Abstract
The functional expression of the G protein– coupled P2Y2 nucleotide receptor (P2Y2R) has been associated with proliferation and migration of vascular smooth muscle cells (SMCs), 2 processes involved in atherosclerosis and restenosis. Activation of the P2Y2R causes dynamic reorganization of the actin cytoskeleton, which transmits biochemical signals and forces necessary for cell locomotion, suggesting that P2Y2Rs may be linked to the actin cytoskeleton. Here, we identified filamin A (FLNa) as a P2Y2R-interacting protein using a yeast 2-hybrid system screen with the C-terminal region of the P2Y2R as bait. The FLNa binding site in the P2Y2R is localized between amino acids 322 and 333. Deletion of this region led to selective loss of FLNa binding to the P2Y2R and abolished Tyr phosphorylation of FLNa induced by the P2Y2R agonist UTP. Using both time-lapse microscopy and the Transwell cell migration assay, we showed that UTP significantly increased SMC spreading on collagen I (6.8 fold; P≤0.01) and migration (3.6 fold; P≤0.01) of aortic SMCs isolated from wild-type mice, as compared with unstimulated SMCs. UTP-induced spreading and migration of aortic SMCs did not occur with cells isolated from P2Y2R knockout mice. Expression of the full-length P2Y2R in SMCs isolated from P2Y2R knockout mice restored both UTP-induced spreading and migration. In contrast, UTP-induced spreading and migration did not occur in SMCs isolated from P2Y2R knockout mice transfected with a mutant P2Y2R that does not bind FLNa. Furthermore, ex vivo studies showed that both ATP and UTP (10 µmol/L) promoted migration of SMCs out of aortic explants isolated from wild-type but not P2Y2R knockout mice. Thus, this study demonstrates that P2Y2R/FLNa interaction selectively regulates spreading and migration of vascular SMCs.
Keywords: cytoskeleton, smooth muscle cell, migration, nucleotide receptor
Migration of vascular smooth muscle cells (SMCs) plays a key role in the development of prominent vascular diseases, including atherosclerosis and restenosis after angioplasty.1–2 Extracellular nucleotides released in the vascular wall from perivascular nerves, activated platelets, and mechanically stretched cells3–5 are the focus of ongoing studies aimed at determining the role of released nucleotides in the development of vascular diseases. Previous studies have indicated that extracellular nucleotides serve as directional cues for the migration of rat SMCs in vitro through activation of G protein– coupled P2Y receptors.6 Upregulation and activation of the P2Y2 receptor (P2Y2R) subtype has been shown to stimulate neointimal growth in the collared rabbit carotid artery,7 suggesting an important role for this receptor in intimal hyperplasia and vascular remodeling.
As with many other cellular processes, the molecular components involved in SMC migration are being identified at a rapid rate but, like most cell functions, the manner in which these components work together as a dynamic, integrated system is less well understood. Although it has been shown that activation of P2Y receptors induces migration of SMCs through increased production of osteopontin,6 an extracellular matrix protein that interacts with integrins, it is not known whether the P2Y2R interacts with intracellular components that modulate the migration process. Activation of P2Y receptors by nucleotides has been shown to induce reorganization of the actin cytoskeleton in vascular SMCs.8 One of the earliest responses to extracellular signals is a rapid reorganization of the actin cytoskeleton, which leads to the formation of motile structures, alterations in cell shape, and changes in cell adhesiveness and locomotion. Actin-binding proteins play a pivotal role in reorganizing the actin cytoskeleton in response to signals exchanged between cells. Accordingly, actin-binding proteins are increasingly a focus of investigations into effectors of cell signaling and the coordination of cellular functions that regulate tissue development. Using the yeast 2-hybrid system, we report for the first time that an interaction occurs between the cytoplasmic domain of the P2Y2R and filamin A (FLNa), an actin-binding protein that crosslinks filamentous actin into either orthogonal or parallel bundles.9 FLNa is required for cell migration,9 and mutations in human FLNa are responsible for impaired migration of cerebral neurons and give rise to periventricular heterotopia, leading to epilepsy and vascular disorders, as well as embryonic lethality.10
FLNa anchors actin filaments to cell– extracellular matrix adhesion sites by binding to β-integrin subunits11 and also provides a scaffold for small GTPases of the Ras and Rho families.12 We characterized the interaction between FLNa and the P2Y2R in biochemical assays and used wild-type (WT) and P2Y2R knockout (KO) mice to show that the loss of this interaction affects spreading and migration of aortic SMCs.
Materials and Methods
Plasmid Constructs
All cDNAs were generated by PCR. The sequence and insert orientation of all constructs were verified by DNA sequencing at the DNA Core Facility of the University of Missouri, Columbia, which are detailed in the online data supplement at http://circres.ahajournals.org.
Mutagenesis and Epitope Tagging of the P2Y2R
The open-reading frame of P2Y2R cDNA was modified to incorporate the hemagglutinin (HA) epitope (YPYDVPDYA) at the amino terminus of the recombinant protein, as described previously.13 Six C-terminal truncation mutants of the P2Y2R were generated by PCR. An expanded description of the mutagenesis can be found in the online data supplement.
Yeast Two-Hybrid Library Screening
A cDNA library from human aorta constructed in the GAL4 activation domain vector pACT2 was purchased from BD Biosciences (Palo Alto, Calif). The cDNA library was cotransformed with the bait plasmid pGBKT7-P2Y2RCTD into Saccharomyces cerevisiae strain AH109 following the instructions of the manufacturer (BD Biosciences). The interaction between bait and prey proteins was determined using the β-galactosidase assay, as described in the Yeast Protocol Handbook (Clontech Laboratories, Palo Alto, Calif). Positive prey plasmids were extracted from yeast and transformed into Escherichia coli TOP10 chemically competent cells (Invitrogen, Carlsbad, Calif). The identity of the prey was determined by DNA sequencing with the 5′-pACT2 primer 5′-CTA TTC GAT GAT GAA GAT ACC CCA CCA AAC CC-3′ and the 3′-pACT2 primer 5′-GTG AAC TTG CGG GGT TTT TCA GTA TCT ACG A-3′ and the Basic Local Alignment Search Tool (BLAST) against the human protein database (tblastx; http://www.ncbi.nlm.nih.gov/BLAST). To confirm the interaction between bait and prey in the yeast 2-hybrid system, purified prey plasmids were retransformed with the bait plasmid or empty pGBKT7 and tested for growth on the same plates.
Glutathione S-Transferase Pull-Down Assay
The glutathione S-transferase (GST) fusion protein pull-down assay was carried out according to the instructions of the manufacturer (Pierce Biotechnology, Rockford, Ill). A brief description can be found in the online data supplement.
Cell Transfections
Transfection of 1321N1 cells with WT of mutant P2Y2R cDNA was performed using Lipofectamine 2000 (Invitrogen) following the instructions of the manufacturer. For transfection of SMCs, full-length and truncated P2Y2R constructs were inserted into the adenoviral vector Ad5mCMV. Virus production was performed at the Gene Transfer Vector Core at the University of Iowa (Iowa City). Mouse aortic SMCs, at 70% to 80% confluence, were incubated for 4 hours with adenoviruses at 200 plaque-forming units per cell in growth medium comprised of DMEM, 2% FBS, 100 IU/mL penicillin, and 100 µg/mL streptomycin. Then, the cells were washed and cultured in fresh growth medium for 24 to 48 hours to allow protein expression. FLNa protein knockdown was performed using the FLNa SMARTpool small interfering (si)RNA (5′-GCUCAGAGGUAGACGUGGAUU-3′; 5′-CCAGCAAGGUGAAGGCGUUUU-3′; 5′-CCAGCAAGGUGAAGGCGUUUU-3′; 5′-CAUUGAGGGUCCAUCUAAAUU-3′) purchased from Dharmacon (Lafayette, Colo) and transfected into mouse aortic SMCs using Nucleofector electroporator and Basic Nucleofector Kit (Primary Smooth Muscle Cells) (Amaxa Inc, Gaithersburg, Md) according to the instructions of the manufacturer. Cells (1×106) were transfected with 0.2 nmol of FLNa siRNA or control scrambled siRNA (5′-AUGAACGUGAAUUGCUCAA-3′; 5′-UAAGGCUAUGAAGAGAUAC-3′; 5′-AUGUAUUGGCCUGUAUUAG-3′; 5′-UAGCGACUAAACACAUCAA-3′). Cells were then cultured in growth medium for 48 hours to allow protein suppression.
Immunoprecipitation and Western Blot Analysis
Methods for immunoprecipitation and Western Blot Analysis are described in detail in the online data supplement.
Cell Culture
Mouse aortic SMCs were obtained from 4- to 6-week-old mice, which are detailed in the online data supplement.
SMC Migration in Aortic Explants
P2Y2R KO mice on a B6D2 genetic background and the corresponding WT mice were generous gifts from Dr B. H. Koller (University of North Carolina, Chapel Hill), and genotypes were verified by a tail-snip PCR. Descending mouse aorta was thoroughly dissected free from connective tissue, cut open longitudinally, and the intima and a thin portion of the subjacent media were removed. The descending aorta was weighed and trimmed to normalize the weight to 5 mg. The aorta was cut into 4 pieces, and each piece was placed in a corner of a chambered slide with the endothelium facing down and cultured in DMEM containing appropriate concentrations of nucleotides or supplemented with 10% (vol/vol) FBS. For the creatine phosphokinase (CPK) regenerating system, cells were pre-incubated with agonist for 10 min, and then 2 U mL−1 CPK and 1 mmol/L phosphocreatine were added. Medium was replaced every day for 5 days before analysis of the explants by confocal microscopy. To count cells that migrated out of the explant, the remaining tissue was removed, and cells that had migrated away from the explant were detached by 0.05% trypsin–EDTA (Invitrogen) and counted under a microscope.
Cell Migration Assay
Cell migration assays were performed on Transwells (Costar), as previously described,6 and are outlined in the online data supplement.
Cell Spreading Assay and Time-Lapse Microscopy
Cell spreading was determined by measuring the number of cells that spread after a defined time, as described previously.14 Coverslips placed in welled plates were coated with type I collagen (20 µg/mL) overnight at 4°C. To suppress nonspecific binding, coverslips were blocked with 1% (wt/vol) BSA in PBS at 37°C for 1 hour. Cells in DMEM containing 0.4% (vol/vol) FCS were added at a concentration of 2000 cells per 100 µL and maintained at 37°C for 1 hour in a POC-R chamber inside a preheated microscope stage insert (LaCon, Staig, Germany). Then, cell spreading was followed by time-lapse microscopy to monitor movement of cells containing nuclei or with a recognizable noncircular shape using an Axiovert 200M inverted microscope equipped with a ×40/0.6 NA LD Plan-Neofluar objective (LSM 510 Meta System, Zeiss, Thornwood, NY). Four fields of view for each coverslip were chosen by visual inspection, and the position of the stage in each field was marked by the microscope control software (Zeiss LSM 5 version 4.0). At different time points, the microscope stage was positioned at each marked location, the fields were focused manually, and cells were imaged by detecting transmitted light while scanning with a 488-nm laser.
Statistical Analysis
ANOVA and paired and unpaired t tests were performed for statistical analysis, as appropriate. Values of P<0.05 were considered to be statistically significant.
Results
FLNa Interacts Directly With Amino Acids 322 to 333 in the C Terminus of the P2Y2R
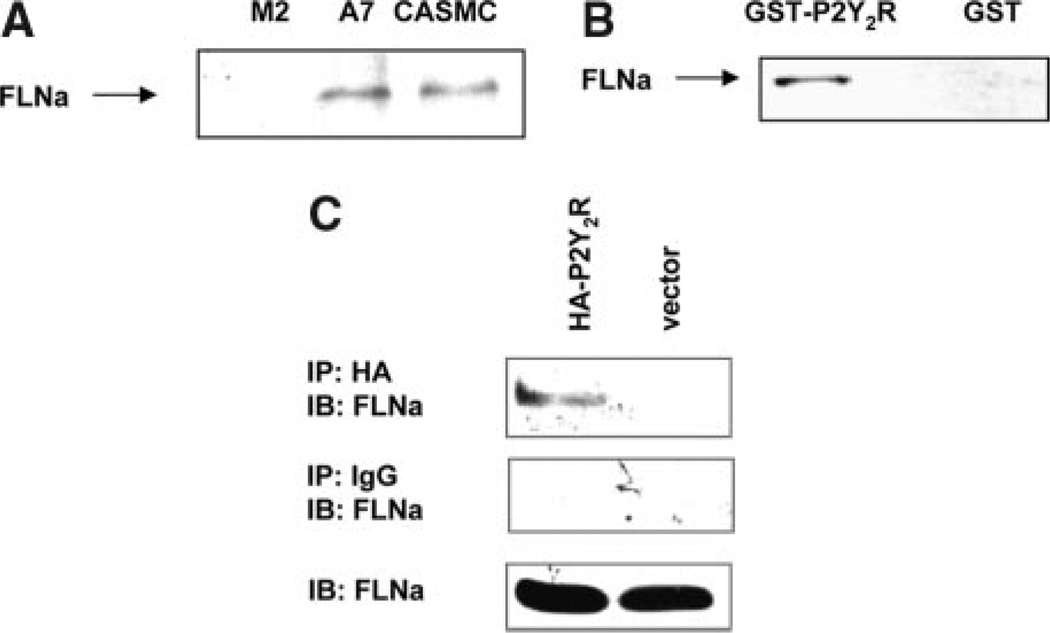
Extracellular nucleotides have been shown to induce reorganization of the actin cytoskeleton,8 a key step in cell migration. Because P2Y2R activation causes SMC migration, we hypothesized that the P2Y2R is directly linked to the actin cytoskeleton. Thus, we used a 45-aa fragment (amino acids 318 to 362) in the intracellular C-terminal tail of the P2Y2R (ie, P2Y2RCTD) as bait to screen for binding of proteins expressed from a human aorta cDNA library using the AH109 yeast 2-hybrid system. Screening of 106 independent clones yielded P2Y2RCTD-binding proteins in several clones expressing an identical 1.2-kb cDNA insert that encoded the C-terminal region of FLNa, a dimeric actin-crosslinking phosphoprotein that promotes orthogonal branching of actin filaments. The specificity of the interaction between P2Y2RCTD and FLNa was verified by 1-on-1 transformation into yeast strain AH109. To further verify that a specific interaction occurs between the P2Y2RCTD and FLNa, we tested the ability of a glutathione S-Transferase (GST) fusion protein containing the C terminus of the P2Y2R (GSTP2Y2RCTD) to associate with endogenously expressed full-length FLNa. Human coronary artery SMC (CASMC) lysates contained a 280-kDa protein that immunoreacted with anti-FLNa antibody (Figure 1A). As shown in Figure 1B, this protein also was detected in pull-down assays when CASMC lysates were incubated with GST-P2Y2RCTD, but not GST, immobilized by glutathione-linked Sepharose. To verify that full-length P2Y2R and FLNa interact in mammalian cells, we transfected P2 receptor–null 1321N1 astrocytoma cells with cDNA encoding the HA-tagged P2Y2R, then immunoprecipitated HA-P2Y2R from cell lysates using anti-HA antibody and detected coprecipitation of FLNa using anti-FLNa antibody (Figure 1C). To identify the domain in the C-terminal tail of the P2Y2R that interacts with FLNa, cDNA encoding progressive deletion mutants of the C-terminal domain (CTD) of the P2Y2R were generated by PCR using specific primers (Figure 2A). All of the P2Y2R mutants expressed in 1321N1 cells showed the same ability as the WT P2Y2R to mediate UTP-induced intracellular calcium mobilization (data not shown). HA-tagged P2Y2R or P2Y2RCTD deletion mutants expressed in 1321N1 cells were immunoprecipitated with anti-HA antibody, and coprecipitation of FLNa was assessed. As indicated in Figure 2B, deletion of amino acids 322 to 333 results in the loss of FLNa binding to the HA-P2Y2R.
Figure 1.
FLNa binds to the P2Y2R CTD and coimmunoprecipitates with the HA-P2Y2R. A, CASMC lysates were analyzed by SDS-PAGE and immunoblotted by goat anti-FLNa antibody. M2 melanoma cells (kindly provided by Dr J.H. Hartwig, Harvard Medical School, Boston, Mass), which are deficient in FLNa, were used in parallel assays as a negative control, and FLNa transfected cells (A7 cells) were used as a positive control. Results are representative of 3 independent experiments. B, Immobilized GST-P2Y2RCTD or GST was incubated with CASMC lysates (107 cells) overnight at 4°C, bound proteins were eluted with 100 mmol/L glutathione, and Western analysis was performed with anti-FLNa antibody. Results are representative of 3 independent experiments. C, Human 1321N1 cells transfected with cDNA encoding the HA-tagged P2Y2R or with the pLXSN vector alone were lysed and immunoprecipitated (IP) with anti-HA affinity matrix or normal IgG, and immunoprecipitates were analyzed by immunoblotting (IB) with anti-FLNa antibody.FLNa levels in whole cell lysates are shown (bottom). Results are representative of 3 independent experiments.
Figure 2.
Identification of the FLNa binding domain of the P2Y2R. A, WT and progressive C-terminal truncation mutants of the HA-tagged P2Y2R were expressed in human 1321N1 astrocytoma cells. B,Lysates from transfected cells were immunoprecipitated (IP) with anti-HA affinity matrix, andimmunoprecipitates were analyzed by immunoblotting (IB) with anti-FLNa antibody. FLNa levels in whole cell lysates were used to normalize FLNa-binding data (means±SEM) from 3 independent experiments (bar graphs). **P<0.01 as compared with WT control, with a representative blot shown on the left.
P2Y2R Activation Mediates FLNa Phosphorylation
Phosphorylation and dephosphorylation events are thought to regulate FLNa activity,15 and therefore we determined whether P2Y2R activation by UTP in mouse aortic SMCs induces the phosphorylation of FLNa. For this purpose, adenoviruses expressing the full-length P2Y2R or the mutant P2Y2R lacking the FLNa binding amino acids 322 to 333 (ie, truncation mutant 5) were transfected in aortic SMCs isolated from P2Y2R KO mice. UTP (10 µmol/L) stimulation of SMCs expressing the full-length P2Y2R, but not truncation mutant 5 P2Y2R, caused a rapid and sustained increase in Tyr phosphorylation of FLNa (Figure 3A). Studies have shown that phosphorylation at Ser/Thr residues, namely at Ser2152, are involved in FLNa activation.23 Therefore, we investigated whether UTP could induce FLNa phosphorylation at Ser2152. Using a specific antibody that recognizes FLNa phosphorylation at Ser2152, we demonstrated that UTP (10 µmol/L) stimulation of SMCs expressing the full-length P2Y2R caused a rapid increase in Ser2152 phosphorylation of FLNa. In contrast, UTP did not cause FLNa phosphorylation of Ser2152 in SMCs expressing the mutant (Figure 3B). Because previous data have indicated that extracellular signal-regulated kinase (ERK) pathways lead to ribosomal protein S6 kinase phosphorylation and potentially to FLNa activation, we sought to determine whether truncated P2Y2R lacking the FLNa-binding site could induce ERK1/2 activation. As shown in Figure 3C, UTP (10 µmol/L) stimulation induces ERK1 activation in SMCs expressing the full-length P2Y2R, but not in SMCs expressing the truncated P2Y2R, indicating that ERK1/2 pathways may be important in UTP-mediated FLNa phosphorylation.
Figure 3.
P2Y2R/FLNa interaction is required for UTP-induced phosphorylation of FLNa and ERK1/2. SMCs from aortas of P2Y2R KO mice were infected with adenoviruses expressing the WT or truncation mutant 5 P2Y2R (Figure 2A). Infected cells were serum starved for 48 hours and stimulated with UTP (10 µmol/L) for the indicated time. A, Cell lysates were immunoprecipitated (IP) with anti–phospho-tyrosine antibody, and immunoprecipitates were analyzed by immunoblotting (IB) with anti-FLNa antibody. B, Cell lysates were immunoblotted with anti–phospho-FLNa (Ser2152) antibody (Millipore Corp). Total FLNa levels in cell lysates were used for normalization. C, Lysates were immunoblotted with antibody against phospho-ERK1 (Millipore Corp). Total ERK1/2 levels in cell lysates were used for normalization. Data represent means±SEM of results from 3 independent experiments. *P<0.05, **P<0.01 as compared with WT P2Y2R at 0 min.
P2Y2R and FLNa Interaction Promotes SMC Spreading
To gain further insight into the role of P2Y2Rs in vascular SMC function, we studied the ability of UTP to induce the spreading of mouse aortic SMCs on collagen I. Treatment of aortic SMCs isolated from WT mice with UTP for 2 hours caused a 6.8-fold increase (P≤0.01) in the spreading of cells on collagen I, as compared to unstimulated cells (Figure 4). In contrast, UTP did not increase spreading of aortic SMCs isolated from P2Y2R KO mice. To test the role of P2Y2R/FLNa interaction in UTP-induced cell spreading, we transfected aortic SMCs obtained from P2Y2R KO mice with adenoviruses expressing either full-length (WT) or truncation mutant 5 P2Y2R cDNA, and successful receptor expression was demonstrated by the appearance of UTP-induced calcium mobilization and P2Y2R mRNA in transfected cells (data not shown). Expression of the full-length P2Y2R in aortic SMCs from P2Y2R KO mice completely restored UTP-induced cell spreading, whereas cells that expressed the truncation mutant 5 P2Y2R exhibited significantly less spreading (Figure 4). These results indicate that the P2Y2R C-terminal tail is required for optimum UTP-induced SMC spreading.
Figure 4.
Spreading of SMCs on collagen-coated coverslips. Aortic SMCs from WT or P2Y2R KO mice were plated on collagen I–coated coverslips and then were incubated for 1 hour in serum-free medium with or without UTP (10 µmol/L). A, Cell spreading was visualized with an Axiovert 200M inverted microscope, and a representative micrograph is shown with arrows indicating spreading cells. B, Aortic SMCs from WT or P2Y2R KO mice were treated as above, and cell spreading was visualized by time-lapse microscopy. Also, aortic SMCs from P2Y2R KO mice were transfected with cDNA encoding WT or truncation mutant 5 P2Y2R. Then, cell transfectants were incubated with or without UTP (10 µmol/L) for 1 hour, and the percentage of cells exhibiting UTP-induced cell spreading was determined by time-lapse microscopy. Data represent means±SEM for 3independent experiments performed in triplicate. **P<0.01, as compared with the indicated condition.
P2Y2R and FLNa Interaction Mediates SMC Migration
UTP increased by 3.4-fold (P≤0.01) the migration of aortic SMCs isolated from WT mice, as compared with unstimulated SMCs or UTP-stimulated SMCs from P2Y2R KO mice (Figure 5A). Adenoviral expression of the full-length P2Y2R in aortic SMCs from P2Y2R KO mice increased UTP-induced cell migration (Figure 5B) to levels seen with SMCs from WT mice (Figure 5A). In contrast, aortic SMCs from P2Y2R KO mice expressing the truncation mutant 5 P2Y2R that is defective in FLNa binding did not exhibit UTP-induced cell migration (Figure 5B), indicating that loss of P2Y2R/FLNa interaction prevents UTP-induced SMC migration.
Figure 5.
Loss of FLNa/P2Y2R interaction affects UTP-induced SMC migration. A, Aortic SMCs from WT or P2Y2RKO mice were added to the upper chamber of Transwells. The lower chamber contained serum-free DMEM with or without UTP (10 µmol/L). Cell migration expressed as the number of cells migrating across the membrane was evaluated after 8 hours. B, Aortic SMCs from P2Y2R KO mice expressing WT or truncation mutant 5 P2Y2R, or vector alone were assayed for UTP-induced cell migration, as described above. Incubation for 8 hours with10% FBS in the lower chamber was used as a positive control. Data with UTP and FBS are expressed as fold increase over untreated control and represent means±SEM of results from 3 independent experiments. **P<0.01.
FLNa Plays a Critical Role in P2Y2R-Mediated Spreading and Migration of SMCs
To determine whether FLNa plays a role in P2Y2R-mediated SMC spreading and migration, we used siRNA to knockdown FLNa levels in these cells. Western blot analysis revealed that cells transfected with FLNa siRNA displayed more than a 90% reduction in FLNa protein expression (Figure 6A) and did not exhibit morphological alterations displayed by M2 FLNa-deficient cells (data not shown).9 In scrambled siRNA–treated aortic SMCs, stimulation with UTP for 2 hours caused a 6.8-fold increase (P≤0.01) in the spreading of cells on collagen I, as compared with unstimulated cells (Figure 6B). In contrast, UTP-induced SMC spreading was almost completely abolished in siRNA-treated cells (Figure 6B). Moreover, UTP did not cause cell migration when FLNa expression was suppressed with FLNa siRNA in SMCs expressing the full-length P2Y2R (Figure 6C). These data demonstrate that FLNa plays a critical role in P2Y2R-mediated SMC spreading and migration.
Figure 6.
FLNa siRNA suppresses UTP-induced SMC spreading and motility. SMCs from aortas of WT mice were transfected with siRNA targeting FLNa or scrambled siRNA. A, The lysates of cell transfectants were immunoblotted with anti-FLNa antibody. B and C,Cell spreading and motility of SMCs in response to UTP or FBS. Data represent means≤SEM for 3 independent experiments. **P<0.01 as compared with the indicated condition.
P2Y2Rs Mediate SMC Migration Out of Mouse Aortic Explants
To validate our in vitro data, we investigated whether extracellular nucleotides serve as directional cues for SMCs within a vessel fragment. Therefore, we examined the ability of SMCs to migrate out of aortic explants isolated from WT and P2Y2R KO mice. The P2Y2R agonists ATP or UTP (10 µmol/L) induced significant migration of SMCs out of aortic explants isolated from WT but not P2Y2R KO mice, as compared to untreated controls (Figure 7A and 7B). ATP and UTP also increased SMC migration out of explants in the presence of 2 U/mL CPK and 1 mmol/L phosphocreatine (Figure 7B), a system for regenerating nucleoside triphosphates from cell-hydrolyzed ATP or UTP, suggesting that ectonucleotidase-mediated nucleoside (ie, adenosine) generation was not responsible for cell migration induced by ATP or UTP. In contrast to ATP and UTP, 5% serum was equally effective in stimulating SMC migration out of aortic explants from both WT and P2Y2R KO mice (Figure 7B). UDP and 2MeS-ADP did not induce SMC migration out of the explants (data not shown). These data indicate that ATP and UTP promote migration of SMCs from aortic tissue via activation of P2Y2Rs.
Figure 7.
Migration of SMCs out of mouse aortic explants. Aortic explants were obtained from WT and P2Y2R KO mice and cultured for 4 hours in DMEM with or without ATP or UTP (10 µmol/L) or 5% (vol/vol) FBS. Some explants were also cultured for 4 hours with a nucleotide regenerating system (CPK). Then, medium was replaced daily with DMEM alone and after 5 days, and cells growing out of the aortic explants were visualized with an Olympus IX70 inverted microscope. A, Micrographs from a representative experiment are shown. Arrows indicate the direction of cell migration. Magnification, ×20. B, SMCs migrating from aortic explants treated as above were removed after 5 days by incubation with trypsin–EDTA solution. The number of cells migrating from explants was determined in 3 independent experiments performed in triplicate. Data with ATP, UTP, FBS, and CPK are expressed as fold increases over untreated control and represent means±SEM of results from 3 independent experiments. **P<0.01.
Discussion
In the present study, we demonstrated that extracellular nucleotides serve as directional cues not only for cultured SMCs but also for SMCs within a vessel segment, suggesting that nucleotides released in vivo at the site of vessel injury could participate in the remodeling process accompanying prominent vascular diseases. We showed that the nucleotides ATP and UTP act directly on P2Y2Rs, because nucleotides were not effective in promoting the motility of SMCs derived from the P2Y2R KO mouse. Furthermore, we showed that ATP and UTP significantly increased the spreading of mouse aortic SMCs on collagen I, a process that involves rearrangement of the cytoskeleton and increased contractility of vascular SMCs.11 Both cytoskeletal rearrangement and contraction of SMCs are essential for the control of vascular remodeling and repair mechanisms that are induced by increased shear stress or vascular damage.15 To date, this is the first report demonstrating that ATP and UTP promote spreading of vascular SMCs.
Nucleotides act on P2Y receptors expressed in vascular SMCs to promote actin cytoskeletal reorganization in a Rho kinase– dependent manner.8 Furthermore, P2Y2R mediates UTP-induced cell migration and Rho/Rac activation.16–17 This prompted us to investigate whether the P2Y2R is directly linked to the actin cytoskeleton. Using a yeast 2-hybrid system screen and in vitro and in vivo binding assays, we demonstrated that the scaffold protein FLNa interacts directly with the P2Y2R. FLNa is an actin-crosslinking protein and is involved in the maintenance of the cytoskeletal architecture.9 FLNa functions as a dimer and is composed of 23 approximately 96-aa-long repeats flanked by an N-terminal actinbinding domain and a C-terminal homodimerization domain that regulate organization of the cortical actin network.9 FLNa is essential for mammalian cell locomotion.9 Human melanoma cell lines that do not express detectable levels of FLNa do not migrate, and expression of FLNa in these cells restores their locomotion.9 FLNa is known to be a phosphoprotein,18 but it is not understood how phosphorylation regulates FLNa function. In the present study, we found that stimulation of quiescent SMCs with UTP results in a rapid increase in the phosphorylation of FLNa (Figure 3A and 3B). FLNa is targeted by a variety of signaling molecules, eg, Src, ribosomal S6 kinase, protein kinase C, and small GTPases of the Rho and Rac families, which thereby implicate FLNa in cell shape modulation and integrin-dependent adhesion and motility.18–22 Previous results have shown that p21-activated kinase (PAK)-1 mediates FLNa phosphorylation in MCF-7 breast cancer cells.23 Rac1, an activator of PAK-1, has been found to be activated by P2Y2R in vascular endothelial cells.24 We have found that UTP induces PAK activity in mouse SMCs (unpublished data), suggesting a possible role of PAK-1 in UTP-induced FLNa phosphorylation. Activation of the P2Y2R can cause Tyr phosphorylation of Src, a kinase family that is known to phosphorylate FLNa on Tyr residues.22,25 Further studies are needed to define the role of Src and PAK-1 in P2Y2R-mediated FLNa phosphorylation. FLNa is also known to be phosphorylated at Ser/Thr residues, namely at Ser2152, which requires ERK1/2 activation.21 Accordingly, we showed that UTP causes FLNa phosphorylation at Ser2152 (Figure 3B) in SMCs expressing the full-length P2Y2R, but not in cells expressing the truncation mutant P2Y2R. Interestingly, our data showed that the ERK1/2 pathway is not activated in SMCs expressing the mutant P2Y2R, which does not bind FLNa. The significance of FLNa phosphorylation at Tyr or Ser/Thr residues is not well understood, although it may be a mechanism by which FLNa regulates different cellular functions such as control of cell motility or interaction with other membrane proteins.
The biological significance of the loss of P2Y2R/FLNa interaction was a major focus of the present study. We have identified amino acids in the CTD of the P2Y2R that are required for P2Y2R/FLNa interaction and UTP-induced SMC migration (Figures 2 and 5). Our data indicate that UTP did not induce spreading and migration of aortic SMCs isolated from P2Y2R KO mice, and expression of the P2Y2R in these cells restored nucleotide-induced SMC spreading and migration (Figures 4 and 5). In further support of a role for P2Y2R/FLNa interaction in nucleotide-induced SMC spreading and migration, expression in SMCs from P2Y2R KO mice of a mutant P2Y2R that does bind FLNa does not restore cell spreading and migration in response to UTP, as compared with expression of the WT P2Y2R (Figures 4 and 5). Because loss of the P2Y2R/FLNa interaction also prevented UTP-induced FLNa phosphorylation in mouse aortic SMCs (Figure 3A and 3B), we conclude that activation of FLNa is a prerequisite for P2Y2R-mediated spreading and migration of SMCs.
Rearrangement of actin filaments plays an essential role in cell growth, survival, locomotion, and shape. For example, cell cycle progression depends on cell spreading and adhesion, whereas apoptosis is initiated after cells are detached.26–27 In cardiovascular diseases, such as hypertension, atherosclerosis, and restenosis after angioplasty, vascular remodeling clearly requires rearrangement of vascular SMC cytoskeleton.15,28 Nucleotides are thought to play important roles in cell remodeling by virtue of their effects on SMC migration and growth.29 P2Y2Rs are highly expressed in rat and rabbit intimal lesions,7,30 and, therefore, P2Y2R interactions with FLNa may play an important role in the regulation of vascular remodeling and tone by modulating SMC migration in response to nucleotides released at the site of vascular injury.
Supplementary Material
Acknowledgments
We thank Dr G. Esteban Fernandez (Molecular Cytology Core, University of Missouri, Columbia) for assistance with time-lapse microscopy.
Sources of Funding
This work was supported by an American Heart Association Predoctoral Fellowship (to N.Y.) and the NIH Program Project grant AG18357 and R01 grants DE07389 and DE017591 (to G.A.W., L.E., and C.I.S.).
Footnotes
Disclosures
None.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RS. Pathophysiology of restenosis: interaction of thrombosis, hyperplasia, and/or remodeling. Am J Cardiol. 1998;81:14E–17E. doi: 10.1016/s0002-9149(98)00191-x. [DOI] [PubMed] [Google Scholar]
- 3.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 4.Hamada K, Takuwa N, Yokoyama K, Takuwa Y. Stretch activates Jun N-terminal kinase/stress-activated protein kinase in vascular smooth muscle cells through mechanisms involving autocrine ATP stimulation of purinoceptors. J Biol Chem. 1998;273:6334–6340. doi: 10.1074/jbc.273.11.6334. [DOI] [PubMed] [Google Scholar]
- 5.Lazarowski ER, Homolya L, Boucher RC, Harden TK. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- 6.Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau AP. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- 7.Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- 8.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, Gadeau AP, Desgranges C, Scalbert E, Chardin P, Pacaud P, Loirand G. P2Y1, P2Y2, P2Y4, and P2Y6 receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1751–H1761. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- 10.Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, Berkovic SF, Huttenlocher PR, Walsh CA. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–1325. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
- 11.Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–22610. doi: 10.1074/jbc.R900037199. [DOI] [PubMed] [Google Scholar]
- 12.Ohta Y, Suzuki N, Nakamura S, Hartwig JH, Stossel TP. The small GTPase RalA targets filamin to induce filopodia. Proc Natl Acad Sci U S A. 1999;96:2122–2128. doi: 10.1073/pnas.96.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrad RC, Otero MA, Erb L, Theiss PM, Clarke LL, Gonzalez FA, Turner JT, Weisman GA. Structural basis of agonist-induced desensitization and sequestration of the P2Y2 nucleotide receptor. Consequences of truncation of the C terminus. J Biol Chem. 1998;273:29437–29444. doi: 10.1074/jbc.273.45.29437. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Yamamoto M. Cell adhesion receptors for native and denatured type I collagens and fibronectin in rabbit arterial smooth muscle cells in culture. Exp Cell Res. 1994;214:258–263. doi: 10.1006/excr.1994.1256. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 16.Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with alphav integrins to activate Go and induce cell migration. J Biol Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- 17.Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with alpha v integrins to access and activate G12. J Cell Sci. 2007;120(pt 9):1654–1662. doi: 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohta Y, Hartwig JH. Phosphorylation of actin-binding protein 280 by growth factors is mediated by p90 ribosomal protein S6 kinase. J Biol Chem. 1996;271:11858–11864. doi: 10.1074/jbc.271.20.11858. [DOI] [PubMed] [Google Scholar]
- 19.Nagano T, Yoneda T, Hatanaka Y, Kubota C, Murakami F, Sato M. Filamin A-interacting protein (FILIP) regulates cortical cell migration out of the ventricular zone. Nat Cell Biol. 2002;4:495–501. doi: 10.1038/ncb808. [DOI] [PubMed] [Google Scholar]
- 20.Tigges U, Koch B, Wissing J, Jockusch BM, Ziegler WH. The F-actin cross-linking and focal adhesion protein filamin A is a ligand and in vivo substrate for protein kinase C alpha. J Biol Chem. 2003;278:23561–23569. doi: 10.1074/jbc.M302302200. [DOI] [PubMed] [Google Scholar]
- 21.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal Sharma C, Goldmann WH. Phosphorylation of actin-binding protein (ABP-280; filamin) by tyrosine kinase p56lck modulates actin filament cross-linking. Cell Biol Int. 2004;28:935–941. doi: 10.1016/j.cellbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 24.Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279:35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem. 2004;279:8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- 26.Assoian RK, Marcantonio EE. The extracellular matrix as a cell cycle control element in atherosclerosis and restenosis. J Clin Invest. 1996;98:2436–2439. doi: 10.1172/JCI119059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meredith JE, Schwartz MA. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7:146–150. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 29.Erlinge D. Extracellular ATP: a central player in the regulation of vascular smooth muscle phenotype. Focus on “dual role of PKA in phenotype modulation of vascular smooth muscle cells by extracellular ATP”. Am J Physiol Cell Physiol. 2004;287:C260–C262. doi: 10.1152/ajpcell.00217.2004. [DOI] [PubMed] [Google Scholar]
- 30.Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.