Abstract
Oxidative stress adaptation or hormesis is an important mechanism by which cells and organisms respond to, and cope with, environmental and physiological shifts in the level of oxidative stress. Most studies of oxidative stress adaption have been limited to adaptation induced by acute stress. In contrast, many if not most environmental and physiological stresses are either repeated or chronic. In this study we find that both cultured mammalian cells, and the fruit fly Drosophila melanogaster, are capable of adapting to chronic or repeated stress by up-regulating protective systems, such as their proteasomal proteolytic capacity to remove oxidized proteins. Repeated stress adaptation resulted in significant extension of adaptive responses. Repeated stresses must occur at sufficiently long intervals, however (12 hours or more for MEF cells and 7 days or more for flies), for adaptation to be successful, and the level of both repeated and chronic stress must be lower than is optimal for adaptation to acute stress. Regrettably, regimens of adaptation to both repeated and chronic stress that were successful for short-term survival in Drosophila, nevertheless also caused significant reductions in lifespan for the flies. Thus, although both repeated and chronic stress can be tolerated, they may result in a shorter life.
Keywords: Oxidative stress, Ubiquitin-Proteasome System, Protein Degradation, Repeated Stress, Chronic Stress, Stress Adaptation
Introduction
Aerobic organisms must live with a multitude of free radicals and related reactive oxygen/nitrogen species. Perhaps the most ubiquitous of these species is hydrogen peroxide (H2O2) which is found at measurable levels in all animal tissues. At nanomolar levels, H2O2 is a stimulant of cell growth and proliferation, whereas micromolar levels cause transient growth arrest and induce protective adaptive alterations in gene expression (1–4). At millimolar levels, and above, H2O2 is clearly a toxic oxidant, causing frank oxidative stress. One of the major results of such oxidative stress is modification of cellular proteins, which can block enzymatic activities, inhibit normal cell functions, and even cause cellular apoptosis or necrosis (5–13). As a primary defense against oxidative stress, cells possess a range of antioxidant compounds and enzymes which function to keep the levels of free-radicals and reactive oxidants low (14–17). Although these primary antioxidant defenses are sufficient under conditions of low or normal oxidant levels, they are overwhelmed during periods of oxidative stress. This results in oxidative protein damage which, if not removed quickly, can accumulate over time and cause deterioration of cell function. It is the role of proteases, such as the 20S Proteasome, the Immunoproteasome (3, 4, 18–28), and the mitochondrial Lon protease (29–32), (as part of a secondary antioxidant response system) to rapidly and selectively remove such damaged proteins, thus preventing their aggregation, cross-linking, and accumulation, as well as the cellular toxicity that would otherwise result.
The degree of oxidative stress which organisms are exposed to is not static but shifts with changes in environment, pollution, metabolism, diet, lifestyle, and age. Both eukaryotic cells and prokaryotes have highly dynamic responses to these changes in oxidative stress levels. It appears that all life forms can transiently adapt to temporary changes in stress levels, by a combination of direct enzyme activation/deactivation and alterations in the level of expression of more than a hundred genes (2–4, 33). Such transient adaptation is also called hormesis (34, 35). Mammalian cells, including humans, can shift their patterns of gene expression over an 18 hour period such that housekeeping genes, growth and proliferation are suppressed, while protective genes are overexpressed; the adapted cell is then significantly more resistant to oxidative stress. If the stress is removed or declines during this 18 hour period, cells will stay adapted only for another 6–12 hours (approximately 24–30 hours in all), after which they gradually de-adapt. Although there are many important enzymes that contribute to this adaptive response, we have previously demonstrated that increased synthesis of the 20S Proteasome, the Immunoproteasome, and the Lon protease is required for efficient clearance of oxidatively damaged proteins, and for greatest stress protection (2–4, 33).
Most of the work to date on transient adaptation to oxidative stress has involved experiments limited to a single acute stress exposure. In previous work, using a wide range of oxidants and cell types, we have described a pre-treatment and challenge model to assay oxidative stress adaptation (2–4). In this system cells were exposed to a single, mild, non-toxic, dose of an oxidant such as H2O2, then permitted to adapt for a period of time (typically 18–24 h). The cells were then exposed to a much higher dose of the oxidant, termed the Challenge dose, which would normally be toxic without the pretreatment and adaptive period. We found that pre-treated cells are considerably more resilient to the toxic challenge level of oxidative stress than are non-pretreated cells. In contrast, as discussed above, both metabolic and environmental sources of oxidative stress are highly variable, and can also occur repeatedly or even continuously for prolonged periods. Thus, it is important that we develop an understanding of stress-adaptive capacities under both repeated and chronic stress conditions that may better reflect real-life experiences. In this study we have examined the ability of both mammalian cells and the invertebrate model organism Drosophila melanogaster to adapt to oxidative stress under an acute oxidative stress exposure and compare it to a more environmentally representative stress of repeated and chronic oxidative stress exposure. In addition we examine the long term repercussions of such oxidative stress adaptation.
Results
Transient Adaptive Response to Acute H2O2 Pretreatment in MEF Cells
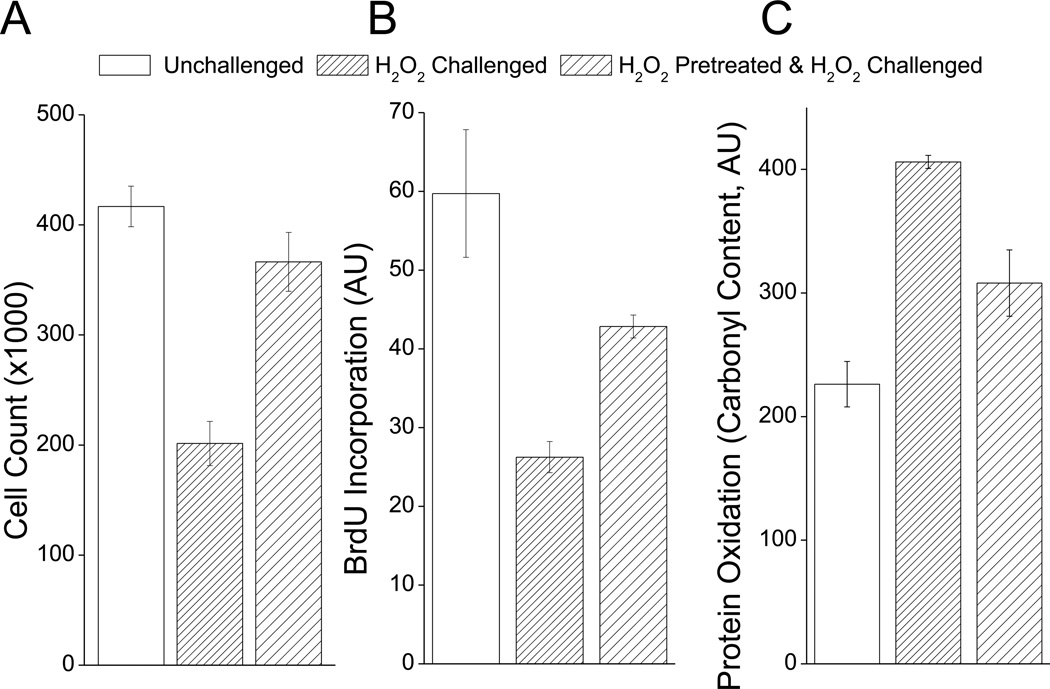
For baseline data on adaptation to a single H2O2 pretreatment, murine embryonic fibroblast (MEF) cells were incubated with 1nmol of H2O2 per 105 cells (1µM H2O2 final concentration), which was found to be the optimum pretreatment condition in preliminary experiments. Cells were then allowed to adapt for 24 h, then challenged for 1 h with 500µmol of H2O2 per 105 cells (1mM H2O2 final concentration) which was optimized to be sufficiently strong to cause almost complete growth arrest, yet with minimal apoptosis. Adaptation was then scored using a variety of techniques, including cell counts 24 h after challenge (Fig. 1A), and BrdU incorporation over the 24 h period following H2O2 challenge (Fig. 1B). In both of these experiments, H2O2 challenge caused a strong decrease in cell division which was partially prevented by H2O2 pretreatment. We next tested whether the pretreatment-induced resistance to H2O2 challenge involved less of an accumulation of oxidatively damaged proteins, as measured by a protein carbonyl assay. H2O2 challenge induced almost a doubling of protein carbonyl content, but this was cut in half if cells were first H2O2 pretreated 24 h before challenge (Fig. 1C). In previous reports, the proteolytic capacity of cellular 20S proteasome has been suggested to be important for surviving an oxidative stress (3, 4, 18, 33, 36, 37). It has been shown for instance that much of the adaptive increase in proteolytic capacity and oxidative stress tolerance is lost by blocking new synthesis of 20S proteasome (3, 4, 38, 39). We, therefore, tested whether the observed increase in oxidative stress tolerance was dependent on 20S proteasome. To do this we briefly exposed cells to siRNA against the β1 subunit of the 20S proteasome 48h prior to H2O2 pre-treatment. We found that this treatment completely abolished the H2O2 pre-treatment induced increase in cell counts (Fig. 1D), and BrdU incorporation (Fig. 1E), as well as the H2O2 pre-treatment induced decrease in protein carbonyl content (Fig. 1F).
Fig. 1. H2O2 Pretreatment Increases Tolerance to H2O2 Challenge.
H2O2 pretreatment enhances resistance to subsequent H2O2 challenge using three different measures of oxidative stress tolerance (Cell counts, BrdU incorporation and protein carbonyl content). In all cases, MEF wells were grown to 10% confluence then some samples were pre-treated with a mild adaptive dose of H2O2 which was 1nmol of H2O2 per 105 cells (1µM H2O2 final concentration) for 1 h. Cells were then challenged 24 h later with a toxic dose of H2O2 which was 500µmol of H2O2 per 105 cells (1mM H2O2 final concentration) for 1 h. A. After challenge, cells were permitted to divide for 24 h then cell counts were taken. B. After H2O2 challenge BrdU was added to cell cultures, cells were permitted to divide for 24 h then BrdU incorporation was measured. C. Protein carbonyl content was measured 6 h after H2O2 challenge. D-E. Cells were treated with either Scrambled or β1 siRNA for 48h then pretreated and assayed as in A-C. In all cases values represent means ± SE, where n = 3.
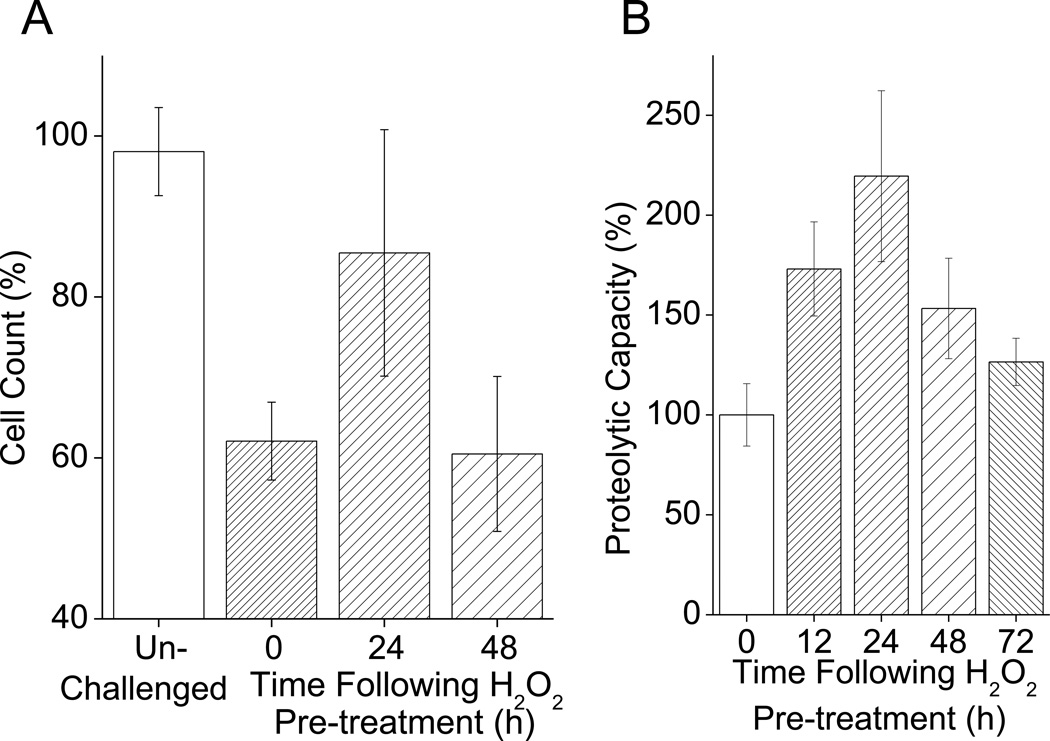
We reasoned that the adaptive response to oxidative stress should be transient, and that oxidative stress tolerance should return back down to normal levels once the stimulatory effect of the H2O2 pretreatment had abated. To test this we ran a cell count time course for oxidative stress tolerance at for up to 48h after H2O2 pre-treatment. Our results show that the adaptive response was indeed transient and reversible over a 48h period (Fig. 2A). At 24 h after pre-treatment, cells had a heightened oxidative stress tolerance, but 48 h after pre-treatment their resistance had returned to baseline levels. Because of the important role of proteolysis in oxidative stress adaptation we also measured proteolytic capacity. To test this, in the current model, we measured proteolytic capacity against the fluoropeptide Suc-LLVY-AMC, which is commonly used as a marker for the chymotrypsin-like activity of the proteasome. We found that proteolytic capacity gradually increased in the first 24h following H2O2 pretreatment, to more than double baseline levels, but then (like overall H2O2 tolerance), it declined over the following 48 h (Fig. 2B).
Fig. 2. H2O2 Pretreatment Causes a Transient Adaptive Response.
H2O2 pretreatment transiently enhances resistant to subsequent H2O2 challenge and increases cellular proteolytic capacity, but cells return to a naïe state 48–72 h after H2O2 pretreatment. A. With H2O2 pretreatment oxidative stress tolerance increases for 24 h, then returns to baseline levels. Cells were prepared and pre-treated as in Fig. 1. Cells were then challenged either 24 or 48 h after pretreatment. Cell counts were taken 24 h after challenge. Values are plotted as a percent of the cell counts of samples which were not exposed to the challenge and are shown as means ± SE, where n = 4. The values for unchallenged samples and 0 h pretreated samples, represent data pooled from cell counts taken 24 and 48 h after pre-treatment. B. With H2O2 pre-treatment, proteolytic capacity increases in the 24 h after pre-treatment but returns to baseline levels over the succeeding 72 h. Cells were prepared and pretreated as in Fig. 1. Samples were then collected 12, 24, 48 and, 72 h after H2O2 pretreatment. Samples were then lysed and proteolytic capacity determined through degradation of Suc-LLVY-AMC. Values are means ± SE, where n = 3. The values for 0 h pretreated samples represent the activity of non pretreated cells taken 12 h after H2O2 pretreatment.
Adaptive Responses to Multiple H2O2 Pretreatments in MEF Cells
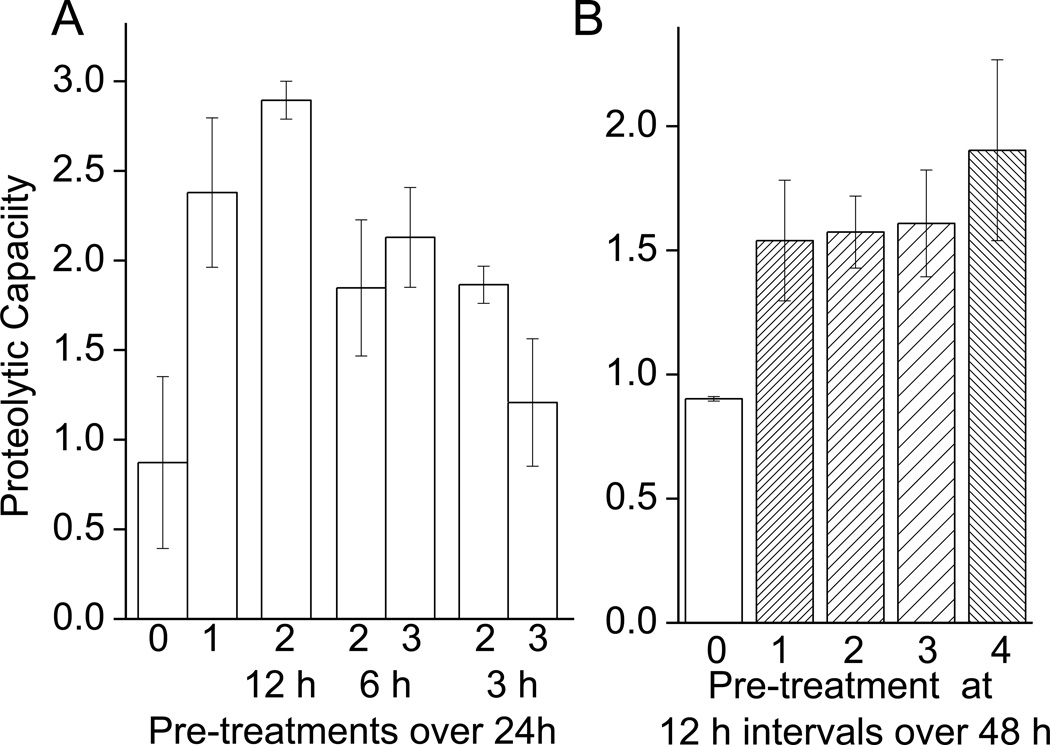
From the results of Figs. 1 and 2, we conclude that a mild, acute H2O2 pretreatment transiently improves oxidative stress tolerance. As pointed out in the Introduction, however, environmental and metabolic oxidative stress exposures are often repeated or even chronic in nature. To begin experiments of changes in proteolytic capacity under repeated stress, we first exposed cells to the adaptive dose of 1nmol of H2O2 per 105 cells and measured proteolytic capacity 24 h later, through degradation of the chymotrypsin like substrate Suc-LLVY-AMC (Fig. 3A). Repeated oxidative stress was then initiated by exposing cells to the adaptive dose of H2O2 a second, or even a third, time after the initial exposure, at intervals of 3, 6 or 12 h after the initial exposure. We observed that a single pretreatment caused an increase in proteolytic capacity. If cells were pre-treated for a second time 12 h after the first treatment, the second pre-treatment was tolerated and proteolytic capacity actually increased slightly more than with a single pretreatment. If, however, samples were pretreated a second or even a third time within 6 h of the initial pretreatment then the proteolytic capacity, was lower than that seen with a single pre-treatment, but still greater than that of naïe (non-pretreated) cells. When cells were given second and third pretreatments at only 3 h intervals, the adaptation was clearly strongly limited (Fig. 3A).
Fig. 3. Capacity to Degrade Suc-LLVY-AMC Following Repeated H2O2 Pretreatments.
A. Additional H2O2 treatments 3 or 6 h following the initial treatment, blunt the adaptive increase in proteolytic activity, while repeated treatments 12 h after the initial treatment seem to be tolerated. Cells were prepared and pretreated as in Fig. 1. Some cells were then pretreated with the same dose of H2O2 up to two additional times after the initial pretreatment. These subsequent pretreatments were performed at 3, 6 or 12 h intervals after the initial pretreatment. Cells were harvested 24 h after initial treatment. Samples were then lysed and proteolytic capacity measured through degradation of Suc-LLVY-AMC. Values are means ± SE, where n = 3. B. Up to 3 additional treatments of H2O2 can be performed on cells with no reduction in the adaptive increase in proteolytic capacity, provided a 12 h recovery time is permitted between treatments. Cells were prepared and pre-treated as in Fig. 1. Some cells were then pretreated with the same dose of H2O2 for up to three additional times after the initial pretreatment at 12 h intervals. Cells were harvested 48 h after initial treatment. Samples were then lysed and proteolytic capacity measured through degradation of Suc-LLVY-AMC. Values are means ± SE, where n = 3. Activity is plotted as μmol of AMC released per minute per gram of lysate.
Having seen that a second pretreatment 12 h after the initial pretreatment was tolerated we wished to test if further 12 h interval pretreatments would also be tolerated, or if there would be a limit to the number of 12h pretreatments that could be borne; in essence, therefore, we actually studied the effects of chronically repeated stress by exposing cells to an adaptive dose of H2O2, then pretreating the cells a further 1–3 times at 12 h intervals. At 48 h after the first pretreatment all cells were harvested and proteolytic capacity measured by degradation of Suc-LLVY-AMC. Our results show that even 4 pre-treatments at adaptive dose of 1nmol of H2O2 per 105 cells were tolerated, and produced similar and consistent increases in proteolytic capacity (Fig. 3B). It should be noted that the results of Fig. 3A were obtained in experiments performed 24h after initial H2O2 pretreatment, whereas the results of Fig. 3B were obtained 48h after initial pretreatment. As shown in Fig. 2A, the adaptive effects of a single pretreatment are lost within 48h, thus the 12h interval repeated pretreatments actually extended the adaptation.
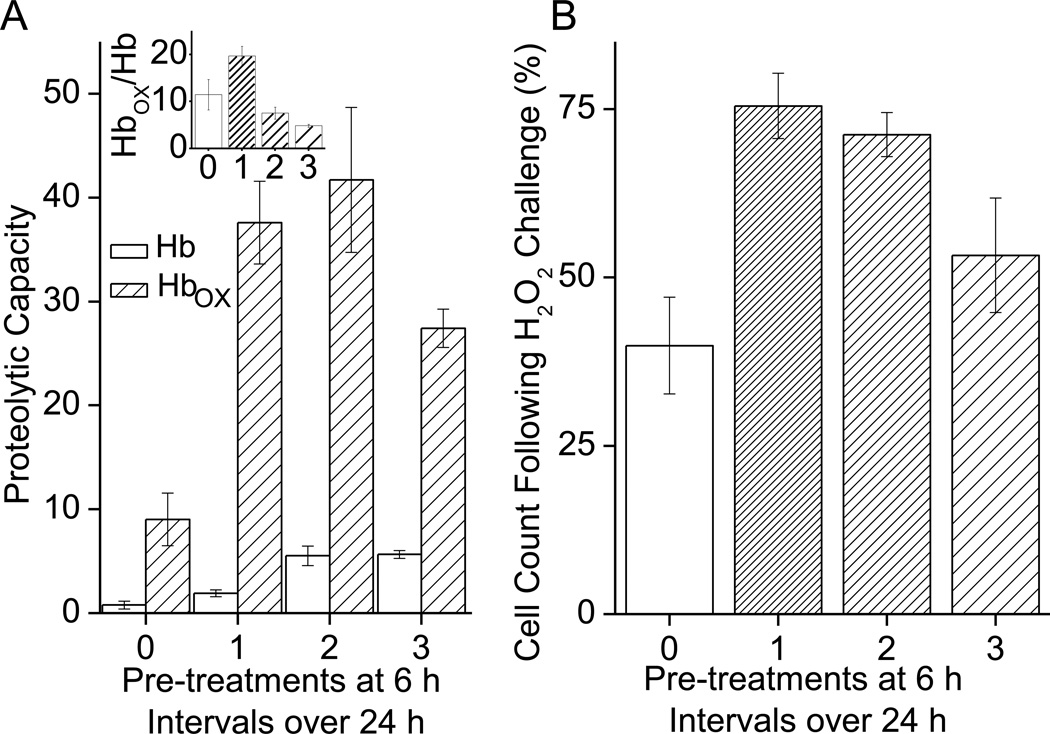
In contrast to the 12h-interval results of Fig 3B, the results of Fig 3A clearly showed that H2O2 treatments at 3h intervals had detrimental effects on adaptation, but the 6h interval data were somewhat equivocal. We decided to further study the effects of multiple 6h interval H2O2 treatments on the capacity of cells to degrade an actual protein (rather than the Suc-LLVY-AMC peptide model substrate) and for this we used both normal and oxidized hemoglobin (Hb), as previously described (3, 4, 18, 40). The capacity of cell extracts to selectively degrade oxidized Hb (Hbox) rose after a single H2O2 pretreatment and was unaffected by a second pretreatment, but was reduced with a third successive H2O2 pretreatment (Fig. 4A). Interestingly, the capacity to degrade native Hb plateaued after two pretreatments, but did not decline even after the third successive treatment. Thus, the selective, preferential degradation of Hbox over that of native Hb actually declined significantly after both the 2nd and 3rd H2O2 pretreatments (Fig. 4A Inset). We also tested tolerance to H2O2 stress, in the same cells, using our pretreatment/challenge model described in Figs. 1 and 2. We observed an increase in oxidative stress tolerance with a single pretreatment, which progressively declined with subsequent pretreatments (Fig. 4B). From this we can conclude that repeated H2O2 pretreatments within in a short time frame (6 h) not only reduced the adaptive increase in proteolytic capacity to degrade oxidized proteins, but also diminished the adaptive increase in oxidative stress tolerance.
Fig. 4. Adaptive Effects of H2O2 Pretreatments at 6h Intervals.
A. The blunting of adaptive response with 6 h repeated H2O2 treatments appears to not just reduce proteolytic capacity, but also to reduce the adaptive increase in capacity to selectively degrade oxidized proteins. Cells were prepared and pretreated as in Fig. 1. Some cells were then pretreated with the same dose of H2O2 up to three additional times after the initial pretreatment, at 6 h intervals. Cells were harvested 24 h after initial treatment. Samples were then lysed and proteolytic capacity measured through degradation of Hb-AMC or oxidized Hb-AMC (Hbox-AMC) as substrates. Values are means ± SE, where n = 6. The Inset to Panel A shows the ratio of degradation of Hbox divided by degradation of Hb, and reveals that the selective degradation of oxidized Hb actually declined significantly after both the 2nd and 3rd H2O2 pretreatments. Activity is plotted as nmol of AMC released per minute per gram of lysate. B. Repeated stress at 6 h intervals appears to blunt the adaptive increase in oxidative stress tolerance as well as blunting the increase in proteolytic capacity. Cells were prepared and treated as in A, Cells were then challenged with a toxic dose of H2O2 which was 500µmol of H2O2 per 105 cells (1mM H2O2 final concentration) 24 h after pre-treatment. Cell counts were taken 24 h after H2O2 challenge. Values are plotted as a percent of the cell counts of samples which were not exposed to the challenge and are shown as means ± SE, where n = 6. Values are plotted as % of unchallenged controls.
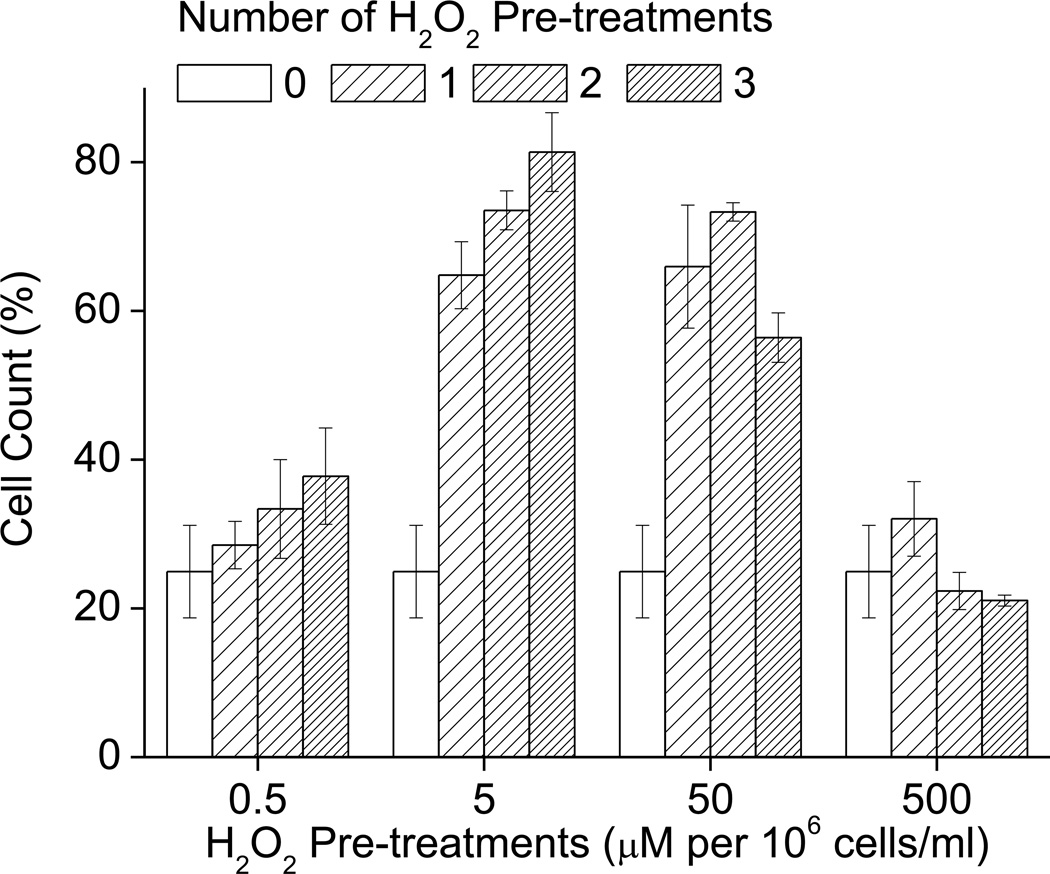
Having found that repeated H2O2 pretreatments at 3h or 6h intervals were detrimental to the adaptive response (Figs 3A and 4AB), but that pretreatments at 12h intervals still allowed a full adaptive increase in proteolytic capacity, we wanted to determine if pretreatments at 12h intervals would also allow a full adaptive increase in tolerance to severe H2O2 challenge. In addition we were also interested in whether the concentration of H2O2 used in the repeated stresses would affect the response; in particular, we wanted to know whether lowering the pretreatment concentration might mitigate against the negative effects of repeated treatments. We found that the original adaptive dose of 1.0nmol H2O2 per 105 cells did not diminish the increase in tolerance to oxidative stress for the first 2 treatments, and had a only a weakly detrimental effect following the 3rd treatment. When we used lower H2O2 pretreatments (e.g. 10–100pmol of H2O2 per 105 cells) than the normally optimal adaptive dose (of 1nmol of H2O2 per 105 cells) the repeated treatments actually exerted a progressively beneficial effect on oxidative stress tolerance. Conversely, H2O2 treatments higher (e.g. 10nmol of H2O2 per 105 cells) than the normal adaptive dose had a strongly detrimental effect on oxidative stress tolerance (Fig. 5). From these results we conclude that repeated H2O2 pretreatments are permissive of oxidative stress adaptation as long as the interval of repeated treatment is 12 hours or more, or if the level of H2O2 pretreatment is lowered.
Fig. 5. Adaptive Effects of H2O2 Pretreatment at 12 h Intervals.
The adaptive increase in oxidative stress tolerance is not affected by repeated H2O2 treatments at the adaptive dose if it is performed at 12 h intervals. If, however, sub-adaptive treatments are performed, they become more effectively adaptive with repeated treatment. If higher pretreatment doses are used then repeated pretreatments have a detrimental effect on oxidative stress tolerance. Cells were grown to 10% confluence then pre-treated with different doses of H2O2 at 0 h, 12 h, 24 h and 36 h after initial treatment. Samples were then challenged with 5mmol of H2O2 per 106 cells (1mM H2O2 final concentration) for 1 h, 24 h later cell counts were taken. Values are plotted as a percent of the cell counts of samples which were not exposed to the challenge and are shown as means ± SE, where n = 4.
Role of the Proteasome in Oxidative Stress Adaptation
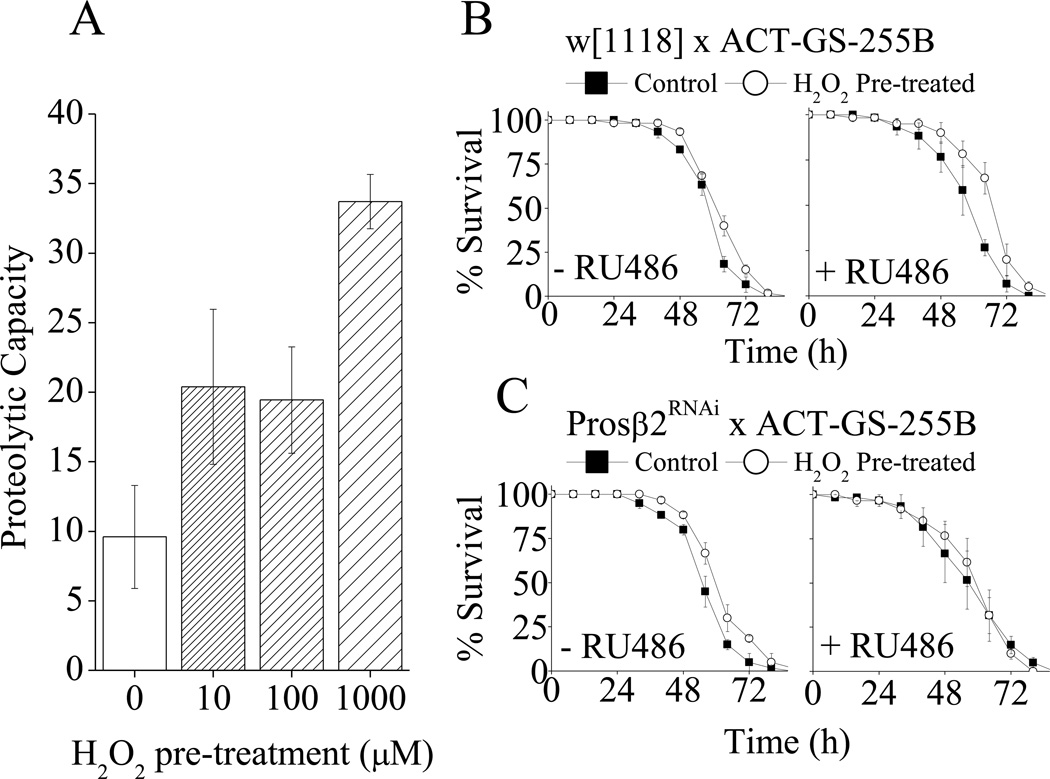
We have previously shown in mammalian cells (3, 4, 33, 41) that the adaptive response to a mild oxidant pretreatment is strongly dependent on Nrf2-induced increases in expression of the 20S proteasome, and the proteasome regulators Pa28αβ and Pa28γ. There have been several reports that mild oxidant exposure of the model organism Drosophila melanogaster the common fruit-fly) will also cause an adaptive increase in oxidative stress tolerance (Fig. 6C and (39, 42–44)). It was, therefore, important to test whether, as in mammalian cells, a mild H2O2 pre-treatment would lead to an increase both in proteolytic capacity and oxidative stress in fruit-flies. Although we have recently investigated this question in both flies and worms (with positive results), the reviewers of the current paper (and the journal’s associate editor) specifically wanted to us to test again for a causal relationship between increased proteasome-dependent proteolytic capacity and increased oxidative stress resistance under the exact conditions of the current experiments. To test this we pre-treated flies with various concentrations of H2O2 pretreatments (as done previously (39, 43)) then measured proteolytic capacity 18 h later. We observed that H2O2 pretreatment caused a 2–4 fold increase in proteolytic capacity (Fig. 6A).
Fig. 6. Adaptation of Drosophila melanogaster to Acute H2O2 Pre-treatment.
A. H2O2 treatment of D. melanogaster increases proteolytic capacity in females. Triplicate vials of w[1118] flies were pre-treated by exposure to ½ a Kim-wipe© soaked in 1ml of 5% sucrose plus the indicated concentrations of H2O2. Proteolytic capacity assays were performed on flies 24 h later as described in materials & methods. Values are shown as nmol of AMC released per min per mg lysate. B - C. H2O2 treatment of D. melanogaster increases oxidative stress tolerance which is lost with knock-down of the proteasome subunit prosβ2. (B) (♂) w[1118] × (♀) Act-GS-255B or (C) (♂)Prosβ2RNAi × (♀) Act-GS-255B Drosophila. In all cases flies were cultured in ± RU486 for 2 days then pre-treated ± 100µM H2O2 (±RU486) for 24 h. After pre-treatment, flies were returned to ± RU486 vials for 16 h then challenged by exposure to ½ a Kim-wipe© soaked in 1ml of 5% sucrose plus 4.4M H2O2. Values are plotted as means ± SE where n = 3.
Next we wished to test if the H2O2 induced increase in proteolytic capacity was dependent upon increases in proteasome, as seen with mammalian cell cultures. This was determined using an RNAi line against the prosβ2 20S proteasome subunits). This line was crossed to the Act-GS-255B strain (45) enabling the RNAi to be conditionally expressed in the presence of the drug RU486 in all the somatic tissues of the adult fly (46). Additionally, the Act-GS-255B strain was crossed with w[1118] flies as controls for potential effects of RU486. The female progeny of these crosses were then cultured ± RU486 for 2 days. Flies were then pre-treated ± 100µM H2O2 for 8 h, and then returned to vials ± RU486 for a further 16 h. Flies were then challenged with by incubation in vials containing kimwipes soaked in 1.0 ml of 5% sucrose plus 4.4M H2O2. We found that H2O2 pretreatment caused an increase in oxidative stress tolerance in control flies which was lost with proteasome RNAi knock-down (Fig 6B,C). The role of H2O2 and proteasome in oxidative stress adaption in flies is explored in more detail in previously published work (39). It should be noted that the experiments of Fig. 6 are confirmatory, not duplicative, of our previous recent results (39).
Adaptive Responses to Multiple H2O2 Pretreatments in Drosophila melanogaster
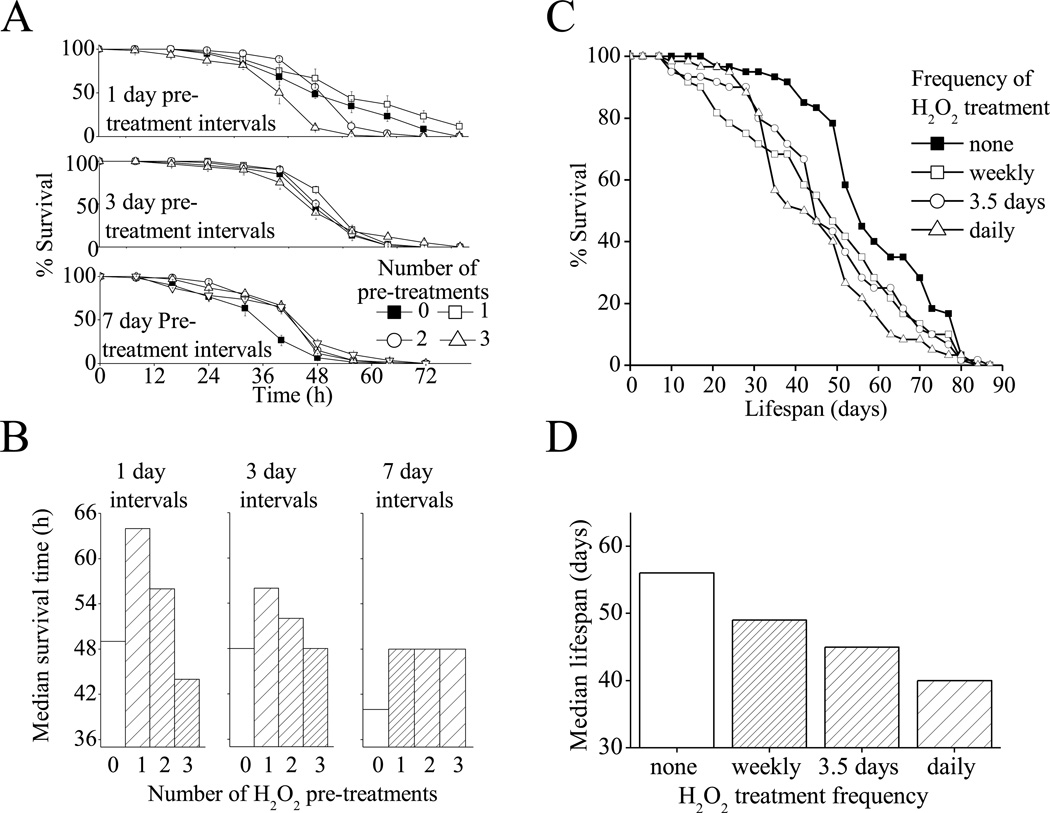
We next wanted to use the Drosophila model of oxidative stress adaptation to test if repeated stress has similar effects at the organismal level as it does at the cellular level. To test this we performed repeated stress adaptation by exposing flies to 8h treatments of the adaptive dose of H2O2, 0, 1, 2, or 3 times. These treatments were performed at 1 day, 3 day, or 7 day intervals. Challenge was performed 16 h after the end of the final pretreatment. In all cases, flies exposed to a single pre-treatment did notably better than control (non-pretreated) flies following the H2O2 challenge (Fig. 7A,B). With a second pre-treatment just one day after the first treatment, however, all of the adaptive benefit was lost, and if a third treatment was given after just one more day the pretreated flies did much worse than controls. Even if three day intervals were allowed between pretreatments the flies exposed to a 2nd or 3rd pretreatment still had a severely blunted adaptive response. Finally, if a 7 day interval was allowed between pretreatments there was no notable difference between flies pretreated once, twice or three times (Fig. 7A,B). From these results it is apparent that for whole flies, as for cultured mammalian cells, multiple pretreatments can be tolerated and still enable a full adaptive response, only with a sufficiently long recovery time between treatments.
Fig. 7. D.melanogaster Adapation to H2O2 Challenge Under Repeated H2O2 Pre-treatment.
A. Like cultured mammalian cells, H2O2 pre-treatment increases Drosophila oxidative stress tolerance and this adaptation is blunted by repeated treatments, though the repeated treatments are tolerated if a sufficiently long recovery period (7 days) is permitted. Experiments were performed using vials of 20, 4–6 day old female w(1118) flies which were cultured in vials containing normal food. Flies were incubated in vials containing ½ a Kim-wipe© soaked in 1ml of 5% sucrose plus 100µM H2O2 0–3 times for 6 h intervals. These pre-treatments were preformed either every day, every 3 days or every 7 days. Then, 1 day after the final pretreatment, all flies were challenged by exposure to ½ a Kim-wipe© soaked in 1ml of 5% sucrose plus a toxic dose of H2O2 (4.4M). Flies were maintained in these vials and survival was scored ever 8 h. Values are plotted as a percent of population before challenge, and are shown as means ± SE, where n = 3. B. Shows values from A plotted as median survival time following initiation of H2O2 challenge, where n = 60. C. Repeated treatment, even when a long recovery time is permitted, appears to reduce fly lifespan. Flies were cultured in vials containing normal food. Flies were then transferred every day, every 3.5 days or every 7 days, to vials containing ½ a Kim-wipe© soaked in 1ml of 5% sucrose plus 100µM H2O2 for 8 h intervals. Fly survival was counted every 3.5 days and treatments were continued until all flies were dead. D. Shows values from C plotted as median survival time following initiation of H2O2 challenge, where n = 60.
In Fig. 7A,B. the assays in all cohorts were started 4–6 days after maturation. Because of the difference in pretreatment intervals, however, flies in the “1 day pre-treatment interval” cohort were challenged at 7–9 days after maturation, while flies in the “3 day pre-treatment interval” cohort were challenged at 13–15 days after maturation, and flies in the “7 day pre-treatment interval” cohort were challenged at 25–27 days after maturation. As has been shown previously (47) there is an age-dependent decline in oxidative-stress-tolerance over the time frame we studied. We also saw this age-associated decline in oxidative-stress-tolerance, with 7–9 day old flies surviving an average of 54 h following H2O2 challenge, while 25–27 day old flies only survived an average of 38 h following H2O2 challenge: a 30% decline in baseline stress tolerance. The older flies were able to mount a significant adaptive response to H2O2 pretreatment but even the most adapted older flies still did not quite reach the median survival times exhibited by the control young flies (compare the right panel of Fig. 7B with the control value in the left panel of Fig. 7B). This suggests that perhaps one of the factors contributing to age-associated deterioration might be a reduced ability to respond to changes in stresses, such as oxidative stress.
In addition to acute oxidative stress tolerance, were also interested in whether repeated oxidative stress adaptation had any effects on the eventual lifespan of flies. We considered this to be something of a measure of the long-term repercussions of oxidative stress adaptation. To explore this question we performed lifespan assays on flies that were repeatedly exposed to oxidative stress. In these lifespan assays, flies were either maintained as naïe (control) flies, or were exposed to 8h treatments of the same dose of H2O2, used for oxidative stress adaptation experiments above, either weekly, twice-weekly, or daily (Fig. 7C,D). In these experiments we found that flies pretreated weekly, twice-weekly, or daily, all had a shorter lifespan than naïe control flies, although the degree of reduction in lifespan was roughly proportional to the frequency of exposure, with flies treated daily living the shortest time.
Chronic H2O2 Adaptation in MEF Cells
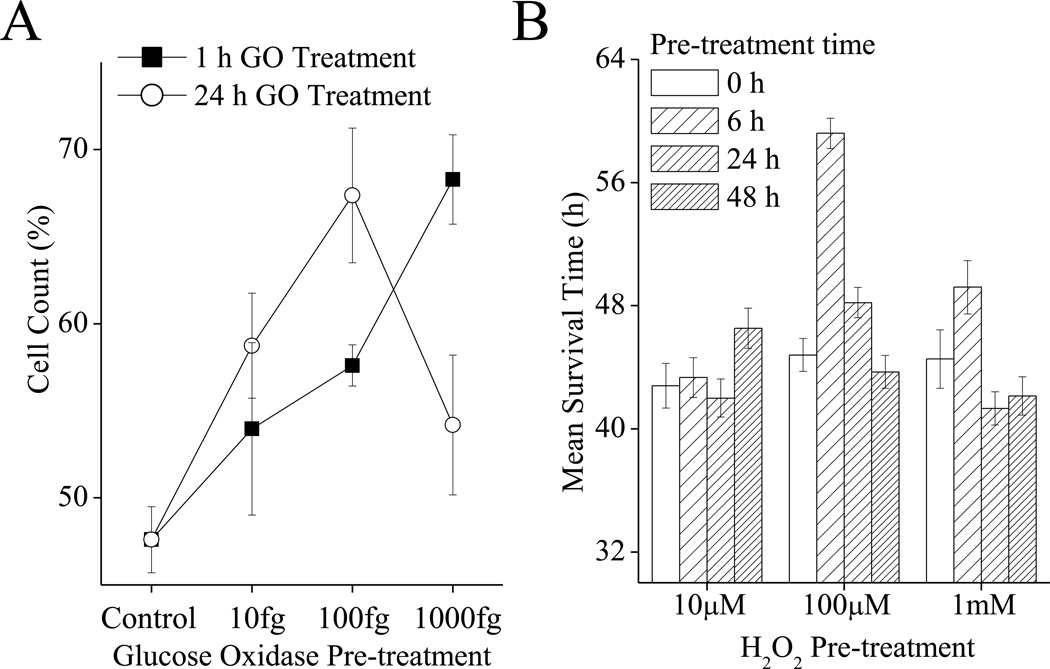
In addition to studying repeated oxidative stress adaptation, a limited set of experiments were performed under chronic oxidative stress conditions. Models of chronic H2O2 stress are particularly difficult since H2O2, and many other oxidants, are rapidly degraded by cells. Thus, it is not possible to use a single H2O2 dose (without removal) to study chronic exposure in cell culture. One solution is the use the enzyme Glucose Oxidase, which catalyzes the direct two-electron reduction of oxygen to of H2O2, using reducing equivalents from the oxidation of glucose. We found that if a sufficiently low dose (10fg/ml-1000fg/ml) of glucose oxidase is used (in the presence of non-limiting glucose) then glucose oxidase treatment can be maintained for 24h with no significant effects on cell division rates for MEF cells. The rate of H2O2 generation by 10fg/ml of Glucose Oxidase was 1fmol of H2O2 per minute. To compare the effects of acute vs chronic oxidant exposure on oxidative stress adaptation, we treated cells with glucose oxidase for either 1h (an acute stress adaptation) or for 24h (our chronic stress adaptation). Cells were challenged 24h after this treatment with a toxic dose of H2O2 and cell counts were taken another 24h later. We found that an acute treatment with glucose oxidase had a progressively stronger adaptive effect on tolerance to oxidative stress in the range from 10fg/ml to 1000fg/ml (Fig. 8A). In comparison, in the chronic cell treatment regime, 10fg/ml and 100fg/ml treatment had stronger beneficial effects than the same dose used as an acute exposure. Chronic 24h exposure to 1000fg glucose oxidase, however, was clearly toxic for the MEF cells, which had a much weaker adaptive response than when the same glucose oxidase concentration was used for only a 1h exposure (Fig. 8A).
Fig. 8. Adaptation to Chronic Oxidative Stress.
A. Chronic oxidative stress exposure of mammalian cell culture produces a stronger adaptive response than acute exposure with weaker oxidant concentrations, but produces a blunted response compared to acute exposures with higher oxidant concentrations. MEF cells were grown to 20% confluence then treated with 10–1000fg/ml of glucose oxidase for either 1 h or 24 h, cells were then washed and permitted to adapt and recover for 24 h. Rates of hydrogen peroxide production were as follows: 1fmol H2O2 per minute for 10fg GO, 10fmol H2O2 per minute for 100fg GO, or 100fmol H2O2 per minute for 1000fg GO. After recovery, cells were challenged with 500µmol of H2O2 per 105 cells for 1 h, and cell counts were taken 24 h later. Values are plotted as a percent of the cell counts of samples which were not exposed to the challenge, and are shown as means ± SE, where n = 3. B. Chronic oxidative stress exposure in Drosophila is beneficial up to a point after which the adaptive response is blunted. The lower the dose of oxidant exposure, the longer the time for which chronic exposure is beneficial. Flies were cultured as in Fig. 5, with 4–6 day old female w(1118) flies being pre-treated with 0, 10, 100 or 1000µM H2O2 for 8, 24 or 48 h. Flies were then challenged with a toxic dose of H2O2 4.4M), at 24 h after the end of the pretreatment. Flies were maintained in these vials and survival was scored ever 8 h. Median survival time was determined, with n = 60.
Chronic H2O2 Adaptation in Flies
Similar results are seen using a fly model of chronic H2O2 adaptation (Fig. 8B). Flies were placed in vials containing Kim-wipes© soaked in 5% sucrose ± three potentially adaptive doses of H2O2 for 0, 6, 24, or 48h. Flies were then permitted to recover on normal food for 24h, then transferred to challenge vials which contained Kim-wipes© soaked in 5% sucrose and a toxic concentration of 4.4M H2O2. Fly survival was then scored over the following 80 h. We found that when 100µM H2O2 was used as an adaptive treatment flies treated for 8 h were considerably more resilient to H2O2 challenge, while flies treated for 24 h were weakly more resilient and flies treated for 48 h had lost the adaptive response and were as sensitive as untreated flies. In comparison when a stronger concentration of 1mM H2O2 was used as the adaptive treatment, then flies treated for 8 h were weakly more resistant to oxidative stress than control flies while flies treated for 24 h or 48 h were no more resilient then control flies. Finally, when a weaker treatment of 10µM H2O2 was used for the adaptive treatment, then 6 h and 24 h exposures had no beneficial effect on oxidative stress tolerance, but 48h exposure had a weakly beneficial effect (Fig. 8B).
Discussion
In previous reports we have shown that a mild exposure of cells to H2O2 or other oxidants produces a transient increase in tolerance to oxidative stress. We have also shown that this transient adaptation is partly a product of enhanced capacity to remove oxidized proteins which occurs through changes in 20S proteasome levels and activity (2–4, 33). These previous studies were, however, limited to acute oxidative stress adaptation rather than repeated or chronic stress, which might be more representative of ‘real-world’ oxidative stress exposures. In the present investigation, we set out to study the ability of cells and flies to adapt to such repeated or chronic oxidative stress exposures.
A single H2O2 pretreatment increased the tolerance of MEF cells to a subsequent H2O2 challenge (as measured though cell counts, BrdU incorporation, proteolytic capacity, and protein carbonyl assays) and this was blocked in cells also treated with an siRNA against the proteasome β1 subunit, to prevent increases in proteasome levels. In contrast with these results for an acute H2O2 exposure, repeated H2O2 pretreatments with short time intervals (3–6 hours) generally blunted the adaptive response. Two modifications to the MEF cell repeated H2O2 pretreatment protocol were effective in permitting an improved adaptive response: increasing the intervals between H2O2 treatments to 12h, or decreasing the H2O2 concentration. When 12h intervals between successive H2O2 treatments were used, at the same H2O2 concentration used for acute adaptation, the MEF cell adaptive response was actually prolonged to at least 48h. Similarly, a concentration of H2O2 5μM) only 10% of that normally used, became a highly effective adaptive treatment, with effects lasting at least 48h, when used for multiple treatments at 12h intervals. Proteolytic capacity and tolerance to oxidative stress tracked very well with one another in these MEF cell experiments, again emphasizing the close relationship between capacity to remove oxidatively damaged proteins and capacity to survive an oxidative stress (3, 33).
Using an H2O2 pretreatment/challenge model we found that Drosophila are capable of a strong H2O2 induced transient adaptive increase in oxidative stress tolerance. Proteolytic capacity and tolerance to oxidative stress again tracked very well with one another in these Drosophila experiments, with RNAi experiments showing that blocking adaptive increases in proteasome levels also blocked adaptive increases in survival, once more emphasizing the close relationship between capacity to remove oxidatively damaged proteins and capacity to survive an oxidative stress. Also, as in MEF cells, the increased resistance of flies to oxidative stress challenge was blunted by repeated H2O2 exposures at short intervals (1–3 days), although if a sufficiently long interval was permitted between treatments (7 days) then the repeated treatments appeared to be tolerated, and generated a significant adaptive increase in oxidative stress tolerance. Conversely, however, all repeated treatments appeared to have a negative long-term effect, since flies which were repeatedly exposed to H2O2 had a shorter lifespans than untreated flies. We also observed a progressive decline in Drosophila lifespan with frequency of H2O2 treatments. When MEF cells were chronically exposed to continuous H2O2 production, generated by the enzyme glucose oxidase, we actually observed an enhanced adaptive response (H2O2 resistance) at low rates of H2O2 generation but an almost complete loss of stress adaptation at higher rates of H2O2 production. In Drosophila, chronic H2O2 exposure had almost universally negative effects, with flies exhibiting little to no adaptive response after 24 or 48 hours.
In conclusion for MEF cells-Repeated MEF cell oxidant exposures appear to prevent adaptive stress responses if the interval between exposures is too short and/or if each exposure is at too high an oxidant level. In contrast, repeated cell exposures at 12h intervals, especially at lower oxidant concentrations, appear to potentiate positive stress adaptations and extend the period of protection. Chronic oxidant exposure at low oxidant levels can actually potentiate and extend adaptive responses, but chronic exposure to higher oxidant levels prevents adaptation. In conclusion for D. melanogaster - Repeated oxidative stress adaptation at intervals of 1 or 3 days is toxic but intervals of 7 days are tolerated in terms of short-term survival. Lifespan, however, appears to be negatively impacted by all repeated stress adaptation regimens. Short-term survival is also negatively impacted by chronic oxidative stress adaptation at all levels.
Materials & Methods
Materials
All materials were purchased from VWR unless otherwise stated. Murine Embryonic Fibroblasts (MEF), catalog #CRL-2214, were purchased from ATCC (Manassas, VA, USA). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM), catalog #10-013-CV, from Mediatech (Manassas, VA) and supplemented with 10% Fetal Bovine Serum (catalog #SH30070.03) from Hyclone (Logan, UT, USA): henceforth referred to as ‘complete media.’ Cells were typically incubated at 37°C under 5% CO2 and ambient oxygen.
Adaptation to Oxidants
MEF cells were grown to 10% confluence (≈100,000 cells per ml) then pretreated with H2O2. The optimum pretreatment condition, causing maximum adaptation, was found to be 1nmol of H2O2 per 105 cells (1µM final concentration) (catalog # H1009-100ml) from Sigma-Aldrich (St Louis, MO, USA), for 1 h at 37°C under 5% CO2. Cells were then washed once with phosphate-buffered saline (PBS), which was finally replaced with fresh complete media.
Fluorpeptide proteolytic assays
Preparation of Mammalian cells
MEF were harvested by scraping in phosphate buffer. Cells were then re-suspended in 50 mM Tris, 25 mM KCl, 10 mM NaCl, 1 mM MgCl21 mM DTT (pH 7.5) and lysed by 3 freeze-thaw cycles.
Preparation of Drosophila
10 flies were collected per sample. Flies were then transferred into a solution containing proteolysis buffer (50 mM Tris, 25 mM KCl, 10 mM NaCl, 1 mM MgCl21 mM DTT, at pH 7.5). Flies were frozen once, then homogenized using a pestle after which lysis was performed by 3 freeze-thaw cycles on dry ice for 5 min each, followed by a room temperature water bath for 5 min. Samples were then centrifuged at 10,000g to remove cuticle fragments and unlysed cells.
Proteolytic assays
Protein content was quantified using a BCA Protein Assay Kit. Samples were transferred, in triplicate, in 5.0 µg of cell lysate aliquots to 96 well plates, and 2 µM of N-succinyl-Leu-Leu-Val-Tyr-AMC (catalog # 80053-860) purchased from VWR (Chester, PA, USA) was added to all samples in the plate. Plates were incubated at 37°C and mixed at 300 rpm for 4 h. Fluorescence readings were taken at 10 minute intervals using an excitation wavelength of 355nm and an emission of 444 nm. Fluoresence units were converted to moles of free AMC, with reference to an AMC standard curve of known amounts of AMC (catalog # 164545) purchased from Merck (Whitehouse Station, NJ, USA). Background readings were then subtracted.
Preparation of AMC labeled hemoglobin and AMC labeled oxidized hemoglobin
Hemoglobin was labeled with the fluorophore AMC (to produce Hb-AMC) as described by Pickering and Davies (40), using 5mg/ml of hemoglobin labeled with 1mM AMC (catalog # 164545) purchased from Merck (Whitehouse Station, NJ, USA). Next, some samples were incubated with 1mM H2O2 for 1h to create AMC-labeled oxidized hemoglobin (Hbox-AMC). Samples were incubated for a further 1 h then extensively dialyzed to remove unbound AMC. Samples were also buffer-exchanged against proteolysis buffer (50 mM Tris, 25 mM KCl, 10 mM NaCl, 1 mM MgCl21 mM DTT, pH 7.5). After buffer exchange, BSA assays were used to adjusted the concentration of the protein substrates.
Hb-AMC or HbOX-AMC proteolytic assays
MEF cells were harvested by cell scraping in phosphate buffer. Cells were then resuspended in 50 mM Tris, 25 mM KCl, 10 mM NaCl, 1 mM MgCl21 mM DTT (pH 7.5) and lysed by 3 freeze-thaw cycles. Protein content was quantified using a BCA Protein Assay Kit. Then 5.0 µg of cell lysate per sample was transferred in triplicate to 96 well plates, and 5µl of either Hb-AMC or HbOX-AMC was added to each plate. Plates were incubated at 37°C and mixed at 300 rpm for 4 h. Fluorescence readings were taken at 10 minute intervals using an excitation wavelength of 355nm and an emission of 444 nm. Fluoresence units were converted to moles of free AMC, with reference to an AMC standard curve of known amounts of AMC (catalog # 164545) purchased from Merck (Whitehouse Station, NJ, USA). Background readings were then subtracted.
siRNA (short interfering RNA) knockdown treatments
β1 (sc-62865) and scrambled (sc-29528) siRNA were purchased from Santa Cruz Biotechnology. For experiments with these siRNAs, MEF cells were seeded at a density of 105 cells/well in six-well plates and grown to 10% confluence. siRNA treatment was then performed over a 48h period as described in the SantaCruz Biotechnology product manual.
Cell counting assay
Cells were seeded in 100μl samples and grown to 20% confluence in 48 well plates. Then, 24 h after seeding, some cells were pre-treated with hydrogen peroxide or glucose oxidase (catalog# 700011-406) purchased from VWR (Chester, PA, USA) for 1 h or 24 h, depending on the assay. Samples were then washed with PBS, and new media was added. At 24 h after treatment, cells were challenged with a toxic dose of 500µmol of H2O2 per 105 cells (1mM H2O2 final concentration) for 1 h, followed by addition of fresh complete media. Cells were harvested 24 h after challenge, using trypsinization. The cell density of 100µl samples of cell suspensions was then obtained in triplicate using a cell counter purchased from Beckman Coulter (Fullerton, CA, USA).
BrdU (bromodeoxyuridine) assay for DNA replication and cell division
BrdU, a synthetic thymidine analogue, can be incorporated into newly synthesized DNA providing a test of DNA replication, as an indirect measure of cell division. The assay was performed as described in the product manual from Millipore. BrdU incorporation was detected by addition of peroxidase substrate.Spectrophotometric detection was performed at a wavelength of 450 nm.
Oxyblot assay for protein carbonyls (protein oxidation
An Oxyblot kit for detection of protein carbonyls was purchased from Millipore and assays were performed as described in the product manual.
Drosophila melanogaster culture
Drosophila were cultured on a standard agar/dextrose/corn meal/yeast media (48) at 25°C. Unless otherwise stated, w(1118) flies were used in all assays. Flies were collected over a 48 h period from pre-cleared bottles, and allowed 4 days to mature so that at initiation of assays, flies were 4–6 days old. All assays were performed exclusively on female flies.
Drosophila H2O2 adaptation
Samples of 20 flies were transferred to vials containing ½ a Kim-wipe© soaked in 1ml of 5% sucrose plus 0µM, 10µM, 100µM or 1mM H2O2 for 8 h. Flies were then returned to normal vials for 16 h to permit adaptation to occur. Flies were then challenged with a toxic dose of H2O2. This procedure has previously been shown to be an effective method of exposing flies to oxidative stress (43).
Drosophila RNAi experiments
Flies expressing RNAi against a required proteasome subunit prosβ2RNAi w[1118]; P[GD10938]v24749) was purchased from the Vienna Drosophila RNAi center (VDRC, Vienna, Austria). Males from this line (or w[1118] as a control) were crossed with virgin females containing the Act-GS-255B driver (45, 49). Parents were removed 4 days after initiation of cross. Progeny were then collected over a 48 h period after eclusion. The Act-GS-255B driver was activated by feeding flies RU486. Flies were cultured in normal vials containing either 50µl of stock RU486 (20mg/ml) or ethanol, which had been added to vials and air dried 24 h prior to the assay. Flies were incubated in these vials for 2 days. For H2O2 adaptation, flies were then pretreated by exposure to ½ a Kim-wipe© soaked in 1ml of 5% sucrose ± 100uM H2O2 for 8 h. then returned to RU486 or ethanol vials for a further 16 h of RU486 treatment.
Drosophila H2O2 challenge assays
Samples of 20 flies were transferred to vials containing ½ a Kim-wipe© soaked in 1ml of 5% sucrose and 4.4M H2O2. Survival was then scored ever 8 h following initiation of challenge. Flies were scored as dead once they became completely immobile.
Highlights.
H2O2 adaptation involves increased proteolytic capacity in mammalian cells & flies
Increased Proteasome expression largely explains increased proteolytic capacity
Repeated or chronic H2O2 exposures must be at low doses for effective adaptation
Repeated H2O2 treatments hinder adaptation unless recovery periods are increased
Either repeated or chronic oxidative stress adaptation decreases lifespan of flies
Acknowledgements
This research was supported by grant #ES03598, and by ARRA Supplement 3RO1-ES 003598-22S2, both from the NIH/NIEHS to KJAD, and a grant from the Department of Health and Human Services (NIH/NIA) to JT (AG011833).
The abbreviations used are
- MEF
murine embryonic fibroblasts
- H2O2
hydrogen peroxide
- Drosophila melanogaster or D. melanogaster
the common fruit fly
- AMC
7-Amino-4-Methylcoumarin
- Suc-LLVY-AMC
the succinylated peptide N-Succinyl-Leucine-Leucine-Valine-Tyrosine-7-Amino-4-Methylcoumarin (used as a peptide substrate to estimate proteolytic capacity
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- Hb
hemoglobin
- Hbox
oxidatively modified hemoglobin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free radical biology & medicine. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 2.Wiese AG, Pacifici RE, Davies KJ. Transient adaptation of oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318:231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 3.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies KJ. Protein damage and degradation by oxygen radicalsIgeneral aspects. J Biol Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- 6.Davies KJ. Protein modification by oxidants and the role of proteolytic enzymes. Biochem Soc Trans. 1993;21:346–353. doi: 10.1042/bst0210346. [DOI] [PubMed] [Google Scholar]
- 7.Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 8.Davies KJ. An overview of oxidative stress. IUBMB Life. 2000;50:241–244. doi: 10.1080/713803723. [DOI] [PubMed] [Google Scholar]
- 9.Davies KJ, Delsignore ME. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J Biol Chem. 1987;262:9908–9913. [PubMed] [Google Scholar]
- 10.Davies KJ, Delsignore ME, Lin SW. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987;262:9902–9907. [PubMed] [Google Scholar]
- 11.Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJ. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919–11927. [PubMed] [Google Scholar]
- 12.Giulivi C, Davies KJ. Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J Biol Chem. 1993;268:8752–8759. [PubMed] [Google Scholar]
- 13.Pacifici RE, Kono Y, Davies KJ. Hydrophobicity as the signal for selective degradation of hydroxyl radical-modified hemoglobin by the multicatalytic proteinase complex, proteasome. J Biol Chem. 1993;268:15405–15411. [PubMed] [Google Scholar]
- 14.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 15.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- 16.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 17.Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 18.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 19.Ding Q, Dimayuga E, Keller JN. Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal. 2006;8:163–172. doi: 10.1089/ars.2006.8.163. [DOI] [PubMed] [Google Scholar]
- 20.Grune T, Reinheckel T, Joshi M, Davies KJ. Proteolysis in cultured liver epithelial cells during oxidative stress. Role of the multicatalytic proteinase complex, proteasome. J Biol Chem. 1995;270:2344–2351. doi: 10.1074/jbc.270.5.2344. [DOI] [PubMed] [Google Scholar]
- 21.Chondrogianni N, Stratford FL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J Biol Chem. 2003;278:28026–28037. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- 22.Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES. Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem. 2005;280:11840–11850. doi: 10.1074/jbc.M413007200. [DOI] [PubMed] [Google Scholar]
- 23.Conconi M, Szweda LI, Levine RL, Stadtman ER, Friguet B. Age-related decline of rat liver multicatalytic proteinase activity and protection from oxidative inactivation by heat-shock protein 90. Arch Biochem Biophys. 1996;331:232–240. doi: 10.1006/abbi.1996.0303. [DOI] [PubMed] [Google Scholar]
- 24.Whittier JE, Xiong Y, Rechsteiner MC, Squier TC. Hsp90 enhances degradation of oxidized calmodulin by the 20 S proteasome. J Biol Chem. 2004;279:46135–46142. doi: 10.1074/jbc.M406048200. [DOI] [PubMed] [Google Scholar]
- 25.Brooks P, Fuertes G, Murray RZ, Bose S, Knecht E, Rechsteiner MC, Hendil KB, Tanaka K, Dyson J, Rivett J. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. Biochem J. 2000;346(Pt 1):155–161. [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrington DA, Hussong SA, Roehrich H, Kapphahn RJ, Kavanaugh SM, Heuss ND, Gregerson DS. Immunoproteasome responds to injury in the retina and brain. J Neurochem. 2008;106:158–169. doi: 10.1111/j.1471-4159.2008.05345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 29.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 30.Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med. 2009;46:1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulteau AL, Szweda LI, Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Bender T, Lewrenz I, Franken S, Baitzel C, Voos W. Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease. Mol Biol Cell. 2011;22:541–554. doi: 10.1091/mbc.E10-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grune T, Catalgol B, Licht A, Ermak G, Pickering AM, Ngo JK, Davies KJ. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med. 2011;51:1355–1364. doi: 10.1016/j.freeradbiomed.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- 35.Przybysz AJ, Choe KP, Roberts LJ, Strange K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mech Ageing Dev. 2009;130:357–369. doi: 10.1016/j.mad.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies KJ, Lin SW. Degradation of oxidatively denatured proteins in Escherichia coli. Free Radic Biol Med. 1988;5:215–223. doi: 10.1016/0891-5849(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 37.Pacifici RE, Davies KJ. Protein degradation as an index of oxidative stress. Methods Enzymol. 1990;186:485–502. doi: 10.1016/0076-6879(90)86143-j. [DOI] [PubMed] [Google Scholar]
- 38.Pickering AM, Davies KJ. Degradation of Damaged Proteins: The Main Function of the 20S Proteasome. Progress in molecular biology and translational science. 2012;109:227–248. doi: 10.1016/B978-0-12-397863-9.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering AM, Staab TA, Tower J, Sieburth DS, Davies KA. A Conserved Role for the 20S Proteasome and Nrf2 Transcription Factor in Oxidative-Stress Adaptation in Mammals, C. elegans and D. melanogaster. J Exp Biol. doi: 10.1242/jeb.074757. (IN PRESS) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering AM, Davies KJ. A simple fluorescence labeling method for studies of protein oxidation, protein modification, and proteolysis. Free Radic Biol Med. 2012;52:239–246. doi: 10.1016/j.freeradbiomed.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickering AM, Davies KJ. Differential roles of proteasome and immunoproteasome regulators Pa28alphabeta, Pa28gamma and Pa200 in the degradation of oxidized proteins. Arch Biochem Biophys. 2012;523:181–190. doi: 10.1016/j.abb.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moskalev A, Shaposhnikov M, Turysheva E. Life span alteration after irradiation in Drosophila melanogaster strains with mutations of Hsf and Hsps. Biogerontology. 2009;10:3–11. doi: 10.1007/s10522-008-9147-5. [DOI] [PubMed] [Google Scholar]
- 43.Grover D, Ford D, Brown C, Hoe N, Erdem A, Tavare S, Tower J. Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS One. 2009;4:e7580. doi: 10.1371/journal.pone.0007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Bourg E. Hormetic effects on longevity of hydrogen peroxide in Drosophila melanogaster flies living on a poorly nutritious medium. Biogerontology. 2007;8:327–344. doi: 10.1007/s10522-006-9077-z. [DOI] [PubMed] [Google Scholar]
- 45.Ford D, Hoe N, Landis GN, Tozer K, Luu A, Bhole D, Badrinath A, Tower J. Alteration of Drosophila life span using conditional, tissue-specific expression of transgenes triggered by doxycyline or RU486/Mifepristone. Exp Gerontol. 2007;42:483–497. doi: 10.1016/j.exger.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen J, Curtis C, Tavare S, Tower J. A screen of apoptosis and senescence regulatory genes for life span effects when over-expressed in Drosophila. Aging (Albany NY) 2009;1:191–211. doi: 10.18632/aging.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minois N. Resistance to stress as a function of age in transgenic Drosophila melanogaster overexpressing Hsp70. J Insect Physiol. 2001;47:1007–1012. doi: 10.1016/s0022-1910(01)00076-2. [DOI] [PubMed] [Google Scholar]
- 48.Ashburner M. Drosophila: A Laboratory Handbook. Plainview, NY: Cold Spring Harbor Lab; 1989. [Google Scholar]
- 49.Fischer JA, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]