Abstract
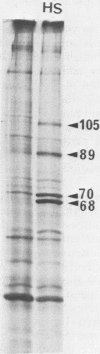
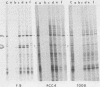
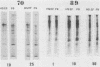
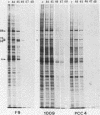
In a previous paper, we have shown that in the absence of stress, mouse embryonal carcinoma cells, like mouse early embryo multipotent cells, synthesize high levels of 89- and 70-kilodalton heat shock proteins (HSP)(O. Bensaude and M. Morange, EMBO J. 2:173-177, 1983). We report here the pattern of proteins synthesized after a short period of hyperthermia in various mouse embryonal carcinoma cell lines and early mouse embryo cells. Among the various cell lines tested, two of them, PCC4-Aza R1 and PCC7-S-1009, showed an unusual response in that stimulation of HSP synthesis was not observed in these cells after hyperthermia. However, inducibility of 68- and 105-kilodalton HSP can be restored in PCC7-S-1009 cells after in vitro differentiation triggered by retinoic acid. Similarly, in the early mouse embryo, hyperthermia does not induce the synthesis of nonconstitutive HSP at the eight-cell stage, but induction of the 68-kilodalton HSP does occur at the blastocyst stage. Such a transition in the expression of HSP has already been described for Drosophila melanogaster and sea urchin embryos and recently for mouse embryos. It may be a general property of early embryonic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bensaude O., Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2(2):173–177. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Capdevila M. P., Garcia-Bellido A. Development and genetic analysis of bithorax phenocopies in Drosophila. Nature. 1974 Aug 9;250(5466):500–502. doi: 10.1038/250500a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Dura J. M. Stage dependent synthesis of heat shock induced proteins in early embryos of Drosophila melanogaster. Mol Gen Genet. 1981;184(3):381–385. doi: 10.1007/BF00352509. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Morgan A. C., Stehlin J. S., Williams L. J. Selective lethal effect of supranormal temperatures on mouse sarcoma cells. Cancer Res. 1973 Nov;33(11):2568–2578. [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982 Jan;79(2):525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Slater M. R., Craig E. A. Saccharomyces cerevisiae contains a complex multigene family related to the major heat shock-inducible gene of Drosophila. Mol Cell Biol. 1982 Nov;2(11):1388–1398. doi: 10.1128/mcb.2.11.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase K., Hahn G. M. Differential heat response of normal and transformed human cells in tissue culture. Nature. 1975 May 15;255(5505):228–230. doi: 10.1038/255228a0. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Türler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983 Jan;3(1):1–8. doi: 10.1128/mcb.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J., Bernier D., Chrétien P., Nicole L. M., Tanguay R. M., Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982 Jun;42(6):2457–2461. [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Avner P., Gaillard J., Guenet J. L., Jakob H., Jacob F. Cell lines derived from teratocarcinomas. Cancer Res. 1976 Nov;36(11 Pt 2):4224–4231. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S. E., Jakob H., Mikoshiba K., Dubois P., Guenet J. L., Nicolas J. F., Gaillard J., Chevance G., Jacob F. Differentiation of a teratocarcinoma line: preferential development of cholinergic neurons. J Cell Biol. 1981 Jan;88(1):57–66. doi: 10.1083/jcb.88.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccheri M. C., Di Bernardo M. G., Giudice G. Synthesis of heat-shock proteins in developing sea urchins. Dev Biol. 1981 Apr 15;83(1):173–177. doi: 10.1016/s0012-1606(81)80020-6. [DOI] [PubMed] [Google Scholar]
- Sirotkin K., Davidson N. Developmentally regulated transcription from Drosophila melanogaster chromosomal site 67B. Dev Biol. 1982 Jan;89(1):196–210. doi: 10.1016/0012-1606(82)90307-4. [DOI] [PubMed] [Google Scholar]
- Strickland S. Mouse teratocarcinoma cells: prospects for the study of embryogenesis and neoplasia. Cell. 1981 May;24(2):277–278. doi: 10.1016/0092-8674(81)90313-5. [DOI] [PubMed] [Google Scholar]
- Wittig S., Hensse S., Keitel C., Elsner C., Wittig B. Heat shock gene expression is regulated during teratocarcinoma cell differentiation and early embryonic development. Dev Biol. 1983 Apr;96(2):507–514. doi: 10.1016/0012-1606(83)90187-2. [DOI] [PubMed] [Google Scholar]