Abstract
Mouse mammary tumor virus (MMTV) is a milk-borne retrovirus that exploits the adaptive immune system. It has recently been shown that MMTV activates B cells via Toll-like receptor 4 (TLR4), a molecule involved in innate immune responses. Here, we show that direct virus binding to TLR4 induced maturation of bone marrow-derived dendritic cells and up-regulated expression of the MMTV entry receptor (CD71) on these cells. In vivo, MMTV increased the number of dendritic cells in neonatal Peyer's patches and their expression of CD71; both these effects were dependent on TLR4. Thus, retroviral signaling through TLRs plays a critical role in dendritic-cell participation during infection.
Mouse mammary tumor virus (MMTV) is a betaretrovirus that causes mammary tumors in mice (29). Exogenous MMTV is transmitted to newborns through milk during the first days of lactation (5, 20). It is well known that MMTV utilizes the host adaptive immune system to establish infection. In the Peyer's patches (PP), milk-borne MMTV particles are thought to first infect B cells, which then present a virus-encoded superantigen (SAg) on their surfaces (9) to T cells expressing SAg-specific T-cell receptor Vβ chains. The resulting T-cell stimulation leads to the amplification of the infected B cells by inducing their proliferation (16).
Although the importance of B and T lymphocytes in MMTV infection is well established, the role of dendritic cells (DCs) in this process is not clear. DCs are professional antigen-presenting cells (APCs) that play a fundamental role in initiating and amplifying both innate and adaptive immune responses (1). In their immature state, DCs are distributed in peripheral tissues, where they take up and process self- and non-self-antigens. Bacterial and viral products, as well as inflammatory cytokines, induce DC maturation through direct interaction with specific surface receptors (15). The differentiation-maturation process down-regulates further antigen processing by DCs but enhances the expression of surface molecules important for successful antigen presentation (major histocompatibility complex I and II, CD40, CD80, and CD86) (8). In addition, maturing DCs secrete cytokines (e.g., tumor necrosis factor alpha [TNF-α], interleukin 1α [IL-1α] and -β, and IL-6) and chemokines (e.g., RANTES, MIP-2, and GROα) that recruit immune cells to sites of infection (2).
DCs express a variety of receptors that recognize microbial products, including Toll-like receptors (TLRs), which have been shown to play an important role in pathogen-induced DC activation. The TLR family mediates recognition of microbial targets in several organisms, including humans, mice, and flies; at least 10 mammalian TLRs have been identified (44). TLRs are evolutionarily conserved receptors; their activation initiates a signaling pathway that leads to activation of NF-κB transcription factors and members of the mitogen-activated protein kinase family (31). In the past few years, dozens of TLR ligands have been identified, most commonly molecules of bacterial origin, such as lipopolysaccharide (LPS) and peptidoglycan, which interact with TLR4 and TLR2, respectively (31). Recently, however, it has been demonstrated that viral proteins, like the measles virus hemagglutinin protein and the respiratory syncytial virus fusion protein, cause activation of monocytic cells by interacting with specific TLRs (6, 22) and that human cytomegalovirus triggers inflammatory-cytokine production in mononuclear cells in a TLR2-dependent manner (10). In addition, the MMTV envelope protein was shown to activate B cells via interaction with TLR4 (38).
Ligand binding to TLRs activates both macrophages and DCs (31) and plays an important role in triggering DC maturation. TLR signaling induces DC up-regulation of major histocompatibility complex and costimulatory molecules and proinflammatory-cytokine expression (4). The recent finding that MMTV infects DCs (26, 45) and that the virus is able to activate B cells through a TLR4-dependent mechanism at an early stage of experimental infection in adult mice (38) led to the present study, in which we show that MMTV interaction with TLR4 is able to induce in vitro maturation of bone marrow-derived dendritic cells (BMDCs), recruitment of DCs to PP (the natural site of initial infection), and increased expression of the MMTV cell entry receptor on these cells in vitro and in vivo.
MATERIALS AND METHODS
Mice.
BALB/cJ mice were bred in the animal facility of the Division Medicina Experimental, Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina. BALB/cJ and congenic C.C3H Tlr4lps-d mice from the Jackson Laboratory and C3H/HeJ and C3H/HeN mice from the National Cancer Institute (Frederick, Md.) were bred at the University of Pennsylvania. TLR2 knockout mice (43) were crossed with C3H/HeN or C3H/HeJ (TLR4Lps-d/TLR4Lps-d) mice, followed by two successive rounds of intercrossing to obtain TLR2−/− and TLR4Lps-d/TLR4Lps-d TLR2−/− H-2k+/H-2k+ mice. TLR2−/− mice were screened by transgene-specific PCR; mice containing the TLR4Lps-d allele were screened by PCR of genomic DNA using primers that flanked the point mutation and sequencing of the product for the C2342-to-A2342 change (36). The mice were housed according to the policies of the University of Pennsylvania and Academia Nacional de Medicina (NIH Guide for the Care and Use of Laboratory Animals) (33a).
Generation of mouse BMDCs.
DCs were generated according to the method of Lutz et al. (25). Femurs and tibiae of mice were removed and freed of muscles and tendons. The bones were placed in 70% ethanol for 120 s and subsequently washed in phosphate-buffered saline. Both bone ends were cut off, and the marrow was flushed out with RPMI medium. The cells were centrifuged for 8 min at 360 × g. The cells were cultured in bacterial petri dishes at a density of 2 × 105/ml in 10 ml of RPMI with 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin, 50 μM 2-mercaptoethanol, and 10% fetal bovine serum (R10 medium) supplemented with 30% mouse granulocyte-macrophage colony-stimulating factor (mGM-CSF)-containing supernatant from a J558 cell line stably transfected with mGM-CSF. On day 3 of culture, another 10 ml of R10 medium with mGM-CSF was added. On days 6 and 8, half of the culture supernatant was collected and centrifuged, and the cell pellet was resuspended in 10 ml of R10 medium with mGM-CSF. On day 10, nonadherent cells were collected by gentle pipetting, centrifuged at 300 × g for 8 min, and resuspended in R10 medium; 90% of these cells were CD11c positive (not shown).
Virus preparation.
MMTV(LA) (14, 35) and MMTV(FM) (38) were isolated from mammary tumors from C3H/HeN mice as previously described and banded on 0 to 60% sucrose gradients (23). Virus obtained from ∼800 mg of tumor tissue was resuspended in 600 μl of phosphate-buffered saline. Dilutions of purified virus were tested for B-cell and SAg-mediated T-cell activation in vivo, as previously described (38), and on C3H/HeN BMDCs in vitro. The highest dilution giving the maximum BMDC activation after 18 h (usually 1:10 to 1:20) was used for all subsequent assays with DCs. 2,2′-Dithiodipyridine (AT-2) (Sigma, Inc., St. Louis, Mo.) and UV treatments were performed as previously described (38).
Stimulation of DCs.
DCs were cultured in R10 medium with LPS from Escherichia coli 0111:B4 (100 ng/ml; Sigma) or diluted virus preparations for 3 h (for reverse transcription [RT]-PCR and RNase protection assays) or 18 h (for enzyme-linked immunosorbent assays [ELISAs] and flow cytometry). For blocking experiments, diluted virus was mixed with a 1:10 dilution of goat anti-MMTV or anti-gp52 antiserum (Quality Biotech, Camden, N.J.). For endotoxin contamination testing, LPS or MMTV preparations were boiled for 1 h prior to their addition to cells. For some experiments, MMTV was pretreated with 150 μM AT-2 for 1 h at 37°C or with UV light for 30 min at 0°C, as previously described (38).
Tolerization.
LPS (10 ng/ml) was added to BMDCs on day 9 of culture. Twenty hours later, the cells were incubated with activating doses of LPS or MMTV as described above.
Flow cytometry.
BMDCs were incubated with Fc-γ block antibody (anti-mouse CD16/32; Pharmingen, San Diego, Calif.) to prevent nonspecific binding of antibodies to Fc-γ receptors. Cells (1.5 × 105 to 5 × 105) were stained with the following monoclonal antibodies (Pharmingen) and subjected to fluorescence-activated cell sorting (FACS) analysis: phycoerythrin-conjugated anti-CD11c (HL3), fluorescein isothiocyanate (FITC)-conjugated anti-CD40 (HM40-3), FITC-conjugated anti-CD80 (16-10A1), and FITC-conjugated anti-CD71 (C2). Cells were acquired on a FACScan cytometer (Becton Dickinson, Mountain View, Calif.). Data were analyzed by using CELLQUEST software (Becton Dickinson Immunocytometry Systems).
ELISA.
Production of TNF-α, IL-6, and IL-12 p40 was detected in the DC supernatant after 18 h of stimulation by using ELISA commercial kits (Quantikine; R&D Systems, Minneapolis, Minn.). The assays were performed as described by the manufacturer. Data are presented as means ± standard deviations (SD) of triplicate observations.
RT-PCR.
Total RNA was isolated from DCs using the RNeasy Mini Kit (Qiagen Inc., Valencia, Calif.) according to the manufacturer's instructions. RT-PCR was performed using the Access RT-PCR System kit (Promega) according to the manufacturer's instructions. The following primers were used: mouse TNF-α, 5′TCTCATCAGTTCTATGGCCC and 5′GGGAGTAGACAAGGTACAAC; mouse IL-6, 5′TTCCTCTCTGCAAGAGACT and 5′TGTATCTCTCTGAAGGACT; and mouse β-actin, 5′TCATGAAGTGTGACGTTGACATC and 5′CCTAGAAGCATTTGCGGTGCAACGATG. The RT-PCR products were analyzed by electrophoresis on 2% agarose gels with ethidium bromide.
RPAs.
Total RNA (2.5 to 6 μg) was analyzed by RNase protection assays (RPAs) using the BD Biosciences-Pharmingen Riboquant kit according to the manufacturer's recommendations. The mCK-3b and mCK-5c multiprobe template sets were used. The bands corresponding to the various chemokines were quantified using a PhosphorImager (Molecular Dynamics, Inc., Sunnyvale, Calif.) and ImageQuant software. For quantitation, chemokine levels were expressed as percentages of the mean levels of the L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping genes for each RNA sample. Data are presented as the increase (n-fold) in mRNA expression in stimulated versus unstimulated cells.
In vitro infection.
DCs were infected by the spinoculation method as previously described (40). In brief, DCs were plated in a 96-well plate at a cell density of 105/100 μl. One microliter of undiluted MMTV(LA) was added to the cells, and the plate was centrifuged at 1,200 × g for 120 min at room temperature. After centrifugation, the supernatant was discarded and fresh medium was added to the cells. The cells were incubated overnight at 37°C, and the DNA was extracted. Semiquantitative PCR was performed using the virus-specific primers TG5 (5′AATTCGGAGAACTCGACCTTCC3′) and TG12 (5′CCCCCATGAGTATATTTGA 3′), which amplify integrated MMTV(LA) DNA. The optimal number of cycles to stay in the linear range was determined as previously described (14). PCR products were analyzed by electrophoresis on 2% agarose gels with ethidium bromide.
PP DCs.
Eight-day-old mice were foster nursed on MMTV(LA)-infected mothers for 3 days. The mice were sacrificed, their PP were dissected, and single-cell suspensions were prepared by homogenization through nylon mesh. The cells were washed once with RPMI-10% fetal bovine serum. FACS staining was performed as described above.
Statistical analysis.
Statistical analysis was performed by one-way analysis of variance for multiple comparisons or by Student’s t test as appropriate. If the analysis of variance yielded significance (P < 0.05), Tukey's multiple-comparison test or Bonferroni's test for multiple comparisons on selected single means was carried out.
RESULTS
Maturation of BMDCs stimulated by MMTV.
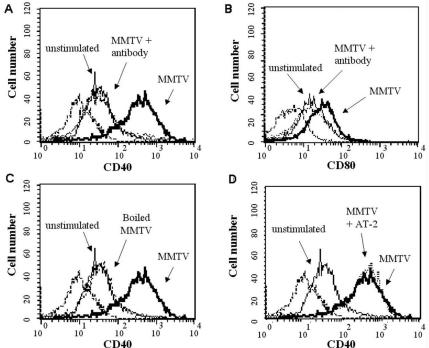
To test whether MMTV had any direct effect on DC function, we incubated BMDCs with MMTV(LA) (14, 35) for 18 h. The maturation status of BMDCs was determined by the expression of cell surface markers, such as CD40 and CD80, on CD11c-positive cells, as well as by the production of proinflammatory cytokines and chemokines. BMDCs incubated with the virus showed increased surface expression of both CD40 and CD80 as assessed by cytofluorometric analysis. Preincubation of the virus with anti-MMTV antisera blocked this effect (Fig. 1A and B), and boiling the virus, but not LPS (not shown), abolished CD40 and CD80 up-regulation (Fig. 1C), thus ruling out endotoxin contamination in the virus preparations.
FIG. 1.
Expression of costimulatory molecules on BMDCs after MMTV stimulation. BMDCs were incubated for 18 h with MMTV(LA), MMTV plus anti-MMTV antibody, boiled MMTV, or AT-2-treated MMTV. Expression of CD40 (A, C, and D) or CD80 (B) on CD11c+ cells was analyzed by FACS. The dashed lines represent isotype antibody control. The histograms depict representative results. The experiment was performed three times with similar results.
To determine whether BMDC maturation depended on viral-gene expression, two different pretreatments were used, AT-2 and UV light, both of which block infection immediately postentry. AT-2 interacts with the zinc finger domains of the nucleocapsid and has been shown to inactivate human immunodeficiency virus (HIV) type 1 (41) and MMTV (38). Untreated and AT-2-treated MMTV(LA) induced similar increases in CD40 and CD80 expression on DCs (Fig. 1D). Similar results were obtained with UV-inactivated MMTV(LA) (not shown).
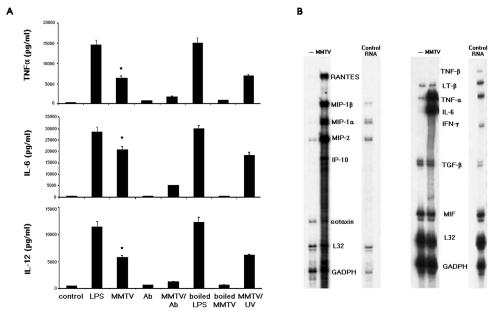
DC maturation is also characterized by the secretion of proinflammatory cytokines and chemokines. We next tested TNF-α, IL-6, and IL-12 p40 levels in the supernatants of BMDCs stimulated with MMTV(LA) for 18 h, using sandwich ELISAs. As shown in Fig. 2A, incubation of DCs with MMTV(LA) greatly increased the production of all three cytokines. Incubation of the virus with anti-MMTV (Fig. 2A) or anti-gp52 (not shown) and virus heat treatment all abolished cytokine production. In contrast, cytokine induction was not affected when UV-treated MMTV(LA) was used (Fig. 2A).
FIG. 2.
Proinflammatory cytokine and chemokine production in MMTV-stimulated BMDCs. Cells (106/100 μl) were incubated with LPS (100 ng/ml) or MMTV. Where indicated, LPS and MMTV were boiled (1 h) or pretreated with UV (30 min) or anti-MMTV antibody (Ab). (A) After 18 h, the concentration of TNF-α, IL-6, or IL-12 in the supernatant was measured by ELISA. The data are presented as means plus SD. One experiment out of three is shown. *, P < 0.001 compared to the control group (unstimulated BMDCs). (B) After 2 h, cytokine and chemokine mRNA levels were measured by RPA, using multiprobe sets. One experiment out of three is shown.
We also performed RPAs to determine if MMTV induced cytokine and chemokine gene transcription in BMDCs. GADPH and L32 housekeeping genes provided internal controls. After 3 h of incubation with virus, there were increased TNF-α, IL-6, IP-10, MIP-1α, MIP-1β, MIP-2, RANTES, and eotaxin mRNA levels in MMTV(LA)-stimulated BMDCs (Fig. 2B). In contrast, migration inhibition factor and transforming growth factor β mRNA levels were not affected.
The same experiments were also performed with BMDCs derived from BALB/cJ mice or with a second MMTV variant, MMTV(FM), and similar results were obtained (not shown). These results showed that MMTV was able to induce murine-BMDC maturation and that this effect was independent of viral-gene expression.
MMTV-induced cytokine production in LPS-tolerized BMDCs.
Ligand binding to TLRs leads to DC maturation and the transcriptional activation of several proinflammatory genes (4). To study whether MMTV activated BMDCs through interaction with TLR4, we first pretreated BMDCs in vitro with low doses of LPS for 20 h to make them hyporesponsive or tolerant to TLR4 ligands (39). Tolerized and nontolerized BMDCs from BALB/cJ or C3H/HeN mice were exposed to MMTV(LA), and the production of TNF-α (Fig. 3) and IL-6 (not shown) was measured by RT-PCR and ELISA. Cytokine production induced by both LPS and MMTV(LA) was impaired in LPS-tolerized BMDCs, suggesting that MMTV interacted with TLR4 molecules on DCs, leading to their maturation.
FIG. 3.
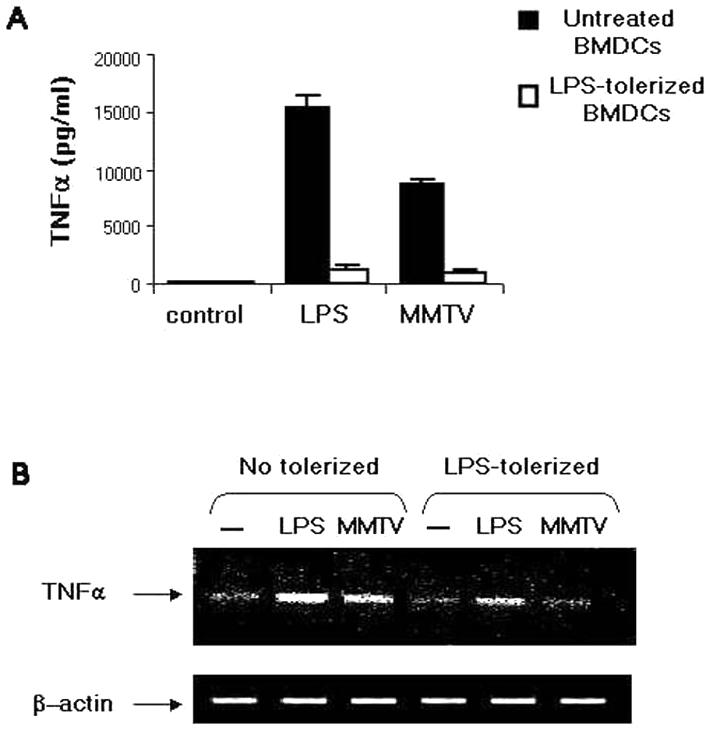
TNF-α production in LPS-tolerized BMDCs. BMDCs from BALB/cJ mice were tolerized for 20 h with LPS (10 ng/ml) and then stimulated with LPS (100 ng/ml) or MMTV for 18 h. The TNF-α concentration in the supernatant was measured by ELISA (A), and total RNA was analyzed by RT-PCR using TNF-α-specific primers (B). The data are representative of three separate experiments with similar results. The error bars indicate SD.
Effects of MMTV on maturation of TLR4Lps-d/TLR4Lps-d BMDCs.
To determine whether MMTV failed to activate LPS-tolerized DCs due to loss of TLR4 signaling, we next studied whether MMTV induced activation of BMDCs from C3H/HeJ (TLR4Lps-d/TLR4Lps-d) mice, which have a point mutation in their TLR4 gene that makes the molecule unable to signal (17, 36, 37). MMTV-induced up-regulation of CD40 and CD80 expression was dramatically reduced on BMDCs from C3H/HeJ mice (Fig. 4).
FIG. 4.
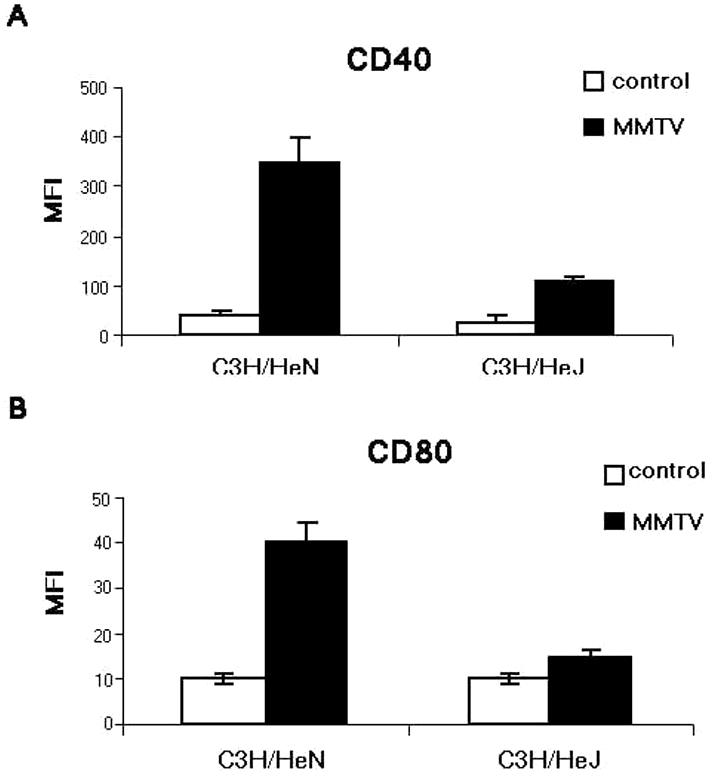
CD40 and CD80 expression on MMTV-induced BMDCs from C3H/HeN and C3H/HeJ mice. BMDCs were incubated for 18 h with MMTV. Expression of CD40 (A) and CD80 (B) on CD11c+ cells was analyzed by FACS. The data represent the mean fluorescence intensity (MFI) (mean ± SD; n = 3). The experiment was performed three times with similar results.
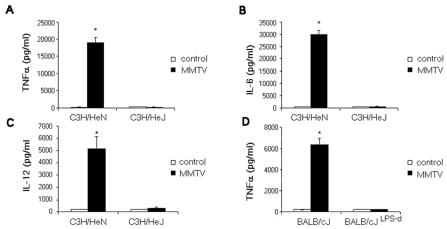
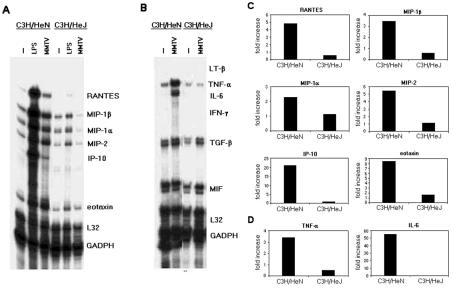
We also analyzed cytokine and chemokine expression in MMTV-stimulated BMDCs obtained from C3H/HeN and C3H/HeJ mice. As shown in Fig. 5A to C, MMTV(LA) did not induce TNF-α, IL-6, or IL-12 secretion by C3H/HeJ BMDCs. Cytokine and chemokine mRNA levels in MMTV-stimulated BMDCs from the different mice were also analyzed by RPAs (Fig. 6). After 3 h of MMTV stimulation, C3H/HeN BMDCs showed increased levels of TNF-α, IL-6, RANTES, MIP-1α, MIP-1β, MIP-2, IP-10, and eotaxin mRNAs. In contrast, none of these mRNAs were induced by virus in C3H/HeJ BMDCs.
FIG. 5.
Cytokine production in MMTV-induced BMDCs from C3H/HeN, C3H/HeJ, BALB/cJ, and C.C3H Tlr4lps-d (BALB/cJLPS-d) mice. BMDCs (106/100 μl) were incubated with MMTV for 18 h. The concentration of TNF-α (A and D), IL-6 (B), or IL-12 (C) in the supernatant was measured by ELISA. The data represent the means ± SD (n = 3). One experiment out of three is shown. *, P < 0.001 compared to the control group (unstimulated BMDCs).
FIG. 6.
Chemokine and cytokine mRNA levels in MMTV-induced BMDCs from C3H/HeN and C3H/HeJ mice. Cells (106/100 μl) were incubated with MMTV or LPS. After 2 h, chemokine (A and C) and cytokine (B and D) mRNA levels were measured by RPA, using multiprobe sets. (A and B) Autoradiographs of RPA gels. −, unstimulated. (C and D) Quantitation of relative expression of chemokine and cytokine mRNAs, as described in Materials and Methods.
BMDCs derived from BALB/cJ mice congenic for the defective LPS response allele from C3H/HeJ mice (C.C3H Tlr4lps-d) (46) also showed abrogated responses to MMTV compared to control BALB/cJ-derived BMDCs (Fig. 5D and not shown). Taken together, these results show that MMTV is able to induce BMDC maturation through interaction with TLR4 molecules.
MMTV cell entry receptor (CD71) expression in BMDCs.
It has long been known that CD71 (also called transferrin receptor 1 [TfR1]) is a lymphocyte activation marker and that the TLR4 ligand, LPS, increases surface expression of the molecule (7, 13). CD71/TfR1 has recently been shown to be the cell entry receptor for MMTV (40). Because measles virus has been shown to increase surface expression of its entry receptor, CD150, on monocytes via TLR2 signaling (6), we tested whether MMTV-mediated stimulation of DCs resulted in increased CD71 expression. BMDCs from C3H/HeN or C3H/HeJ mice were cultured with MMTV(LA) for 18 h. BMDCs from both strains of mice showed increased CD71 expression, although this increase was significantly smaller in C3H/HeJ than in C3H/HeN mice (Fig. 7). Pretreatment of the virus with AT-2 or UV light did not alter the increases in CD71 (not shown).
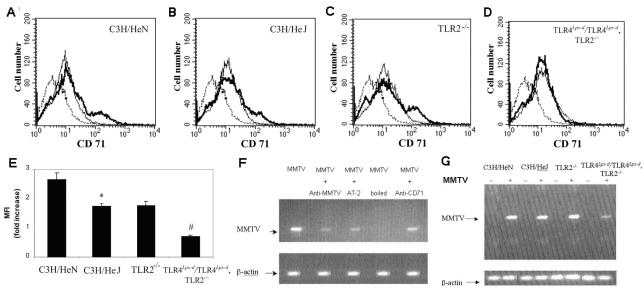
FIG. 7.
Expression of the MMTV receptor on BMDCs and infection with MMTV. (A to D) BMDCs derived from C3H/HeN (A), C3H/HeJ (B), TLR2−/− (C) and TLR4Lps-d/TLR4Lps-d TLR2−/− (D) mice were incubated for 18 h with MMTV. Expression of CD71+/CD11c+ cells was analyzed by FACS. The histograms depict representative results. Dashed lines represent isotype antibody; solid lines, unstimulated BMDCs; bold lines, MMTV. (E) Data are presented as the increase (n-fold) in the mean fluorescence intensity (MFI) (plus SD) compared to unstimulated cells for each mouse strain. The experiment was performed three times with similar results. *, P < 0.01 (C3H/HeN versus C3H/HeJ); #, P < 0.01 (TLR2−/− versus TLR4Lps-d/TLR4Lps-d TLR2−/−). (F and G) BMDCs were infected with MMTV as described in Materials and Methods. After overnight incubation, DNA was extracted and PCR was performed to look for integrated viral DNA in the cellular genome. The data are representative of three separate experiments with similar results.
Recent work in our laboratory has demonstrated that MMTV may also interact with TLR2 molecules and that B cells lacking functional TLR2 and TLR4 molecules show almost no response to MMTV (J. Rassa, unpublished data). We therefore tested whether CD71 would be up-regulated in BMDC from TLR2−/− and TLR4Lps-d/TLR4Lps-d TLR2−/− mice. There was an approximately twofold increase in CD71 on DCs from TLR2−/− mice, similar to that seen with cells from C3H/HeJ mice. Interestingly, there was no increase in CD71 expression on DCs from TLR4Lps-d/TLR4Lps-d TLR2−/− mice (Fig. 7). These results indicate that MMTV interaction with TLR molecules on DCs results in increased expression of the virus entry receptor.
MMTV infection of BMDCs.
Because MMTV was able to up-regulate the expression of its cell entry receptor on BMDCs and this effect was abolished in the absence of functional TLR4 and TLR2, we next examined MMTV infection of BMDCs from the different TLR-defective mice. C3H/HeN BMDCs were infected in vitro with MMTV(LA), and infection was substantially blocked when anti-MMTV antiserum was added to the virus (Fig. 7F). Likewise, infection was inhibited by AT-2 or heat treatment of MMTV. A monoclonal antibody against CD71 that is known to down-regulate surface expression on lymphocytes also blocked the infection of BMDCs with MMTV(LA) (Fig. 7F), as was previously demonstrated for mammary epithelial and B lymphoma tissue culture cells (40).
Infection of BMDCs with MMTV(LA) was found to be similar in BMDCs from C3H/HeN, C3H/HeJ, and TLR2−/− mice. However, correlating with the lack of increased CD71 expression, the level of infection of BMDCs from TLR4Lps-d/TLR4Lps-d TLR2−/− mice was decreased (Fig. 7G).
Percentage of CD11c+ cells and CD71 expression in neonatal PP.
It has recently been reported that the number of DCs in popliteal lymph nodes dramatically increases after subcutaneous injection of MMTV into the footpads of BALB/c mice (26). To determine whether natural milk-borne infection induced similar changes in the number of CD11c+ cells in the PP and whether these increases were the result of interaction of the virus with TLR4, C3H/HeN and C3H/HeJ pups were foster nursed on MMTV(LA)-infected mothers. After 3 days of foster nursing, the percentage of CD11c+ cells in the PP was determined by FACS. As shown in Table 1, MMTV(LA) induced a twofold increase in the number of DCs in the PP of C3H/HeN pups that was not observed in C3H/HeJ mice.
TABLE 1.
Changes in PP DCs after MMTV infectiona
| Mouse | MMTV | % CD11c+ | % CD71+/CD11c+ |
|---|---|---|---|
| C3H/HeN | − | 2.3 ± 0.2 | 25.1 ± 2.0 |
| C3H/HeN | + | 5.6 ± 1.0b | 38.4 ± 0.9b |
| C3H/HeJ | − | 1.9 ± 0.5 | 30.0 ± 3.0 |
| C3H/HeJ | + | 1.7 ± 0.4 | 27.5 ± 1.8 |
C3H/HeN or C3H/HeJ pups were foster nursed on uninfected or MMTV(LA)-infected C3H/HeN mothers (MMTV − or +, respectively). After 3 days, the percentage of CD11c+ cells in the PP and their expression of CD71 were analyzed by FACS. The experiment was performed three times with similar results. The data are presented as means ± SD.
P < 0.01 compared to the respective control group.
To determine whether MMTV also had an effect on the level of expression of its entry receptor during natural virus acquisition, we next analyzed the expression of CD71 in PP DCs following milk-borne MMTV infection. A significant increase in the percentage of CD71+ cells in the CD11c+-DC population from C3H/HeN pups foster nursed for 3 days on MMTV(LA)+ mothers was observed, whereas this increase was not detected in the DCs of C3H/HeJ pups nursed on the same mothers (Table 1). In addition, the mean fluorescence intensity of CD71 in DCs was increased in C3H/HeN but not in C3H/HeJ mice (not shown). Thus, MMTV also activated DCs in PP during milk-borne infection, and this activation was dependent on functional TLR4 molecules.
DISCUSSION
DCs are one of the major sensors of microbial infection. These cells interact with T lymphocytes, as well as other cells involved in pathogen recognition, and play a role in the clearance of infectious agents through the secretion of mediators of inflammatory response, such as cytokines and chemokines, and through their ability to serve as APCs. Thus, TLR-mediated activation of DCs has profound effects not only on innate immune responses but also on subsequent adaptive responses. Because it has recently been shown that MMTV binds TLR4 and activates B cells (38) and that DCs can be infected at levels comparable to those of B cells shortly after subcutaneous MMTV injection (26, 45), we investigated TLR4 involvement in (i) MMTV-induced BMDC maturation and (ii) the consequences of MMTV infection on PP DCs.
First, we showed that BMDCs from C3H/HeN mice were stimulated to mature upon exposure to MMTV; CD40 and CD80 costimulatory molecule expression was up-regulated, the mRNA levels of several chemokines and cytokines were increased, and proinflammatory-cytokine secretion was induced. This maturation process was shown to be independent of viral-gene expression, since AT-2 and UV pretreatment of MMTV did not block MMTV-induced BMDC maturation. These data indicate that virus binding to DCs rather than actual infection initiates maturation. Similar results were obtained when BMDCs from BALB/c mice were used, suggesting that the MMTV-induced maturation was not unique to C3H/HeN BMDCs but was a general response to MMTV infection. Moreover, two variants of MMTV [MMTV(LA) and MMTV(FM)] induced BMDC maturation, showing that this effect was not specific to MMTV(LA).
LPS, a major cell wall component of gram-negative bacteria, also induces the production of proinflammatory cytokines, such as TNF-α, IL-6, and IL-12, by DCs, monocytes, and macrophages through interaction with TLR4. Preexposure to LPS results in a loss of sensitivity to subsequent challenge with LPS, including reduced production of proinflammatory cytokines (32, 48). This phenomenon, called tolerance, hyporesponsiveness, or refractoriness, is observed in vivo and in vitro (21, 47, 49) and is thought to occur through alterations in the LPS signaling pathway (30) or through down-regulation of TLR4 surface expression (34). Here, we showed that in vitro tolerization of BMDCs with LPS impaired the production of TNF-α and IL-6 upon subsequent exposure to MMTV. Moreover, BMDCs isolated from C3H/HeJ mice, which have a mutation in their TLR4 gene (TLR4Lps-d), showed impaired DC maturation in response to MMTV. These data indicate that direct virus binding to TLR4 induces DC maturation. Other retroviruses could also interact with TLRs, as has been shown for murine leukemia virus (38). It would be of interest to determine whether HIV is also able to activate DCs through interaction with TLR4, since it has recently been shown that DC maturation induced through TLR4 signaling by LPS increases the ability to recruit HIV to DC- T-cell junctions and to enhance HIV infectivity (28).
In addition to causing the maturation of BMDCs from C3H/HeN mice, MMTV was able to induce significant increases in the expression of its cellular entry receptor, TfR1 or CD71. These increases were independent of viral-gene expression. When BMDCs from C3H/HeJ mice were tested, the increase in the expression of CD71 was significantly smaller, although under the experimental conditions used, it was not completely abolished. MMTV-induced increases in CD71 expression were completely abolished in BMDCs from mice lacking both functional TLR2 and TLR4 molecules. This result reinforces the case for participation of TLR4 in the increase of CD71 in BMDCs and raises the possibility of participation by TLR2 molecules in the response to virus. This agrees with our observation that the MMTV Env protein coimmunoprecipitates with TLR2 (Rassa, unpublished) and that MMTV-induced BMDC maturation is slightly reduced with cells isolated from TLR2−/− mice (D. Burzyn, unpublished data).
Previous work had shown that DCs are infected at levels similar to those in B220+ B cells at the early stages of MMTV infection in acutely infected adult mice and in BMDCs from BALB/c mice (26, 45). In agreement with these studies, we showed here that BMDCs from C3H/HeN mice were efficiently infected by MMTV. Correlating with the lack of increased CD71 on MMTV-stimulated BMDCs from mice lacking both functional TLR2 and TLR4, infection of their BMDCs was decreased. Thus, MMTV interaction with TLR2 and TLR4 leads to increased expression of its cell entry receptor, which in turn may favor BMDC infection. Similarly, measles virus was recently shown to up-regulate its cellular entry receptor on human monocytic cells through interaction with TLR2 (6, 33).
It is well known that MMTV uses the adaptive immune system to establish viral infection. It has recently been suggested that the retrovirus also uses the innate immune system to alter the virus-specific adaptive immune response (19). Indeed, Jude and colleagues observed that MMTV-mediated activation of C3H/HeN but not C3H/HeJ splenocytes led to production of IL-10, a cytokine known to repress induction of Th1-type cellular immune responses (19). They proposed that MMTV was able to subvert an antiviral adaptive Th1 response through TLR4 signaling in C3H/HeN but not C3H/HeJ mice. Interestingly, IL-10 production was induced in C3H/HeN B cells, although these cells did not require TLR4 expression themselves. Instead, the B cells secreted IL-10 in response to signaling from a TLR4-positive macrophage-DC population (19). Here, we demonstrated that MMTV-TLR4 interaction in BMDCs leads to the production of proinflammatory cytokines and chemokines. Functional differences among different tissue DCs have been extensively reported (24). For example, PP DCs have been shown to be particularly adept at priming naïve T cells to secrete IL-10 following microbial stimulation (18). Although the pattern of cytokine and chemokine production induced by MMTV in PP DCs during natural infection and its ultimate influence on the adaptive immune response to the virus remain to be elucidated, our results show alterations in PP DCs from infected neonatal mice that are compatible with the in vitro effects of MMTV on BMDCs.
During experimental infection with MMTV in adult mice, a significant increase in lymph node DCs was observed (26). This increase was attributed to CD62-L-dependent recruitment of blood DC precursors (27). We report here that MMTV is able to induce BMDC release of chemokines, such as MIP-1α, MIP-1β, and RANTES, which are known to attract immature DCs into the immediate vicinity of pathogens (11, 42), and that milk-borne MMTV infection induced a TLR4-dependent recruitment of DCs to neonatal PP. Furthermore, MMTV was able to induce an increase in the expression of its own cell entry receptor in PP DCs. The absence of functional TLR4 was sufficient to abrogate this MMTV-induced CD71 up-regulation. Additional experiments are being conducted in our laboratory to further investigate the consequences of MMTV-TLR interaction for neonatal PP DCs during milk-borne infection.
DCs may be the first cells to be activated and infected by MMTV. MMTV interaction with TLR4 on DCs would induce them to secrete cytokines and chemokines, leading to the chemoattraction of additional DC precursors and further activation of more immature DCs. In addition to their role in SAg presentation to T cells (3, 45), DCs could also produce chemokines responsible for the massive recruitment of naïve B and T cells that occurs in PP on day 2 of infection (12; I. Piazzon, unpublished results). The presence of large numbers of B and T cells in a microenvironment which could favor cell activation would increase B-cell infection and their subsequent interaction with SAg-reactive T cells. Additionally, the interaction of MMTV with DCs through TLRs would favor their infection by increasing the expression of the viral cell entry receptor. Finally, infected DCs might eventually constitute a viral reservoir that allows virus persistence. Thus, in addition to inducing DC maturation, retrovirus signaling through TLR4 molecules ultimately has the potential to facilitate and increase virus spread.
Acknowledgments
This work was supported by NIH grants PHS R01 CA45954 and PHS R01 CA73746 (S.R.R.); Fogarty International Research Collaboration Award PHS RO3 TW01103 (S.R.R and I.P.); and ANPCYT PICT 506305, CONICET, and FUNDALEU (I.P.).
We thank C. D. Pasqualini for helpful discussions. We also thank G. Lomabardi and R. Gargini for helping throughout the study and the preparation of the manuscript. We are grateful to Héctor Costa, Antonio Morales, and Juan Portaluppi for efficient technical assistance.
REFERENCES
- 1.Banchereau, J., and R. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Baribaud, F., I. Maillard, S. Vacheron, T. Brocker, H. Diggelman, and H. Acha-Orbea. 1999. Role of dendritic cells in the immune response induced by mouse mammary tumor virus superantigen. J. Virol. 73:8403-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton, G. M., and R. Medzhitov. 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380-383. [DOI] [PubMed] [Google Scholar]
- 5.Beutner, U., E. Kraus, D. Kitamura, K. Rajewsky, and B. T. Huber. 1994. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J. Exp. Med. 179:1457-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. Paul Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemmaglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brekelmans, P., P. van Soest, J. Voerman, P. P. Platenburg, P. J. Llenen, and W. van Ewijk. 1994. Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell. Immunol. 159:331-339. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z., J. R. Gordon, X. Zhang, and J. Xiang. 2001. Analysis of gene expression profiles of immature versus mature bone marrow-derived dendritic cells using DNA arrays. Biochem. Biophys. Res. Commun. 290:66-72. [DOI] [PubMed] [Google Scholar]
- 9.Choi, Y., J. W. Kappler, and P. Marrack. 1991. A superantigen encoded in the open reading frame of the 3′ long terminal repeat of the mouse mammary tumor virus. Nature 350:203-207. [DOI] [PubMed] [Google Scholar]
- 10.Compton, T., E. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cravens, P. V., and P. E. Lipsky. 2002. Dendritic cells, chemokine receptors and autoimmune inflammatory diseases. Immun. Cell. Biol. 80:497-505. [DOI] [PubMed] [Google Scholar]
- 12.Czarneski, J., P. Berguer, P. Bekinschtein, D. C. Kim, P. Hakimpour, N. Wagner, I. Nepomnaschy, I. Piazzon, and S. R. Ross. 2002. Neonatal infection with a milk-borne virus is independent of β7 integrin- and l-selectin-expressing lymphocytes. Eur. J. Immunol. 32:945-956. [DOI] [PubMed] [Google Scholar]
- 13.Futran, J., J. D. Kemp, E. H. Field, A. Vora, and R. F. Ashman. 1989. Transferrin receptor synthesis is an early event in B cell activation. J. Immunol. 143:787-792. [PubMed] [Google Scholar]
- 14.Golovkina, T. V., I. Piazzon, I. Nepomnaschy, V. Buggiano, M. de Olano Vela, and S. R. Ross. 1997. Generation of a tumorigenic milk-borne mouse mammary tumor virus by recombination between endogenous and exogenous viruses. J. Virol. 71:3895-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Théry, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 16.Held, W., G. A. Waanders, A. N. Shakhov, L. Scarpellino, H. Acha-Orbea, and H. R. MacDonald. 1993. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell 74:529-540. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 18.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jude, B. A., Y. Pobezinskaya, J. Bishop, S. Parke, R. M. Medzhitov, A. V. Chervonsky, and T. V. Golovkina. 2003. Subversion of the innate immune system by a retrovirus. Nat. Immunol. 4:573-578. [DOI] [PubMed] [Google Scholar]
- 20.Karapetian, O., A. N. Shakhov, J. P. Kraehenbuhl, and H. Acha-Orbea. 1994. Retroviral infection of neonatal Peyer's patch lymphocytes: the mouse mammary tumor virus model. J. Exp. Med. 180:1511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karp, C. L., M. Wysocka, X. Ma, M. Marovich, R. E. Factor, T. Nutman, M. Armant, L. Wahl, P. Cuomo, and G. Trinchieri. 1998. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur. J. Immunol. 28:3128-3136. [DOI] [PubMed] [Google Scholar]
- 22.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Trip, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 5:398-401. [DOI] [PubMed] [Google Scholar]
- 23.Le Bon, A., W. C. Wache, and M. Papiernik. 1999. In vivo elimination of viral superantigen-activated CD4+ T cells: apoptosis occurs at a distance from the activation site. Int. Immunol. 11:373-382. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585-588. [DOI] [PubMed] [Google Scholar]
- 25.Lutz, M. P., N. Kukutsch, A. L. J. Ogilvie, S. Röβner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 26.Martin, P., S. Ruiz Ruiz, G. Martinez del Hoyo, F. Anjuère, H. Hernández Vargas, M. López-Bravo, and C. Ardavín. 2002. Dramatic increase in lymph node dendritic cell number during infection by the mouse mammary tumor virus occurs by a CD62L-dependent blood-borne DC recruitment. Blood 99:1282-1288. [DOI] [PubMed] [Google Scholar]
- 27.Martínez del Hoyo, G., P. Martín, H. Hernández Vargas, S. Ruiz, C. Fernández Arias, and C. Ardavín. 2002. Characterization of a common precursor population for dendritic cells. Nature 415:1043-1047. [DOI] [PubMed] [Google Scholar]
- 28.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 29.McGrath, C. M., S. Nandi, and L. Young. 1972. Relationship between organization of mammary tumors and the ability of tumor cells to replicate mammary tumor virus and to recognize growth-inhibitory contact signals in vitro. J. Virol. 9:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medvedev, A., K. M. Kopydlowski, and S. N. Vogel. 2000. Inhibition of lipopolysaccharide signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine and toll-like receptor 2 and 4 gene expression. J. Immunol. 164:5564-5574. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 32.Mengozzi, M., and P. Ghezzi. 1993. Cytokine down-regulation in endotoxin tolerance. Eur. Cytokine Netw. 4:89-98. [PubMed] [Google Scholar]
- 33.Murabayashi, N., M. Kurita-Taniguchi, M. Ayata, M. Matsumoto, H. Ogura, and T. Seya. 2002. Susceptibility of human dendritic cells (DCs) to measles virus (MV) depends on their activation stages in conjunction with the level of CDw150: role of Toll stimulators in DC maturation and MV amplification. Microbes Infect. 4:785-794. [DOI] [PubMed] [Google Scholar]
- 33a.National Academy Press. 1996. Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources. Commission on Life Sciences. National Academy Press, Washington, D.C.
- 34.Nomura, F., S. Akashi, Y. Sakao, S. Sato, T. Kawai, M. Matsumoto, K. Nakanishi, M. Kimoto, K. Miyake, K. Takeda, and S. Akira. 2000. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J. Immunol. 164:3476-3479. [DOI] [PubMed] [Google Scholar]
- 35.Piazzon, I., A. Goldman, S. Torello, I. Nepomnaschy, A. Deroche, and G. Dran. 1994. Transmission of an Mls-1a-like superantigen to BALB/c mice by foster-nursing on F1 Mls-1 bxa mothers. Sex-influenced onset of clonal deletion. J. Immunol. 153:1553-1562. [PubMed] [Google Scholar]
- 36.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rassa, J. C., J. L. Meyers, Y. Zhang, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieser, C., C. Papesh, M. Herold, G. Böck, R. Ramoner, H. Klocker, G. Bartsch, and M. Thurner. 1998. Differential deactivation of human dendritic cells by endotoxin desensitization: role of Tumor Necrosis Factor-α and prostaglandin E2. Blood 91:3112-3117. [PubMed] [Google Scholar]
- 40.Ross, S. R., J. J. Schofield, C. J. Farr, and M. Bucan. 2002. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 99:12386-12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. Bess, Jr., G. M. Vazquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattenau, L. O. Arthur, L. E. Henderson, and J. D. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sozzani, S., P. Allavena, A. Vechi, and A. Mantovani. 1999. The role of chemokines in the regulation of dendritic cell trafficking. J. Leukoc. Biol. 66:1-9. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 44.Underhill, D. M., and A. Ozinsky. 2002. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 14:103-110. [DOI] [PubMed] [Google Scholar]
- 45.Vacheron, S., S. A. Luther, and H. Acha-Orbea. 2002. Preferential infection of immature dendritic cells and B cells by mouse mammary tumor virus. J. Immunol. 168:3470-3476. [DOI] [PubMed] [Google Scholar]
- 46.Vogel, S. N., J. S. Wax, P. Y. Perera, C. Padlan, M. Potter, and B. A. Mock. 1994. Construction of a BALB/c congenic mouse, C.C3H-Lpsd, that expresses the Lpsd allele: analysis of chromosome 4 markers surrounding the Lps gene. Infect. Immun. 62:4454-4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wysocka, M., S. Robertson, H. Riemann, J. Caamano, C. Hunter, A. Mackiewicz, L. J. Montaner, G. Trinchieri, and C. L. Karp. 2001. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 166:7504-7513. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler-Heitbrock, H. W. 1995. Molecular mechanism in tolerance to lipopolysaccharide. J. Inflamm. 45:13-26. [PubMed] [Google Scholar]
- 49.Zuckerman, S. H., and E. Evans. 1992. Endotoxin tolerance: in vivo regulation of tumor necrosis factor and interleukin-1 synthesis is at the transcriptional level. Cell. Immunol. 140:513-519. [DOI] [PubMed] [Google Scholar]