Abstract
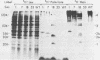
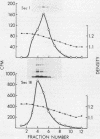
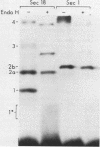
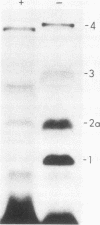
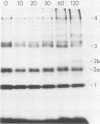
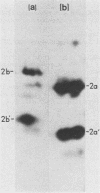
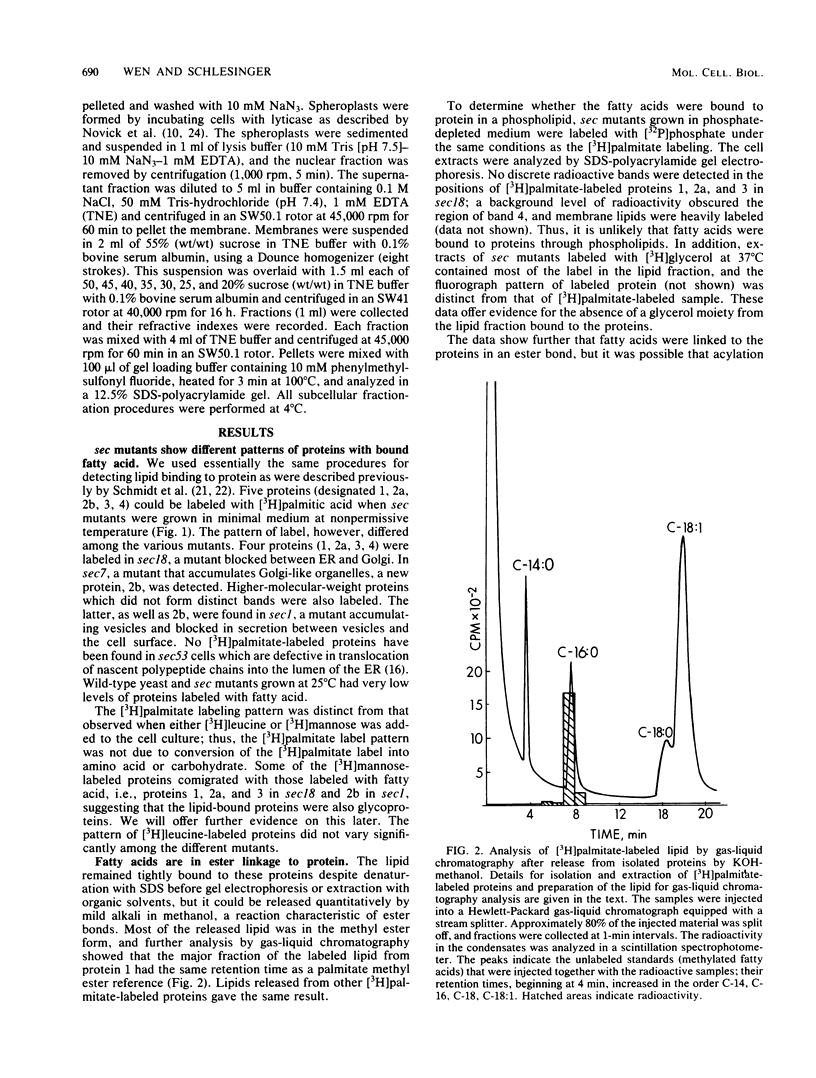
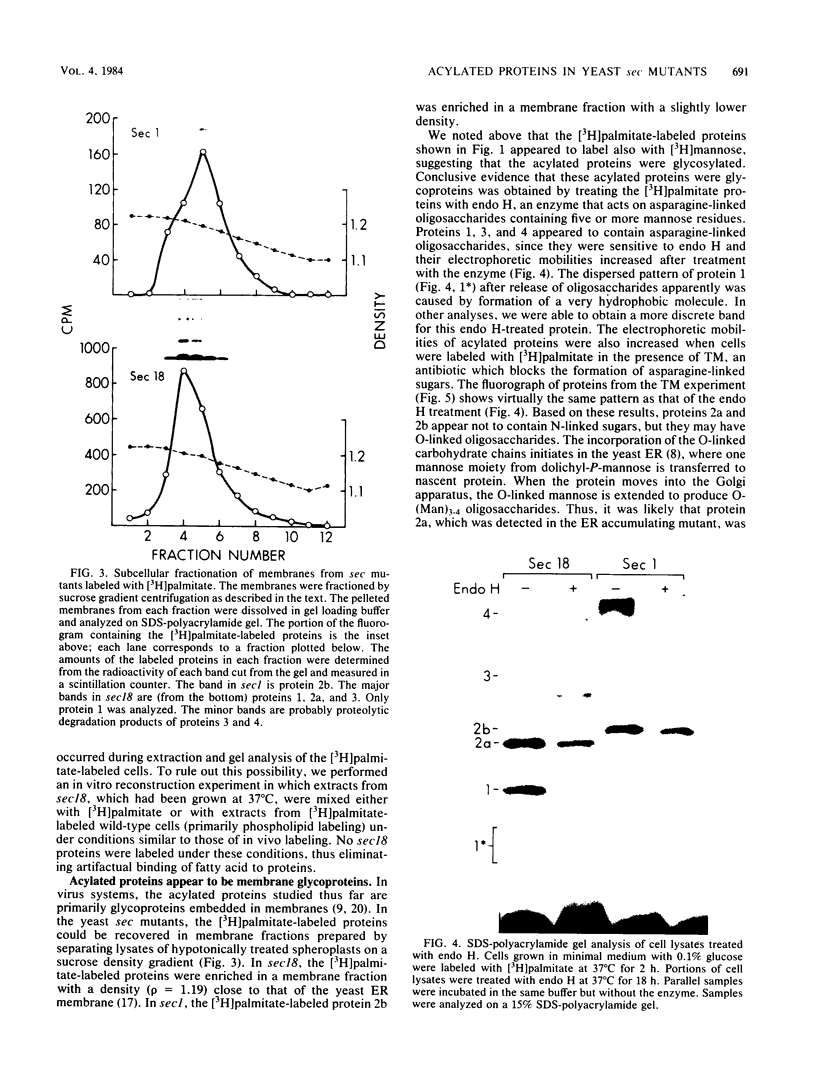
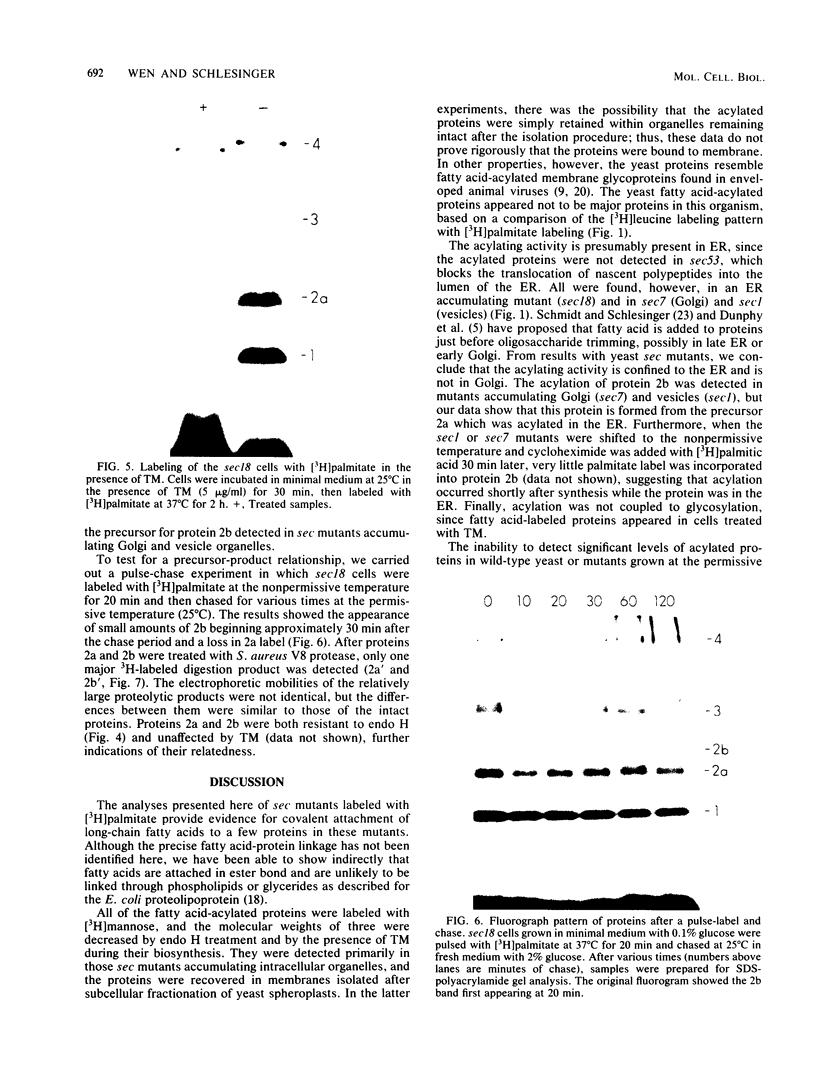
Yeast secretory (sec) mutants that are blocked in the transport of secretory proteins and accumulate membrane organelles were used to study the biosynthesis of fatty acid-acylated proteins. Four proteins were labeled with [3H]palmitate in sec mutants accumulating endoplasmic reticulum membranes. Three of these (molecular weights approximately equal to 20,000, 50,000, and 120,000) were N-linked glycoproteins, based on their ability to be labeled with [3H]mannose and their sensitivity to endoglycosidase H. The fourth protein (molecular weight approximately equal to 30,000) also was labeled with [3H]mannose but was insensitive to endoglycosidase H; it appeared to contain O-linked sugars. In sec mutants accumulating Golgi membranes or post-Golgi vesicles, a 35-kilodalton protein was labeled with [3H]palmitate. Analysis of Staphylococcus aureus protease V8 digests and pulse-chase experiments indicated that the 30-kilodalton protein was a precursor of 35 kilodaltons. None of these proteins was labeled with [3H]palmitate in a sec mutant that blocked the penetration of nascent polypeptides into endoplasmic reticulum; thus, acylation occurred in endoplasmic reticulum. All four proteins could be recovered from fractions enriched for yeast membranes. Fatty acids were not released from proteins by boiling in sodium dodecyl sulfate or extraction with organic solvents but were recovered as methyl esters after proteins were treated with KOH-methanol, a reaction characteristic of an acyl ester linkage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal H. C., Randle C. L., Agrawal D. In vivo acylation of rat brain myelin proteolipid protein. J Biol Chem. 1982 Apr 25;257(8):4588–4592. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dunphy W. G., Fries E., Urbani L. J., Rothman J. E. Early and late functions associated with the Golgi apparatus reside in distinct compartments. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7453–7457. doi: 10.1073/pnas.78.12.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Bauer F., Tanner W. The formation of glycosidic bonds in yeast glycoproteins. Intracellular localisation of the reactions. Arch Microbiol. 1977 Jul 26;114(1):77–81. doi: 10.1007/BF00429634. [DOI] [PubMed] [Google Scholar]
- Magee A. I., Schlesinger M. J. Fatty acid acylation of eucaryotic cell membrane proteins. Biochim Biophys Acta. 1982 Nov 30;694(3):279–289. doi: 10.1016/0304-4157(82)90008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983 Feb;96(2):541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Magee A. I., Schmidt M. F. Fatty acid acylation of proteins in cultured cells. J Biol Chem. 1980 Nov 10;255(21):10021–10024. [PubMed] [Google Scholar]
- Schlesinger M. J. Proteolipids. Annu Rev Biochem. 1981;50:193–206. doi: 10.1146/annurev.bi.50.070181.001205. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F. Acylation of viral spike glycoproteins: a feature of enveloped RNA viruses. Virology. 1982 Jan 15;116(1):327–338. doi: 10.1016/0042-6822(82)90424-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Bracha M., Schlesinger M. J. Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1687–1691. doi: 10.1073/pnas.76.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Park F. J., Jones E. W. Mutations in PEP4 locus of Saccharomyces cerevisiae block final step in maturation of two vacuolar hydrolases. Proc Natl Acad Sci U S A. 1983 Jan;80(2):510–514. doi: 10.1073/pnas.80.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]