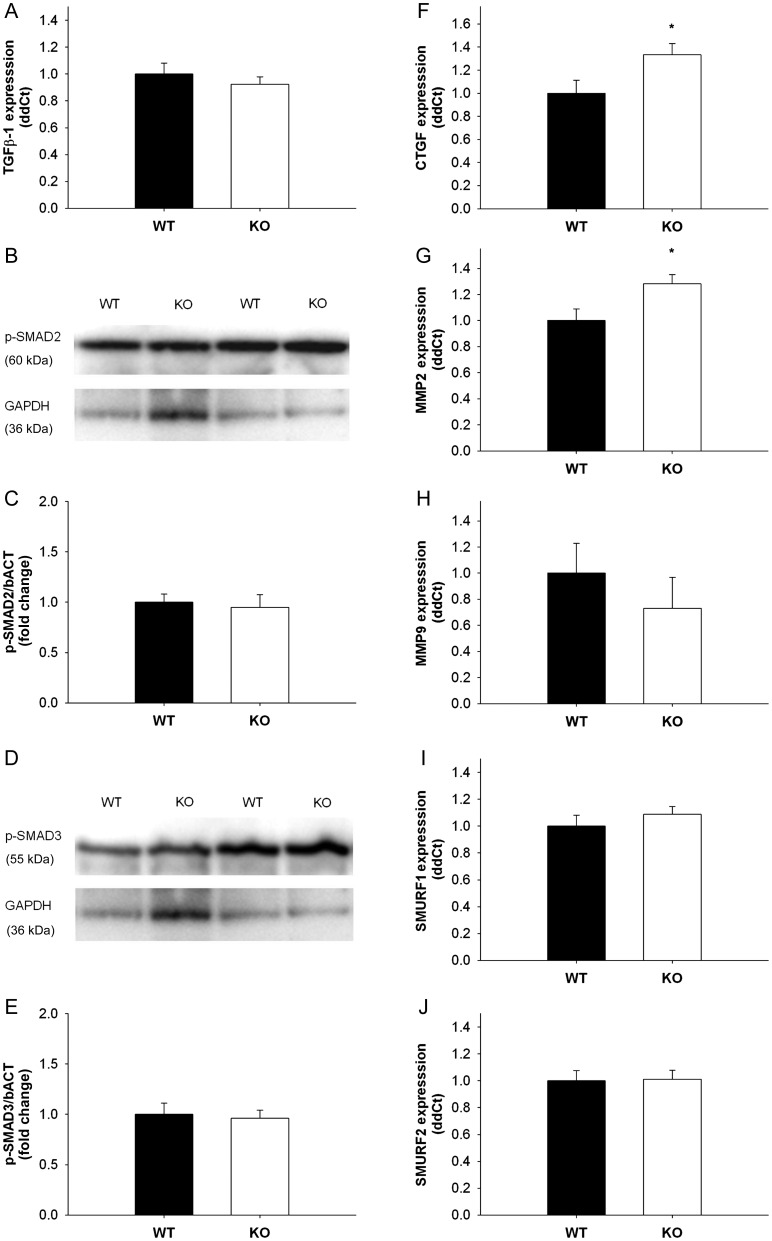
3.6. Expression of TGF-β1 and SMAD2/3 phosphorylation in SOD1-deficient mice
TGF-β1 expression was unaltered in mitral valves from SOD1-deficient mice compared with wild-type littermates (Figure 3A). Protein levels of p-SMAD2, 3 were also comparable between groups (Figure 3B–E).
Figure 3.
TGF-β1 mRNA expression and p-SMAD2/3 levels in mitral valves from SOD1 knockout mice compared with wild-type littermates. (A) TGF-β1 expression was unaltered in SOD1-deficient mitral valves (n= 9 wild-type valves, n= 13 knockout valves). (B–E) Immunoblots showing SMAD2/3 phosphorylation (B and D) and subsequent quantitation using densitometry (C and E). Note that similar to TGF-β1 expression, SMAD2/3 phosphorylation is not changed in mitral valves with SOD1 deficiency (n= 4 wild-type valves, n= 4 knockout valves). (F–H) Pro-fibrotic and matrix remodelling gene expression in mitral valves from wild-type and SOD1-deficient mice. Note that CTGF and MMP-2 are significantly increased in SOD1 knockout mice (n= 9 wild-type valves, n= 13 knockout valves). *P < 0.05 in all figures.
3.7. TGF-β1 target gene expression in mice with SOD1 deficiency
Expression of CTGF and MMP2 was increased in mitral valves from SOD1 knockout mice compared with wild-type littermate controls (Figure 3F and G). Expression of MMP9, SMURF1, and SMURF2 was not significantly changed in SOD1-deficient mice (Figure 3H–J).
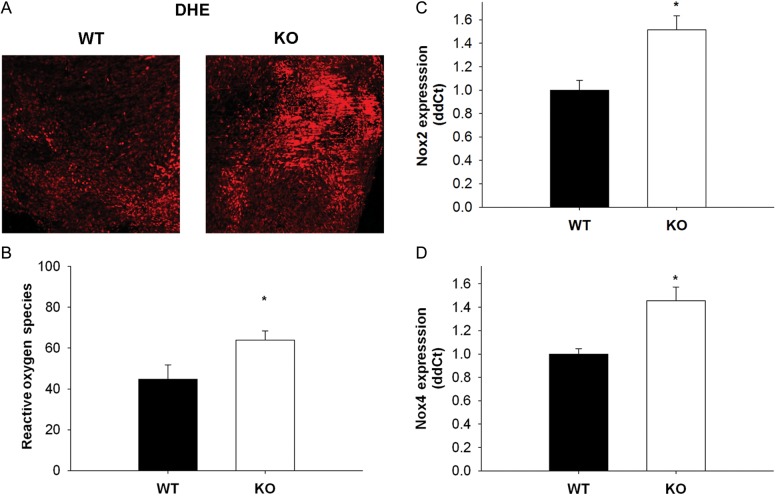
3.8. Oxidative stress in SOD-1-deficient mitral tissue
DHE fluorescence was low in wild-type mitral valves, but elevated in SOD1-deficient mitral valves (Figure 4A and B).
Figure 4.
ROS and pro-oxidative gene expression in wild-type and SOD1-deficient mice mitral valves. (A and B) Micrographs and quantitation of dihydroethidium staining in wild-type and SOD1-deficient mitral valves. Note that ROS are significantly increased in valve tissue from mice lacking two copies of SOD1 (n= 4 wild-type valves, n= 4 knockout valves). (C and D) Pro-oxidative gene expression in mitral valves from wild-type and SOD1-deficient mice. Note that both Nox 2 and 4 are significantly increased in SOD1-deficient mice (n= 9 wild-type valves, n= 13 knockout valves). *P < 0.05 in all figures.
3.9. Pro-oxidant and antioxidant gene expression in SOD1 deficiency
As expected, expression of SOD1 was undetectable in mitral valves from SOD1-deficient mice (see Supplementary material online). Expression of Nox2 and 4, however, was increased in mitral valves from SOD1 knockout mice (Figure 4C and D).
3.10. Effects of exogenous antioxidants on SMAD2 phosphorylation and SMURF expression in response to TGF-β1 in mouse mitral valve interstitial cells in vitro
SMAD2 phosphorylation levels in mouse mitral valve interstitial cells (mMVICs) were elevated after 24 h of TGF-β1 treatment (10 nmol). However, addition of PEG-SOD had no effect on induction of SMAD2 phosphorylation in TGF-β1-treated cells (Figure 5A and B). Gene expression of SMURF1 and SMURF2 in mMVICs was increased following TGF-β1 treatment, but unchanged by PEG-SOD stimulation (Figure 5I and J).
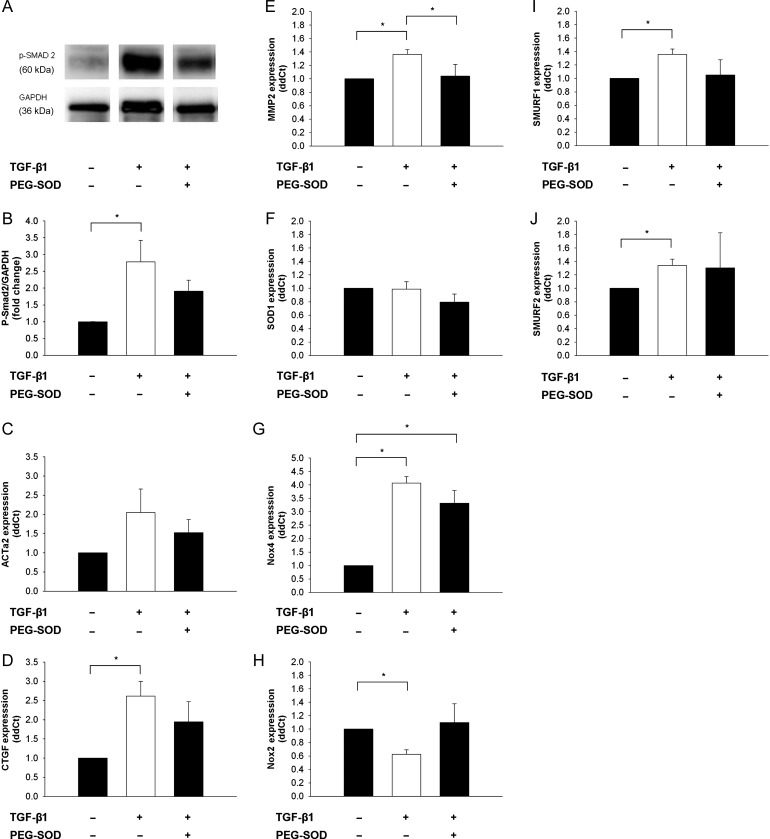
Figure 5.
Effects of reducing oxidative stress on TGF-β1-induced gene expression (10 ng/mL TGF-β1 for 24 h) in mMVICs in vitro. (A–E) Effects of antioxidant treatment on induction of TGF-β1 signalling molecules and target genes related to myofibroblast activation, fibrosis, and matrix remodelling. Note that PEG-SOD (200 U/mL) tends to reduce SMAD phosphorylation levels and TGF-β1-induced myofibroblast activation (SM-α-actin) and CTGF expression, and abrogates TGF-β1-induced MMP-2 expression. (G and H) Regulation of anti/pro-oxidant enzyme expression in TGF-β1-treated mMVICs. Note that SOD1 is relatively unchanged and Nox4 is significantly increased in mMVICs after treatment with TGF-β1, whereas Nox2 is significantly reduced by TGF-β1. Furthermore, induction of Nox4 is relatively unaffected by treatment with PEG-SOD, whereas TGF-β1-induced suppression of Nox2 is eliminated by PEG-SOD. (I and J) Regulation of endogenous inhibitors of canonical TGF-β1 signalling by oxidative stress. Note that increases in SMURF1 and SMURF2 expression with TGF-β1 treatment were attenuated (or no longer significant) following treatment with PEG-SOD (n= 5 cell lines in each condition). *P < 0.05 for all figures.
3.11. Effects of exogenous antioxidants on pro-fibrotic and matrix remodelling gene responses to exogenous TGF-β1 in mMVICs in vitro
Treatment of mMVICs with TGF-β1 (10 nmol, 24 h) resulted in induction of CTGF and MMP2 mRNA levels, whereas SM α-actin was not significantly increased (Figure 5C–E). Co-incubation of TGF-β1-treated mMVICs with PEG-SOD did not significantly alter expression of SM α-actin or CTGF (Figure 5C and D). TGF-β1-induced expression of MMP2, however, was abrogated by co-treatment with PEG-SOD (Figure 5E).
3.12. Effects of TGF-β1 and antioxidants on oxidative stress in vitro
SOD1 expression was unchanged in mMVICs following TGF-β1 treatment for 24 h (Figure 5F). Nox4 mRNA, however, was significantly increased in response to exogenous TGF-β1 (Figure 5G). Addition of PEG-SOD did not significantly reduce TGF-β1-induced changes in Nox4 gene expression. Interestingly, Nox2 expression was decreased with treatment of TGF-β1, and slightly elevated upon co-incubation with PEG-SOD (Figure 5H).
4. Discussion
The major findings of this study are: (i) canonical TGF-β signalling is increased in MMVD despite increases in inhibitory molecules, (ii) oxidative stress is increased in human myxomatous mitral valves due to increases in Nox2- and Nox4-dependent oxidases, and occur despite up-regulation of SOD1 expression, (iii) reducing SOD1 in mice in vivo increases oxidative stress and pro-fibrotic/matrix remodelling gene expression in mitral valve leaflets, and (iv) increasing antioxidant capacity in mMVICs in vitro reduces expression of matrix remodelling genes in response to TGF-β1. Collectively, these data support a working model in which cytosolic oxidative stress is a key modulator of pro-fibrotic and matrix remodelling genes in humans with MMVD.
4.1. Canonical TGF-β signalling is increased in MMVD
In the current study, TGF-β1 expression and phosphorylated SMAD2/3 protein were both significantly increased in MMVD, indicative of increases in canonical TGFβ signalling. These data are consistent with previous reports of increases in TGF-β1 expression in myxomatous mitral valves.12,13 This up-regulation of canonical TGF-β1 signalling in human MMVD was associated with elevated expression of several known TGF-β1 target genes, including CTGF and MMP2. Empirical evidence suggesting that inhibition of TGF-β signalling may be a viable therapeutic target in MMVD is derived from mouse models of Marfan syndrome, where mitral valve prolapse can be largely prevented by parenteral administration of TGF-β neutralizing antibodies.31
Interestingly, increased TGF-β1 expression and SMAD2/3 phosphorylation in myxomatous mitral valves occurred despite up-regulation of the E3 ubiquitin ligases SMURF1 and SMURF2. Along with SMAD6 and SMAD7, the promoter regions of both SMURF1 and SMURF2 are rich in SMAD-binding elements and play a critical role in the negative feedback regulation of SMAD signalling.32–37 Along these lines, multiple in vitro studies have shown increased SMURF1/2 expression with exogenous TGF-β1 stimulation,17,34 and knockdown of either of these inhibitory molecules dramatically increases TGF-β1 target gene expression in multiple cell types.38 Collectively, these data suggest that increases in SMURF1/2 expression play a role in limiting increases in canonical Smad signalling, but are not sufficient to abrogate increases in pro-fibrotic or matrix remodelling genes.
4.2. Mechanisms of increased oxidative stress in MMVD
To our knowledge, this is the first report demonstrating that oxidative stress is increased in mitral valve tissue from humans with MMVD. Furthermore, our data suggest that Nox2 and Nox4 are likely contributors to increased oxidative stress in myxomatous mitral valves, as expression of both enzymes was markedly increased in MMVD. Previous reports using myocardial tissue have established a clear link between up-regulation of TGF-β signalling and Nox4 activation, suggesting that Nox4-derived ROS are critical for SMAD2/3 phosphorylation in response to TGF-β1.26 While increases in Nox2 may increase overall cellular oxidative stress and contribute to amplification of TGF-β signalling20 and matrix remodelling protein activation,21 our observation that exogenous TGF-β results in robust down-regulation of Nox2 in vitro strongly suggests that multiple signalling mechanisms contribute to NAD(P)H oxidase isoform expression and activity in MMVD.
Our data also suggest that oxidative stress is increased in valve tissue from patients with MMVD despite significant increases in CuZnSOD expression. Expression of CuZnSOD is highly responsive to increases in oxidative stress and inflammation due to the presence of several NFκB-binding sites in its promoter region, and has been shown to play a critical role in suppression of inflammation and fibrosis in a number of disease states.39–43 Interestingly, this pattern of oxidant-related gene expression markedly differs from that observed in calcific aortic valve stenosis, where uncoupled NOS and reduced antioxidant capacity are the primary contributors to increases in ROS levels.44
4.3. SOD1 plays a role in the up-regulation of pro-fibrotic/matrix remodelling genes in vivo
In support of the concept that SOD1 is a tonic suppressor of TGF-β signalling in vivo, we demonstrated that genetic deletion of SOD1 in mice resulted in significant up-regulation of pro-fibrotic and matrix remodelling genes. More specifically, we found that CTGF and MMP2—both target genes of canonical TGF-β signalling45–49—are up-regulated in mitral valves from SOD1-deficient mice, despite our observation that TGF-β expression and p-SMAD2/3 protein were relatively unchanged. These data suggest that even in the absence of changes in canonical TGF-β signalling, SOD1 is likely to be a protective/compensatory antioxidant mechanism that prevents fibrosis and excessive matrix turnover/remodelling in non-myxomatous mitral valves.
Our data also suggest that SOD1 activity serves as a tonic suppressor of NAD(P)H oxidase expression and activity, as genetic deletion of SOD1 resulted in increased expression of Nox2 and Nox4 in mitral valves. Previous work in other cell types suggested that activation of NAD(P)H oxidase may self-perpetuate through a phenomenon called ROS-induced ROS release (or RI-RR).50,51 Our data from SOD1-deficient mice are consistent with a working model in which SOD1 suppresses RI-RR in non-myxomatous mitral valve interstitial cells (at least, with regard to Nox2- and Nox4-derived ROS), and inadequate antioxidant responses may contribute to exacerbation of RI-RR and amplification of pro-fibrotic signalling cascades in MMVD.
4.4. Role of increased oxidative stress in mitral valve interstitial cell responses to TGF-β1
Our observation that induction of matrix remodelling gene expression in response to exogenous TGF-β1 was attenuated by pre-treatment of mitral valve interstitial cells in vitro with cell-permeable SOD strongly suggests that a cellular redox state is an important determinant of TGF-β1-mediated MMP2 expression, and may be a useful complementary treatment to slow progression of MMVD in vivo. It is important to note, however, that not all TGF-β1-induced changes in gene expression were abrogated by PEG-SOD. First, up-regulation of Nox4 was not affected by this treatment, suggesting that Nox4 is tightly coupled to TGF-β1 signalling in an ROS-independent manner. Up-regulation of SMURF1/2 and CTGF by TGF-β1, however, no longer achieved statistical significance after treatment with PEG-SOD, suggesting fibrosis and matrix remodelling gene expression induced by TGF-β1 are dependent upon generation of intracellular ROS. Furthermore, PEG-SOD eliminated suppression of Nox2 by TGF-β1, implying that some of the potentially beneficial, anti-inflammatory aspects of TGF-β1 are also mediated by ROS. Thus, successful application of antioxidant therapies in MMVD require further investigation of the context dependence of ROS-related signalling in mitral valve interstitial cells.
4.5. Conclusions
These studies are the first to demonstrate that oxidative stress is increased in MMVD, which likely augments TGF-β-induced fibrosis and matrix remodelling. Furthermore, cytosolic antioxidant capacity plays a role in tonic suppression of fibrogenic and matrix remodelling gene expression in mitral valves. Our data suggest that future studies examining the utility of antioxidant therapy as a complementary modality to slow or prevent progression of myxomatous mitral valve prolapse are warranted.
4.6. Limitations
One limitation to the current study is that the amount of tissue available for molecular analyses is very limited (as a relatively small amount of tissue is removed during mitral valve repair procedures). Thus, we were not able to perform all measurements on all samples (especially, those requiring a relatively large amount of tissue).
A second limitation compounding the small amount of tissue removed relates to the resection of almost exclusively myxomatous tissue during surgical mitral valve repair. More specifically, we generated correlations between regurgitant volume, left atrial volume, and key molecular changes we observed in tissue from patients with MMVD, but did not find significant correlations between indices of mitral valve function and the consequent molecular changes within each group (i.e. non-myxomatous or myxomatous valves; data not shown). The lack of a strong correlation within each group can likely be attributed to two factors: (i) the severity of the disease (i.e. a cohort of patients with disease severe enough to warrant surgery), and (ii) the fact that predominantly diseased tissue is removed at the time of surgery (i.e. only myxoid regions of the valve). When combined, these two factors make the variance of both measurements relatively low within each group. We would presume that performing correlations between valve function, valve thickness/fibrosis (difficult to measure consistently in frozen sections), and molecular markers would be significant in animal models of MMVD, in which molecular and functional changes could be measured over a greater continuum and across greater regions of the entire mitral valve structure. Future studies in appropriate animal models of mitral valve disease will be critical to confirming these working hypotheses and advancing our understanding of the pathogenesis of MMVD.
Common to our current report and many others utilizing human valve tissue, our non-myxomatous valves were taken from patients undergoing transplant surgery. Thus, while the mitral valves were not myxomatous, the leaflet tissue was likely exposed to an ‘abnormal’ metabolic and neurohormonal milieu. Despite this, we observed significant differences between frankly myxomatous mitral leaflets and those explanted as bystanders to other cardiac diseases, suggesting that the molecular changes in MMVD are not processes that occur secondary to cardiac disease in general, but are instead primary drivers of myxomatous valve remodelling and disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This study was supported by funding from the National Institutes of Health (HL092235 and HL111121 to J.D.M.), the Mayo Clinic Center for Regenerative Medicine, and the Mayo Clinic Kogod Center on Aging.
Supplementary Material
Acknowledgements
The authors thank Bin Zhang for assistance with microdissection and isolation of mouse mitral valve tissue for these studies.
Conflict of interest: none declared.
References
- 1.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. doi: 10.1056/NEJM199907013410101. doi:10.1056/NEJM199907013410101. [DOI] [PubMed] [Google Scholar]
- 2.Freed LA, Benjamin EJ, Levy D, Larson MG, Evans JC, Fuller DL, et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40:1298–1304. doi: 10.1016/s0735-1097(02)02161-7. doi:10.1016/S0735-1097(02)02161-7. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Gotlieb AI. Transforming growth factor-beta regulates the growth of valve interstitial cells in vitro. Am J Pathol. 2011;179:1746–1755. doi: 10.1016/j.ajpath.2011.06.007. doi:10.1016/j.ajpath.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. doi:10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrov VV, Fagard RH, Lijnen PJ. Transforming growth factor-beta(1) induces angiotensin-converting enzyme synthesis in rat cardiac fibroblasts during their differentiation to myofibroblasts. J Renin Angiotensin Aldosterone Syst. 2000;1:342–352. doi: 10.3317/jraas.2000.064. doi:10.3317/jraas.2000.064. [DOI] [PubMed] [Google Scholar]
- 6.Liu AC, Gotlieb AI. Transforming growth factor-beta regulates in vitro heart valve repair by activated valve interstitial cells. Am J Pathol. 2008;173:1275–1285. doi: 10.2353/ajpath.2008.080365. doi:10.2353/ajpath.2008.080365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 8.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- 9.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. doi:10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. doi:10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- 12.Aupperle H, Marz I, Thielebein J, Schoon HA. Expression of transforming growth factor-beta1, -beta2 and -beta3 in normal and diseased canine mitral valves. J Comp Pathol. 2008;139:97–107. doi: 10.1016/j.jcpa.2008.05.007. doi:10.1016/j.jcpa.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, et al. Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation. 2012;126:S189–S197. doi: 10.1161/CIRCULATIONAHA.111.082610. doi:10.1161/CIRCULATIONAHA.111.082610. [DOI] [PubMed] [Google Scholar]
- 14.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. doi:10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piek E, Heldin CH, Ten Dijke P. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999;13:2105–2124. [PubMed] [Google Scholar]
- 16.Lin X, Liang M, Feng XH. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. J Biol Chem. 2000;275:36818–36822. doi: 10.1074/jbc.C000580200. doi:10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 17.Tan R, He W, Lin X, Kiss LP, Liu Y. Smad ubiquitination regulatory factor-2 in the fibrotic kidney: regulation, target specificity, and functional implication. Am J Physiol Renal Physiol. 2008;294:F1076–F1083. doi: 10.1152/ajprenal.00323.2007. doi:10.1152/ajprenal.00323.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. doi:10.1016/S1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Chang C, Gehling DJ, Hemmati-Brivanlou A, Derynck R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc Natl Acad Sci USA. 2001;98:974–979. doi: 10.1073/pnas.98.3.974. doi:10.1073/pnas.98.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li PF, Dietz R, von Harsdorf R. Superoxide induces apoptosis in cardiomyocytes, but proliferation and expression of transforming growth factor-beta1 in cardiac fibroblasts. FEBS Lett. 1999;448:206–210. doi: 10.1016/s0014-5793(99)00370-1. doi:10.1016/S0014-5793(99)00370-1. [DOI] [PubMed] [Google Scholar]
- 21.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Huang H, Xia W, Tang Y, Li H, Huang C. NADPH oxidase inhibition ameliorates cardiac dysfunction in rabbits with heart failure. Mol Cell Biochem. 2010;343:143–153. doi: 10.1007/s11010-010-0508-4. doi:10.1007/s11010-010-0508-4. [DOI] [PubMed] [Google Scholar]
- 23.Ruetten H, Dimmeler S, Gehring D, Ihling C, Zeiher AM. Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload. Cardiovasc Res. 2005;66:444–453. doi: 10.1016/j.cardiores.2005.01.021. doi:10.1016/j.cardiores.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, et al. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. doi:10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 25.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, et al. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol. 2010;21:93–102. doi: 10.1681/ASN.2009020146. doi:10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. doi:10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 27.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. doi:10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 28.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, et al. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. doi:10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. doi:10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arocha F, Diez-Ewald M, Durango AI, Sulbaran T. Platelet activity in mitral valve prolapse: a study of platelet aggregation, malondialdehyde production, and plasma beta-thromboglobulin. Am J Hematol. 1985;19:21–25. doi: 10.1002/ajh.2830190104. doi:10.1002/ajh.2830190104. [DOI] [PubMed] [Google Scholar]
- 31.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagarajan RP, Zhang J, Li W, Chen Y. Regulation of Smad7 promoter by direct association with Smad3 and Smad4. J Biol Chem. 1999;274:33412–33418. doi: 10.1074/jbc.274.47.33412. doi:10.1074/jbc.274.47.33412. [DOI] [PubMed] [Google Scholar]
- 33.Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, et al. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275:6075–6079. doi: 10.1074/jbc.275.9.6075. doi:10.1074/jbc.275.9.6075. [DOI] [PubMed] [Google Scholar]
- 34.Ohashi N, Yamamoto T, Uchida C, Togawa A, Fukasawa H, Fujigaki Y, et al. Transcriptional induction of Smurf2 ubiquitin ligase by TGF-beta. FEBS Lett. 2005;579:2557–2563. doi: 10.1016/j.febslet.2005.03.069. doi:10.1016/j.febslet.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 35.Denissova NG, Pouponnot C, Long J, He D, Liu F. Transforming growth factor beta-inducible independent binding of SMAD to the Smad7 promoter. Proc Natl Acad Sci USA. 2000;97:6397–6402. doi: 10.1073/pnas.090099297. doi:10.1073/pnas.090099297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stopa M, Anhuf D, Terstegen L, Gatsios P, Gressner AM, Dooley S. Participation of Smad2, Smad3, and Smad4 in transforming growth factor beta (TGF-beta)-induced activation of Smad7. The TGF-beta response element of the promoter requires functional Smad binding element and E-box sequences for transcriptional regulation. J Biol Chem. 2000;275:29308–29317. doi: 10.1074/jbc.M003282200. doi:10.1074/jbc.M003282200. [DOI] [PubMed] [Google Scholar]
- 37.von Gersdorff G, Susztak K, Rezvani F, Bitzer M, Liang D, Bottinger EP. Smad3 and Smad4 mediate transcriptional activation of the human Smad7 promoter by transforming growth factor beta. J Biol Chem. 2000;275:11320–11326. doi: 10.1074/jbc.275.15.11320. doi:10.1074/jbc.275.15.11320. [DOI] [PubMed] [Google Scholar]
- 38.Tang LY, Yamashita M, Coussens NP, Tang Y, Wang X, Li C, et al. Ablation of Smurf2 reveals an inhibition in TGF-beta signalling through multiple mono-ubiquitination of Smad3. EMBO J. 2011;30:4777–4789. doi: 10.1038/emboj.2011.393. doi:10.1038/emboj.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HT, Kim YH, Nam JW, Lee HJ, Rho HM, Jung G. Study of 5′-flanking region of human Cu/Zn superoxide dismutase. Biochem Biophys Res Commun. 1994;201:1526–1533. doi: 10.1006/bbrc.1994.1877. doi:10.1006/bbrc.1994.1877. [DOI] [PubMed] [Google Scholar]
- 40.Molla M, Gironella M, Salas A, Closa D, Biete A, Gimeno M, et al. Protective effect of superoxide dismutase in radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2005;61:1159–1166. doi: 10.1016/j.ijrobp.2004.11.010. doi:10.1016/j.ijrobp.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka M, Mokhtari GK, Terry RD, Balsam LB, Lee KH, Kofidis T, et al. Overexpression of human copper/zinc superoxide dismutase (SOD1) suppresses ischemia-reperfusion injury and subsequent development of graft coronary artery disease in murine cardiac grafts. Circulation. 2004;110:II200–II206. doi: 10.1161/01.CIR.0000138390.81640.54. [DOI] [PubMed] [Google Scholar]
- 42.Lijnen P, Petrov V, van Pelt J, Fagard R. Inhibition of superoxide dismutase induces collagen production in cardiac fibroblasts. Am J Hypertens. 2008;21:1129–1136. doi: 10.1038/ajh.2008.242. doi:10.1038/ajh.2008.242. [DOI] [PubMed] [Google Scholar]
- 43.Vozenin-Brotons MC, Sivan V, Gault N, Renard C, Geffrotin C, Delanian S, et al. Antifibrotic action of Cu/Zn SOD is mediated by TGF-beta1 repression and phenotypic reversion of myofibroblasts. Free Radic Biol Med. 2001;30:30–42. doi: 10.1016/s0891-5849(00)00431-7. doi:10.1016/S0891-5849(00)00431-7. [DOI] [PubMed] [Google Scholar]
- 44.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. doi:10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- 46.Abraham DJ, Shiwen X, Black CM, Sa S, Xu Y, Leask A. Tumor necrosis factor alpha suppresses the induction of connective tissue growth factor by transforming growth factor-beta in normal and scleroderma fibroblasts. J Biol Chem. 2000;275:15220–15225. doi: 10.1074/jbc.275.20.15220. doi:10.1074/jbc.275.20.15220. [DOI] [PubMed] [Google Scholar]
- 47.Richiert DM, Ireland ME. Matrix metalloproteinase secretion is stimulated by TGF-beta in cultured lens epithelial cells. Curr Eye Res. 1999;19:269–275. doi: 10.1076/ceyr.19.3.269.5316. doi:10.1076/ceyr.19.3.269.5316. [DOI] [PubMed] [Google Scholar]
- 48.Lin SW, Lee MT, Ke FC, Lee PP, Huang CJ, Ip MM, et al. TGFbeta1 stimulates the secretion of matrix metalloproteinase 2 (MMP2) and the invasive behavior in human ovarian cancer cells, which is suppressed by MMP inhibitor BB3103. Clin Exp Metastasis. 2000;18:493–499. doi: 10.1023/a:1011888126865. doi:10.1023/A:1011888126865. [DOI] [PubMed] [Google Scholar]
- 49.Wiercinska E, Naber HP, Pardali E, van der Pluijm G, van Dam H, ten Dijke P. The TGF-beta/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Cancer Res Treat. 2011;128:657–666. doi: 10.1007/s10549-010-1147-x. doi:10.1007/s10549-010-1147-x. [DOI] [PubMed] [Google Scholar]
- 50.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. doi:10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 51.Zinkevich NS, Gutterman DD. ROS-induced ROS release in vascular biology: redox–redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647–H653. doi: 10.1152/ajpheart.01271.2010. doi:10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.