Abstract
Background:
Transforaminal lumbar interbody fusion (TLIF) has been preferred to posterior lumbar interbody fusion (PLIF) for different spinal disorders but there had been no study comparing their outcome in lumbar instability. A comparative retrospective analysis of the early results of TLIF and PLIF in symptomatic lumbar instability was conducted between 2005 and 2011.
Materials and Methods:
Review of the records of 102 operated cases of lumbar instability with minimum 1 year followup was done. A total of 52 cases (11 men and 41 women, mean age 46 years SD 05.88, range 40-59 years) underwent PLIF and 50 cases (14 men and 36 women, mean age 49 years SD 06.88, range 40-59 years) underwent TLIF. The surgical time, duration of hospital stay, intraoperative blood loss were compared. Self-evaluated low back pain and leg pain status (using Visual Analog Score), disability outcome (using Oswestry disability questionnaire) was analyzed. Radiological structural restoration (e.g., disc height, foraminal height, lordotic angle, and slip reduction), stability (using Posner criteria), fusion (using Hackenberg criteria), and overall functional outcome (using MacNab's criteria) were compared.
Results:
Pain, disability, neurology, and overall functional status were significantly improved in both groups but PLIF required more operative time and caused more blood loss. Postoperative hospital stay, structural restoration, stability, and fusion had no significant difference but neural complications were relatively more with PLIF.
Conclusions:
Both methods were effective in relieving symptoms, achieving structural restoration, stability, and fusion, but TLIF had been associated with shorter operative time, less blood loss, and lesser complication rates for which it can be preferred for symptomatic lumbar instability.
Keywords: Lumbar instability, posterior lumbar interbody fusion, transforaminal lumbar interbody fusion
INTRODUCTION
Spinal stability is the vertebral ability to maintain their relationship and limit their relative displacements during physiologic postures and loads.1 Instability develops when the spinal stabilizing system fails to maintain the physiological limit of spinal “neutral zone”, which may result in progressive deterioration of the structural components of the spine leading to incapacitating symptoms [e.g., low back pain (LBP) with or without sciatica, increasing disability and progressive deterioration of quality of life]. The concept of “lumbar segmental instability (LSI)” is not new and degenerative and lytic spondylolisthesis comprises the principal aetiology. The clinical symptoms and proposed clinical tests had limited diagnostic significance,2 hence the radiological criteria has been emphasized.3 Although the initial treatment is conservative (e.g., patient education, exercise, bracing, physical therapy), surgery is the last resort for symptomatic instability.4
Spinal fusion procedures are indicated with severe disabling symptoms and radiographic evidence of increased segmental motion that fails to respond to adequate conservative trial.5 Segmental fusion provides solid fixation, restores the spinal stability, and maintains loadbearing capacity of spine.6 Considering all these advantages, posterior lumbar interbody fusion (PLIF) has long been the “gold standard” surgical technique for LSI,7,8 but since transforaminal lumbar interbody fusion (TLIF) (a modification of PLIF by Harms9) has been introduced, it has been found to be better technique for different other spinal disorders.10,11,12 To the best of our knowledge, there is no study comparing these two techniques in spinal instability in English language literature. Our aim was to assess and compare the early clinical and radiological outcome of PLIF and TLIF in the two most common causes of symptomatic lumbar instability.
MATERIALS AND METHODS
A retrospective review of the records of 52 patients, 11 men and 41 women aged 40 to 59 years (mean 46.73, SD 05.88) who underwent PLIF (Group-I), and 50 patients, 14 men and 36 women aged 40 to 59 years (mean 49.04, SD 06.88) who underwent TLIF (Group-II) were reviewed between January 2005 and December 2011. Patients with lumbar instability (degenerative and lytic) with uni/bilateral radiculopathy who failed adequate conservative therapy (e.g., bracing, physiotherapy, and exercises) for 6-15 months (mean 9 months, SD 2.75) were included, but patients with (i) symptomatic radiological instability in > 1 segment, (ii) previous history of spondylodiscitis; and (iii) pathological condition of lumbar spine (e.g., trauma, tumor) were excluded. Higher grades (>Grade-II) of spondylolisthesis were also excluded because of unavailability of reduction pedicle screws and in situ reduction devices.
Preoperative X-ray lumbosacral (L/S) spine antero/posterior (A/P), lateral [Figure 1] and dynamic views were done to assess the instability by the radiographic criteria of Posner3 and Meyerding's13 grading was done to assess forward slip. Magnetic resonance imaging (MRI) of the L/S spine was done routinely to delineate the intra-spinal pathoanatomy. The operative time, intraoperative blood loss, postoperative hospital stay, improvement of neurological status was recorded. Preoperative and postoperative pain status was recorded by self-evaluated Visual Analog Score (VAS)14 and disability by Oswestry Disability Index (ODI).15 Followup was done at 6 weeks, 3 months, 6 months, and then every yearly16 for radiological fusion,17 structural restoration (disc height, foraminal height, angle of total lumbar lordosis and slip reduction) in standing lateral films18 [Figure 2], and maintenance of stability.3 Computed tomography (CT) scan had been reserved for cases where radiological fusion was doubtful or delayed or suspected of pseudarthrosis, as recommended.19 The overall functional outcome was assessed by Macnab's20 criteria.
Figure 1.
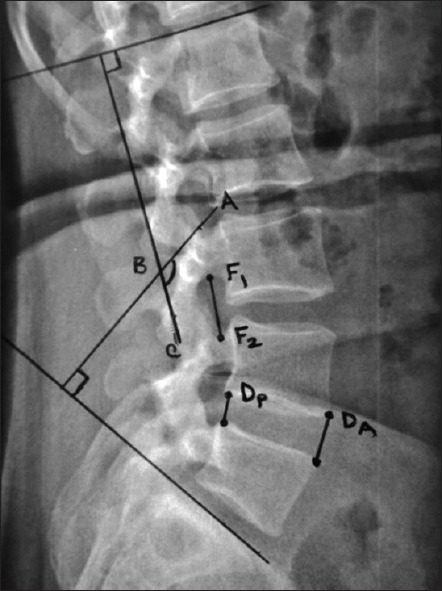
X-ray lumbosacral spine lateral view showing radiological assessment of structural restoration (Disc height, foraminal height and angle of total lumbar lordosis). Disc height has been measured as (DA+DP) / 2; The foraminal height has been measured as the distance between the midpoint of the superior and inferior neural arch (F1-F2); The angle of total lumbar lordosis has been measured by the angle formed by the perpendicular lines from the two lines drawn along the superior end plate of L1 and superior end plate of S1 (angle ABC)
Figure 2.
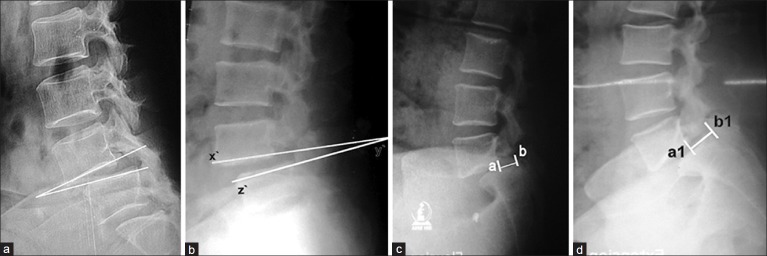
X-ray lumbosacral spine lateral view showing (a) Measurement of instability according to posner;3 Dynamic lateral film (flexion) in standing posture showing >10° sagittal rotation (angular displacement); measurement is done by the angle formed between the two adjacent end-plates. (b) Dynamic film in extension. (c) The dynamic films producing anterior translation measured as the distance between a and b= d (in flexion). (d) The distance of a1 and b1 = d1 (in extension). The distance difference (D) in flexion (d) and extension (d1) calculated as the percentage (D = d – d1 above vertebral width ×100) of the width of the above vertebra
Operative procedure
After proper explanation of the management options and surgical technique, an informed written consent was taken, The patients were operated under general anaesthesia. Patients of both groups i.e. PLIF and TLIF, were placed in prone position and a routine posterior midline approach was used. The paraspinal muscles were exposed by subperiosteal dissection till the intended segmental levels. The pedicle screws were inserted using a standard “free hand targeting” technique under fluoroscopic control. In PLIF [Figure 3], decompression was commenced by laminectomy and removal of interspinous ligaments and ligamentum flavum with sufficient decompressive laminotomy superiorly and inferiorly. But in TLIF [Figure 4], unilateral laminotomy and partial facetectomy were performed on the side consistent with the patient's symptoms or anatomical abnormalities. Unlike most other literature, we used to excise the spinous process to collect adequate autografts for the prepared disc space intended to achieve good fusion. The osteophytes and bony spurs were removed. Discectomy was done bilaterally in PLIF but unilaterally in TLIF.
Figure 3A.
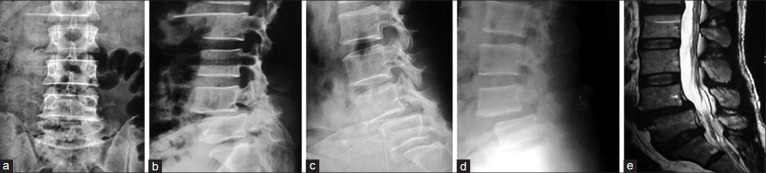
Degenerative instability managed by PLIF; (a) X-Ray lumbosacral (L/S) spine Anteroposterior view. (b) X-Ray L/S spine lateral view showing degenerative spondylolisthesis at L4 over L5. (c) Dynamic film in flexion showing anterior translation and angular displacement. (d) Dynamic film in extension showing differences in translation and angular motion. (e) T2W MRI scan of that patient with degeneration and spondylolisthesis at L4/5 level.
Figure 3B.
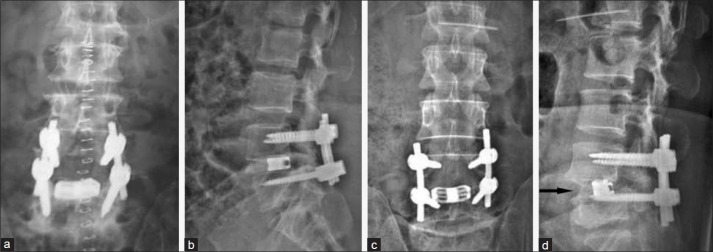
(a) Postoperative X-ray lumbosacral spine anteroposterior view and (b) lateral view showing good implant position. (c) 1 year followup X-ray anteroposterior view and (d) lateral view showing listhesis reduction and radiological fusion (arrow)
Figure 4A.
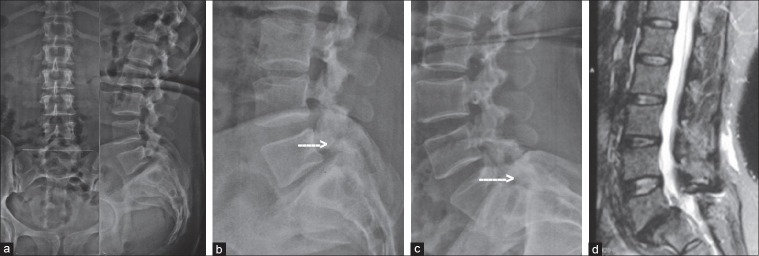
Spondylolytic instability managed by TLIF: X-ray lumbosacral spine anteroposterior view (a) and lateral view (b) showing grade-I spondylolytic spondylolisthesis instability at L5/S1 level. (c) Dynamic lateral view (flexion) showing spondylolysis (arrow) and anterior translation. (d) Dynamic lateral view (extension) showing spondylolysis (arrow) and angular motion at L5/S1 level. (e) T2W MRI scan of that patient showing involvement of L5/S1 level
Figure 4B.
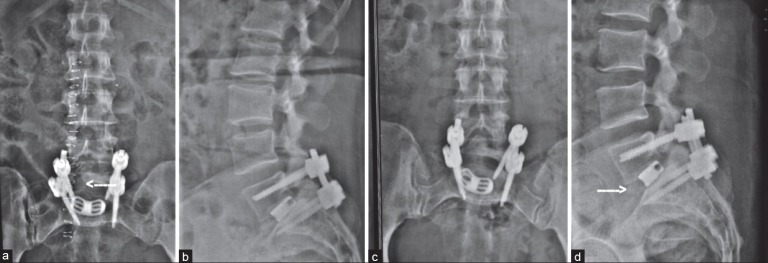
(a) Postoperative X-ray lumbosacral spine anteroposterior view and (b) lateral view showing good implant position and the transforaminal approach (arrow). (c) 1 year followup X-Ray anteroposterior view and (d) lateral view showing listhesis reduction and radiological fusion (arrow)
Disc space preparation was done by unilateral distractor instrumentation with bilateral curettage in PLIF; however, in TLIF we used unilateral curettage by using special curved curettes to remove the end plates. The morcelized bone grafts taken from the excised spinous process and/or laminar bone were introduced to the anterior part of the disc space and impacted with an L-shaped impactor. In both techniques, we used single banana cage (Titanium, size 9 mm, 10 mm, or 11 mm) which was packed with cortico-cancellous autografts and inserted in the prepared space from the surgeon's side. The final position of the cage was confirmed fluoroscopically or by check X-ray. Cortico cancellous bone grafts were placed to the prepared posterolateral decorticated beds. Two rods were contoured and fixed to the pedicle screw heads. Final tightening of the nuts was performed under compression. Patients were mobilized on the 2nd or 3rd postoperative day with a brace support which was continued for minimum 6 weeks postoperaively.
During the followup period, the clinical and radiological parameters were measured by the same assessor and the statistical analysis was performed using the SPSS statistical software where results were achieved from the chi square test and z test where applicable.
RESULTS
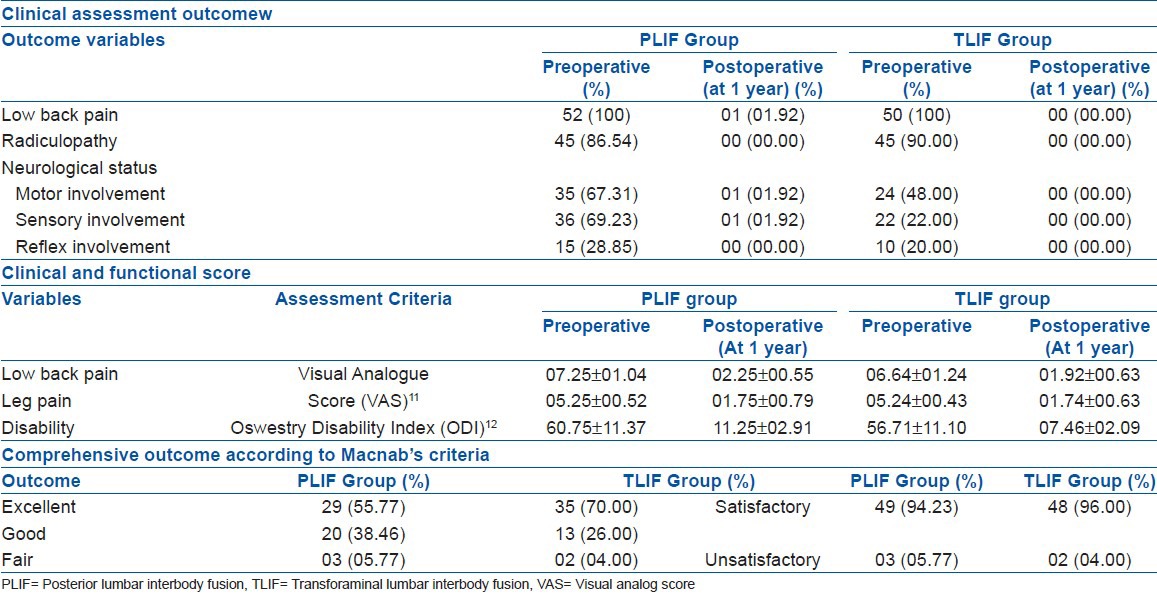
In both the groups, female patients of the 40-49 years age group were significant (Chi-square test, P < 0.05). Maximum cases had spondylolytic instability [PLIF = 27 (51.92%), TLIF = 24 (48.00%)] and L4/5 level involved most commonly [PLIF = 27 (51.92%), TLIF = 28 (56.00%)] in both groups. Grade-II slip [PLIF = 41 (78.85%), TLIF = 40 (80.00%)] had been significant (Chi-square test, P < 0.05) in both groups [Table 1]. Preoperative LBP and radiculopathy (leg pain) was significantly present in both groups (Chi-square test, P < 0.05) having a highly significant improvement of their VAS scores (z test, P < 0.001) at 1 year. LBP score improved from 07.25 ± 01.04 to 02.25 ± 00.55 in the PLIF group and from 06.64 ± 01.24 to 01.92 ± 00.63 in the TLIF group. Similar significant improvement of the leg pain status was achieved from 05.25 ± 00.52 to 01.75 ± 00.79 in the PLIF group and from 05.24 ± 00.43 to 01.74 ± 00.63 in the TLIF group. The ODI score revealed highly significant (z test, P < 0.001) improvement from 60.75 ± 11.37 to 11.25 ± 02.91 in the PLIF group and 56.71 ± 11.10 to 07.46 ± 02.09 in the TLIF group. The neurological status (e.g., motor and sensory) of both groups also had highly significant recovery (z test, P < 0.001). None of the pain score, disability score, and neurological recovery had significant difference between the groups (z test, P > 0.05). Although overall satisfactory outcome [PLIF = 49 (94.23%), TLIF = 48 (96.00%)] had no significant difference, there had been more excellent results with TLIF [35 (70.00%)] than that of PLIF [29 (55.77%)] [Table 2].
Table 1.
Demographics of the patients (n=102)
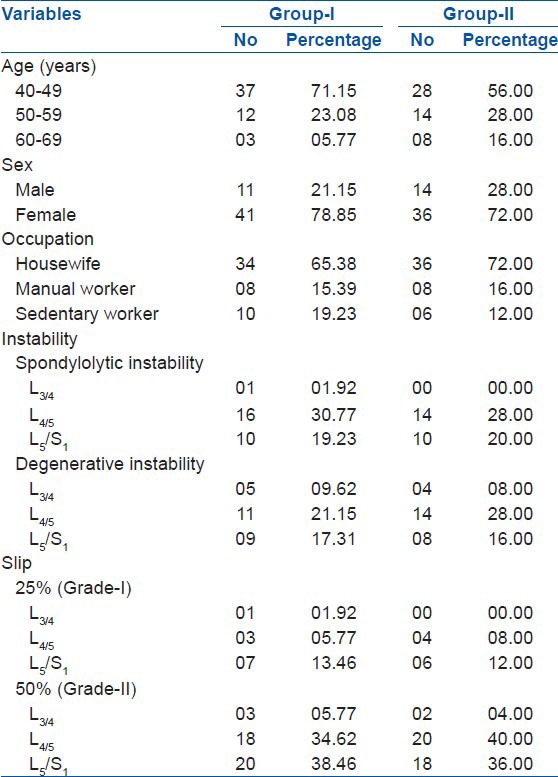
Table 2.
Clinical, functional, and overall outcome of the patients (n=102)
Assessment of radiological restoration of structural components revealed a significant (z test, P < 0.05) rise of mean disc height (MDH) from 07.50 ± 02.73 to 12.00 ± 01.97 mm in the PLIF group and from 07.76 ± 02.77 to 12.24 ± 01.89 mm in the TLIF group. The mean foraminal height (MFH) increase was recorded from 13.25 ± 01.61 to 17.50 ± 01.85 mm in PLIF and from 13.30 ± 1.55 to 17.50 ± 01.87 mm in the TLIF group, which was also significant (z test, P < 0.05). The preoperative value of mean total lumbar lordosis (MTLL) increased to 24.60 ± 00.85° (from 18.30 ± 03.75°) in cases the PLIF group and from 18.60 ± 03.16° to 24.00 ± 02.00° in the TLIF group. The mean anterior slip (MAS) in the PLIF group was 30.25 ± 05.75% and in the TLIF group it was 34.80 ± 03.25%. There had been a significant slip reduction in both the groups (PLIF = 90 ± 03.85% for grade-I and 81 ± 05.24% for grade-II, TLIF = 92 ± 03.63% for grade-I and 84 ± 01.50% for grade-II), which had been maintained even at their last followup. The preoperative mean anterior translation (MAT) was 09.50 ± 0.87% at L3/4, 09.21 ± 1.03% at L4/5, and 07.75 ± 1.93% at L5/S1 in the PLIF group and 09.22 ± 1.07% at L3/4, 09.00 ± 1.12% at L4/5, and 08.26 ± 1.07% at L5/S1 in the TLIF group. The preoperative mean angular displacement (MAD) was 10.75 ± 01.58° at L3/4, 12.40 ± 00.70° at L4/5, and 04.50 ± 01.98° at L5/S1 in the PLIF group and 11.12 ± 01.70° at L3/4, 12.14 ± 00.95° at L4/5, and 04.20 ± 01.10° at L5/S1 in the TLIF group [Table 3]. But all these variables of structural restoration (e.g., MDH, MFH, MTLL, MAS, MAT, MAD) had no statistical significant differences (z test, P > 0.05) between the groups. Postoperative dynamic films did not reveal abnormal range of movement in patients of either of the group, hence achieving radiological stability. Time required for achieving signs of radiological fusion [PLIF (17.23 ± 02.95 weeks), TLIF (16.80 ± 03.94 weeks)] also had no significant difference (z test, P > 0.05) [Table 4].
Table 3.
Radiological outcome of the patients (n=102)
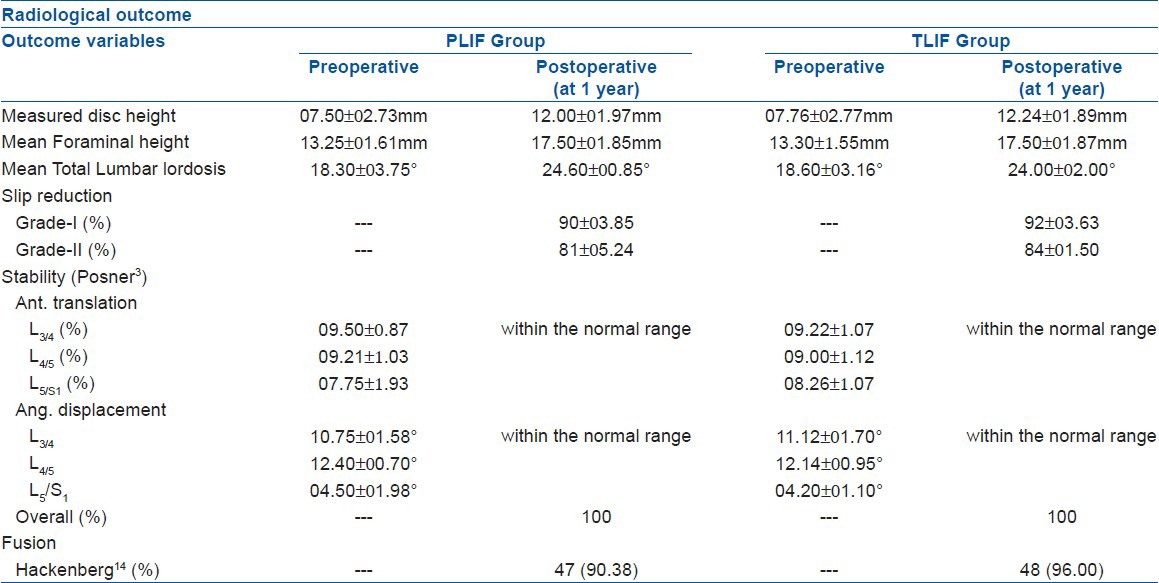
Table 4.
Operative outcome and complications in both groups (n=102)
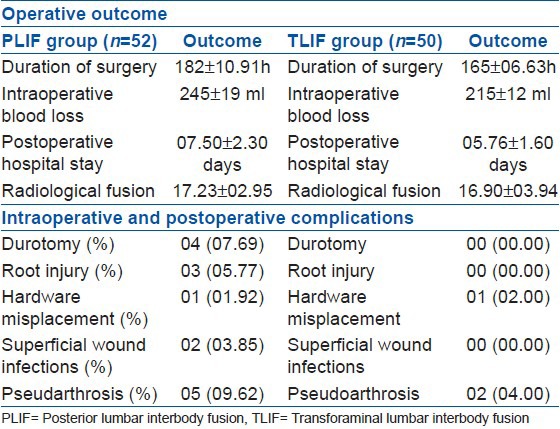
PLIF surgery took longer time (182 ± 10.91 minutes) and higher intraoperative bleeding (245 ± 19 ml) than TLIF (165 ± 06.63 minutes and 215 ± 12 ml). The postoperative hospital stay (07.50 ± 02.30 days for PLIF versus 05.76 ± 01.60 days for TLIF) and the intraoperative complication [PLIF (09.61%), TLIF (02.00%)] and postoperative complication [PLIF (13.47%), TLIF (04.00%)] had no significant difference between the groups. Accidental durotomy had occurred in four (07.69%) cases - all of which could be repaired primarily and none of them had any postoperative CSF leakage. Three (05.77%) cases had intraoperative root injury (during the insertion of interbody cage), among which one case developed foot drop. Five (09.62%) cases in PLIF and two (04.00%) cases in TLIF developed pseud-arthrosis (Chi-square test, P > 0.05). These cases were managed conservatively and had their self-assessed VAS pain score ranged within 02 to 04, ODI disability score from 19.80% to 28.20% (moderate disability) and none of them had any signs of neurological compromise, hence did not require revision surgery. There were no cases of superficial wound infection in the TLIF group but two (03.85%) cases with PLIF developed superficial wound infection, which were managed conservatively [Table 4].
DISCUSSION
The concept of LSI has received an increased attention from the clinicians and researchers as a potential cause of chronic LBP, which has been commonly associated with spondylolisthesis and spondylolysis.21,22 Restoration of the segmental stability by adequate neural decompression, fusion, and stabilization helps to improve clinical symptoms and achieve normal spinal anatomy.23 Failure of restoration can result in inadequate clinical improvement potentially leading to poor long term results.24 Significant clinical improvement was observed in both PLIF and TLIF techniques in different spinal disorders25,26 and found to be superior due to proper neural decompression, structural restoration, and segmental stabilization that ultimately lead to improved pain, disability, and functional capability. In our study, both the techniques resulted in significant clinical and functional improvement, structural restoration, fusion, and stability but PLIF had been associated with higher rates of intraoperative neural complications.
There are some limitations in this study. Firstly, the diagnosis was solely depended upon the radiological findings which may have misinterpretations.27 Secondly, T2-weighted kinetic MRI and three-dimensional CT reconstruction had been recommended for a precise diagnosis of LSI,27,28 but we could not perform these due to unavailability of expertise. Thirdly, foraminal widening and fusion assessment needs CT evaluation,29 but was ignored due to patients financial constraints. The cases with multi-segmental instability were not included with an assumption of bias. Fourthly, we did not intend to assess whether there is any biomechanical relationship between pseudarthrosis and postoperative instability. Lastly, the study population was not large enough and followup period was short, as a result we could not evaluate the long term complications, pseudarthrosis requiring revision, adjacent segment degeneration, implant failure, or even the cases with failed back syndrome.
In literatures, comparison of PLIF and TLIF in spondylolisthesis and degenerative lumbar spine had been done. Both Yan26 and Audat30 showed highly significant improvement pain and disability status. The improvement of VAS score of the initial series [PLIF (07.08 ± 01.13 to 02.84 ± 0.89) and TLIF (07.18 ± 01.09 to 02.84 ± 0.91)] was comparable to ours. According to Audat30 excellent outcome had been observed around 60% cases in PLIF and around 70% cases in TLIF, which was also comparable to ours. The overall satisfactory clinical outcome was not measured by the same criteria in different literatures26,30 but even then, the overall outcome had also been similar.
Interbody cages are used to restore the disc height, foraminal height and stabilize the affected segment.31 These parameters have significant correlation regarding structural restoration and maintenance of stability.32 The cage was inserted from the patients left side irrespective of neurological involvement but decompression was done by changing the side with contralateral involvements. We observed a significant increase of disc and foraminal height as well as neurological improvement comparable to other studies.33,34 The increased foraminal height effectively decompresses the nerve roots32 and restores lumbar lordosis which ultimately maintains the lumbar sagittal profile.33 Restoration of local and regional lordosis ultimately achieves clinical and biomechanical stability.35 Kim36 recommended to place the graft anterior to the cage and Hsieh31 recommended to apply compression using the graft and cage as a fulcrum to achieve the desired lordosis. In our study, we were able to achieve 06.50°±0.55° and 05.70°±0.75° improvement of lordotic angles in PLIF and TLIF, that were comparable to other studies.33,34 Despite of no statistical difference of postoperative lordotic angles between the groups, we observed lesser degree of correction in TLIF which assumed to be due to intact posterior structures which had also been observed by Hsieh.31 The correction of forward slip restores sagittal alignment and physiological transmission of weight. Inadequate restoration and abnormal lordosis is the primary predisposing factor for adjacent segment degeneration.37 Moreover, it shifts the spinal vertical axis anteriorly to induce further degeneration and results in chronic LBP.38 The percentage of correction of slip in our study had been significant by both techniques which was comparable to Yan26 [PLIF (72.60 ± 05.20%), TLIF (72.40 ± 05.40%)]. But the reports of increased chance of postoperative instability with PLIF due to loss of integrity of posterior structures should not be abandoned.32
Interbody fusion with cage has been well accepted for its superior fusion results,39,40 and has been reported to be significant by both these techniques.25,26 Theoretically, TLIF is advantageous to PLIF, as it provides a full 360° fusion because of intact contralateral laminar surface that increases the surface area for new bone to grow and bridge the gap.41 We had observed signs of radiological union at 17.23 ± 02.95 weeks in PLIF and 16.90 ± 03.94 weeks in TLIF. But these signs might not conclude regarding the achievement of fusion as because, Kim et al.42 showed around 35% patients achieved solid fusion at 1 year despite of radiological union signs and it even took minimum 4 years to achieve solid fusion in 82% cases. But Brantigan16 compared radiological fusion with exploratory fusion and stated >97% sensitivity, >94% positive predictive value and >93% accuracy of the radiological parameters. Autografts had been the gold standard for achieving fusion, but there are recent reports of graft substitutes for fusion enhancement and has become a new arena of research. We placed autografts anteriorly and impacted before the introduction of cage in all the cases of PLIF and TLIF with a theoretical background of anterior column load transmission (80%)39 and enhancement of fusion.43 The radiological fusion in both the groups had no statistical difference as like other studies.11,12,30 The biomechanical concept of “fusion stability” is assessed postoperatively by dynamic films to determine the achievement of stability even after fusion. Although posterior instrumentations enhances the stability and fusion,8 biomechanically stable spine is achieved only when solid fusion is achieved.44 We achieved the target motion of stability in all the cases of PLIF and TLIF at 1 year according to Posner et al.,3 but according to Kumar et al.,45 postoperative segmental stability is achieved only when radiological motion is < 2° in dynamic films. Development of pseudarthrosis is one of the most common (range, 05-45%) complications of interbody fusion.46 it can be managed conservatively and does not necessarily need revision surgery.47 Pseud arthrosis was present in two (2.60%) and two (4.60%) patients with PLIF or TLIF in a series of Mehta10 which is comparable to our results.
In different literatures, PLIF had been reported to be associated with more neural complications.10,11,12,26 The excess medial retraction of the dura has been blamed to be the cause of such injuries during the placement of the cage.11 On the contrary, TLIF approaches the disc space through far lateral portion of the vertebral foramen, which ultimately reduces the thecal manipulation and the chances of complications. In this series, we had iatrogenic durotomy in four (7.69%) cases and root injury in three (5.77%) cases. There were no such complications in the TLIF group. Intraoperative dural repair were sufficient to control the leaks but one case with root injury ultimately developed foot drop, whereas other two cases were transient and were under the process of gradual recovery. There was a case each in both groups with hardware misplacement which we identified as an instrumental default. Both the cases were reexplored within a week for reinsertion of the implant. Two (3.85%) cases in PLIF had superficial wound infection that had been managed with intravenous antibiotics following culture sensitivity (Staphylococcus aureus) and regular dressing, and the wound was later healed with secondary intention.
To conclude, both methods are effective in relieving symptoms, achieving stability and fusion, but TLIF can be preferred for its shorter operative time, less blood loss, and lesser complication rates in surgical management of symptomatic lumbar instability.
Footnotes
Source of Support: Nil
Conflict of Interest: None
REFERENCES
- 1.Guillot M, Fournier J, Scheye T, Escande G, Chazal J, Tanguy A, et al. Mechanics of the characteristic geometry of the human spine undergoing vertical pressure. Bull Assoc Anat (Nancy) 1990;74:7–8. [PubMed] [Google Scholar]
- 2.Alqarni AM, Schneiders AG, Hendrick PA. Clinical tests to diagnose lumbar segmental instability: A systematic review. J Orthop Sports Phys Ther. 2011;41:130–40. doi: 10.2519/jospt.2011.3457. [DOI] [PubMed] [Google Scholar]
- 3.Posner I, White AA, 3rd, Edwards WT, Hayes WC. A biomechanical analysis of the clinical stability of the lumbar and lumbosacral spine. Spine (Phila Pa 1976) 1982;7:374–89. doi: 10.1097/00007632-198207000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Fritz JM, Erhard RE, Hagen BF. Segmental instability of the lumbar spine. Phys Ther. 1998;78:889–96. doi: 10.1093/ptj/78.8.889. [DOI] [PubMed] [Google Scholar]
- 5.Sonntag VK, Marciano FF. Is fusion indicated for lumbar spinal disorders? Spine (Phila Pa 1976) 1995;20:138s–42s. [PubMed] [Google Scholar]
- 6.Stonecipher T, Wright S. Posterior lumbar interbody fusion with facet-screw fixation. Spine (Phila Pa 1976) 1989;14:468–71. doi: 10.1097/00007632-198904000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461–72. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 8.West JL, 3rd, Bradford DS, Ogilvie JW. Results of spinal arthrodesis with pedicle screw-plate fixation. J Bone Joint Surg Am. 1991;73:1179–84. [PubMed] [Google Scholar]
- 9.Harm J, Jeszensky D. The unilateral transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol. 1998;6:88–99. [Google Scholar]
- 10.Mehta VA, McGirt MJ, Garcés Ambrossi GL, Parker SL, Sciubba DM, Bydon A, et al. Trans-foraminal versus posterior lumbar interbody fusion: Comparison of surgical morbidity. Neurol Res. 2011;33:38–42. doi: 10.1179/016164110X12681290831289. [DOI] [PubMed] [Google Scholar]
- 11.Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976) 2001;26:567–71. doi: 10.1097/00007632-200103010-00023. [DOI] [PubMed] [Google Scholar]
- 12.Park J, Kim Y, Hong H, Hwang S. Comparison between posterior and transforaminal approaches for lumbar interbody fusion. J Korean Neurosurg Soc. 2005;37:340–4. [Google Scholar]
- 13.Meyerding HW. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371–80. [Google Scholar]
- 14.Guyatt GH, Townsend M, Berman LB, Keller JL. A comparison of Likert and visual analogue scales for measuring change in function. J Chronic Dis. 1987;40:1129–33. doi: 10.1016/0021-9681(87)90080-4. [DOI] [PubMed] [Google Scholar]
- 15.Walsh TL, Hanscom B, Lurie JD, Weinstein JN. Is a condition-specific instrument for patients with low back pain/leg symptoms really necessary? The responsiveness of the Oswestry Disability Index, MODEMS, and the SF-36. Spine (Phila Pa 1976) 2003;28:607–15. doi: 10.1097/01.BRS.0000050654.97387.DF. [DOI] [PubMed] [Google Scholar]
- 16.Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM. Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: Two-year results from a food and drug administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 2000;25:1437–46. doi: 10.1097/00007632-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 17.Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U. Transforaminal lumbar interbody fusion: A safe technique with satisfactory three to five year results. Eur Spine J. 2005;14:551–8. doi: 10.1007/s00586-004-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang S, Xu W. Does disc space height of fused segment affect adjacent disc degeneration in anterior lumbar interbody fusion? A radiological study. Iran Red Crescent Med J. 2012;14:139–45. [PMC free article] [PubMed] [Google Scholar]
- 19.Siambanes D, Mather S. Comparison of plain radiographs and CT scans in instrumented posterior interbody fusion. Orthopedics. 1998;21:165–7. doi: 10.3928/0147-7447-19980201-09. [DOI] [PubMed] [Google Scholar]
- 20.Macnab I. Negative disc exploration. J Bone Joint Surg. 1971;53:891–901. [PubMed] [Google Scholar]
- 21.Fujiwara A, Tamai K, An HS, Kurihashi T, Lim TH, Yoshida H, et al. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J Spinal Disord. 2000;13:444–50. doi: 10.1097/00002517-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan PB. Lumbar segmental “instability”: Clinical presentation and specific stabilizing exercise management. Man Ther. 2000;5:2–12. doi: 10.1054/math.1999.0213. [DOI] [PubMed] [Google Scholar]
- 23.Kraft CN, Krauspe R. Spondylolisthesis. In: Boos N, Aebi M, editors. Spinal Disorders: Fundamentals of Diagnosis and Treatment. Berlin: Springer; 2008. pp. 733–96. [Google Scholar]
- 24.Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol. 2003;13:371–9. doi: 10.1016/s1050-6411(03)00044-0. [DOI] [PubMed] [Google Scholar]
- 25.Lund T, Oxland TR, Jost B, Cripton P, Grassmann S, Etter SC, et al. Interbody cage stabilisation in the lumbar spine biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80-B:351–9. doi: 10.1302/0301-620x.80b2.7693. [DOI] [PubMed] [Google Scholar]
- 26.Yan D, Pei F, Li J, Soo C. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17:1311–6. doi: 10.1007/s00586-008-0739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvorak J, Panjabi MM, Chang DG, Theiler R, Grob D. Functional radiographic diagnosis of the lumbar spine: Flexion-extension and lateral bending. Spine (Phila Pa 1976) 1991;16:562–71. doi: 10.1097/00007632-199105000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang S, Kong M, Hymanson HJ, Jin TK, Song KY, Jeffrey C, et al. Radiographic parameters of segmental instability in lumbar spine using kinetic MRI. J Korean NeurosurgSoc. 2009;45:24–31. doi: 10.3340/jkns.2009.45.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang P, Chafetz N, Genant HK, Morris JM. Lumbar spinal fusion. Assessment of functional stability with magnetic resonance imaging. Spine (Phila Pa 1976) 1990;15:581–8. doi: 10.1097/00007632-199006000-00028. [DOI] [PubMed] [Google Scholar]
- 30.Audat Z, Moutasem O, Yousef K, Mohammad B. Comparison of clinical and radiological reslts of posterolateral fusion, posteror lumbar interbody fusion and transforaminal lumbar interbody fusion techniques in the treatment of degenerative lumbar spine. Singapore Med J. 2012;53:183–7. [PubMed] [Google Scholar]
- 31.Hsieh PC, Koski TR, O’shaughnessy BA, Sugrue P, Salehi S, Ondra S, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: Implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine. 2007;7:379–86. doi: 10.3171/SPI-07/10/379. [DOI] [PubMed] [Google Scholar]
- 32.Osman SG, Nibu K, Panjabi MM, Marsolais EB, Chaudhary R. Transforaminal and posterior decompressions of the lumbar spine. A comparative study of stability and intervertebral foramen area. Spine (Phila Pa 1976) 1997;22:1690–5. doi: 10.1097/00007632-199708010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Rahn KA, Shugart RM, Wylie MW, Reddy KK, Morgan JA. The effect of lordosis, disc height change, subsidence, and transitional segment on stand-alone anterior lumbar interbody fusion using a nontapered threaded device. Am J Orthop. 2010;39:E124–9. [PubMed] [Google Scholar]
- 34.Kim M, Chung H, Kim D, Kim S, Jeon S. The clinical and radiological outcomes of minimally invasive transforaminal lumbar interbody single level fusion. Asian Spine J. 2011;5:111–6. doi: 10.4184/asj.2011.5.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klemme WR, Owens BD, Dhawan A, Zeidman S, Pollv DW., Jr Lumbar sagittal contour after posterior inter-body fusion: Threaded devices alone versus vertical cages plus posterior instrumentation. Spine (Phila Pa 1976) 2001;26:534–7. doi: 10.1097/00007632-200103010-00017. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Jeon T, Heo Y, Lee W, Yi J, Kim T, et al. Radiographic results of single level transforaminal lumbar interbody fusion in degenerative lumbar spine disease: Focusing on changes of segmental lordosis in fusion segment. Clin Orthop Surg. 2009;1:207–13. doi: 10.4055/cios.2009.1.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djurasovic MO, Carreon LY, Glassman SD, Dimar JR, 2nd, Puno RM, Johnson JR. Sagittal alignment as a risk factor for adjacent level degeneration: A case-control study. Orthopedics. 2008;31:546. [PubMed] [Google Scholar]
- 38.Gelb DE, Lenke LG, Bridwell KH, Blanke K, McEnery KW. An analysis of saggital spinal alignment in 100 asymptomatic middle and older aged volunteers. Spine (Phila Pa 1976) 1995;20:1351–8. [PubMed] [Google Scholar]
- 39.Jeon JH, Kim SM, Jung DJ, Moon SM, Hwang HS, Choi SK. PLIF using cages at the instability level and additional transpedicular instrumental fusion in multilevel degenerative lumbar disease. J Korean Neurosurg Soc. 2004;35:372–8. [Google Scholar]
- 40.Kuslich SD, Danielson G, Dowdle JD, Sherman J, Fredrickson B, Yuan H, et al. Four-year followup results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine (Phila Pa 1976) 2000;25:2656–62. doi: 10.1097/00007632-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 41.Chastain CA, Eck JC, Hodges SD, Humphreys SC, Levi P. Transforaminallumbar interbody fusion: A retrospective study of long term pain relief and fusion outcomes. Orthopedics. 2007;30:389–92. doi: 10.3928/01477447-20070501-18. [DOI] [PubMed] [Google Scholar]
- 42.Kim KS, Yang TK, Lee JC. Radiological changes in the bone fusion site after posterior lumbar interbody fusion using carbon cages impacted with laminar bone chips: Followup study over more than 4 years. Spine (Phila Pa 1976) 2005;30:655–60. doi: 10.1097/01.brs.0000155421.07796.7f. [DOI] [PubMed] [Google Scholar]
- 43.Brodke DS, Dick JC, Kunz DN, McCabe R, Zdeblick TA. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine (Phila Pa 1976) 1997;22:26–31. doi: 10.1097/00007632-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 44.Tsantrzos A, Baramki HG, Zeidman S, Steffen T. Segmental stability and compressive strength of posterior lumbar interbody fusion implants. Spine (Phila Pa 1976) 2000;25:1899–907. doi: 10.1097/00007632-200008010-00007. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Kozak JA, Doherty BJ, Dickson JH. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine (Phila Pa 1976) 1993;18:2393–400. doi: 10.1097/00007632-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop. 1992;284:80–90. [PubMed] [Google Scholar]
- 47.Thalgott JS, LaRocca H, Aebi M, Dwyer AP, Razza BE. Reconstruction of the lumbar spine using AO DCP plate internal fixation. Spine (Phila Pa 1976) 1989;14:91–5. doi: 10.1097/00007632-198901000-00018. [DOI] [PubMed] [Google Scholar]


