Abstract
Species of the genus Hypericum contain a rich array of unusual polyketides, however, only a small proportion of the over 450 Hypericum species, other than the popular medicinal supplement St. John’s Wort (H. perforatum), have even been chemically characterized. H. gentianoides, a small annual used medicinally by Cherokee Americans, contains bioactive acylphloroglucinols. Here, we identify acylphloroglucinol constituents of H. gentianoides and determine a potential pathway to their synthesis. Liquid chromatography/electrospray ionization-mass spectrometry (LC/ESI-MS) and HPLC-UV indicate that the level of accumulation and profile of acylphloroglucinols in H. gentianoides vary little seasonally when grown in a greenhouse, but do vary with development and are highly dependent on the accession, highlighting the importance of the selection of plant material for study. We identify the chemical structures of the nine prevalent polyketides, based on LC/ESI-MS and hybrid quadrupole orthogonal time-of-flight mass (Q-TOF) spectrometry; these metabolites include one monomeric phlorisobutyrophenone (PIB) derivative and eight dimeric acylphloroglucinols. Q-TOF spectrometry was used to identify eight additional PIB derivatives that were not detected by LC/ESI-MS. These data lead us to propose that diacylphloroglucinols are synthesized via modification of PIB to yield diverse phloroglucinol and filicinic acids moieties, followed by dimerization of a phloroglucinol and a filicinic acid monomer to yield the observed complement of diacylphloroglucinols. The metabolomics data from H. gentianoides are accessible in PMR (http://www.metnetdb.org/pmr), a public metabolomics database with analysis software for plants and microbial organisms.
Introduction
Specialized phytochemicals provide complex defense strategies to combat insects, fungi, animals, or abiotic stressors, as well as attractants for pollination or dispersal. Such phytochemicals encompass tremendous structural diversity, from terpene alkaloids as in Digitalis purpurea (Chappell 2008), to polyketide alkylamides as in Echinacea (Wu et al. 2009), to waxes and scents (Fatland et al. 2002, Klingauf et al. 2005). Often, specialized metabolites are contained in discreet compartments. For example, peltate glands in the young leaves of sweet basil (Ocimum basilicum) accumulate monoterpenes (Iijima et al. 2004); 2-carene, α-terpinene, and limonene are concentrated in the glandular trichomes of tomato (Solanum lycopersicum) (Schilmiller et al. 2009); and the toxic dianthrone hypericin accumulates in nodular glands of H. perforatum (Piovan et al. 2004, Košuth et al. 2011).
Hypericum encompasses over 450 species, few of which have been chemically profiled (Crispin and Wurtele 2013), and is the largest genus within Clusiaceae, fossils of which date to the Late Cretaceous (90 million years ago) (Gustafsson et al. 2002). H. perforatum (St. John’s Wort) is commonly used by humans medicinally (Ernst 2003, Birt et al. 2009). Extracts of H. perforatum, when applied to bacterial, viral or animal systems, induce complex, context-dependent changes to cellular and physiological functions. Medicinally “desirable” bioactivities include: anti-viral (Axarlis et al. 1998, Maury et al. 2009), anti-inflammatory (Hammer et al. 2007), anti-depressive (Mennini and Gobbi 2004, Butterweck and Schmidt 2007, Kasper et al. 2008), and anti-bacterial (Franklin et al. 2009, Saddiqe et al. 2010). Furthermore, these compounds have been used to treat obsessive compulsive disorder (Camfield et al 2011). Potentially negative effects of H. perforatum include: cytotoxicity in HaCaT human keratinocytes (Schmitt et al. 2006); photosensitivity in mammals (Traynor et al. 2005, Onoue et al. 2011); decreased serum concentrations of anti-retroviral and anti-cancer pharmaceuticals in humans (De Maat et al. 2001, Caraci et al. 2011); and increased blood pressure and heart rate in humans taking MAOIs (Tachjian et al. 2010).
A single chemical compound in H. perforatum, the acylphloroglucinol hyperforin, is believed to be the source of the anti-depressive activity (Mennini and Gobbi 2004, Brenner et al. 2000, Tu et al. 2010, Carpenter 2011). Hyperforin is also toxic to gram-positive bacteria (Reichling et al. 2001, Avato et al. 2004, Joray et al. 2011), has been shown to be a potential anticarcinogen (Schempp et al. 2002) and is an angiogenesis inhibitor (Schempp et al. 2005). Four less studied, acylphloroglucinols, saroaspidin A, saroaspidin B, uliginosin A, and uliginosin B have also been shown to have a variety of biological activities (Ishiguro et al. 1987, Rocha et al. 1995, Stein et al. 2012)
H. gentianoides (Orange grass, Pineweed), is a small annual that grows throughout the central and eastern United States and Canada (USDA Natural Resources Conservation Service http://plants.usda.gov/java/profile?symbol=HYGE). Cherokee Native Americans used this plant to cure ailments as mild as nosebleeds and sores, and as severe as fever and venereal diseases (Hamel and Chiltoskey 1975). Interestingly, neither hypericin nor hyperforin have been detected in this species (Crockett et al. 2005, Hillwig et al. 2008). Overall, H. gentianoides has been characterized by a few studies of its biological activities (Hillwig et al. 2008, Huang et al. 2011) and in relation to its geographical distribution (Bliss et al. 2002). Despite lacking hypericin or hyperforin, extracts from H. gentianoides, and non-polar fractions of these extracts that are highly enriched in putative acylphloroglucinols, have anti-inflammatory bioactivity in mouse macrophage cells (Hillwig et al. 2008). H. gentianoides accumulates nine unusual specialized phytochemicals that have similar UV spectra, and are postulated to be acylphloroglucinols (Hillwig et al. 2008). Three of these compounds have been identified as saroaspidin A, uliginosin A, and hyperbrasilol C by NMR (Hillwig 2008).
Identifying the range of existing acylphloroglucinols and the routes to their synthesis will enhance our understanding of the development of diversity in biology, and inform the evolutionary origin of this branch of the polyketide pathway. In part because of their high-energy content and diverse chemical functionalities, polyketides in general provide a major potential as biorenewable chemicals to replace petroleum as a feedstock for polymer production (Nikolau et al. 2008, Oliver et al. 2009). Acylphloroglucinol research has particular potential for practical application in medicinal venues. For example, given the complex effects of hyperforin and other acylphloroglucinols on cellular and viral function, the availability of a spectrum of acylphloroglucinols would better enable researchers to define structure-function relationships in humans and other animals.
Here, we determine the structures of the six previously unidentified major accumulating constituents in H. gentianoides as a monomeric and five dimeric acylphloroglucinols. We identify nine additional monomeric acylphloroglucinols that are implicated as precursors. Finally, we compare the accumulation patterns of these acylphloroglucinols between vegetative and reproductive phases of growth, and across two standardized accessions.
Materials and methods
Plant material
H. gentianoides germplasm (as seeds), accessioned in the US National Germplasm System as PI 664838 (and for studies in Figure 3a, Ames 27657), was obtained from the USDA North Central Regional Plant Introduction Station, Ames, Iowa. Accession PI 664838 of H. gentianoides provides the advantage that both vegetative and reproductive developmental phases are present on a given plant simultaneously under the growth conditions used in this study. For determinations of relative concentrations of acylphloroglucinols across shoot (leaf and stem, with flower buds for reproductive stage) material, plants were grown in randomized block design. Seeds were planted in a growing medium (Sunshine LC1 SUN-COIR, Sun Gro Horticulture, Canada) in a greenhouse with natural light supplemented by an 8/16 hr photoperiod at 120 µmol m−2 s−1, in temperatures between 19–25 degrees Celsius. Plants were watered daily, and fertilized bi-weekly. During each harvest, samples were collected beginning at 11 am. Vegetative shoots and reproductive shoots that had differentiated but had not begun to flower were collected from individual plants in triplicate (approximately 5–8 shoots per biological replicate). In experiments to evaluate seasonal variation, H. gentianoides seeds were planted in the greenhouse under the conditions described at four-month intervals, and grown 6 months, and harvested. (Plantings in September and January; harvests in February and June)
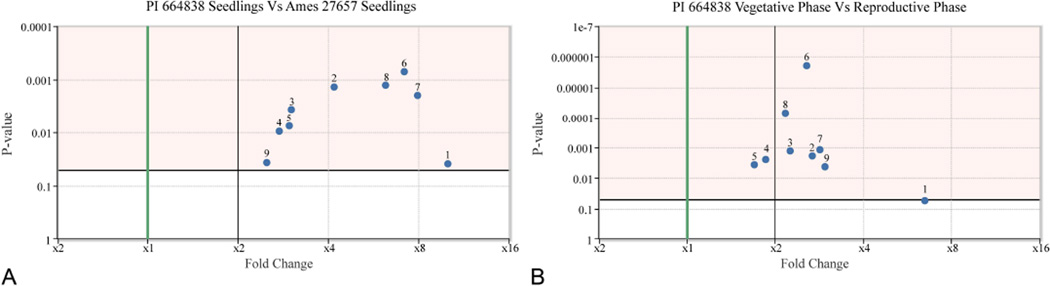
Figure 3.
Accumulation of acylphloroglucinols in Hypericum gentianoides varies with developmental stage and accession. HPLC analysis was used for relative quantification of the nine major acylphloroglucinol metabolites. Three biological replicates were used for each sample. Data is analyzed and visualized in the interactive Plant Metabolomics Resource (www.metnetdb.org/pmr). Complete data is shown in Table S1. Volcano plots illustrates a comparison of two of the most diverse materials. In this plot, the Y-axis represents the p-value (lower p-values indicate a more significant difference); the X-axis represents the fold change (the farther from 1, the greater the fold-change). p-values are determined using a student T-test after log (base 2) transformation. 1-1’3’pren45’me4’oxoPIB; 2-Uliginosin B; 3-Saroaspidin A; 4-Saroaspidin B; 5-[3’mePIB]-[1’pren3’me4’oxoPIB]; 6-[3’3’4me6’oxoPIB]-[3’prenPIB]; 7-Uliginosin A; 8-Hyperbrasilol C; 9-[1’3’pren45’me4’oxoPIB]-[3’prenPIB]. (a) Accumulation of 9 major acylphloroglucinols is between 2–7.5 fold greater in young seedlings of standardized accession PI 664838 compared to Ames 27657 young seedlings. (n=3, p values range from 0.0009 to 0.05). (b) Accumulation of 9 major acylphloroglucinols (is between 1.7–10.9 fold greater in vegetative phase shoots than in reproductive phase shoots (preflowering) in the standardized accession PI 664838). (n=3, p values range from 1.9×10−6 to 0.03)
Plant samples were placed in a 1.5 mL Eppendorf tubes, and immediately stored in liquid nitrogen to minimize degradation of polyketides. Samples were kept in liquid nitrogen until they were extracted.
Preparation of extracts
Before extraction of each sample, liquid nitrogen was allowed to evaporate; then, the plant material was weighed without thawing and placed in a liquid nitrogen cooled mortar. 10 µL of apigenin in methanol at a concentration of 1 mg mL−1 was added to each sample as an internal standard. More liquid nitrogen was added to prevent thawing, and the mixture was finely ground with a pestle. 2 mL of methanol was added, the mixture was further ground, and then vortexed for 30 s, and centrifuged at 13 000 rpm for 2 min. The supernatant was decanted, filtered through a 0.45 micron nylon syringe filter, and the filtrate was placed under nitrogen gas to minimize oxidation of compounds that might be present at low concentrations and detected by mass spectrometry but not by a UV detector. Extracts were stored in a −80°C freezer until analysis.
High pressure liquid chromatography (HPLC)
HPLC conditions resembled those of Hillwig et al (2008). A Beckman Coulter Gold HPLC System (Fullerton, CA) with a Detector 168 photo diode array detector was used for determination of metabolite levels. A Synergi Max-RP 4 micron 150 × 4.6 mm column (Phenomenex, Torrance, CA 90501) was used for separation. A 10 mM ammonium acetate (solvent A) and 9:1 v/v acetonitrile/methanol (solvent B) mobile phase was used. The gradient consisted of 87% A/13% B in 10 min to 83% A/17% B, then to 100% B in 25 min and held for 5 min. The flow rate was 1.0 mL min−1, the solvent gradient and flow rate was initially described by (Ganzera et al. 2002). All solvents and the apigenin internal standard used were HPLC grade (Sigma, St. Louis, MO).
Liquid chromatography/electrospray ionization-mass spectrometry (LC/ESI-MS)
Samples were placed under nitrogen gas to minimize oxidation of low-accumulating compounds that might be detected by mass spectrometry but not by a UV detector. An Agilent Technologies Ion Trap 1100 was used for LC/ESI-MS analysis. Analytical separation was accomplished using a Synergi Max-RP 4 micron 150 × 4.6 mm column (Phenomenex, Torrance, CA 90501) with a 10 mM ammonium acetate (solvent A) and 9:1 v/v acetonitrile/methanol (solvent B) mobile phase, with a gradient from 85% A/15% B to 80% A/20% B over a 10 min time, to 100% B over 25 min, and held at 100% B for 5 minutes. The flow rate was 0.75 mL min−1. All solvents were HPLC grade (Sigma, St. Louis, MO). This LC/ESI-MS analysis detected the nine final acylphloroglucinols but only two of eight precursors.
Metabolite quantification based on an internal standard
The relative concentrations of the acylphloroglucinols were quantified using HPLC-photo diode array UV, against 0.05 g (185.020 µmol) of apigenin (MW 270.24) as internal standard. Apigenin was added at the time of extraction to each biological replicate. Extracts derived from three independent biological replicates for each sample were analyzed, data normalized, converted to a relative scale of nmol based on the apigenin standard, and further normalized by dividing µmol of compound by the fresh weight of the plant material (µmol g−1 FW).
Q-TOF mass spectrometry and spectral interpretation
High resolution mass spectrometric analysis was performed on a hybrid quadrupole orthogonal time-of-flight mass spectrometer (Waters SYNAPT G2 HDMS, Manchester, U.K.) equipped with an automatic chip-based nanoelectrospray source NanoMate HD (Advion BioSciences, Ithaca, NY). Ionization voltage was set to 1.09 kV, gas pressure to 1.45 psi in the negative-ion mode, and the source was controlled by Chipsoft 8.3.1 software (Advion BioSciences). All samples were diluted with 5 mM ammonium acetate in CHCl3:MeOH:IPA (1:2:4, v/v/v) and infused. The system was equipped with an integral Advion LockSpray unit with its own reference sprayer that was controlled automatically by the acquisition software to collect a reference scan every 10 s, for 1 s. Reference internal calibrant (leucine enkephalin) was introduced into the lock mass sprayer at a constant flow rate of 3 µL min−1 using an internal pump connected to the LockSpray. A single lock mass calibration at m/z 554.2615 in the negative-ion mode was used for the complete analysis. Spectral interpretation was performed with MassLynx™ v.4.1 Mass Spectrometry Software (Waters Co., Milford, MA, USA). After peak picking, elemental formulas were calculated and assigned on the basis of m/z values within a 6 ppm error range. Standard conditions for plant metabolomics data (Cc Hh Oo, c ≤ 500, h ≤ 1000, o ≤ 20; −1.5 ≤ Double Bond Equivalent ≥ 50.0) were used in these calculations.
Results
Liquid chromatography/electrospray ionization-mass spectroscopy (LC/ESI-MS)-based structural identification of nine acylphloroglucinols of H. gentianoides
H. gentianoides (accessions Ames 27657 and Ames 28015) has been shown to contain multiple metabolites with retention times, mass spectra, and distinctive three-peaked UV spectra indicative of acylphloroglucinols (Hillwig et al. 2008). Three of these compounds (MWs 446 D, 500 D, 554 D; ret times 23.418, 24.820, 24.993 min) have been previously identified in our lab as saroaspidin A, uliginosin A, and hyperbrasilol C (Hillwig, 2008). These spectra were also compared to the 2-D NMR of the same compounds isolated from other species of Hypericum: saraospidin A, in H. japonicum (Ishiguro et al. 1987); uliginosin A, in H. uliginosum (Taylor and Brooker 1969); and hyperbrasilol C, in H. brasilense (Rocha et al. 1995).
To further identify the chemical nature of the acylphloroglucinol-like compounds in H. gentianoides, plants of the accession PI 664838 were germinated from seeds, and grown in a greenhouse under natural plus supplemental light. Shoots in the vegetative phase of development were harvested from 11 months old plants (Figure 1). To minimize potential alteration or degradation of the polyketide constituents, we reduced the exposure of plant material and extracts to oxygen and Hypericum enzymes during pulverization, extraction and separation (Hillwig et al. 2008). The methanolic extract was analyzed by HPLC-UV and LC/ESI-MS (Figure S1, S2).
Figure 1.
Developmental phases of H. gentianoides life cycle. In some accessions of H. gentianoides, including PI 664838, both vegetative and reproductive phases often occur concurrently on a single plant.
HPLC-UV of the H. gentianoides methanolic extract shows nine non-polar compounds with the characteristic three-peaked UV spectra with maxima at 220, 300, and 350 nm, indicative of acylphloroglucinols (Figure S1). Three of these nine compounds are saraospidin A, uliginosin A, and hyperbrasilol C, based on molecular mass, retention time, UV spectra, molecular fragmentation products, and NMR (Hillwig 2008). The extract was analyzed by LC/ESI-MS to further determine the structures of the nine acylphloroglucinols observed at m/z’s of 359, 497, 445, 459, 513, 499, 553, 567. Table 1 shows fragment ions and retention times from each MS/MS spectrum, of which are shown in Figure S1. Throughout this paper, there are several compounds that are newly identified, and do not have commonly used names; rather than make up a series of new names, these compounds will be referred to by abbreviations according to their chemical properties (Table 1 and Table 2).
Table 1.
Molecular and MS/MS ions of acylphloroglucinols from H. gentianoides using LC/ESI-MS. ![]()
![]()
| Relative Retention Time (min) |
Abbreviation/common name | m/z | Fragment Ions |
|---|---|---|---|
| 21.566 | 1’3’pren45’me4’oxoPIB | 359 | 222 |
| 23.32 | Uliginosin B | 497 | 223, 235, 261 |
| 23.418 | Saroaspidin A | 445 | 209, 223, 235 |
| 23.791 | Saroaspidin B | 459 | 223, 235 |
| 24.090 | [3’mePIB]-[1’pren3’me4’oxoPIB] | 499 | 209, 221, 277, 289 |
| 24.510 | [3’3’4me6’oxoPIB]-[3’prenPIB] | 513 | 237, 249, 263, 275 |
| 24.820 | Uliginosin A | 499 | 223, 235, 263, 275 |
| 24.993 | Hyperbrasilol C | 553 | 263, 277, 289 |
| 25.265 | [1’3’pren45’me4’oxoPIB]-[3’prenPIB] | 567 | 263, 291, 303 |
LC/ESI-MS analysis. Molecular ions measured in negative-ion mode. Vegetative phase shoots from 11-month-old plants of H. gentianoides accession PI 664838. RT, retention time.
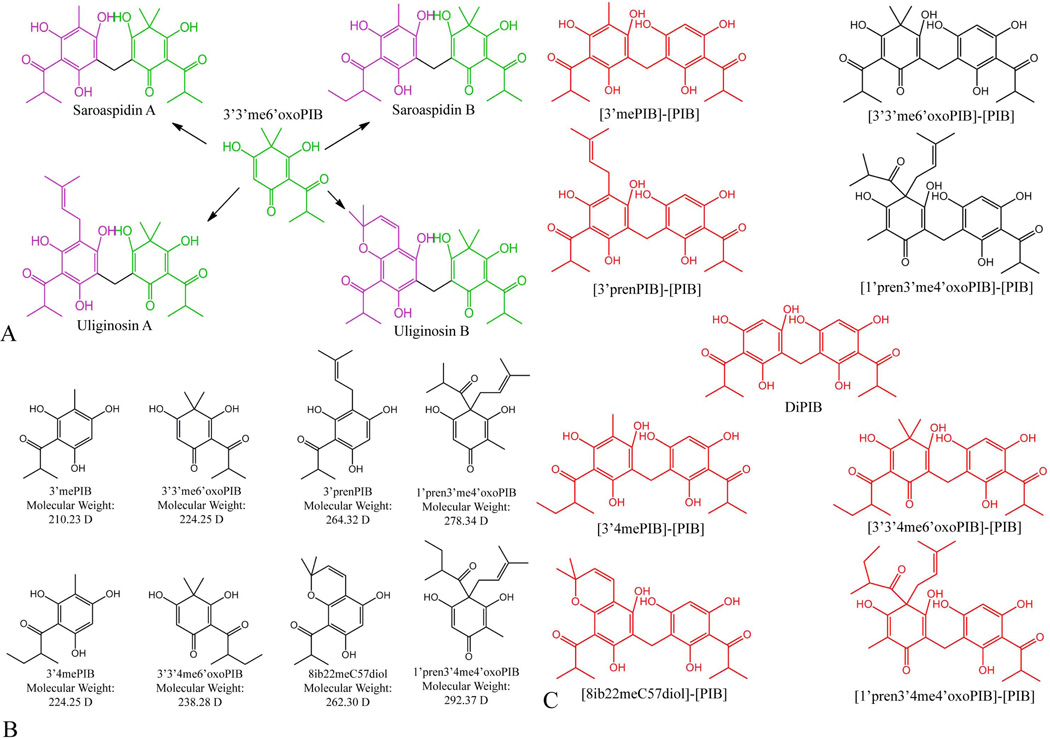
Saroaspidin B and japonicin A have the same MW and MS/MS spectra and cannot be formally distinguished by our analysis. Japonicin A is a homodimer of two filicinic acid moieties, while saroaspidin B is a heterodimer. Because the other seven diacylphloroglucinols are heterodimers of a phloroglucinol and filicinic acid moiety we believe saroaspidin B to be the more likely compound.
Table 2.
Molecular ions, predicted empirical formulas, and designations of acylphloroglucinols and their putative precursors present in H. gentianoides, as identified by high-resolution Q-TOF mass analysis. ![]()
![]() .
.
| Relative Abundance (µmol g−1 FW)* |
Predicted m/z |
Measured m/z |
Predicted Empirical Formula |
Mass Difference (ppm) |
Systematic Name | Compound Abbreviation/Common Name |
|---|---|---|---|---|---|---|
| .0706 | 195.07 | 195.0657 | C10 H11 O4 | 0.0 | 2’,4’,6’-trihydroxy-isobutyrophenone | PIB/Phlorisobutryophenone 1,2 this study |
| .3844 | 209.08 | 209.0816 | C11 H13 O4 | 1.0 | 3’-methyl-phlorisobutyrophenone | 3’mePIB this study |
| .3516 | 223.10 | 223.0972 | C12 H15 O4 | 0.9 | 3’,3’-dimethyl-6’-oxo- phlorisobutyrophenone or 3’4-dimethyl- phlorisobutyrophenone |
3’3’me6’oxoPIB or 3’4mePIBthis study |
| .0756 | 237.11 | 237.1128 | C13 H17 O4 | 0.4 | 3’,3’,4-trimethyl-6’-oxo- phlorisobutyrophenone |
3’3’4me6’oxoPIB this study |
| .0693 | 261.11 | 261.1219 | C15 H17 O4 | --- | 8-isobutyryl-2,2-dimethyl-chromene-5,7- diol |
8ib22meC57diol this study |
| .5344 | 263.13 | 263.1285 | C15 H19 O4 | 0.8 | 3’-prenyl-phlorisobutyrophenone | 3prenPIB 4 this study |
| .4525 | 277.14 | 277.1442 | C16 H21 O4 | 0.7 | 1’-prenyl −3’-methyl-4’-oxo- phlorisobutryophenone |
1’pren3’me4’oxoPIB this study |
| .0429 | 291.16 | 291.0848 | C17 H23 O4 | --- | 1’-prenyl-3’,4-dimethyl-4’-oxo- phlorisobutyrophenone |
1’pren3’4me4’oxoPIB this study |
| .0403 | 359.22 | 359.0747 | C22 H31 O4 | --- | 1’,3’-diprenyl-4,5’-dimethyl-4’-oxo- phlorisobutyrophenone |
1’3’pren45’me4’oxoPIB this study |
| .7890 | 445.18 | 445.1862 | C24 H29 O8 | 0.0 | 3,5-dihydroxy-2-isobutyryl-4,4- dimethyl-6-(2,4,6-trihydroxy-3- isobutyryl-5-methylbenzyl)cyclohexa- 2,5-dienone |
Saroaspidin A 2,5, this study |
| .2344 | 459.20 | 459.2019 | C25 H31 O8 | 0.0 | 3,5-dihydroxy-2-isobutyryl-4,4- dimethyl-6-(2,4,6-trihydroxy-3-methyl- 5-(2-methylbutanoyl)benzyl)cyclohexa- 2,5-dienone |
Saroaspidin B 2, this study |
| .0706 | 497.22 | 497.2226 | C28 H33 O8 | −1.6 | 2-((5,7-dihydroxy-8-isobutyryl-2,2- dimethyl-2H-chromen-6-yl)methyl)-3,5- dihydroxy-6-isobutyryl-4,4- dimethylcyclohexa-2,5-dienone |
Uliginosin B 1, 6, this study |
| .0416 | 499.23 | 499.0497 | C28 H35 O8 | --- | 3,5-dihydroxy-4-isobutyryl-2-methyl-4- (3-methylbut-2-enyl)-6-(2,4,6- trihydroxy-3-I sobutyryl-5- methylbenzyl)cyclohexa-2,5-dienone |
[3’mePIB]- [1’pren3’4me4’oxoPIB] this study |
| 3.437 | 499.23 | 499.2330 | C28 H35 O8 | −0.4 | 3,5-dihydroxy-2-isobutyryl-4,4- dimethyl-6-(2,4,6-trihydroxy-3- isobutyryl-5-(3-methylbut-2- enyl)benzyl)cyclohexa-2,5-dienone |
Uliginosin A 1, 5, this study |
| .5092 | 513.25 | 513.2330 | C29 H37 O8 | −1.0 | 3,5-dihydroxy-4,4-dimethyl-2-(2- methylbutanoyl)-6-(2,4,6-trihydroxy-3- isobutyryl-5-(3-methylbut-2- enyl)benzyl)cyclohexa-2,5-dienone |
[3’3’4me6’oxoPIB]-[3’prenPIB] this study |
| 2.103 | 553.28 | 553.2800 | C32 H41 O8 | −0.2 | 3,5-dihydroxy-4-isobutyryl-2-methyl-4- (3-methylbut-2-enyl)-6-(2,4,6- trihydroxy-3-isobutyryl-5-(3-methylbut-2- enyl)benzyl)cyclohexa-2,5-dienone |
Hyperbrasilol C 3, this study |
| .0756 | 567.29 | 567.2965 | C33 H43 O8 | 1.2 | 3,5-dihydroxy-2-methyl-4-(3- methylbut-2-enyl)-4-(2- methylbutanoyl)-6-(2,4,6-trihydroxy-3- isobutyryl-5-(3-methylbut-2- enyl)benzyl)cyclohexa-2,5-dienone |
[1’3’pren45’me4’oxoPIB]- [3’prenPIB] this study |
Relative abundance based on an apigenin internal standard.
Abbreviation: phloroisobutyrophenone=PIB; methyl=me; pren=prenyl; oxo=oxo; ib=isobutyryl; C=chromene
MS/MS spectra, in negative ion mode, of H. gentianoides extract constituents saraospidin A, uliginosin A, and hyperbrasilol C indicate that the methylene bridge that connects the two decorated PIB molecules is the main fragmentation target (Table 1). The six additional acylphloroglucinols in H. gentianoides detected by LC/ESI-MS, which had not been previously characterized, were identified based on the following criteria: the MS/MS spectra for a diacylphloroglucinol should reveal the m/z of the two decorated PIB molecules composing said diacylphloroglucinol. For example, the MS/MS spectra in negative ion mode of hyperbrasilol C is composed of ions at 263, 277, and 289 m/z, corresponding to 3’-prenyl-PIB (3’prenPIB), 1’-prenyl-3’-methyl-4’-oxo-PIB (1’pren3’me4’oxoPIB), and 1’pren3’me4’oxoPIB with a methyl group fragment of the methylene bridge that connected the two monomers together, respectively. The fragmentation pattern of the three NMR identified compounds were used to establish an additional four compounds identity as: uliginosin B, 3,5-dihydroxy-4-isobutyryl-2-methyl-4-(3-methylbut-2-enyl)-6-(2,4,6-trihydroxy-3-isobutyryl-5-methylbenzyl)cyclohexa-2,5-dienone, (abbreviated as [3’mePIB]-[1’pren3’me4’oxoPIB]); 3,5-dihydroxy-4-4-dimethyl-2-(2-methylbutanoyl)-6-(2,4,6-trihydroxy-3-isobutyryl-5-(3-methylbut-2-enyl)benzyl)cyclohexa-2,5-dienone (abbreviated as [3’3’4me6’oxoPIB]-[3’prenPIB]); and 3,5-dihydroxy-2-methyl-4-(3-methybut-2-enyl)-4-(2-methylbutanoyl)-6-(2,4,6-trihydroxy-3-isobutyryl-5-(3-methylbut-2-enyl)benzyl)cyclohexa-2,5-dienone (abbreviated as [1’pren3’4me4’oxoPIB]-[3’prenPIB] ) (Table 1). The compound at RT=23.761 min is a diacylphloroglucinol which could be either saroaspidin B or japonicin A; both compounds have the same molecular weight and MS/MS spectral pattern, and thus cannot be formally distinguished by MS/MS. NMR analysis was not performed on this compound, or the other five previously unidentified acylphloroglucinols in H. gentianoides, because of the difficulty in obtaining purified compound necessary for crystallization. Saroaspidin B is a dimer of a phloroglucinol moiety (3’,4-dimethyl-PIB (3’4mePIB), m/z=223) and a filicinic acid moiety (3’,3’-dimethyl-6’-oxo-PIB (3’3’me6’oxoPIB), m/z=223), while japonicin A is a dimer of two filicinic acid moieties (3’3’me6’oxoPIB). Given that the other seven diacylphloroglucinols in H. gentianoides are each heterodimers composed of one phloroglucinol moiety (3’-methyl-PIB (3’mePIB), 3’4mePIB, 8-isobutyryl-2,2-dimethyl-chromene-5,7-diol (8ib22meC57diol), 3’prenPIB) and one filicinic acid moiety (3’3’me6’oxoPIB, 3’,3’,4-trimethyl-6’-oxo-PIB (3’3’4me6’oxoPIB), 1’pren3’me4’oxoPIB, 1’-prenyl-4,5’-dimethyl-4-oxo-PIB (1’pren3’4me4’oxoPIB), we view saroaspidin B as the more likely identity (Figure 2a). The first-eluting compound of the nine major acylphloroglucinols in H. gentianoides has the typical characteristic acylphloroglucinol UV absorbance spectrum, eluted at 21.566 minutes, and has a molecular weight of 360 D, [M-H]− ion at m/z 359. This compound, unlike the other eight predominant acylphloroglucinols, should be a monoacylphloroglucinol, because the minimum weight possible for a diacylphloroglucinol is 404 D. A modification to 1’pren3’4me4’oxoPIB, specifically a 3’-prenylation as observed in 3’prenPIB, could account for a molecular weight of 360. We predicted this compound to be 1’,3’-diprenyl-4,5’-dimethyl-4’-oxo-PIB (1’3’pren45’me4’oxoPIB). Consistent with this interpretation, the MS/MS spectrum of this compound shows an intense fragmented ion at 222 m/z; an m/z of 222 corresponds to the core cyclic ring of this compound with 4 and 5’ methyl groups but without 1’ and 3’ prenyl groups. We also expected a peak at m/z=69 corresponding to the fragmented prenyl group. However, possibly because of the low abundance of this ion (Table 1, Figure S2), we were unable to detect this peak through Q-TOF or LC.
Figure 2.
Diacylphloroglucinols in Hypericum gentianoides. A) Each diacylphloroglucinol identified in H. gentianoides (see Table 1) is composed of one phloroglucinol (purple) and one filicinic acid (green); many of these diacylphloroglucinols share a common phloroglucinol or filicinic acid. For example, Saroaspidin A, Saroaspidin B, Uliginosin A, and Uliginosin B, all share the filicinic acid 3’,3’-dimethyl-6’-oxo-phlorisobutyrophenone (3’3’me6’oxoPIB). B) Four PIB derivatives from H. gentianoides identified by Q-TOF. Methylation and/or prenylation of PIB would give rise to each of these compounds. Additional methylation and/or cyclization of the resultant compounds would give rise to four more monomers, creating a pool of eight monomers sufficient to explain the synthesis of all nine highly accumulated acylphloroglucinols, without any post-dimerization modifications. C) Nine diacylphloroglucinols would be possible if diacylphloroglucinol biosynthesis occurred via a “diacylphloroglucinol decoration” route. In black are compounds that were identified through our Q-TOF analyses, in red are compounds that were not detected in our Q-TOF analysis of H. gentianoides extracts. The fully undecorated dimer (diPIB), and six of the eight partially decorated dimers that would be required if synthesis followed the “diacylphloroglucinol decoration” route were not detected. We assume the two partially decorated dimers present in H. gentianoides are 1) degradation products of diacylphloroglucinol end products or 2) products resulting from dimerization of a decorated monomer to a PIB molecule.
One possible synthetic route for diacylphloroglucinols is that PIB is first decorated, followed by dimerization of the resultant monomer pool to form the diacylphloroglucinols. In this case, eight acylphloroglucinol monomers (synthesized from PIB) would be sufficient to account for all of the H. gentianoides diacylphloroglucinols, as a variety of combinations can be made (exemplified in Figure 2a). Furthermore, if the “monomer pool” biosynthetic route were correct, we might expect that there would be low levels of the eight acylphloroglucinol monomer precursors in H. gentianoides (Figure 2b).
An alternate process that could account for biosynthesis of the observed diacylphloroglucinols is that a single diacylphloroglucinol might be formed and decorated to form the other diacylphloroglucinols. If this “diacylphloroglucinol decoration” route were correct, we would anticipate the presence of the precursors: partially decorated diacylphloroglucinols, and the fully undecorated dimer (di-PIB) (Figure 2c).
Acylphloroglucinol Accumulation Differs Between Life Phases and Accessions of H. gentianoides
To facilitate detection of acylphloroglucinol precursors, which might be present at lower abundance or be more difficult to detect by the methods used, we wanted H. gentianoides material with high acylphloroglucinol concentrations and that was relatively easy to obtain. To evaluate which plant material might have higher levels of acylphloroglucinols, the concentrations of major acylphloroglucinols were compared under different conditions of plant growth. For these analyses, we used accessions of H. gentianoides (Ames 27657 and PI 664838) whose seeds are being maintained within the US National Plant Germplasm System.
First, we determined whether acylphloroglucinol accumulation in H. gentianoides varied with the season of the year, using the greenhouse growth conditions we had previously established for optimal plant growth. Under these conditions, supplemental light was used to create similar 12-hour daily photoperiods throughout the year. To determine whether the season affected the level of accumulation of acylphloroglucinols, batches of H. gentianoides were planted at four-month intervals, allowed to grow six months, and vegetative shoots were harvested (harvests in February and June). The growth and morphology of these greenhouse-grown H. gentianoides plants was not visibly distinguishable, irrespective of the growth season. A comparison of acylphloroglucinol accumulation in February versus June is shown in Figure S3. The slope of the regression between the two time points was 1.01 and an R-squared value of 0.994. Thus, under these growth conditions, both the development of the H. gentianoides and the relative concentrations of each of its predominant acylphloroglucinols are similar across these dates.
Relative concentrations of acylphloroglucinols in H. gentianoides accession PI 664838 was compared at three stages of development (seedling, mature vegetative, and flowering reproductive) (Figure 1, 3a; Table S1). Reproductive phase shoots (stem, leaves and flower buds) and vegetative phase shoots (stem and leaves) were harvested from plants that had both of these stages simultaneously. Thus, levels of acylphloroglucinol accumulation were determined for vegetative and reproductive phase shoots from the same plants. The vegetative phase shoots accumulate higher amounts of all diacylphloroglucinols (p<1.9×10−6 to p<0.03) in µmol per gram fresh weight tissue (µmol g−1 FW) (Figure 3b); the least abundant major accumulating constituent, 1’3’pren45’me4’oxoPIB, is more than 10-fold higher in the vegetative phase (p<0.055).
Seedling shoots from the two standardized accessions Ames 27657 and PI 664838 were evaluated for acylphloroglucinols (Figure 3b, Table S1). PI 664838 accumulated between 2 to 7.5 fold higher amounts of each of the nine major acylphloroglucinols (p<0.0005 to p<0.039) than did Ames 27657. The greater acylphloroglucinol accumulation in seedling shoots and in the mature vegetative shoots of PI 664838, together with the shorter time to harvest of seedlings, led us to use seedling shoots from accession PI 664838 for further identification of H. gentianoides acylphloroglucinols.
Hybrid quadrupole orthogonal time-of-flight mass spectrometry (Q-TOF) detects PIB and eight other monoacylphloroglucinols
Determination of the complement of mono- and diacylphloroglucinols present in H. gentianoides could provide evidence for the biosynthetic route to the major accumulating acylphloroglucinols. Because LC/ESI-MS did not reveal potential diacylphloroglucinol intermediates, we chose a more sensitive method, hybrid quadrupole orthogonal time-of-flight mass (Q-TOF) spectrometry. This method not only detects molecules that might be undetectable using LC/ESI-MS, but also yields precise data on mass (up to 10−4 of a dalton).
To experimentally determine whether the eight acylphloroglucinol monomers that we postulated to be precursors of the diacylphloroglucinols in H. gentianoides are actually present in that species, high diacylphloroglucinol-accumulating seedlings of PI 664838 were extracted in methanol, and these extracts were diluted with 5 mM ammonium acetate in CHCl3:MeOH:IPA and analyzed by Q-TOF. All nine of the acylphloroglucinols detected by LC/ESI-MS (Table 1) were also detected by Q-TOF spectrometry (Table 2).
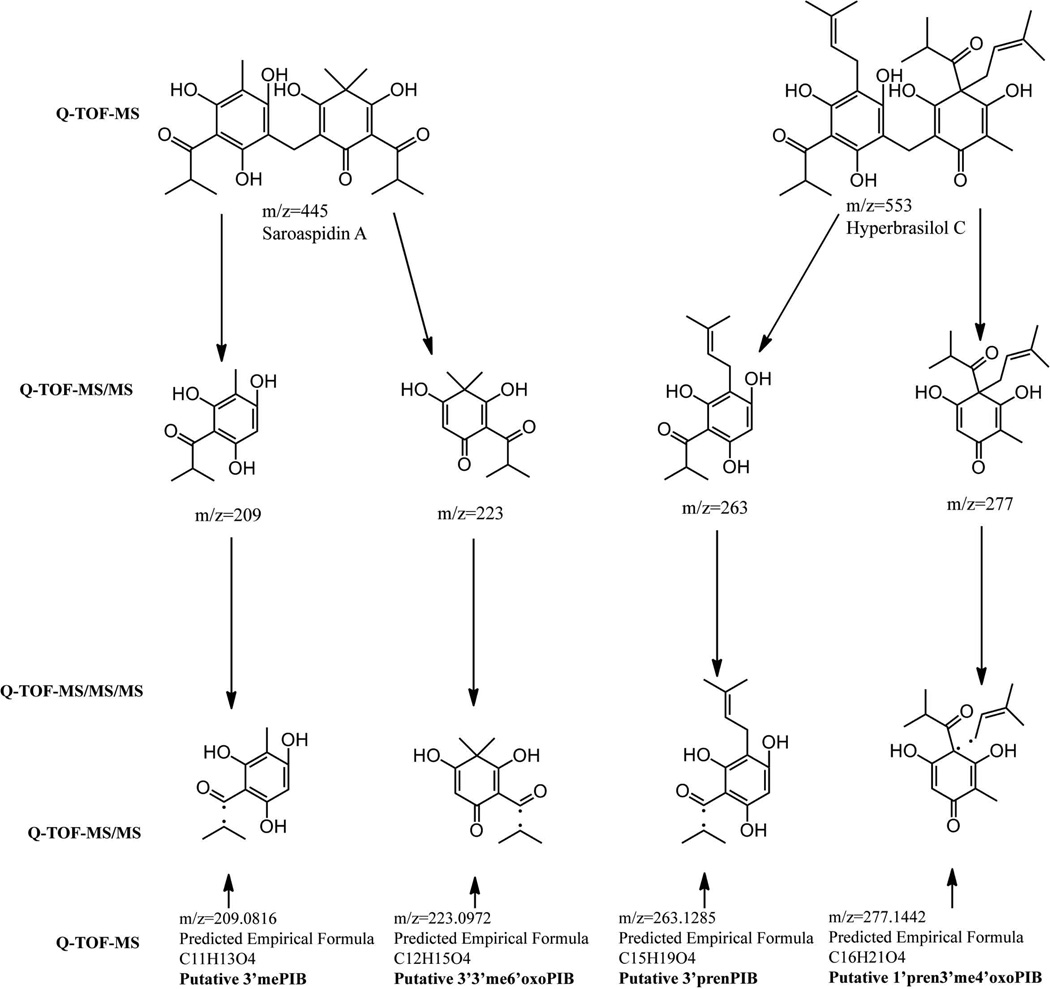
Q-TOF spectrometry also showed compounds that could not be detected by LC/ESI-MS. Notably, molecular weights and empirical formulas corresponding to all eight of the monoacylphloroglucinols that could explain synthesis of the H. gentianoides major accumulating acylphloroglucinols were identified, as was the precursor PIB and two putative partially decorated dimers (Figure 2). To provide additional evidence as to the identity of the putative monomers and partially decorated dimers, we employed Q-TOF-MS/MS on these masses (Figure S4). Because of the low abundance of MS/MS fragmentation ions at m/z values corresponding to the predicted monomers 3’4mePIB and 8ib22meC57diol, these two compounds were not included in this analysis. Using MS/MS itself does not positively identify these compounds, however, we have been able to leverage NMR data from saroaspidin A and hyperbrasilol C, which are composed of monomers 3’mePIB, 3’3’me6’oxoPIB, 3’prenPIB, and 1’pren3’me4’oxoPIB collectively (Ishiguro et al. 1987, Rocha et al. 1996; Hillwig 2008), for the identification. Our MS/MS analyses indicate the methylene bridge is the primary fragmentation target, yielding 3’mePIB and 3’3’me6’oxoPIB from saroaspidin A as well as 3’prenPIB and 1’pren3’me4’oxoPIB from hyperbrasilol C (Figure S2C, 2H). These ions were each fragmented again, and Q-TOF-MS/MS/MS was employed to identify the fragmentation patterns of 3’mePIB, 3’3’me6’oxoPIB, 3’prenPIB, and 1’pren3’me4’oxoPIB. These, in turn, were directly compared to the Q-TOF-MS/MS profiles corresponding to the predicted 3’mePIB, 3’3’me6’oxoPIB, 3’prenPIB, and 1’pren3’me4’oxoPIB. Identical fragmentation patterns confirm that the Q-TOF-MS derived masses are indeed 3’mePIB, 3’3’me6’oxoPIB, 3’prenPIB, and 1’pren3’me4’oxoPIB (Figure 4, Table 3).
Figure 4.
Q-TOF-MS/MS/MS of H. gentianoides extract to validate the identity of the predicted monomers of Table 2. The fragmentation pattern corresponding to 3’mePIB,3’3’me6’oxoPIB,3’prenPIB, and 1’pren3’me4’oxoPIB was compared to that of the previously identified uliginosin A, saroaspidin A, and hyperbrasilol C. Identical fragmentation patterns indicate that these masses in fact correspond to these four monomers and not any other isomer. MS/MS spectra at the molecular weights of the putative monomers 3’3’4me6’oxoPIB and 1’pren3’4me4’oxoPIB were analyzed (Table 3). None of the previously identified acylphloroglucinols contain either of these two monomers. Therefore these fragmentation patterns are consistent with the presence of 3’3’4me6’oxoPIB and 1’pren3’4me4’oxoPIB in H. gentianoides.
Table 3.
Molecular ions and MS/MS of putative monomers and partially decorated diacylphloroglucinols from H. gentianoides detected using Q-TOF. With the exception of 3’3’4me6’oxoPIB and 1’pren3’me4’oxoPIB, an isobutane is fragmented from the isobutyl group on all monomers. MS/MS of 3’prenPIB and 1’pren3’me4’oxoPIB show a fragmentation on the prenyl group as well. 3’4mePIB and 8ib22meC57diol are not shown due to low ion abundance. The partially decorated dimers (3’3’me6’oxoPIB and PIB and 1’pren3’me4’oxoPIB and PIB) are fragmented at the methylene bridge connecting them, similar to what is observed in the full decorated dimers. ![]()
![]()
| Compound Abbreviation | m/z | Fragment Ions (m/z) |
|---|---|---|
| PIB | 195 | 111, 151 |
| 3’mePIB | 209 | 165, 209 |
| 3’3’me6’oxoPIB | 223 | 179, 223 |
| 3’3’4me6’oxoPIB | 237 | 193, 237 |
| 3’prenPIB | 263 | 151, 194, 219, 263 |
| 1’pren3’me4’oxoPIB | 277 | 166, 208, 233 |
| 1’pren3’4me4’oxoPIB | 291 | 222 |
| [3’3’me6’oxoPIB]-[PIB] | 431 | 195, 223 |
| [1’pren3’me4’oxoPIB]-[PIB] | 485 | 195, 277 |
MS/MS analysis of these monomers indicates an isobutane is fragmented from the isobutyryl group in 3’mePIB, 3’3’me6’oxoPIB, 3’prenPIB, and 1’pren3’me4’oxoPIB. This is the only fragmentation, with the exception of the molecular ion, present in MS/MS spectra of 3’mePIB, and 3’3’me6’oxoPIB. MS/MS spectra for 3’prenPIB have four ions present at m/z=151, 194, 219, and 263 (the molecular ion). The ion at 151 m/z corresponds to fragmentation of the isobutane and prenyl group, while 194 m/z corresponds to fragmentation of only the prenyl group, and 219 m/z is a fragmentation of only the isobutane. MS/MS spectra of 1’pren3’me4’oxoPIB follow the same pattern of fragmentation as 3’prenPIB, in which the isobutyl and prenyl groups are the primary target, yielding ions at 166, 208, and 233 m/z (Figure S4). We cannot identify 3’4mePIB, 3’3’4me6’oxoPIB, 8ib22meC57diol, or 1’pren3’4me4’oxoPIB with as great a certainty as 3’mePIB, 3’3’me6’oxoPIB, 3’prenPIB, and 1’pren3’me4’oxoPIB, but the ion at m/z=291 is likely 1’pren3’4me4’oxoPIB, given the primary fragmentation ion of this compound is at m/z=222, exactly the same mass as seen in MS/MS spectra of 1’3’pren45’me4’oxoPIB. This is expected given m/z=222 would correspond to a fragmentation of one prenyl group from 1’pren3’4me4’oxoPIB and two fragmented prenyl groups from 1’3’pren45’me4’oxoPIB. Suggestive MS/MS spectra for 1’pren3’4me4’oxoPIB along with the confirmed presence of 3’mePIB, 3’3’me6’oxoPIB, 3’prenPIB, and 1’pren3’me4’oxoPIB indicate the m/z do indeed correspond to the monomers. The monomers that we observed were probably not detected in previous studies of Hypericum spp that contain diacylphloroglucinols (Taylor et al. 1969, Ishiguro et al. 1987, Rocha et al. 1996, Huang et al. 2011) because of the detection method or their lower abundance. Also identified by Q-TOF-MS/MS were the partially decorated dimers [3’3’me6’oxoPIB]-[PIB] and [1’pren3’me4’oxoPIB]-[PIB] (Table 3, Figure 2C). These partially decorated dimers are fragmented at the methylene bridge in the same way as observed for the fully decorated dimers; yielding fragmentation ions at 195 and 223 m/z for [3’3’me6’oxoPIB]-[PIB] and 195 and 277 m/z for [1’pren3’me4’oxoPIB]-[PIB] (Figure S4).
Discussion
The mono- and diacylphloroglucinols thus far identified from Hypericum spp. display a wealth of structural variety (Avato et al. 2004, Gibbons et al. 2005, Manning et al. 2011). A number of these compounds have been shown to induce diverse bioactivities when applied to other organisms (Schempp et al. 2002, Avato et al. 2004, Mennini et al. 2004, Beerhues 2006, Hillwig et al. 2008, Henry et al. 2009, Saddiqe et al. 2010, Wang et al. 2011, Stein et al. 2012, Zaher et al. 2012). St. John’s Wort contains a single monoacylphloroglucinol that has been the subjects of hundreds of medicinal studies (hyperforin), but no known diacylphloroglucinols. The biological activities of diacylphloroglucinols are a recent research focus. Uliginosin B has anti-depressive effects when given to mice before a forced swim stress test (Stein et al. 2012). Saroaspidin A, B, uliginosin A, B, and hyperbrasilol C have anti-bacterial activity properties in Staphylococcus aureus, and Bacillus cereus assays (Ishiguro et al. 1987, Rocha et al. 1995, Rocha et al. 1996, Winkelmann 2001, França and Kuster 2009). Diacylphloroglucinols can show specificity with respect to a given activity; for example, saroaspidin A is active in anti-inflammatory assays with mouse macrophage cells, while uliginosin A has little anti-inflammatory activity in these assays (Hillwig et al. 2008). These multiple bioactive effects, combined with the specificity of the action of individual acylphloroglucinols, emphasize the scope of potential medical applications for these compounds.
The major acylphloroglucinols of H. gentianoides are a single monoacylphloroglucinol and eight diacylphloroglucinols. Three of the diacylphloroglucinols, [3’mePIB]-[1’pren3’me4’oxoPIB], [3’3’4me6’oxoPIB]-[3’prenPIB], [1’pren3’4me4’oxoPIB]-[3’prenPIB], had not been previously described in any species. Identifying the acylphloroglucinol constituents of H. gentianoides is a prerequisite to deciphering the pathways by which they are biosynthesized. Ultimately, such data will be critical for identifying the primary sequences and three-dimensional structures of enzymes and regulatory proteins that control this pathway, and will provide an important step towards understanding that pathway in the context of its evolution (Schmidt et al. 2003, O’Maille et al. 2008, Ngaki et al. 2012).
From an applied aspect, the determination of the chemical nature of Hypericum polyketide constituents is important for those who use extracts of this genus as medical supplements. Furthermore, the availability of a wide variety of acylphloroglucinols will provides researchers with tools to investigate the mechanisms by which members of this class of compounds affect other organisms, and to dissect the enzymes and regulatory factors that lead to these compounds in plants. This information sets the stage for genetic engineering of custom acylphloroglucinols (Crispin and Wurtele 2013; http://www.metnetdb.org/pmr/) and other polyketides, which is significant because of the potential medicinal applications.
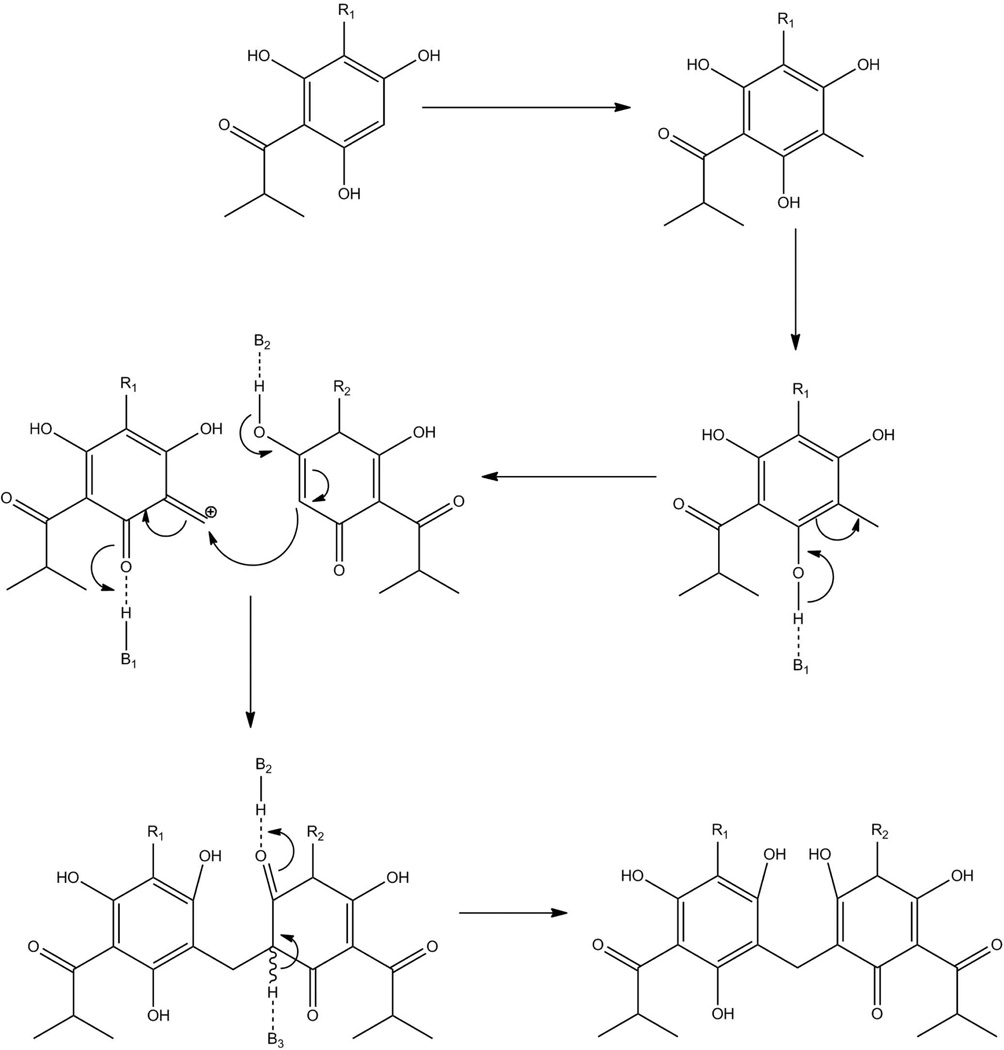
Virtually nothing had been reported previously about the pathway leading to the biosynthesis of diacylphloroglucinols. Here, we combine previous knowledge and our new understanding of the acylphloroglucinols in H. gentianoides to propose a pathway leading to the biosynthesis of diacylphloroglucinols (Figure 5) and describe a potential mechanism for dimerization (Figure 6).
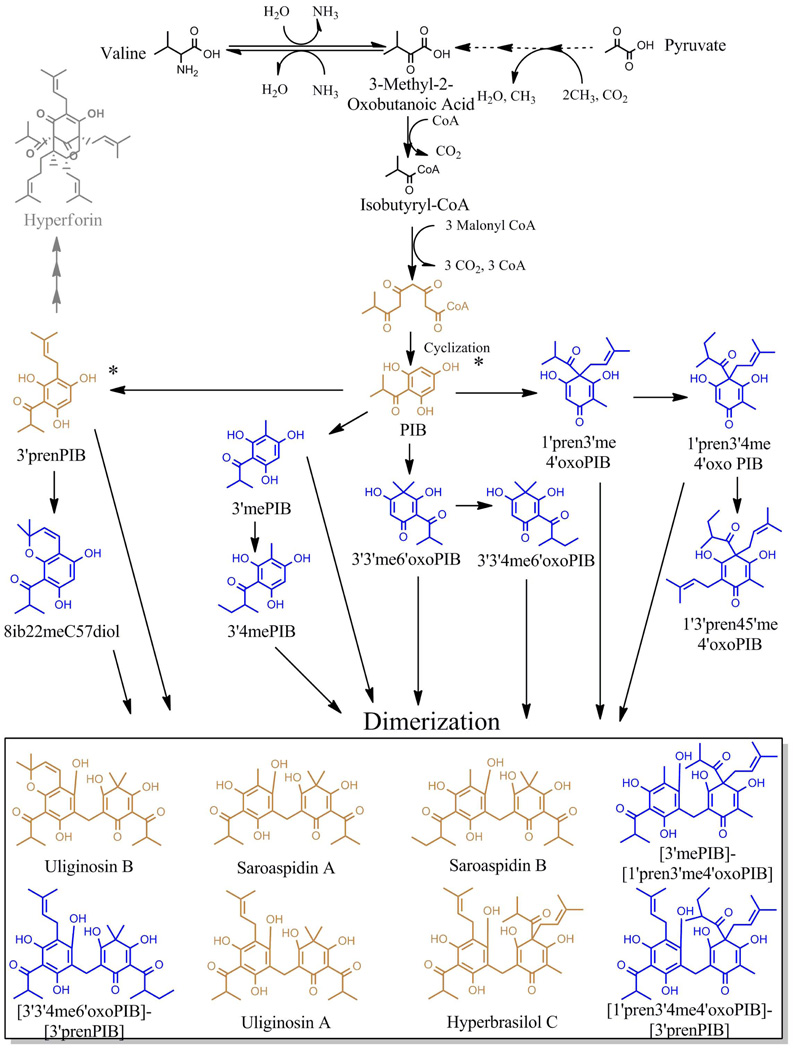
Figure 5.
Proposed biosynthetic pathway of acylphloroglucinols in Hypericum gentianoides as detected by Q-TOF-MS, Q-TOF-MS/MS and LC/ESI-MS analysis. All acylphloroglucinols involved in the biosynthesis of the nine acylphloroglucinol products are shown in this schematic. Valine and possibly pyruvate are likely primary metabolite precursors of acylphloroglucinols in H. perforatum (Karppinen 2010). Q-TOF and LC/ESI analysis indicates that H. gentianoides contains PIB and PIB derivatives that are decorated by prenylation or methylation, or are cyclized (in the case of 8ib22meC57diol). Every acylphloroglucinol dimer in H. gentianoides can be accounted for as a dimerization product of one of the detected phloroglucinol monomers and one of the detected filicinic acid monomers. Thus, the complement of monomers in H. gentianoides is consistent with the concept that monomers are first decorated and then dimerized to yield the diacylphloroglucinols . Blue, metabolites identified for the first time in this study; rust, identified in H. gentianoides (this study) and other Hypericum species (see Table 2). Hyperforin (grey) accumulates in H. perforatum (Soelberg et al. 2007). PIB* and 3’prenPIB* are postulated to be a hyperforin precursor in H. perforatum (Adam et al. 2002).
Figure 6.
Proposed mechanism of methylene bridge formation in diacylphloroglucinol biosynthesis. The formation of a diacylphloroglucinol from two monoacylphloroglucinols proceeds via a multi-step process similar to that of reticuline oxidase (Kutchan and Dittrich 1995). First one of the monomers would have to be methylated, that methyl group would become charged. A nucleophilic attack of this charged methyl group by the other monomer would form the observed methylene bridge. A third basic residue would assist in stabilizing the second monomer completing the observed diacylphloroglucinols. R1 and R2 are chemical groups corresponding to PIB decorations. B1, B2, and B3 are proposed basic residues of the enzyme involved in diacylphloroglucinol formation.
Biosynthesis of the monoacylphloroglucinol hyperforin in H. perforatum is becoming better understood (Beerhues 2003, Soelberg et al. 2007, Karppinen 2010) and provides a possible template for the initial steps of diacylphloroglucinol biosynthesis (Figure 5). PIB could be derived from either pyruvate or valine in H. perforatum (Adam et al. 2002). Isotopically labeled L-[U-13C6]-valine was preferentially incorporated into the acyl chain of hyperforin in liquid cultures of 12 day old H. perforatum, indicating that valine is likely the major precursor under this condition (Karppinen 2010). In H. perforatum, PIB is an intermediate of hyperforin (Paniego et al. 1999, Adam et al. 2002, Soelberg et al. 2007, Karppinen 2010) via its prenylation to form 3’prenPIB (Adam et al. 2002, Karppinen et al. 2007). Presumably the early pathway to acylphloroglucinols is common among Hypericum spp; consistent with this supposition, we identified PIB and 3’prenPIB in H. gentianoides. Either of two processes could yield the array of diacylphloroglucinols present in H. gentianoides. 1) PIB monomers might be decorated, and the pool of decorated monomers then dimerized (decoration of monomer pool); and/or 2) PIB monomers might be dimerized and then the dimers might be further modified (decoration of dimers)
Our observations, taken together, indicate that the synthesis of diacylphloroglucinols likely occurs via a pool of decorated monomers (Figure 5). PIB and eight other monoacylphloroglucinols, each a derivative of PIB, were detected in H. gentianoides. The H. gentianoides monomers, in different combinations, would exactly explain the presence of each of the diacylphloroglucinols. Thus, the particular complement of acylphloroglucinol monomers supports the “monomer pool” concept, in which the monomers are first decorated and then dimerized. We could not detect any completely undecorated dimers, nor six of the eight partially decorated dimers that would be expected if diacylphloroglucinol biosynthesis occurred by a process in which an “undecorated” acylphloroglucinol dimer is biosynthesized, followed by a stepwise derivatization. (We did, however, detect low concentrations of two of the eight partially decorated dimers, that would be anticipated if the “decoration of dimers” process took place, [3’3’me6’oxoPIB]-[PIB] and [1’pren3’me4’oxoPIB]-[PIB].) The presence of substantial concentrations of 1’3’pren4’5’me4’oxoPIB lends further support for the “monomer pool” concept. This acylphloroglucinol is detectable through every method we have tested, and is not able to be dimerized as the available carbons are derivatized. It would be rather convoluted to consider that, as would be predicted by the decoration of dimer model, the plant would first dimerize two PIB molecules, decorate them, break them apart, and then prenylate the fragmented half as would be necessary to synthesize this monoacylphloroglucinol. The idea that one monomer becomes fully decorated and thus accumulates as such, as would be predicted by the decoration of monomer pool model, is much more direct.
A notable difference between hyperforin and diacylphloroglucinol biosynthesis is the creation of a methylene bridge in diacylphloroglucinols. There is no reported mechanism describing this reaction, however, the mechanism for EC 1.21.3.3, which forms a methylene bridge during the formation of (S)-scoulerine from (S)-reticuline (Kutchan and Dittrich 1995), has been described. EC 1.21.3.3 has features in common with the dimerization of acylphloroglucinols, as both involve formation of a methylene bridge between two phenolic rings. Applying the EC 1.21.3.3 mechanism to diacylphloroglucinol formation, the enzymatic synthesis of a methylene bridge between two PIB molecules could proceed as shown in Figure 6.
In summary, we characterize the mono- and diacylphloroglucinols of H. gentianoides, and describe their accumulation in different biological accessions and developmental stages. The data is deposited in the public PMR metabolomics database (http://metnetdb.org/PMR/, Hur et al. 2013), enabling accessibility and extension by the research community. The data lead us to propose a network for acylphloroglucinol biosynthesis in which PIB is decorated to yield the observed monomers, followed by condensed in various combinations, giving rise to the observed major constituents. These data enhance our understanding of the diversity of this medicinal species, and set the stage for characterization of the genes and enzymes involved in acylphloroglucinol metabolism.
Supplementary Material
Acknowledgements
We thank Dr. Ann Perera, manager of the WM Keck Metabolomics Research Laboratory for her insights in liquid chromatography technologies, Dr. Mark Widrlechner for helpful advice and seeds of H. gentianoides, accession PI 664838 (http://www.ars-grin.gov/cgi-bin/npgs/acc/display.pl?1668188) and Ames 27657 (http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=Ames+27657), and Dr. Heather Babka for advice on H. gentianoides growth conditions. We are grateful to WM Keck Metabolomics Research Laboratory for use of LC/ESI-MS equipment, and to the Korea Basic Science Institute (KBSI) for high resolution mass profiling using Q-TOF mass spectrometer. This publication was made possible in part by grant NIGM-99511 from National Institute of General Medical Sciences, National Institutes of Health (NIH), and grant MCB-0951170 from the National Science Foundation.
Glossary
Abbreviations
- PIB
phlorisobutyrophenone
- 1’pren3’me4’oxoPIB
1’-prenyl-3’-methyl-4’-oxo-phlorisobutyrophenone
- 1’pren3’4me4’oxoPIB
1’-prenyl-3’,4-dimethyl-4’-oxo-phlorisobutyrophenone
- [1’pren3’4me4’oxoPIB]-[3’prenPIB]
3,5-dihydroxy-2-methyl-4-(3-methylbut-2-enyl)-4-(2-methylbutanoyl)-6-(2,4,6-trihydroxy-3-isobutyryl-5-(3-methylbut-2-enyl)benzyl)cyclohexa-2,5-dienone
- 1’3’pren45’me4’oxoPIB
1’,3’-diprenyl-4,5’-dimethyl-4’-oxo-phlorisobutyrophenone
- 3’mePIB
3’-methyl-phlorisobutyrophenone
- [3’mePIB]-[1’pren3’me4’oxoPIB]
3,5-dihydroxy-4-isobutyryl-2-methyl-4-(3-methylbut-2-enyl)-6-(2,4,6-trihydroxy-3-isobutyryl-5-methylbenzyl)cyclohexa-2,5-dienone
- 3’4mePIB
3’,4-dimethyl-phlorisobutyrophenone
- 3’3’me6’oxoPIB
3’,3’-dimethyl-6’-oxo-phlorisobutyrophenone
- 3’3’4me6’oxoPIB
3’,3’,4-trimethyl-6’-oxo-phlorisobutyrophenone
- [3’3’4me6’oxoPIB]-[3’prenPIB]
3,5-dihydroxy-4,4-dimethyl-2-(2-methylbutanoyl)-6-(2,4,6-trihydroxy-3-isobutyryl-5-(3-methylbut-2-enyl)benzyl)cyclohexa-2,5-dienone
- 3’prenPIB
3’-prenyl-phlorisobutyrophenone
- LC/ESI-MS
liquid chromatography/electrospray ionization-mass spectrometry
- MW
molecular weight
- RT
retention time
- Q-TOF
quadrupole orthogonal time-of-flight
- FW
fresh weight
References
- Adam P, Arigoni D, Bacher A, Eisenreich W. Biosynthesis of hyperforin in Hypericum perforatum . J Med Chem. 2002;45:4786–4793. doi: 10.1021/jm0209782. [DOI] [PubMed] [Google Scholar]
- Avato P, Raffo F, Guglielmi G, Vitali C, Rosato A. Extracts from St. John’s Wort and their antimicrobial activity. Phytother Res. 2004;18:230–232. doi: 10.1002/ptr.1430. [DOI] [PubMed] [Google Scholar]
- Axarlis S, Mentis A, Demetzos C, Mitaku S, Skaltsounis AL, Marselos M, Malamas M. Antiviral activity of Hypericum perforatum L. extract on the human cytomegalovirus (HCMV) Phytother Res. 1998;12:507–511. [Google Scholar]
- Beerhues L. Hyperforin. Phytochem. 2006;67:2201–2207. doi: 10.1016/j.phytochem.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Birt DF, Widrlechner MP, Hammer KDP, Hillwig ML, Wei J, Kraus GA, Murphy PA, McCoy J, Wurtele ES, Neighbors JD, Wiemer DF, Maury WJ, Price JP. Hypericum in infection: identification of anti-viral and anti-inflammatory constituents. Pharm Biol. 2009;47:774–782. doi: 10.1080/13880200902988645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss KM, Jones RH, Mitchell RJ, Mou PP. Are competitive interactions influenced by spatial nutrient heterogeneity and root foraging behavior? New Phytol. 2002;154:409–417. doi: 10.1046/j.1469-8137.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- Brenner R, Azbel V, Madhusoodanan S, Pawlowska M. Comparison of an extract of Hypericum (LI 160) and sertraline in the treatment of depression: a double-blind, randomized pilot study. Clin Ther. 2000;22:411–419. doi: 10.1016/S0149-2918(00)89010-4. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Schmidt M. St. John’s Wort: role of active compounds for its mechanism of action and efficacy. Wien Med Wochenschr. 2007;157:356–361. doi: 10.1007/s10354-007-0440-8. [DOI] [PubMed] [Google Scholar]
- Camfield DA, Sarris J, Berk M. Nutraceuticals in the treatment of obsessive compulsive disorder (OCD): a review of mechanistic and clinic evidence. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:887–895. doi: 10.1016/j.pnpbp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Caraci F, Crupi R, Drago F, Spina E. Metabolic drug interactions between antidepressants and anticancer drugs: focus on selective serotonin reuptake inhibitors and Hypericum extract. Curr Drug Metab. 2011;12:570–577. doi: 10.2174/138920011795713706. [DOI] [PubMed] [Google Scholar]
- Carpenter DJ. St. John’s Wort and S-adenosyl methionine as “natural” alternatives to conventional antidepressants in the era of the suicidality boxed warning: what is the evidence for clinically relevant benefit? Altern Med Rev. 2011;16:17–39. [PubMed] [Google Scholar]
- Chappell J. Production platforms for the molecular pharming of alkaloid diversity. Proc Natl Acad Sci USA. 2008;105:7897–7898. doi: 10.1073/pnas.0803930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispin MC, Wurtele ES. Use of metabolomics and transcriptomics to gain insights into the regulation and biosynthesis of medicinal compounds: Hypericum as a model. In: Chandra S, Lata H, Varma A, editors. Biotechnology for Medicinal Plants. Berlin Heidelberg: Springer-Verlag, HAS Pages; 2013. pp. 395–411. [Google Scholar]
- Crockett SL, Schaneberg B, Khan IA. Phytochemical profiling of new and old world Hypericum (St. John’s Wort) species. Phytochem Anal. 2005;16:479–485. doi: 10.1002/pca.875. [DOI] [PubMed] [Google Scholar]
- De Maat MMR, Hoetelmans RMW, Mathôt RAA, van Gorp ECM, Meenhorst PL, Mulder JW, Beijnen JH. Drug interaction between St. John’s Wort and nevirapine. AIDS. 2001;15:420–421. doi: 10.1097/00002030-200102160-00019. [DOI] [PubMed] [Google Scholar]
- Ernst E. Hypericum: the genus Hypericum. Boca Raton: Taylor & Francis, CRC Press; 2003. [Google Scholar]
- Fatland BL, Ke J, Anderson MD, Mentzen WI, Cui LW, Allred CC, Johnston JL, Nikolau BJ, Wurtele ES. Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis. Plant Physiol. 2002;130:740–756. doi: 10.1104/pp.008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França HS, Kuster RM. Antibacterial activity of the phloroglucinols and hexanic extract from Hypericum brasiliense Choysi. Quim Nova. 2009;32:1103–1106. [Google Scholar]
- Franklin G, Conceição LFR, Kombrink E, Dias ACP. Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry. 2009;70:60–68. doi: 10.1016/j.phytochem.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Ganzera M, Zhao J, Khan IA. Hypericum perforatum-chemical profiling and quantitative results of St. John’s Wort products by an improved high-performance liquid chromatography method. J Pharmaceut Sci. 2002;91:623–630. doi: 10.1002/jps.10057. [DOI] [PubMed] [Google Scholar]
- Gibbons S, Moser E, Hausmann S, Stavri M, Smith E, Clennett C. An anti-staphylococcal acylphloroglucinol from Hypericum foliosum . Phytochemistry. 2005;66:1472–1475. doi: 10.1016/j.phytochem.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Gustafsson MHG, Bittrich V, Stevens PF. Phylogeny of Clusiaceae based on rbcL sequences. Int J Plant Sci. 2002;163:1045–1054. [Google Scholar]
- Hamel PB, Chiltoskey MU. Cherokee plants and their uses-a 400 year history. Dublin: Herald Publishing Co; 1975. [Google Scholar]
- Hammer KDP, Hillwig ML, Solco AKS, Dixon PM, Delate K, Murphy PA, Wurtele ES, Birt DF. Inhibition of prostaglandin E2 production by anti-inflammatory Hypericum perforatum extracts and constituents in RAW 264.7 mouse macrophage cells. J Agric Food Chem. 2007;55:7323–7331. doi: 10.1021/jf0710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry GE, Campbell MS, Zelinsky AA, Liu Y, Bowen-Forbes CS, Li L, Nair MG, Rowley DC, Seeram NP. Bioactive acylphloroglucinols from Hypericum densiflorum . Phytother Res. 2009;23:1759–1762. doi: 10.1002/ptr.2845. [DOI] [PubMed] [Google Scholar]
- Hillwig ML. Understanding the biological activity of Hypericum species through metabolomic studies. Ames, Iowa: Iowa State University; 2008. PhD thesis. [Google Scholar]
- Hillwig ML, Hammer KDP, Kirt DF, Wurtele ES. Characterizing the metabolite fingerprint and anti-inflammatory activity of Hypericum gentianoides . J Agric Food Chem. 2008;56:4359–4366. doi: 10.1021/jf800411v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Rizshsky L, Hauck CC, Nikolau BJ, Murphy PA, Birt DF. Identification of anti-inflammatory constituents in Hypericum perforatum and Hypericum gentianoides extracts using RAW 264.7 mouse macrophages. Phytochemistry. 2011;72:2015–2023. doi: 10.1016/j.phytochem.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Rizshsky L, Hauck CC, Nikolau BJ, Murphy PA, Birt DF. The inhibition of lipopolysaccharide-induced macrophage inflammation by 4 compounds in Hypericum perforatum extracts is partially dependent on the activation of SOCS3. Phytochemistry. 2012;76:106–116. doi: 10.1016/j.phytochem.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur M, Cambell AA, Almeida-de-Macedo M, Li L, Ransom N, Jose A, Crispin MC, Nikolau BJ, Wurtele ES. A global approach to analysis and interpretation of metabolic data for plant natural product discovery. Nat Prod Rep. 2013 doi: 10.1039/c3np20111b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima Y, Gang DR, Fridman E, Lewinsohn E, Pichersky E. Characterization of geraniol synthase from the peltate glands of sweet basil. Plant Physiol. 2004;134:370–379. doi: 10.1104/pp.103.032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Yamaki M, Kashihara M, Takagi S. Saroaspidin A, B, and C: additional antibiotic compounds from Hypericum japonicum . Planta Med. 1987;53:415–417. doi: 10.1055/s-2006-962760. [DOI] [PubMed] [Google Scholar]
- Joray MB, Rollán MDR, Ruiz GM, Palacios SM, Carpinella MC. Antibacterial activity of extracts from plants of Central Argentina-isolation of an active principle from Achyrocline satureioides . Planta Med. 2011;77:95–100. doi: 10.1055/s-0030-1250133. [DOI] [PubMed] [Google Scholar]
- Karppinen K. Biosynthesis of hypericins and hyperforins in Hypericum perforatum L. (St. John’s Wort)-precursors and genes involved. Oulu: University of Oulu; 2010. DPhil thesis. [Google Scholar]
- Karppinen K, Hokkanen J, Tolonen A, Mattila S, Hohtola A. Biosynthesis of hyperforin and adhyperforin from amino acid precursors in shoot cultures of Hypericum perforatum . Phytochemistry. 2007;68:1038–1045. doi: 10.1016/j.phytochem.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Kasper S, Volz HP, Moller HJ, Dienel A, Kieser M. Continuation and log-term maintenance treatment with Hypericum extract WS5570 after recovery from an acute episode of moderate depression – a double-blind, randomized, placebo controlled long-term trial. Eur Neuropsychopharmacol. 2008;18:803–813. doi: 10.1016/j.euroneuro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Košuth J, Smelcerovic A, Borsch T, Zuehlke S, Karppinen K, Spiteller M, Hohtola A, Čellárová E. The hyp-1 gene is not a limiting factor for hypericin biosynthesis in the genus Hypericum . Funct Plant Biol. 2011;38:35–43. doi: 10.1071/FP10144. [DOI] [PubMed] [Google Scholar]
- Klingauf P, Beuerle T, Mellenthin A, El-Moghazy SAM, Boubakir Z, Beerhues L. Biosynthesis of the hyperforin skeleton in Hypericum calycinum cell cultures. Phytochemistry. 2005;66:139–145. doi: 10.1016/j.phytochem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Kutchan TM, Dittrich H. Characterization and mechanism of the berberine bridge enzyme, a covalently flavinylated oxidase of benzophenanthridine alkaloid biosynthesis in plants. J Biol Chem. 1995;270:24475–24481. doi: 10.1074/jbc.270.41.24475. [DOI] [PubMed] [Google Scholar]
- Manning K, Petrunak E, Lebo M, González-Sarrías A, Seeram NP, Henry GE. Acylphloroglucinol and xanthones from Hypericum ellipticum . Phytochemistry. 2011;72:662–667. doi: 10.1016/j.phytochem.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Maury W, Price JP, Brindley MA, Oh C, Neighbors JD, Wiemer DF, Wills N, Carpenter S, Hauck CC, Murphy PA, Widrlechner MP, Delate K, Kumar G, Kraus GA, Rizshsky L, Nikolau BJ. Identification of light-independent inhibition of human immunodeficiency virus-1 infection through bioguided fractionation of Hypericum perforatum . Virus J. 2009;6:101–113. doi: 10.1186/1743-422X-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum . Life Sci. 2004;75:1021–1027. doi: 10.1016/j.lfs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Ngaki M, Louie GV, Phillipe RN, Manning G, Pojer F, Bowman ME, Li L, Larsen E, Wurtele ES, Noel JP. Evolution of the chalcone isomerase fold from fatty acid-binding to stereospecific enzyme. Nature. 2012;485:530–533. doi: 10.1038/nature11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau BJ, Perera MADN, Brachoval L, Shanks B. Platform biochemicals for a biorenewable chemical industry. Plant J. 2008;54:536–545. doi: 10.1111/j.1365-313X.2008.03484.x. [DOI] [PubMed] [Google Scholar]
- Oliver DJ, Nikolau BJ, Wurtele ES. Acetyl-CoA-life at the metabolic nexus. Plant Sci. 2009;176:597–601. [Google Scholar]
- O’Maille PE, Malone A, Dellas N, Hess BA, Smentek L, Sheehan I, Greenhagen BT, Chappell J, Manning G, Noel JP. Quantitative exploration of the catalytic landscape separating divergent plant sesquiterpene synthases. Nature Chem Biol. 2008;4:617–623. doi: 10.1038/nchembio.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue S, Seto Y, Ochi M, Inoue R, Ito H, Hatano T, Yamada S. In vitro photochemical and phototoxicological characterization of major constituents of St. John’s Wort (Hypericum perforatum) extracts. Phytochemistry. 2011;72:1814–1820. doi: 10.1016/j.phytochem.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Paniego NB, Zuurbier KW, Fang SY, van der Heijden R, Scheffer JJ, Verpoorte R. Phlorisovalerophenone synthase, a novel polyketide synthase from hop (Humulus lupulus L.) Cones. Eur J Biochem. 1999;262:612–616. doi: 10.1046/j.1432-1327.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- Piovan A, Filippini R, Caniato R, Borsarini A, Maleci LB, Cappelletti EM. Detection of hypericins in the ‘red glands’ of Hypericum elodes by ESI-MS/MS. Phytochemistry. 2004;65:411–414. doi: 10.1016/j.phytochem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Reichling J, Weseler A, Saller R. A current review of the antimicrobial activity of Hypericum perforatum L. Pharmacopsychiat. 2001;34:116–118. doi: 10.1055/s-2001-15514. [DOI] [PubMed] [Google Scholar]
- Rocha L, Marston A, Potterat O, Kaplan MAC, Stoeckli-Evans H, Hostettmann K. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense . Phytochemistry. 1995;40:1447–1452. doi: 10.1016/0031-9422(95)00507-4. [DOI] [PubMed] [Google Scholar]
- Rocha L, Marston A, Potterat O, Kaplan MAC, Hostettmann K. More phloroglucinols from Hypericum brasiliense . Phytochemistry. 1996;42:185–188. doi: 10.1016/0031-9422(95)00507-4. [DOI] [PubMed] [Google Scholar]
- Saddiqe Z, Naeem I, Maimoona A. A review of the antibacterial activity of Hypericum perforatum L. J Ethnopharmacol. 2010;131:511–521. doi: 10.1016/j.jep.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Schempp CM, Kirkin V, Simon-Haarhaus B, Kersten A, Kiss J, Termeer CC, Glib B, Kaufmann T, Borner C, Sleeman JP, Simon JC. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s Wort that acts by induction of apoptosis. Oncogene. 2002;21:1242–1250. doi: 10.1038/sj.onc.1205190. [DOI] [PubMed] [Google Scholar]
- Schempp CM, Kiss J, Kirkin V, Averbeck M, Simon-Haarhaus B, Kremer B, Termeer CC, Sleeman J, Simon JC. Hyperforin acts as an angiogenesis inhibitor in vitro and in vivo. Pharmacol. 2005;71:999–1004. doi: 10.1055/s-2005-871303. [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Schauvinhold I, Larson M, Xu R, Charbonneau AL, Schmidt A, Wilkerson C, Last RL, Pichersky E. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc Natl Acad Sci USA. 2009;106:10865–10870. doi: 10.1073/pnas.0904113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Sunyaev S, Bork P, Dandekar T. Trends Biochem Sci. Metabolites: a helping hand for pathway evolution? 2003;28:336–341. doi: 10.1016/S0968-0004(03)00114-2. [DOI] [PubMed] [Google Scholar]
- Schmitt LA, Liu Y, Murphy PA, Birt DF. Evaluation of the light-sensitive Cytotoxicity of Hypericum perforatum extracts, fractions, and pure compounds. J Agric Food Chem. 2006;54:2881–2890. doi: 10.1021/jf052344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soelberg J, Jørgensen LB, Jäger AK. Hyperforin accumulates in the translucent glands of Hypericum perforatum . Ann Bot. 2007;99:1097–1100. doi: 10.1093/aob/mcm057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AC, Viana AF, Müller LG, Nunes JM, Stolz ED, Do Rago JC, Costentin J, von Poser GL, Rates SMK. Uliginosin B, a phloroglucinol derivative from Hypericum polyanthemum: a promising new molecular pattern for the development of antidepressant drugs. Behav Brain Res. 2012;228:66–73. doi: 10.1016/j.bbr.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Tachjian A, Maria V, Jahangir A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J Am Coll Cardiol. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HL, Brooker RM. The isolation of Uliginosin A and Uliginosin B from Hypericum uliginosum . J Nat Prod. 1969;32:217–219. [PubMed] [Google Scholar]
- Traynor NJ, Beattie PE, Ibbotson SH, Moseley H, Ferguson J, Woods JA. Photogenotoxicity of hypericin in HaCaT keratinocytes: implication for St. John’s Wort supplements and high dose UVA-1 therapy. Toxicol Lett. 2005;158:220–224. doi: 10.1016/j.toxlet.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Tu P, Gibon J, Bouron A. The TRPC6 channel activator hyperforin induces the release of zinc and calcium from mitochondria. J Neurochem. 2010;112:204–213. doi: 10.1111/j.1471-4159.2009.06446.x. [DOI] [PubMed] [Google Scholar]
- Wang YL, Zhang YB, He J, Zhang HD, Xiao L, Nazarali A, Zhang ZJ, Zhang D, Tan QR, Kong JM, Li XM. Hyperforin promotes mitochondrial function and development of oligodendrocytes. J Neurochem. 2011;119:555–568. doi: 10.1111/j.1471-4159.2011.07433.x. [DOI] [PubMed] [Google Scholar]
- Winkelmann KP. Phytochemical and biological investigations on Hypericum papuanum, emphasizing on structure elucidation of acylphloroglucinol derivatives by NMR spectroscopy. Zurich, Switzerland: Swiss Federal Institute of Technology Zurich; 2001. PhD thesis. [Google Scholar]
- Wu L, Dixon PM, Nikolau BJ, Kraus GA, Widrlechner MP, Wurtele ES. Metabolic profiling of Echinacea genotypes and a test of alternative taxonomic treatment. Planta Med. 2009;75:178–183. doi: 10.1055/s-0028-1112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaher M, Tang R, Bombarda I, Merhi F, Bauvois B, Billard C. Hyperforin induces apoptosis of chronic lymphocytic leukemia cells through upregulation of the BH3-only protein Noxa. Int J Oncol. 2012;40:269–276. doi: 10.3892/ijo.2011.1206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.