Abstract
Animals reared in an enriched environment are less vulnerable to abuse-like behavior and exhibit less persistent drug seeking, perhaps due to a decrease in the incentive value of stimuli associated with reward. The present study investigated the effects of environmental enrichment on Pavlovian conditioned approach (PCA) performance, a measure of incentive salience attribution. Rats were first reared from postnatal day 21 to postnatal day 51 in either an enriched environment with large cages, social cohorts and novel objects, or in an isolated environment with small, hanging cages, no social cohorts and no novel objects. Rats were then trained on a PCA task for 5 consecutive days, where a retractable lever was predictive of a food reward. Isolated rats predominantly exhibited sign-tracking responses directed toward the reward-predicted lever (indicative of incentive salience attribution), while enriched rats predominantly exhibited goal-tracking responses directed toward the location of food delivery. Both groups learned their respective response type at equal rates. The results indicate that environmental enrichment reduces the readiness to attribute incentive value to reward-associated cues, which may explain the enrichment-induced protection against addiction-like behaviors.
Keywords: enrichment, sign tracking, goal tracking, incentive salience, autoshaping
Incentive salience speaks to the notion that stimuli consistently and contiguously paired with reward garner motivational value themselves, making them “wanted” stimuli and “motivational magnets” [19]. Incentive salience attribution to reward-associated stimuli has been suggested as a mechanism underlying individual differences in the development and persistence of addictive behavior [18, 19, 26]. In support of this hypothesis, relative to rats that do not attribute incentive salience to a reward-associated cue, rats prone to attribute incentive salience show greater cocaine-induced psychomotor sensitization [7], faster acquisition of cocaine self-administration [2], higher breakpoints and cocaine infusions under a progressive ratio schedule [21], and greater cocaine- and cue-induced reinstatement of cocaine seeking [20, 21].
One method to protect against vulnerability to and persistence of addiction-like behavior in animals is environmental enrichment [22, 24]. Relative to rats reared in an isolated condition (IC), rats reared in an enriched condition (EC) are less likely to initiate drug self-administration [10, 12], are less willing to work for drug reinforcement [1, 11], show more rapid extinction of drug seeking [12, 23], and are less susceptible to both drug- [12] and cue-induced reinstatement, when carried out before [23] or after [25] initial drug exposure. Although much is known about the effects of enrichment on abuse-like behaviors, the effects of enrichment on the attribution of incentive salience to reward-associated cues is unknown. Given the established relationship between incentive salience and abuse-like behaviors, we investigated the effect of environmental enrichment on the attribution of incentive salience to a food-associated stimulus using a Pavlovian conditioned approach task (PCA).
Ten male Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN, USA) at postnatal day 21. Five rats were housed in an enriched environment and the other five in an isolated environment according to methods described by Gipson et al. [10]. Briefly, EC housing consisted of a large steel wire cage (122 × 61 × 45.5 cm), with a solid steel floor covered with pine bedding. An assortment of 14 hard plastic toys were arranged throughout the cage and changed daily. The objects varied in color, size and shape. EC Rats were housed together and handled daily. IC housing consisted of stainless steel hanging cages (17 × 24 × 20 cm). IC rats were single-housed without exposure to objects or handling. All rats were reared in their corresponding conditions and remained within these conditions throughout experimentation. All rats were housed in a temperature- and humidity-controlled colony room on a 12:12 h light-dark cycle. All rats had ad libitum access to food and water. All experimental protocols were in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
All behavioral training took place in operant conditioning chambers (ENV-008, MED Associates, St. Albans, VT, USA), housed within sound attenuation chambers (ENV-018M, MED Associates), and operated via a personal computer interface (SG-502, MED Associates). Each operant chamber was equipped with a 5 × 4.2 cm recessed food receptacle (ENV-200R2M, MED Associates) located on the response panel, 2 retractable levers (ENV-112CM, MED Associates) mounted 7.3 cm above the metal rod floor on either side of the food receptacle, two white cue lights (ENV-221M, MED Associates) mounted above each lever, and a house-light (ENV-227M, MED Associates) mounted at the top of the panel opposite to the response panel.
PCA was carried out according to methods based on Flagel et al. [6] and described by Beckmann et al. [2]. Briefly, all rats were trained initially to retrieve pellets (45 mg Noyes Precision Pellets; Research Diets, Inc., New Brunswick, NJ, USA), delivered via a food hopper (ENV-203, MED Associates), from the food receptacle for 2 consecutive days. Following consistent pellet retrieval, 8-s presentations of the retractable lever (counterbalanced for side across animals) were followed by lever retraction and non-contingent delivery of a single 45-mg food pellet into the receptacle. Each lever presentation was spaced by a 90-s variable time inter-trial interval (ITI) that began immediately after pellet delivery. Each session consisted of 25 lever insertion trials and the house-light was illuminated throughout each session. Sign-tracking responses were recorded as lever presses and goal-tracking responses were recorded as photo beam breaks via head-poke responses into the food receptacle. Training continued for 5 daily sessions.
The top panel of Fig 1 illustrates the average response rate (responses/second/trial) for both sign- and goal-tracking responses during conditioning trials and goal-tracking responses during the ITI for both EC and IC rats, collapsed over the 5 training sessions. A one-way ANOVA, with rearing condition as a between-subject variable, demonstrated that, IC rats emitted more sign tracking (F(1, 8) = 13.52, p < 0.05) and less goal tracking (F(1, 8) = 6.88, p < 0.05), relative to EC rats, with no differences between EC and IC rats in goal-tracking responses during the ITI (F(1, 8) = 2.24, p > 0.05). In addition, planned comparisons between sign- and goal-tracking responses during conditioning trials and goal-tracking responses during the ITI demonstrated that IC rats emitted more sign-tracking responses during the conditioning trial than goal-tracking responses during the ITI (t(24) = 3.34, p < 0.05), and EC rats emitted more goal-tracking responses during the conditioning trial than goal-tracking responses during the ITI (t(24) = 3.21, p < 0.05), indicating that both EC and IC rats learned their respective response types equally well.
Fig 1.
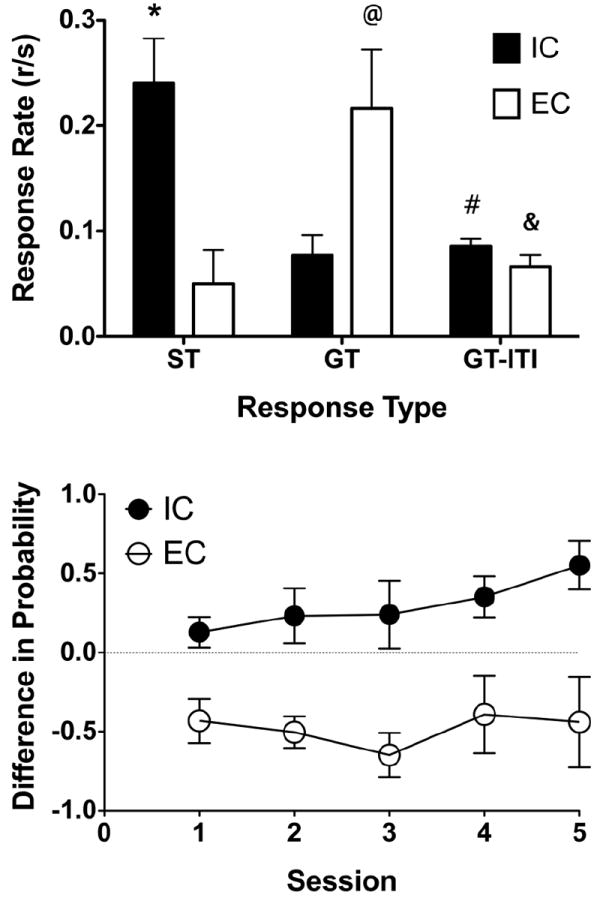
Top panel: Response rate (responses/sec) as a function of sign-tracking (ST) and goal-tracking (GT) responses during conditioning trials and GT responses during the ITI from EC rats (n = 5) and IC rats (n = 5) * = significant difference in ST between EC and IC rats; @ = significant difference in GT between EC and IC rats; # = significant difference between ST and GT-ITI for IC rats; & = significant difference between GT and GT-ITI for EC rats. p < 0.05. Bottom panel: Difference score (probability of a sign-tracking response – probability of a goal-tracking response) as a function of training session from EC and IC rats. IC rats expressed an overall greater propensity to sign track and EC rats a greater propensity to goal track (F(1, 8) = 15.57, p < 0.05).
The bottom panel of Fig 1 illustrates the difference between the probability of sign tracking and goal tracking (i.e., sign-tracking probability – goal-tracking probability; 1 = exclusive sign tracking; 0 = indifference; -1 = exclusive goal tracking) over the course of training for both EC and IC rats. A two-way, mixed-factors ANOVA, with rearing condition as a between-subject variable, and training session as a within-subject variable, demonstrated a significant main effect of rearing condition (F(1, 8) = 15.57, p < 0.05), but no significant main effect of training session or rearing condition × training session interaction, indicating that IC rats expressed an overall greater propensity to sign track and EC rats expressed a greater propensity to goal track.
Figure 2 illustrates the changes in conditioning trial sign-tracking rate (panel A), conditioning trial goal-tracking rate (panel B), ITI goal-tracking rate (panel C), and response ratio (panel D); response ratio was calculated as the probability of preferred response type during conditioning trials for IC (sign tracking) and EC (goal tracking) divided by the probability of responding (i.e., goal tracking) during the ITI. A two-way mixed-factors ANOVA with rearing condition as a between-subject variable and training session as a within-subject variable revealed that EC rats exhibited less sign-tracking behavior throughout training, relative to IC rats (F(1, 8) = 13.52, p < 0.05); sign tracking increased with training (F(4, 32) = 3.78, p < 0.05), and the overall difference between groups in sign tracking did not change with continued training. The same analysis demonstrated that EC rats exhibited more goal tracking throughout training (F(1, 8) = 7.57, p < 0.05), with no significant changes in goal tracking with continued training for either EC or IC rats. Additionally, goal tracking during the ITI decreased equally with continued training for both EC and IC rats (F(4, 32) = 7.87, p < 0.05). Finally, the same analysis indicated that response ratio increased equivalently over training (F(4, 32) = 9.63, p < 0.05) for both EC and IC rats, indicating that both groups learned their respective preferential response types at the same rate.
Fig 2.
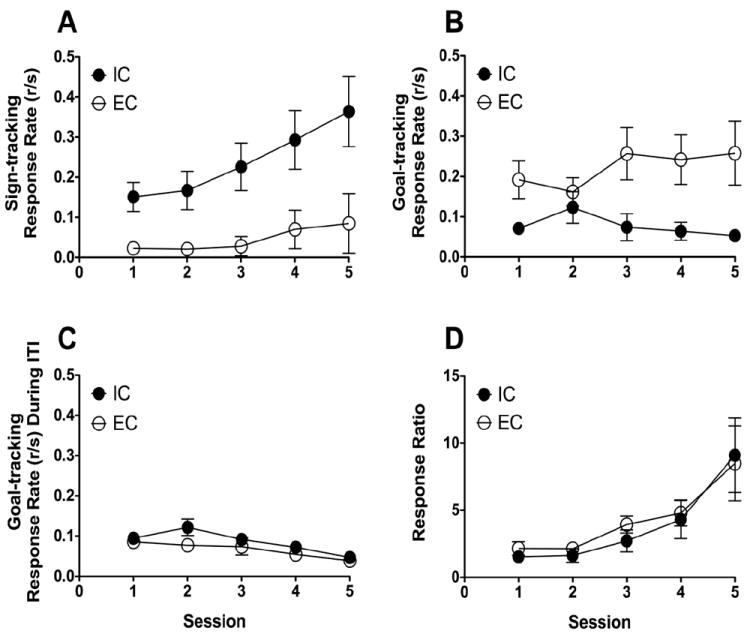
Top panel: A) Sign-tracking response rate (responses/sec) during conditioning trials as a function of training session for EC rats (n = 5) and IC rats (n = 5). EC rats exhibited less sign-tracking behavior, relative to IC rats (F(1, 8) = 13.52, p < 0.05). B) Goal-tracking response rate (responses/sec) during conditioning trials as a function of training session for EC and IC rats. EC animals exhibited more goal tracking, relative to IC rats (F(1, 8) = 7.57, p < 0.05). C) Goal-tracking response rate (responses/sec) during the intertrial interval (ITI) as a function of training session for EC and IC rats. D) Response ratio [probability of preferred response type during conditioning trials for IC (sign tracking) and EC (goal tracking) divided by the probability of responding (i.e., goal tracking) during the ITI] as a function of training session for EC and IC rats. *Significant difference from IC rats, p < 0.05.
Overall, the results of the present experiment indicate that environmental enrichment prevents the attribution of incentive salience to a reward related cue, without affecting learning about the same cue. Although past research has indicated that EC rats exhibit superior learning relative to IC rats [17], the present study found that response ratios increased equally over training for both EC and IC rats (Fig 2, panel D), indicating that both EC and IC rats learned their preferred response types (sign tracking for IC and goal tracking for EC) at the same rate. Thus, the primary difference observed here between EC and IC rats was in the readiness to attribute incentive salience to the reward-predictive cue.
In preclinical models, environmental enrichment has been demonstrated to have a protective effect against the initiation of abuse-like behavior [1, 11, 12, 23], the transition from regulated to dysregulated drug use [10], and the likelihood of relapse [23, 25]. Conversely, when rats from a random population are categorized as sign–trackers, rather than goal trackers, they more readily initiate self-administration [2], are more willing to work for drug reinforcement [21], and exhibit elevated relapse to drug seeking [20, 21]. Taken together, the protective effects of environmental enrichment could be mediated by its ability to reduce the attribution of incentive salience to reward-associated cues. The present finding that EC rats tend to goal track, while IC rats tend to sign track, supports this hypothesis.
The present result is part of a growing body of evidence suggesting that early developmental experience affects the readiness to attribute incentive salience to reward-related cues. For example, Lomanowska et al. [14] demonstrated that artificially reared rats attributed greater incentive salience to a food-related cue, relative to animals that were maternally reared. Interestingly, both the artificially reared animals in Lomanowska et al. [14] and the IC animals in the present experiment were reared without regular social interaction, and both of these groups demonstrated enhanced incentive salience attribution. Furthermore, lower basal corticosterone levels have been reported in EC animals [24], while sign trackers exhibit elevated corticosterone levels [8, 27]. These results suggest that early social experience may be crucial in the development and control of incentive salience attribution to reward-related stimuli through a reduction of hypothalamo-pituitary-adrenal (HPA) stress axis activity.
Environmental enrichment has a variety of effects on dopamine signaling within the mesocorticolimbic pathway, including increased total dopamine transporter (DAT) protein expression within the nucleus accumbens [Nac; 28], decreased DAT binding in the striatum [3], decreased DAT function and cell-surface expression in prefrontal cortex [29, 30], decreased D1 receptor expression in prefrontal cortex [5], and elevated drug-induced dopamine synthesis and metabolisim in Nac and striatum [4]. Sign trackers have reduced DAT and tyrosine hydroxylase mRNA expression in ventral tegmental area [6], reduced D2 receptor mRNA expression in Nac [6], and elevated dopamine and 3,4-dihydroxyphenylacetic acid (DOPAC) tissue levels in Nac [27]. Sign trackers also exhibit greater Nac DA release when exposed to the food-related cue during PCA training [9]. Thus, the effects of environmental enrichment and individual differences in incentive salience may be mediated by common dopaminergic mechanisms within the mesocorticolimbic pathway, and these differences may be responsible for their effects on differential vulnerability to abuse-like behavior.
In addition to dopamine, environmental enrichment is known to affect glutamatergic signaling within the mesocorticolimbic pathway. For example, EC rats have elevated mGluR2-mediated glutamatergic tone in prefrontal cortex [15]. Furthermore, Rahman and Bardo [16] demonstrated that, relative to IC rats, EC rats exhibit elevated drug-induced glutamate release in Nac. Given the role of glutamatergic tone in abuse-like behavior [13], the effects of environmental enrichment observed here may be mediated by differences in glutamate functioning. However, despite the importance of glutamatergic functioning within the mesocorticolimbic pathway, the role of such signaling in individual differences in incentive salience attribution is unknown.
In conclusion, environmental enrichment reduces the readiness to attribute incentive salience to a reward-related cue, measured by a reduction in sign-tracking behavior; this reduction in incentive salience attribution by enrichment may be mediated by its effects on dopaminergic signaling, as sign-tracking behavior has been demonstrated to be dopamine-dependent. Furthermore, the elevation of corticosterone levels in sign trackers suggests that activity of the HPA stress axis is involved in the attribution of incentive salience, and a reduction in HPA stress activity by environmental enrichment and other social experiences offers another possible mechanism by which enrichment reduces the incentive salience of reward-related cues. Finally, given that enrichment and individual differences in incentive salience attribution have been linked to differential abuse-like behavior, future research is needed to determine the role of other transmitter systems known to underlie abuse-related behavior, like glutamate.
Research Highlights.
Environmental enrichment reduced incentive salience attribution.
Enriched and impoverished animals learned their respective response types equally.
Enrichment may protect against addiction via reduction in incentive salience.
Acknowledgments
We thank Travis McCuddy for his technical assistance. This work was supported by NIH grants T32 DA007304, P50 DA05312 and R01 DA12964.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155(3):278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 2.Beckmann JS, Marusich JA, Gipson CD, Bard MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216(1):159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezard E, Dovero S, Belin D, Duconger S, Jackson-Lewis V, Przedborski S, Piazza PV, Gross CE, Jaber M. Enriched environment confers resistance to 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine and cocaine: involvement of dopamine transporter and trophic factors. J Neurosci. 2003;23(35):10999–11007. doi: 10.1523/JNEUROSCI.23-35-10999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32(9):885–893. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- 5.Del Arco A, Segovia G, Canales JJ, Garrido P, de Blas M, Garcia-Verdugo JM, Mora F. Environmental enrichment reduces the function of D1 dopamine receptors in the prefrontal cortex of the rat. J Neural Transm. 2007;114(1):43–48. doi: 10.1007/s00702-006-0565-8. [DOI] [PubMed] [Google Scholar]
- 6.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 7.Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186(1):48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role of dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gipson CD, Beckmann JS, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 2011;214(2):557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162(4):373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- 12.Green TA, Alinhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67(1):28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 14.Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res. 2011;220:91–99. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Melendez RI, Gregory ML, Bardo MT, Kalivas PW. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the preforntal cortex. Neuropsychopharmacology. 2004;29(11):1980–1987. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- 16.Rahman S, Bardo MT. Environmental enrichment increases amphetamine-induced glutamate neurotransmission in the nucleus accumbens: a neurochemical study. Brain Res. 2008;1197:40–46. doi: 10.1016/j.brainres.2007.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renner MJ, Rosenzweig MR. Enriched and impoverished environments: effects on brain and behavior. New York: Springer-Verlag; 1987. [Google Scholar]
- 18.Robinson TE, Berridge KC. The neural basis of drug craving: and incentiv-sensitization theory of addiction. Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TE, Berridge KC. Incentive sensitization theory of addiction: some current issues. Phil Trans R Soc B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67(8):730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36(8):1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92(4):572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Stairs DJ, Klein ED, Bardo MT. Effects of environmental enrichment on extinction and reinstatement of amphetamine self-administration and sucrose-maintained responding. Behav Pharmacol. 2006;17(7):597–604. doi: 10.1097/01.fbp.0000236271.72300.0e. [DOI] [PubMed] [Google Scholar]
- 24.Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92(3):377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL. Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol. 2009;12(9):1151–1156. doi: 10.1017/S1461145709990472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomie A, Tirado AD, Yu L, Pohorecky LA. Pavlovian autoshaping procedures increase plasma corticosterone and levels of norepinephrine and serotonin in prefrontal cortex in rats. Behav Brain Res. 2004;153:97–105. doi: 10.1016/j.bbr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163(2):890–897. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances senistization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148(1-2):107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93(6):1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]


