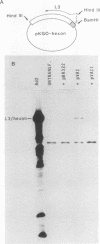
Abstract
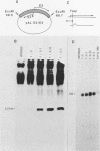
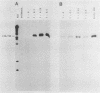
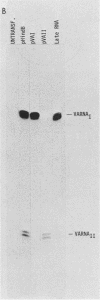
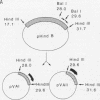
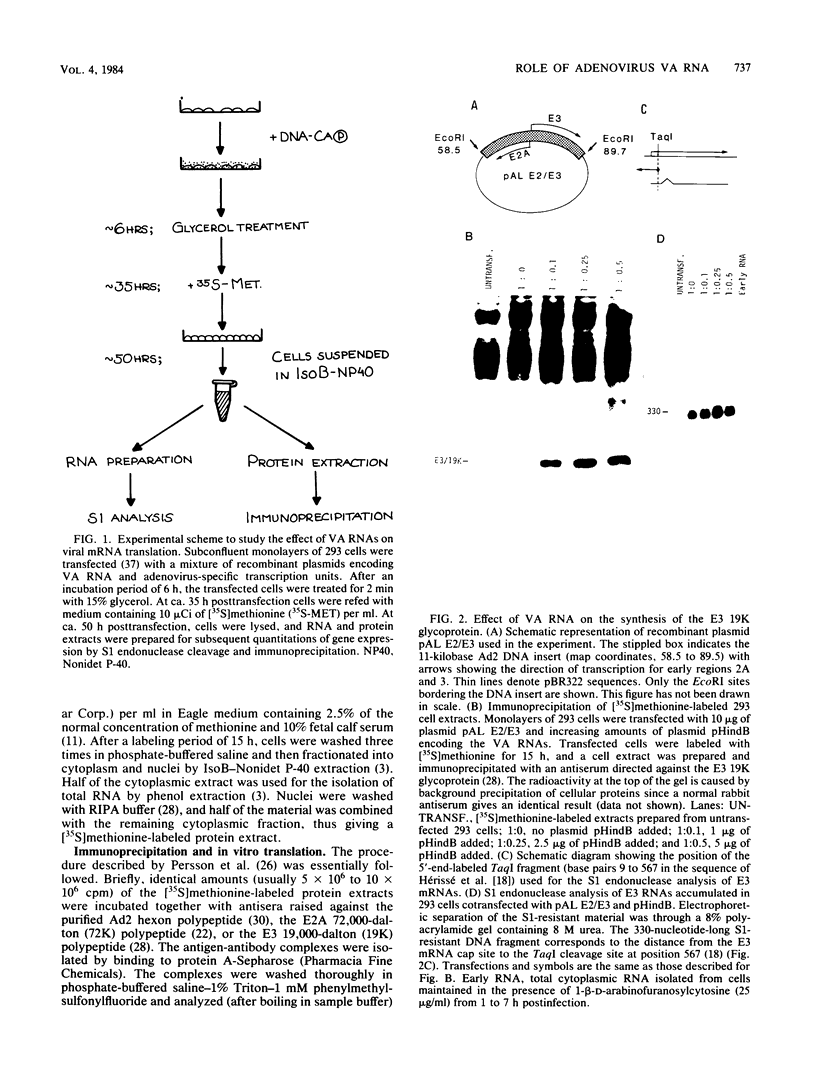
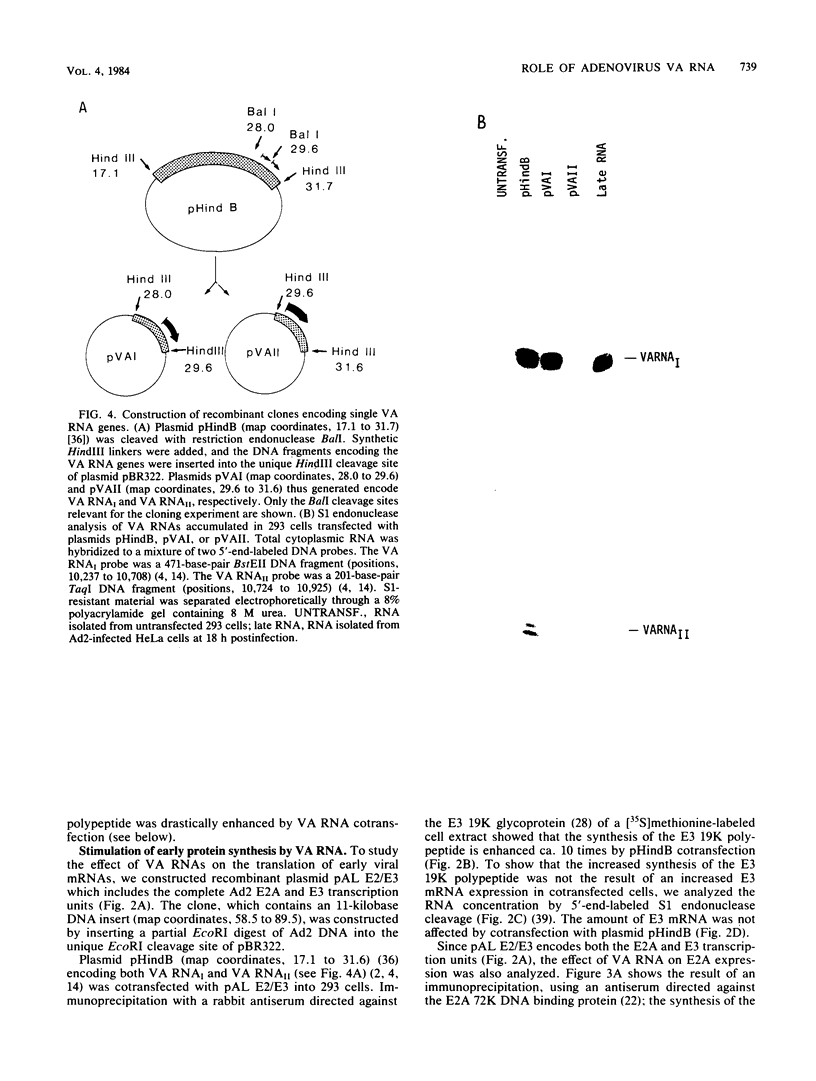
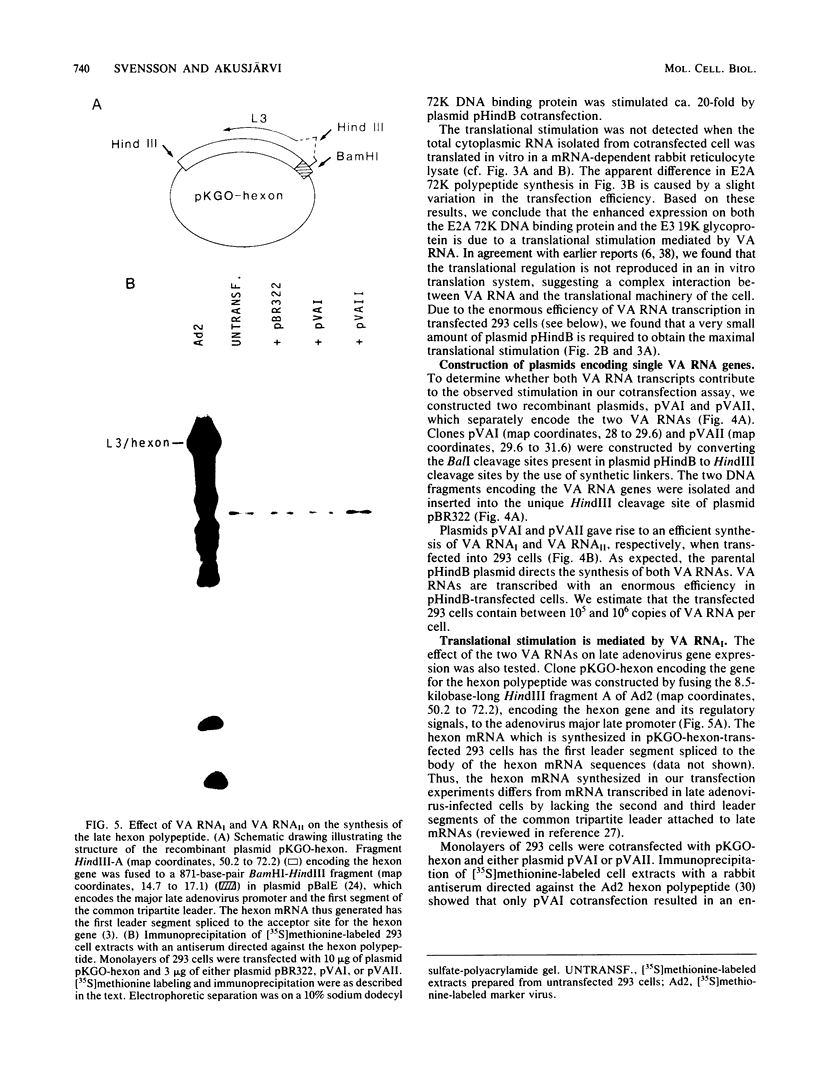
We have developed a sensitive transient expression assay in 293 cells to study the effect of VA RNAs on the translation of adenovirus mRNAs. Monolayers of 293 cells were transfected with mixtures of recombinant plasmids encoding adenovirus-specific transcription units and plasmids encoding VA RNAs. Transfected cells were labeled with [35S]methionine for ca. 15 h, and labeled cell extracts were prepared. Changes in the protein expression caused by VA RNA cotransfection were measured by immunoprecipitation, using monospecific antisera prepared against adenovirus-specific polypeptides. Using this experimental design, we demonstrate that VA RNAI stimulates the translation of both early and late adenovirus mRNAs. Synthesis of the E3 19,000-dalton glycoprotein and the E2A 72,000-dalton DNA binding protein was stimulated between 10 and 20 times by VA RNAI cotransfection. Synthesis of the late hexon polypeptide was also stimulated, although translation of hexon was from an aberrant mRNA lacking the second and third segments of the common tripartite leader attached to late adenovirus mRNAs. VA RNAII, although very homologous to VA RNAI, does not function as a translational stimulator.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello L., Guilfoyle R., Huebner K., Weinmann R. Adenovirus 5 DNA sequences present and RNA sequences transcribed in transformed human embryo kidney cells (HEK-Ad-5 or 293). Virology. 1979 Apr 30;94(2):460–469. doi: 10.1016/0042-6822(79)90476-8. [DOI] [PubMed] [Google Scholar]
- Akusjärvi G., Mathews M. B., Andersson P., Vennström B., Pettersson U. Structure of genes for virus-associated RNAI and RNAII of adenovirus type 2. Proc Natl Acad Sci U S A. 1980 May;77(5):2424–2428. doi: 10.1073/pnas.77.5.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akusjärvi G., Pettersson U. Nucleotide sequence at the junction between the coding region of the adenovirus 2 hexon messenger RNA and its leader sequence. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5822–5826. doi: 10.1073/pnas.75.12.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleström P., Akusjärvi G., Pettersson M., Pettersson U. DNA sequence analysis of the region encoding the terminal protein and the hypothetical N-gene product of adenovirus type 2. J Biol Chem. 1982 Nov 25;257(22):13492–13498. [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich A., Feldman L. T., Nevins J. R., Darnell J. E., Jr, Weinberger C. Effect of adenovirus on metabolism of specific host mRNAs: transport control and specific translational discrimination. Mol Cell Biol. 1983 Jul;3(7):1212–1221. doi: 10.1128/mcb.3.7.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J Virol. 1967 Oct;1(5):843–850. doi: 10.1128/jvi.1.5.843-850.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz G. A., Flint S. J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J Mol Biol. 1979 Jun 25;131(2):353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Bhat R. A., Thimmappaya B. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4789–4793. doi: 10.1073/pnas.80.15.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes D. M., Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980 Nov;22(2 Pt 2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guilfoyle R., Weinmann R. Control region for adenovirus VA RNA transcription. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3378–3382. doi: 10.1073/pnas.78.6.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérissé J., Courtois G., Galibert F. Nucleotide sequence of the EcoRI D fragment of adenovirus 2 genome. Nucleic Acids Res. 1980 May 24;8(10):2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Persson H., Philipson L., Peterson P. A. Molecular association between transplantation antigens and cell surface antigen in adenovirus-transformed cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5674–5678. doi: 10.1073/pnas.75.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Andrews N. C., Miller G., Steitz J. A. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1981 Feb;78(2):805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Linné T., Jörnvall H., Philipson L. Purification and characterization of the phosphorylated DNA-binding protein from adenovirus-type-2-infected cells. Eur J Biochem. 1977 Jun 15;76(2):481–490. doi: 10.1111/j.1432-1033.1977.tb11618.x. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Genes for VA-RNA in adenovirus 2. Cell. 1975 Oct;6(2):223–229. doi: 10.1016/0092-8674(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Persson H., Monstein H. J., Akusjärvi G., Philipson L. Adenovirus early gene products may control viral mRNA accumulation and translation in vivo. Cell. 1981 Feb;23(2):485–496. doi: 10.1016/0092-8674(81)90144-6. [DOI] [PubMed] [Google Scholar]
- Persson H., Philipson L. Regulation of adenovirus gene expression. Curr Top Microbiol Immunol. 1982;97:157–203. doi: 10.1007/978-3-642-68318-3_4. [DOI] [PubMed] [Google Scholar]
- Persson H., Signäs C., Philipson L. Purification and characterization of an early glycoprotein from adenovirus type 2-infected cells. J Virol. 1979 Mar;29(3):938–948. doi: 10.1128/jvi.29.3.938-948.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Philipson L., Höglund S. Structural proteins of adenoviruses. I. Purification and characterization of the adenovirus type 2 hexon antigen. Virology. 1967 Dec;33(4):575–590. doi: 10.1016/0042-6822(67)90057-8. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Philipson L. Location of sequences on the adenovirus genome coding for the 5.5S RNA. Cell. 1975 Sep;6(1):1–4. doi: 10.1016/0092-8674(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. Transcription of the adenovirus genome by an -amanitine-sensitive ribonucleic acid polymerase in HeLa cells. J Virol. 1972 Apr;9(4):621–626. doi: 10.1128/jvi.9.4.621-626.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Forget B. G., Weissman S. M. RNA of low molecular weight in KB cells infected with adenovirus type 2. J Mol Biol. 1966 Jun;17(2):428–439. doi: 10.1016/s0022-2836(66)80153-5. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Jones N. C., Smith L. D. Stimulation of Xenopus oocyte protein synthesis by microinjected adenovirus RNA. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3789–3793. doi: 10.1073/pnas.79.12.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signäs C., Katze M. G., Persson H., Philipson L. An adenovirus glycoprotein binds heavy chains of class I transplantation antigens from man and mouse. Nature. 1982 Sep 9;299(5879):175–178. doi: 10.1038/299175a0. [DOI] [PubMed] [Google Scholar]
- Stenlund A., Perricaudet M., Tiollais P., Pettersson U. Construction of restriction enzyme fragment libraries containing DNA from human adenovirus types 2 and 5. Gene. 1980 Jun;10(1):47–52. doi: 10.1016/0378-1119(80)90142-0. [DOI] [PubMed] [Google Scholar]
- Svensson C., Pettersson U., Akusjärvi G. Splicing of adenovirus 2 early region 1A mRNAs is non-sequential. J Mol Biol. 1983 Apr 15;165(3):475–495. doi: 10.1016/s0022-2836(83)80214-9. [DOI] [PubMed] [Google Scholar]
- Söderlund H., Pettersson U., Vennström B., Philipson L., Mathews M. B. A new species of virus-coded low molecular weight RNA from cells infected with adenovirus type 2. Cell. 1976 Apr;7(4):585–593. doi: 10.1016/0092-8674(76)90209-9. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R., Brendler T. G., Raskas H. J., Roeder R. G. Low molecular weight viral RNAs transcribed by RNA polymerase III during adenovirus 2 infection. Cell. 1976 Apr;7(4):557–566. doi: 10.1016/0092-8674(76)90206-3. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]