Abstract
Background and Aim:
Bronchiolitis obliterans (BO) is the main pulmonary involvement resulting from sulfur mustard (SM) gas exposure that was used against Iranian civilians and military forces during the Iran-Iraq war. The present study aimed to investigate the prevalence of gastro-esophageal reflux (GER) and gastric micro-aspiration in SM gas injured patients with chronic pulmonary diseases and recurrent episodes of exacerbations.
Materials and Methods:
This cross-sectional study was done at Baqiyatallah University of Medical Sciences, Tehran, Iran. Gastric micro-aspiration and GER were assessed in the enrolled patients by assessing bile acids, pepsin and trypsin in their bronchoalveolar lavage fluid.
Results:
Our result showed that bile acids were found to be high in 21.4% patients, and low in 53.6% of patients. Only in 16% patients, no bile was detected in the BALF. Trypsin and pepsin were detected in BAL fluid of all patients.
Conclusion:
Most of BO patients after exposure to SM suffer GER, while none the etiologic factors of GER in post lung transplant BO are present. It would be hypothesized that GER per se could be considered as an aggregative factor for exacerbations in patients. Further studies will provide more advances to better understanding of pathophysiological mechanism regarding GER and BO and treatment.
Keywords: Bronchiolitis obliterans, gastro-esophageal reflux, sulfur mustard
INTRODUCTION
The association between chronic pulmonary diseases and gastro-esophageal reflux disease (GERD) has been recognized for a long time.[1,2] In fact GERD is shown to be highly prevalent in patients with a variety of lung diseases; in particular, patients with chronic cough, asthma, Chronic obstructive pulmonary disease, cystic fibrosis, and idiopathic pulmonary fibrosis.[3,4,5] Furthermore, GER disease is suggested to be a risk factor for acute exacerbation of COPD.[6] In fact it is shown that COPD patients with GERD symptoms have more COPD exacerbations and subsequent hospitalization and drug usage.[6,7,8] The pathophysiology of the associations between GERD and various pulmonary diseases including COPD is not clear. The proposed mechanisms are microaspiration of the gastric contents leading to airway irritation and increased resistance, vagally mediated bronchoconstriction from esophago-bronchial reflex, and increased bronchial reactivity to other stimuli induced by esophageal acid exposure. Recent study indicated that abnormal swallowing reflexes would frequently occur in COPD patients and predisposes them to exacerbation and this abnormality might be affected by comorbidity of GERD.[9] Disrupted breathing–swallowing coordination and impaired swallowing response could increase the risk of aspiration, and may contribute to exacerbations of COPD. Cvejic et al.[10] demonstrated the aspiration of liquid material using sub-mandible video fluoroscopy during swallowing in COPD patient.
Sulfur mustard (SM) was used against Iranian civilians and military forces during the Iran-Iraq war; it leads to the development of acute and long term pulmonary complication. Previous studies have shown that bronchiolitis obliterans (BO) is the most frequent and the main pulmonary involvement resulting from SM gas exposure.[11] Anecdotal evidence indicates that the prevalence of GERD in mustard-gas exposed patients is higher than that in the normal population.[12] In this study we have aimed to investigate the prevalence of gastric micro-aspiration in SM gas injured patients with chronic pulmonary diseases as the cause of recurrent episodes of exacerbations, while they are under the treatment of GERD by proton pomp inhibitor, by assaying bile acids, pepsin, and trypsin in bronchoalveolar lavage fluid (BALF).
MATERIALS AND METHODS
Subjects
This cross-sectional study was done at Baqiyatallah University of Medical Sciences, Tehran, Iran between January 2009 and December 2010. The studied populations were the sulfur mustard gas injured patients with chronic pulmonary disease who experienced pulmonary exacerbation more than twice per year or had persistent pulmonary symptoms in spite of receiving full medical treatment. The exclusion criteria were as follows: patients with accompanied chronic lung disease rather than SM intoxication, patients with the history of smoking, patients with liver disorders and raised level of serum bilirubin, patients who were high risk to undergo bronchoscopy. All patients were interviewed to take a complete medical history regarding gastro-esophageal reflux symptoms. Pulmonary function test (PFT) was performed without bronchodilator administration and patients were classified according to Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (updated 2009). Chest high resolution computerized tomography (HRCT) was obtained from all patients and their findings were registered.
Bronchoscopy
All included patients underwent bronchoscopy. Subjects were pre-medicated with intravenous midazolam. 4% lignocaine was applied topically to the nose, pharynx, and larynx and below the vocal cords in 1-ml aliquots, as required, up to a maximum dose of 7 mg/kg body weight. Bronchoscopy was carried out with patients in a semi-reclined position. Bronchoalveolar lavage was done with oxygen saturation routinely measured during the procedure. The bronchoalveolar fluids were collected and split to assess the clinical microbiology and differential cell counts on Giemsa-stained cyto-centrifuge preparation. Aliquots of the BAL fluid were also collected and snap frozen by immersion in liquid N2, stored at -80°C. The samples were clarified by centrifugation at 1,500 rpm for 10 minutes. The resulting supernatant was assayed for bile acids. The samples were also tested for IL-8 and IL-15.
The study was approved by the Research Ethics Committee of Baqiyatallah Medical Science University and informed consent was obtained from every patient after presenting enough information about the study.
Bile acids assay
The bile acid determination in BALF was performed with a spectrophotometric enzymatic assay. The assay was conducted according to a commercially available kit (Sigma Diagnostics, Inc, St Louis, Mo). BALF bile acids levels were considered high when they were greater than 8 μmol/L, lower if they were between 0 to 8, and absent when they were equal to 0.
Statistical analysis
The statistical analysis was carried our using the statistical package for the Social Sciences, version 16.0 (SPSS Inc., Chicago, IL, USA). Deviations from Gaussian distribution were tested using the Kolmogorov–Smirnov test. T student test, kruskal-wallis test and Mann-whitney test were used as needed. A P-value under 0.05 was considered to be statistically significant.
RESULTS
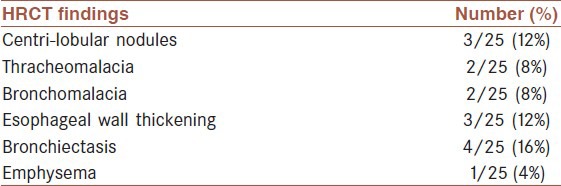
Samples from 25 patients (mean age, 43.19; age range, 30-59) were assayed and included in the analysis. Of the 25 patients, 92% were male and only 2 patients (8%) were female. In 92% patients, pulmonary function was successfully tested using spirometry. Sixteen percent of patients were smoker and none of them were alcohol drinker. The findings are revealed in Table 1. According to GOLD guideline, updated 2009, patients were divided into 4 groups: 3 patients stage I, 7 patients stage II, 9 patients stage III and 4 patients stage IV. In all patients chest HRCT was performed, air trapping was the most frequent finding of HRCT in the patients; 52% had 6-12% air trapping, 40% had 1-5% and 8% had 13-24% air trapping in their HRCT. Other HRCT findings of the patients are summarized in Table 2. The mean bile acid concentration detected in BALF of patients was 5.3 μmol/L, range 0-14 μmol/L. The average levels of bile acids quantified within the BALF were found to be high (more than 8 μmol/L) in 21.4% patients, low (greater than 0 and less than 8) in 53.6% of patients. In 16%of patients, no bile was detected in the BALF. Trypsin (4.55 ± 0.24 ng/ml) and pepsin (21.75 ± 7.62 ng/ml) were detected in BAL fluid of all patients.
Table 1.
Results of the pulmonary function test (PFT) in 23 patients

Table 2.
Chest HRCT findings of 25 patients were the sulfur mustard gas injured patients with chronic pulmonary disease
The overall mean level of IL-8 within the BALF of patients was 298 pg/ml with a range of 193- 797 pg/ml. There was not any significant statistical difference considering the quantity of IL-8 measured in patients with high levels of bile acids within the BALF (mean, 346 pg/ml; range, 240- 797), compared with patients with low or absent bile acids (mean, 283 pg/ml; range, 193-557 pg/ml), although the mean level of IL-8 was higher in patients with the high levels of bile acids (P= 0.642). No statistically significant difference was detected comparing the levels of bile acids among four different groups of GOLD classification (P = 0.946) but the mean level of bile acid in stage III was the highest. In both groups, smoker and nonsmoker, there was not any significant relationship between bile acid, pepsin, trypsin, and IL8 of BALF and smoking.
DISCUSSION
In the present study, we found that a considerable number of patients with chronic pulmonary disease and frequent exacerbations had gastro-duodenal micro-aspiration showed in their BAL fluid. As we found, in 21.4% of patients the level of bile acid in BALF was more than 8 μmol/L and also in 53.6% of patients, bile acid was present in BAL fluid with a level of less than 8 μmol/L. Only in 16% of patients, bile acid was not present in BAL fluid. Also, trypsin and pepsin were detected in BAL fluid of all patients. Air trapping was the most frequent finding of HRCT in the studied patients. It has been noted that air trapping is the most sensitive and accurate radiological indicator of BO.[13] Considering the HRCT findings of the studied patients, BO is the main underlying pathology in these patients. This study is the first one in evaluation of the frequency of gastro-duodenal micro-aspiration in patients with chronic pulmonary disease and frequent exacerbations due to sulfur mustard gas inhalation. Unfortunately we did not any control group to compare our result.
Aguilar et al. in a study on COPD patients concluded that patients who have COPD and reflux symptoms simultaneously are more likely to have an increased number of COPD exacerbations compared to patient who are either asymptomatic or have reflux symptoms less than once a week.[8] In another study by Rogha et al. they have found that patients who had daily or weekly symptoms of GERD not only were more likely to experience acute exacerbations of COPD but they showed more severe COPD, increased number of hospitalizations, and increased drug usage.[7] Although the exact mechanism of COPD exacerbations by GERD is not clear but damage to the pulmonary tree following direct exposure to acid refluxate (reflux theory), or through bronchial constriction as a result of stimulation of vagal nerve endings in the esophagus (reflex theory) is suggested.[14] In the present study, although all patients were under treatment of GER disease using proton pomp inhibitors, they had experienced pulmonary exacerbation more than twice per year or had persistent pulmonary symptoms in spite of receiving full medical treatment. Previous studies showed that aspiration secondary to GER has been suggested to be a potential contributor to lung allograft dysfunction and the development of BO. In the D’Ovidio et al. study,[15] elevated BALF bile acids were measured in 17% of 120 patients. They also demonstrated an association between the presence of bile acids in BALF and the onset of BOS. Blondeau et al.[16] in a cross-sectional study showed that gastric aspiration occurs frequently in lung transplantation patients, as shown by the presence of pepsin in BALF of all patients and bile acids in BALF of 50% of the patients. The studied population of the mentioned articles was lung transplant patients. In lung allograft patients, significant damage to vagal innervation of the gastrointestinal tract would occur. Consequently, defense mechanisms, such as cough reflex and mucociliary activity, are frequently altered in these patients. Furthermore, mucociliary clearance is reduced in up to 15% of normal values in this population, a fact that can explain why even short exposure of the graft to gastric content can result in an intense inflammatory reaction and fibrosis.[17] In addition, the use of immunosuppressant drugs (cyclosporin and tacrolimus) in lung transplant patients would reduce gastric motility and LES dysfunction.[18] Mentioned mechanisms are not responsible for occurring GER in SM gas injured patients. A variety of respiratory symptoms are associated with GER because the impaired swallowing reflex perturbs the inspiratory expiratory transition during deglutition in COPD patients. As a result, GER-related symptoms and aspiration may be increased in these patients.[19] Cvejic et al. in a study using sub-mandibular video fluoroscopy during swallow of graduated volumes of barium demonstrated convincing aspiration of liquid material in stable COPD.[10] It is supposed to be due to altered airway protective mechanisms in COPD patients possibly through reduced coordination of breathing with swallowing. The prevalence of abnormal swallowing reflexes and its relationship with COPD exacerbation was assessed in a study by Terada et al. They concluded that abnormal swallowing reflexes frequently occurred in COPD patients and predisposed them to exacerbations.[9] Gastroesophageal reflux and silent microaspiration has been suggested as a cause of chronic bronchiolar and interstitial lung disease. Recently, a strong association between gastroesophageal reflux and idiopathic pulmonary fibrosis has been reported but there are no direct data demonstrating that microaspiration leads to pulmonary fibrosis in humans. It has been suggested that occult aspiration may be a cause of acute exacerbations of idiopathic pulmonary fibrosis, as aspiration of gastric contents is a known cause of diffuse alveolar damage.[20] As mentioned in previous studies, the best available method to demonstrate that GER disease may have a role in pulmonary diseases is to treat the GER and observe if the pulmonary disease improves.[4] In a review by Schnatz et al., 42 of 54 patients with chronic cough or asthma suspected to be due to reflux had abnormal acid reflux documented by dual channel, ambulatory pH monitoring. In 71% of these documented reflux patients, pulmonary symptoms relieved when treated with anti-reflux therapy.[21] Recently, in a randomized, prospective study, authors demonstrated that treatment of GER with a proton pump inhibitor (PPI) significantly improved the quality of life and the symptom scores of asthma patients with GER.[22] PPI only changes the acidity of the refluxate; they do not prevent reflux or micro-aspiration of gastric contents. Studies have suggested an increased risk of community-acquired pneumonia in association with current use of PPI. PPIs also have been associated with an increased risk of hip fracture.[20] At this time, a diagnostic gold standard for micro-aspiration remains unknown. However, based on the current data, the specificity of pepsin and bile salt in the gastrointestinal tract makes this diagnostic approach highly appealing.
In an in vitro study, a component of bile acid, chenodeoxycholic acid, has been shown to induce TGF-beta production from human airway epithelial cells via a p38 MAP-kinase dependent pathway. Fibroblast cell proliferation also was increased with exposure to chenodeoxycholic acid.[23] In a study by Ghanei et al., they indicated that levels of TGF-beta 1 and TGF-beta 3 mRNAs were significantly higher in chemical gas-injured patients than non-injured group. The authors concluded that TGF-beta1 and TGF-beta3, but not TGF-beta2, secretion is a result of efficient efferocytosis in chemically injured patients, playing a protective role by improving airway remodeling and lung homeostasis in this group. These properties of TGF-beta are consistent with long-time survival of chemical-injured people suffering from BO.[24]
The exact mechanism of gastric micro-aspiration and GER in patients exposed to SM gas is not explained but high acute dose-exposure may damage the gastrointestinal tract severely.[12] It was shown that the frequency of GER and esophagitis was higher in the patients with BO due to SM exposure than the control group.[12] It should be considered that we are encountering to BO in SM gas injured patients where patients suffer from GER disease but none the etiologic factors of GER in lung transplant patients such as immunosuppressant drugs and damage to vagal innervation are present. All together, it would be hypothesized that GER per se could be considered as an aggregative factor for exacerbations in BO and one of important cause of resistance to therapy in such patients. It is of note that all patients were receiving proton pomp inhibitor but it seems has no inhibitory effect on GER and related outcomes. Whether BO and GER have developed simultaneously after exposure to SM or one of them occurred at first and led to another is remained unknown. In conclusion, our study has shown that a considerable number of patients with chronic pulmonary disease and frequent exacerbations due to exposure to SM, have gastro-duodenal micro-aspiration studying their BALF. Gastric aspiration and GER may be not only an etiologic factor of their exacerbations and weak response to pulmonary disease treatment but also it could be a result of underlying pathophysiological mechanism behind BO. Further studies are needed to reveal if treatment of gastrointestinal disease in these patients might be able to improve their pulmonary symptoms. In addition, more studies in this setting will provide more advances to provide better understanding of pathophysiological mechanism regarding GER and BO, e.g., neurogenic inflammation after SM exposure.
Footnotes
Source of Support: Research Center of Chemical Injuries, Baqiyatallah University of Medical Sciences, Tehran, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Pearson JE, Wilson RS. Diffuse pulmonary fibrosis and hiatus hernia. Thorax. 1971;26:300–30. doi: 10.1136/thx.26.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belsey R. The pulmonary complications of dysphagia. Thorax. 1948;4:44–56. doi: 10.1136/thx.4.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigelson J, Girault F, Pecau Y. Gastro-oesophageal reflux and esophagitis in cystic fibrosis. Acta Paediatr Scand. 1987;76:989–90. doi: 10.1111/j.1651-2227.1987.tb17283.x. [DOI] [PubMed] [Google Scholar]
- 4.Tobin RW, Pope CE, 2nd, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158:1804–8. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 5.Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119:1043–8. doi: 10.1378/chest.119.4.1043. [DOI] [PubMed] [Google Scholar]
- 6.Takada K, Matsumoto S, Hiramatsu T, Kojima E, Iwata S, Shizu M, et al. Relationship between chronic obstructive pulmonary disease and gastroesophageal reflux disease defined by the Frequency Scale for the Symptoms of gastroesophageal reflux disease. Nihon Kokyuki Gakkai Zasshi. 2010;48:644–8. [PubMed] [Google Scholar]
- 7.Rogha M, Behravesh B, Pourmoghaddas Z. Association of gastroesophageal reflux disease symptoms with exacerbations of chronic obstructive pulmonary disease. J Gastrointestin Liver Dis. 2010;19:253–6. [PubMed] [Google Scholar]
- 8.Rascon-Aguilar IE, Pamer M, Wludyka P, Cury J, Coultas D, Lambiase LR, et al. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130:1096–101. doi: 10.1378/chest.130.4.1096. [DOI] [PubMed] [Google Scholar]
- 9.Terada K, Muro S, Ohara T, Kudo M, Ogawa E, Hoshino Y, et al. Abnormal swallowing reflex and COPD exacerbations. Chest. 2010;137:326–32. doi: 10.1378/chest.09-0482. [DOI] [PubMed] [Google Scholar]
- 10.Cvejic L, Harding R, Churchward T, Turton A, Finlay P, Massey D, et al. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology. 2010;16:269–75. doi: 10.1111/j.1440-1843.2010.01875.x. [DOI] [PubMed] [Google Scholar]
- 11.Ghanei M, Mokhtari M, Mohammad MM, Aslani J. Bronchiolitis obliterans following exposure to sulfur mustard: Chest high resolution computed tomography. Eur J Radiol. 2004;52:164–9. doi: 10.1016/j.ejrad.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Ghanei M, Khedmat H, Mardi F, Hosseini A. Distal esophagitis in patients with mustard-gas induced chronic cough. Dis Esophagus. 2006;19:285–8. doi: 10.1111/j.1442-2050.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 13.Kraft M, Mortenson RL, Colby TV, Newman L, Waldron JA, Jr, King TE., Jr Cryptogenic constrictive bronchiolitis. A clinicopathologic study. Am Rev Respir Dis. 1993;148:1093–101. doi: 10.1164/ajrccm/148.4_Pt_1.1093. [DOI] [PubMed] [Google Scholar]
- 14.Castell DO, Schnatz PF. Gastroesophageal reflux disease and asthma. Reflux or reflex? Chest. 1995;108:1186–7. doi: 10.1378/chest.108.5.1186. [DOI] [PubMed] [Google Scholar]
- 15.D’Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–52. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 16.Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, et al. Nocturnal weakly acidic reflux promotes aspiration of bile acids in lung transplant recipients. J Heart Lung Transplant. 2009;28:141–8. doi: 10.1016/j.healun.2008.11.906. [DOI] [PubMed] [Google Scholar]
- 17.Hartwig MG, Appel JZ, Davis RD. Antireflux surgery in the setting of lung transplantation: Strategies for treating gastroesophageal reflux disease in a high-risk population. Thorac Surg Clin. 2005;15:417–27. doi: 10.1016/j.thorsurg.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Maes BD, Vanwalleghem J, Kuypers D, Ghoos Y, Rutgeerts PJ, Vanrenterghem YF. Differences in gastric motor activity in renal transplant recipients treated with FK-506 versus cyclosporine. Transplantation. 1999;68:1482–5. doi: 10.1097/00007890-199911270-00009. [DOI] [PubMed] [Google Scholar]
- 19.Teramoto S, Kume H, Ouchi Y. Altered swallowing physiology and aspiration in COPD. Chest. 2002;122:1104–5. doi: 10.1378/chest.122.3.1104. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Collard HR, Raghu G, Sweet MP, Hays SR, Campos GM, et al. Does chronic microaspiration cause idiopathic pulmonary fibrosis? Am J Med. 2010;123:304–11. doi: 10.1016/j.amjmed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnatz PF, Castell JA, Castell DO. Pulmonary symptoms associated with gastroesophageal reflux: Use of ambulatory pH monitoring to diagnose and to direct therapy. Am J Gastroenterol. 1996;91:1715–8. [PubMed] [Google Scholar]
- 22.dos Santos LH, Ribeiro IO, Sánchez PG, Hetzel JL, Felicetti JC, Cardoso PF. Evaluation of pantoprazol treatment response of patients with asthma and gastroesophageal reflux: A randomized prospective double-blind placebo-controlled study. J Bras Pneumol. 2007;33:119–27. doi: 10.1590/s1806-37132007000200004. [DOI] [PubMed] [Google Scholar]
- 23.Perng DW, Chang KT, Su KC, Wu YC, Wu MT, Hsu WH, et al. Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: A relation to transforming growth factor-beta1 production and fibroblast proliferation. Chest. 2007;132:1548–56. doi: 10.1378/chest.07-1373. [DOI] [PubMed] [Google Scholar]
- 24.Zarin AA, Behmanesh M, Tavallaei M, Shohrati M, Ghanei M. Overexpression of transforming growth factor (TGF)-beta1 and TGF-beta3 genes in lung of toxic-inhaled patients. Exp Lung Res. 2010;36:284–91. doi: 10.3109/01902140903578868. [DOI] [PubMed] [Google Scholar]


