Abstract
Nitric oxide, a unique messenger in biological system, is ubiquitously present virtually in all tissues revealing its versatile nature of being involved in diverse physiological functions such as vascular tone, inhibition of platelet aggregation, cell adhesion, neurotransmission and enzyme and immune regulation. The tremendous advancements made in the past few decades in this area suggests that the nitric oxide modulation either by its exogenous release through nitric oxide donors or inhibition of its synthesis by nitric oxide synthase inhibitors in physiological milieu may provide newer clinical strategies for the treatment of some diseases. In this review, an attempt is made to document and understand the biological chemistry of different classes of nitric oxide modulators that would prove to be a fruitful area in the years to come.
Keywords: Nitric oxide, nitric oxide donor, nitric oxide synthase inhibitor
Ever since the discovery of endothelium-derived relaxing factor (EDRF)[1] in 1980 and its subsequent identification, as simple nitric oxide (NO) molecule[2], the area of biological sciences never remained the same and NO attained a status by occupying the central position in biological science.
Later, it was shown that, NO is biosynthesised from the amino acid L-arginine by the enzyme nitric oxide synthase (NOS)[2], that exist in different isoforms; endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS) and are located in many cells[3]. All three NOS isoforms catalyse five electron oxidation of one of the two equivalent nitrogen atoms of the guanidino group of L-arginine using NADPH as the source of electrons and (6R)-5,6,7,8-tetrahydrobiopterin (BH4), flavin adenine dinucleotide, flavin mononucleotide and iron protoporphorin IX (heme) as cofactors. It occurs through hydroxylation of l-arginine to L-hydroxyarginine, which is further oxidised to L-citrulline and NO[4] (fig. 1). Because of its low molecular weight and lipophilic properties, NO penetrates cell membranes very easily and can exert its effects on the luminal sites e.g. on platelets, that are deactivated, as well as in the abluminal site, where it dilates smooth muscle cells by increasing cyclic guanosine mono phosphate (cGMP) levels[5].
Fig. 1.
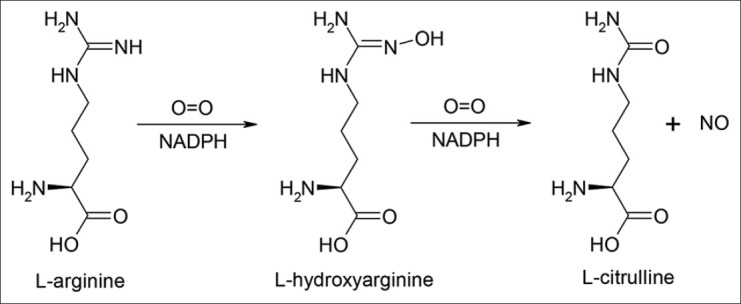
Biosynthesis of nitric oxide from L-arginine
Regarded as gaseous signalling molecule from the body, NO acts as an intercellular messenger molecule regulating physiological functions such as vascular tone, platelet aggregation, immune response and neurotransmission in the brain and in the periphery in the nonadrenergic noncholinergic nerves. In addition, NO synthesised in high amounts by activated macrophages possesses cytotoxic properties implicated in the ability of these cells to kill bacteria, viruses and protozoa as well as tumour cells. Although this function seems to be an important mechanism in host defence, it is also a harmful and destructive action involved in the pathogenesis of autoimmune diseases and several other pathological conditions[6,7] making NO a double-edged sword.
NITRIC OXIDE MODULATORS
The diverse and important physiological roles of NO suggest that modulation of NO in physiological system by increasing its concentration through exogenous release by NO donors or lowering its level by NOS inhibitors may be useful for the treatment of some diseases. NO modulators can act as research tools involving NO in laboratory animals. In this regard, there has been a renewed effort to identify and therapeutically exploit NO modulators.
Depending on the amount produced by the different isozymes and in different cell systems, NO can exert beneficial as well as toxic effects. At physiological concentrations produced by constitutive enzymes eNOS and nNOS in response to agonists such as acetylcholine in vascular endothelium, glutamate in brain or collagen acting on platelets, NO has beneficial effects like vasodilation and antiaggregatory effects on platelets. Such receptor-mediated response result in an increase in the intracellular concentrations of calcium which is critical for activation of constitutive NOS[7,8]. Hence, it makes sense to administer NO donors that are capable of releasing NO slowly compensating certain conditions where the endogenous NO production is insufficient[5].
The iNOS is virtually independent of free intracellular calcium concentrations and is expressed in cell types, including macrophages, chondrocytes, neutrophils, hepatocytes and smooth muscle cells after exposure to diverse stimuli such as inflammatory cytokines (e.g., interleukin-1 (IL-1), tissue necrosis factor (TNF), interferon-g and lipopolysaccharide (LPS) either alone or in combination. Once expressed, the iNOS generates significantly larger and sustained amounts of NO than does the constitutive isoforms, leading to dangerous conditions of hypotension and cell damage[9,10]. In such cases, it would be beneficial to reduce physiologic NO production by NOS inhibitors.
NO donors:
All NO donors are prodrugs and apparently rely on the generation of NO in vivo for their pharmacological activities. NO donors have a long history of use in the treatment of cardiovascular diseases. The antianginal effects of glyceryl trinitrate (GTN) were discovered as early as late last century. The various NO donors currently available are also used for the management of acute myocardial infarction, acute and chronic congestive heart failure and surgical control of blood pressure[11,12].
As additional information about the physiological role of NO is accumulated, new ideas and strategies for the use of NO donors are emerging. NO donors may be useful in erectile dysfunction stroke and cerebral ischemia and play an important role in regulating several cellular interactions, including platelet aggregation, neutrophil adhesiveness and vascular cell growth[11].
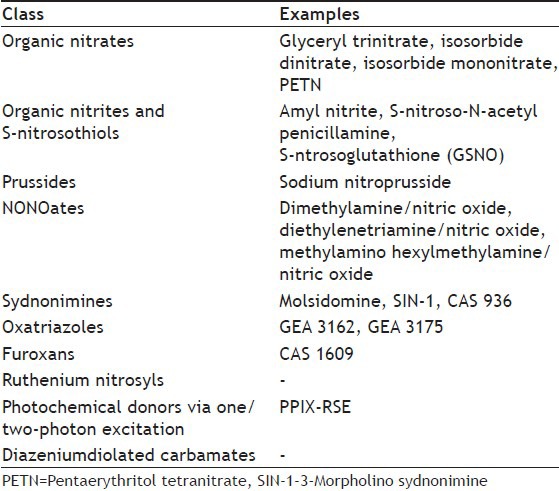
NO donors belong to diverse chemical classes (Table 1) and since their structure and reactivities are different, it is perhaps not surprising that they apparently require diverse enzymatic systems for bioactivation. Pertaining to NO donors one has to consider several points[5], such as (1)The site of NO release: Intracellular or extracellular; (2) Kinetics: Short acting compounds for acute treatment, slow acting for prevention and to avoid toxicity; (3) Chemical properties allowing solutions for intravenous administration or sufficient half-life for oral preventive treatment; (4) The redox state of the NO species released: NO, NO+ and NO-: Their interconversion and nitrosating potential; (5) Cofactors necessary for NO formation: Thiols, oxygen, enzymes and pH; (6) Stability towards light, heat and pH; (7) Development of tolerance.
TABLE 1.
DIFFERENT CLASS OF NITRIC OXIDE DONORS
Organic nitrates:
They are the oldest known NO donors and represent esters of nitric acid. Clinically used compounds include GTN, isosorbide dinitrate, isosorbide 5-mononitrate and pentaerythritol tetranitrate (PETN) (fig. 2). GTN and related vasodilators release NO in vivo, thereby enhancing the production of cGMP and relaxing smooth muscles[13]. Organic nitrates require either enzymatic or nonenzymatic bioactivation to release NO. It is likely that multiple intracellular and extracellular pathways contribute to NO formation from these compounds, but the relative importance of individual metabolic systems is poorly understood[14]. The activity of glutathione S-transferase and cytochrome P450 related enzymes is thought to be involved in the bioactivation of organic nitrates[15]. Thiols present in the cytosol are likely to account for nonenzymatic nitrate metabolism and in both cases an unstable thionitrate may be the common intermediate. Certain structural prerequisites of the thiol are thought to account for the finding that under physiological conditions only a limited number of sulphahydryl containing compounds (e.g., cystiene) react with organic nitrates to form NO, whereas virtually all thiols inactivate organic nitrates to nitrite[16]. A diminished efficacy of nitrates after a prolonged administration, called nitrate tolerance, was observed and is explained by depletion or decrease in the thiol content of the cells leading to autoinhibition of their metabolism to NO[17].
Fig. 2.
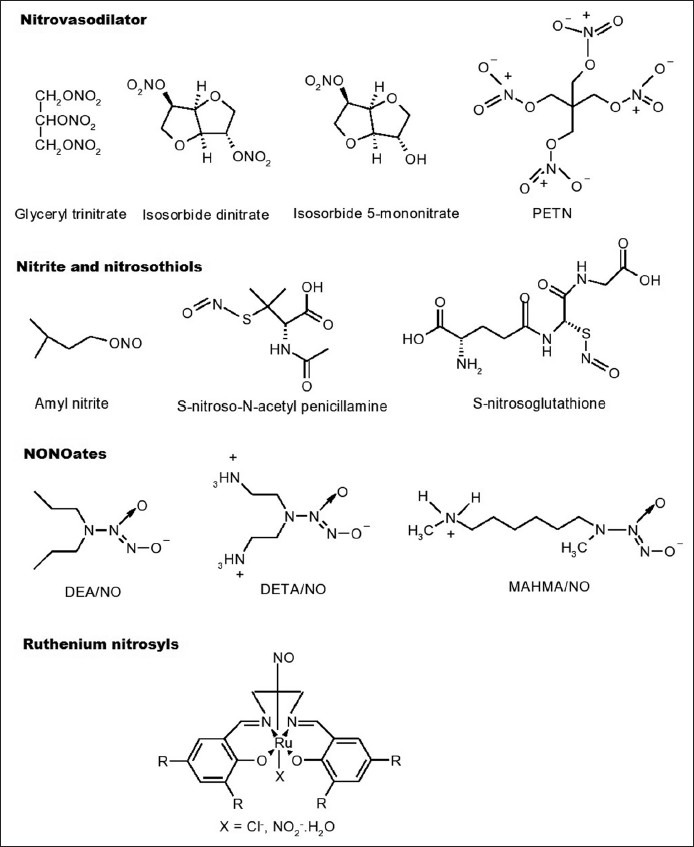
Chemical structures of nitric oxide donors
Organic nitrites and S-nitrosothiols:
Organic nitrites such as amyl nitrite are esters of nitrous acid. They react with thiol groups to form S-nitrosothiols which, decompose to form NO[16]. S-nitrosothiols are sulphur analogues of organic nitrites. S-nitroso-N-acetyl penicillamine and S-nitrosoglutathione have been prepared as stable solids and characterised[18] (fig. 2). In physiological buffers, S-nitrosothiols decompose to yield the corresponding disulphide and NO. This process is enhanced by heat, light and metal ions[19]. S-nitrosothiols are also capable of carrying transnitrosation, also implicated in their biological activity[14].
Sodium nitroprusside (SNP), Na2Fe (CN)5NO:
Vascular tissues, as well as the reducing agents catalyses the production of NO from sodium nitroprusside (SNP, Na2Fe(CN)5NO). Many of the reducing agents tested (e.g., thiols, hemoproteins and possibly ascorbate) are abundant in most biological tissues. Their nonspecific nature and wide spread presence are sufficient to release NO spontaneously from SNP[20]. It was showed that in addition to chemical degradation, SNP was readily metabolised to NO in the cellular subfractions of the bovine coronary artery smooth muscle cell and the dominant metabolic activity was membrane associated[21].
NONOates:
NO/nucleophile complexes known as NONOates are the promising compounds that are capable of spontaneously generating NO in vitro and in vivo[22] without any requirement of electron transfer, cofactors, metabolic activation or redox activation[11]. NONOates can be formed by exposing different nucleophilic compounds (usually an amine, e.g., diethyl amine or spermine) to a few atmospheres of NO gas[23,24]. NONOates may generate NO by acid catalysed dissociation with the regeneration of nucleophile and NO (fig. 3), although enzymatic metabolism in vivo cannot be ruled out[14]. The members of this group are dimethylamine (DEA)/NO, diethylenetriamine (DETA)/NO and methylamino hexylmethylamine (MAHMA)/NO (fig. 2). It has been reported that, there is a linear correlation between the NO release by NONOates and their vasodilating properties[22].
Fig. 3.

NONOates formation and generation of nitric oxide. X=Nucleophile
Sydnonimines:
The best-known compound of mesoionic sydnonimines is 3-morpholino sydnonimine (SIN-1), which is a hepatic metabolite of molsidomine that is in the European market as an antianginal drug since 1977. Sydnonimines rapidly decompose to release NO without the need for any cofactors such as thiols. The first step in the transformation of SIN-1 is the pH dependant conversion to the open ring form SIN-1A. This compound releases NO via a radical process following reaction with molecular oxygen (fig. 4). Superoxide that is formed in this reaction can combine with NO to form peroxynitrite, which is an active oxidant and a nitrating agent responsible for some of the adverse effects of SIN-1[25,26,27]. However, oxidants other than oxygen, and certain enzymes can promote oxidation of NO release from SIN-1 in biological set up and no peroxynitrite is formed in such cases[14,28].
Fig. 4.
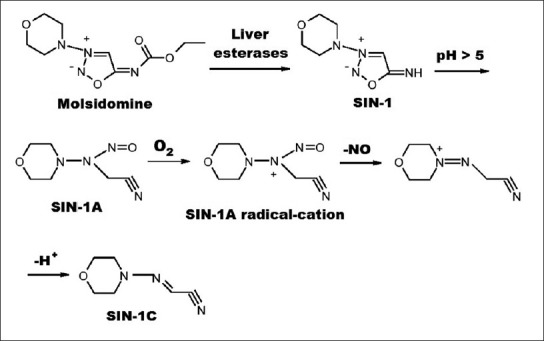
Metabolism and degradation of molsidomine
Two other sydnonimines that showed promising pharmacological activities are CAS 936 (prisidomine) and C87-3754 (fig. 5). CAS 936 represents an acyclated sydnonimine of the molsidomine type with a prolonged duration of action[29]. The enzymatic degradation product of CAS 936, the C87-3754 has been shown to produce dilation of noradrenaline-contracted rabbit aorta and femoral arteries which was found to be endothelium-independent[30].
Fig. 5.
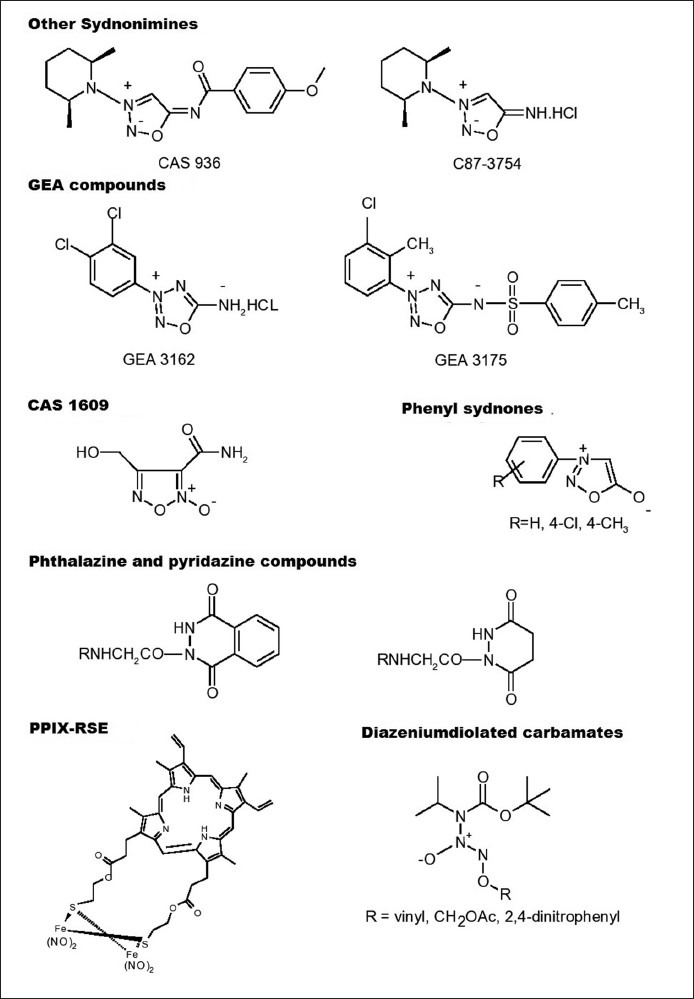
Chemical structures of some other nitric oxide donors
Oxatriazoles:
Replacement of C-4 atom in sydnonimines by nitrogen affords 1,2,3,4-oxatriazolium-5-imidates, a further interesting hypotensive mesoionic structure capable of releasing NO[31]. A series of new NO-releasing mesoionic oxatriazole derivatives has been synthesised at GEA's Chemical Laboratories, Denmark. The active compounds GEA 3162 and GEA 3175 (fig. 5) have been shown to produce NO-dependant pharmacological effects such as vasodilator, antiplatelet, fibrinolytic[32] and antibacterial activities[33] as well as inhibit neutrophil functions[34], suppress tumor cell growth[35], regulate glycosaminoglycan synthesis in articular cartilage[36], inhibit oxidation of low density lipoprotein[37] and induce relaxation of bronchioles[38] and trachea[39]. GEA compounds consume oxygen only when used at high concentrations and thereby an oxygen dependant mechanism of NO release, similar to that found in SIN-1, is unlikely in these cases[40]. GEA 3162 has been reported to produce only negligible amounts of super oxide and peroxynitrite, and releases NO spontaneously in buffer solutions[41]. The sulfonamide derivative, GEA 3175 is only a weak NO donor in phosphate buffer. However, in the presence of living cells or plasma GEA 3175 releases comparable amounts of NO[42], indicating a need for enzymatic degradation or the presence of thiols[40].
Furoxans:
Heterocyclic N-oxides, like furoxans (1,2,5-oxadiazole-2-oxides) are another class of NO donors that have been extensively investigated with regard to their chemistry, stability and activity[43,44,45,46]. Furoxans are thermally very stable compounds and also stable against acids and electrophiles, but less stable towards bases and nucleophiles[5]. The most probable mechanism of NO release by them is via nucleophilic attack of thiolates[47,48]. However, new furoxan derivatives have been reported to release NO spontaneously independent of thiols and without requiring enzymatic metabolism[49]. The most promising furoxan derivative has been CAS 1609 (fig. 5). Furoxans exhibit a very desirable pharmacological profile such as slow onset and long duration of action with no development of tolerance.
Ruthenium nitrosyls:
These are new NO donors for use as photopharmaceuticals in the treatment of infections and malignancies and exhibit exceptional stability in biological media. A variety of ruthenium nitrosyls complexed with dye molecules have been synthesised that strongly absorb light in the 400-600 nm range and rapidly release NO under such illumination (fig. 2). The resultant fluorescence of the dye ligands makes them ‘trackable’ within cellular matrices. Selected ruthenium nitrosyls have been used to deliver NO to cellular targets to induce apoptosis. Alteration of the ligands in terms of (i) donor atoms, (ii) extent of conjugation and (iii) substituents on the ligand frames sensitizes the final ruthenium nitrosyls toward visible light in a predictable fashion[50,51].
Photochemical donors:
NO was shown to be generated by decomposition via two photon excitation of the thermostable porphyrin complex, PPIX-RES ([mu-S,mu-S'-protoporphyrin-IX-bis(2-thioethyl)diester]tetranitrosyl-diiron, fig. 5) upon irradiation with near IR light (800-1100 nm) that has greatest penetration into the mammalian tissue[52].
Diazeniumdiolated carbamates:
These novel diazeniumdiolated carbamate prodrugs (fig. 5) upon activation releases NO similar to their secondary amine counterparts. They are also efficient sources of intracellular NO[53].
Others:
We have studied in vitro NO donor activity of mesoionic 3-phenylsydnones (fig. 5) that resemble sydnonimines in structure, by Griess reagent method[54]. The NO release by these compounds was found to be thiol, pH and time dependent. N-nitrosophenyl glycines, the alkaline hydrolytic products of 3-phenylsydnones have also released NO. The 3-phenylsydnones were found to be slow and weak releasers of NO.
Phthalazine and pyridazine compounds (fig. 5) synthesised by us were subjected to in vitro NO release[55]. In general, phthalazines released higher amount of NO than pyridazines in presence of thiol and have also been shown to exhibit increased cardiac output and stroke volume on an isolated rat heart. The release of NO was well correlated with vasodilation produced by these compounds.
NO synthase inhibitors:
Because of the involvement of all the three NOS isozymes in various aspects of signal transduction, NOS inhibitors have gained prominence in the management of ischemic reperfusion injury, hypotensive effects of drugs, and inflammatory response to cytokines. There are bewildering arrays of NOS inhibitors described in the literature which are still under investigation for clinical application and in use as pharmacological tools[56]. Most of the animal models of human diseases in which the occurrence of large amounts of iNOS-derived NO has been observed to make use of NOS inhibitors to study the involvement of NO overproduction as either the cause or the consequence of the particular disease[57]. Since NO is biosynthesised by L-arginine, several L-arginine analogues such as NG-monomethyl-L-arginine (L-NMMA), NG-nitro-L-arginine methyl ester (L-NAME) and N-iminoethyl-L-ornithine (L-NIO) (fig. 6) were synthesised and characterised as nonselective competitive NOS inhibitors[58]. Whereas, L-NMMA and L-NAME appear to be nonselective inhibitors of the various NOS enzymes, L-NIO has shown some selectivity towards the iNOS[8,59]. However, none of the arginine analogues discriminate sufficiently to enable them to be used to target a single NOS isoform.
Fig. 6.
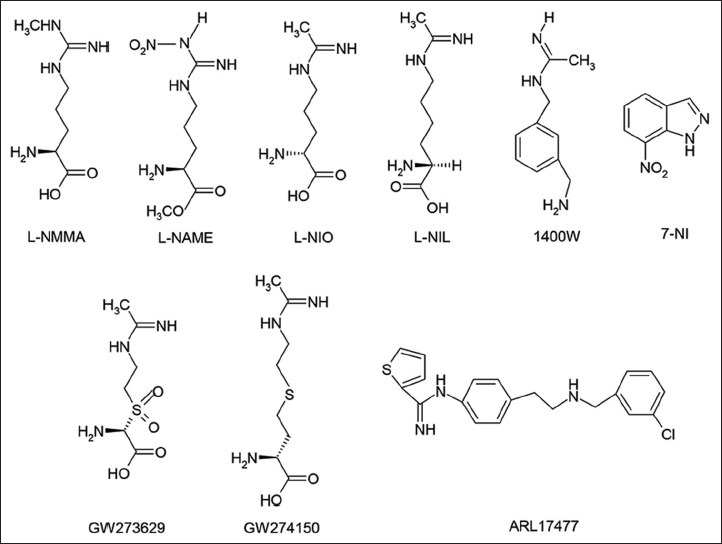
Chemical structures of nitric oxide synthase inhibitors
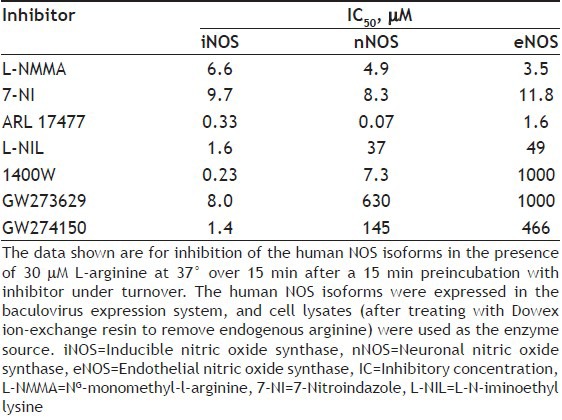
Recently, most efforts to find an iNOS selective inhibitor are being pursued, largely because of the importance of NO derived from iNOS in mediating harmful reactions and cell injury. In particular, selective inhibition of iNOS should be advantageous in septic shock and in chronic inflammatory diseases such as arthritis. This led to the development of more selective inhibitors of iNOS like, L-N-iminoethyl lysine (L-NIL)[60], 1400W[61], and sulphur-substituted acetamidine amino acids, GW273629 and GW274150[62] (fig. 6). 7-Nitroindazole (7-NI) and analogues have been reported to exhibit selectivity towards nNOS[63]. The non-amino acid ARL 17477[64] (fig. 6) has been reported to be a selective nNOS inhibitor in vitro and effective in vivo in animal models of brain damage in stroke. S-ethyl and S-methyl thiocitrulline and vinyl L-NIO have also exhibited specificity towards nNOS. The selectivity of some NOS inhibitors toward different human NOS isoforms[56] is shown in Table 2.
TABLE 2.
SELECTIVITY OF NITRIC OXIDE SYNTHASE INHIBITORS TOWARDS DIFFERENT HUMAN NOS ISOFORMS
CLINICAL POTENTIAL OF NITRIC OXIDE MODULATORS
Antiinflammatory corticosteroids inhibit iNOS expression in some[65] but not in all cell types and have been reported to do so by down regulating transcription factor, nuclear factor kappa B (NF-κB)[66] and post transcriptional regulation[67]. Glucocorticoids also inhibit NO production by limiting BH4 and L-arginine availability[68].
Antiinflammatory and immunosuppressive drug cyclosporine A has been reported to inhibit induction of iNOS[69]. Methotrexate, an antineoplastic agent inhibits the formation of BH4, an essential cofactor required for NOS[70].
Addition of NO releasing moieties to non-steroidal antiinflammatory drugs (NSAIDs) resulted in the development of NO-NSAIDs with markedly reduced ulcerogenic properties, and have been shown to be safe and effective alternatives to conventional NSAIDs[71,72,73,74]. Nitroaspirins such as NCX-4016, NCX-4215[72,73,74] and S-nitrosodiclofenac[75] (fig. 7) have been shown to produce uncompromised antiinflammatory and analgesic properties with gastric-sparing compared to the parent NSAIDs in experimental animals. The success of NO-NSAID approach has led to this strategy being applied to other classes of drugs that could benefit from addition of NO-releasing moiety, such as glucocorticoids and 5-aminosalicylic acid[57]. The effect of NO modulation on the healing of an existing peptic ulcer induced by indomethacin using a NO precursor L-arginine, NO donor GTN and a NOS inhibitor, L-NAME was assessed in rats. L-arginine and GTN almost completely healed ulceration, restored normal levels of NO and glutathione and significantly attenuated the increase in PGE2 and lipid peroxides whereas, L-NAME was found to exacerbate mucosal damage[76].
Fig. 7.
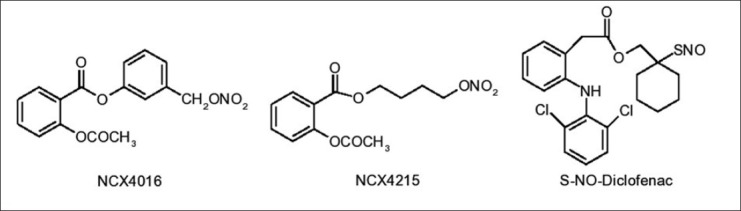
Structures of nitric oxide-nonsteroidal antiinflammatory drugs
In a recent study[77], short-chain fatty acids (SCFAs) such as sodium acetate, sodium propionate and sodium butyrate have been shown to reduce production of pro inflammatory factors, including, TNFα, IL-1β, IL-6 and NO. SCFAs also inhibited vitality of iNOS and enhanced the production of antiinflammatory cytokine IL-10 in lower concentrations. Sodium acetate and sodium butyrate significantly inhibited LPS-induced NF-κB activation in RAW264.7 cells.
NO modulators both NO donors (molsidomine and SNP) and NOS inhibitors (L-NAME and 7-NI) were shown to reverse apomorphine (dopamine D1/D2 mixed receptor agonist) induced disrupted short term recognition memory in rats, suggesting cognitive deficit produced by dopamine dysfunction is sensitive to modulation of NO[78]. But it was not clear whether high or low concentration of NO is responsible in this case.
Cardioprotective effects of BRL37344, a β3 adrenergic receptor agonist, in pressure overload hypertrophy and heart failure was studied. It was found that, BRL37344 upregulated by 2-fold nNOS protein expression in nNOS knockout mice, indicating nNOS as the primary downstream NOS isoform in maintaining NO and reactive oxygen species balance in the failing heart[79].
In a recent investigation, the role of NO on the neurogenic effects of neuropeptide Y (NPY), that is widely expressed in the central and peripheral nervous systems to play a key role in regulating adult hippocampal neurogenesis in vivo under both basal and pathological conditions was studied[80]. In postnatal rat hippocampal cultures, the proliferative effect of NPY on (+) nestin precursor cells is NO-dependent and is mediated via an NO/cGMP/cGMP-dependent protein kinase and extracellular signal-regulated kinase 1/2 signalling pathway. By contrast, extracellular NO had an opposite, inhibitory effect on proliferation.
Recombinant human serum albumin S-nitrosated AL-dimer containing AL-dimer as a carrier, and NO as (i) an anticancer therapeutic drug/cell death inducer and (ii) an enhancer of the enhanced permeability and retention (EPR) effect was developed[81]. Treatment with it induced apoptosis of C26 tumour cells in vitro depending on the concentration of NO and it was found to specifically deliver large amounts of cytotoxic NO into tumour tissue but not into normal organs in vivo in C26 tumour-bearing mice. Intriguingly, S-nitrosation improved the uptake of AL-dimer in tumour tissue through augmenting the EPR effect.
Recognising endogenous gaseous mediators NO and hydrogen sulphide (H2S) can increase mucosal defence mechanisms, NOSH-aspirin, a new hybrids of aspirin, bearing both NO- and H2S-releasing areas was developed with an intention of increased safety profiles. NOSH-aspirin inhibited cell proliferation, induced apoptosis, and caused G0/G1 cell cycle block. It also inhibited ovine cyclooxygenase-1 (COX-1) more than ovine COX-2. Treatment of NOSH-aspirin on mice bearing a human colon cancer xenograft caused 85% reduction in volume. Preliminary studies have found that four NOSH-aspirin variants, evaluated in eleven different human cancer cell lines, were effective in inhibiting the growth of these cell lines[82,83].
The effect of NO modulators on cardiovascular risk factors in mild hyperhomocysteinaemic rats induced by methionine was assessed in a study using SNP and Nω-nitro-l-arginine (LNNA) as NO donor and NOS inhibitor, respectively[84]. It was found that, LNNA significantly increased the level of cholesterol in aorta while SNP significantly suppressed the activity of HMG-CoA reductase. The mRNA levels of caveolin, P2X and P2Y showed a significant decrease in rats administered with SNP. LNNA showed significant induction in the expression of caveolin and P2Y expression and no remarkable change in the level of P2X.
CONCLUSION
Over three decades of tremendous and matured research on NO clearly established it as signalling molecule and also made us understand a rather complex biological chemistry of NO as it stands today. Modulation of NO concentration in vivo could be an important key to the treatment of a variety of disease conditions. A tiny free radical, once considered only as an environmental pollutant, NO now turned out as an attractive therapeutic target. Compounds that release small amounts of NO (insufficient to cause hypotension or motility disorders) over a prolonged period of time have great promise in the management of cardiovascular, inflammatory, neurological, neoplasmic and other diseases. Invention of specific NOS isoform inhibitors to deal with the harmful actions of NO can obviously make NO therapy safer. A better understanding of the complex chemistry, biochemistry, and molecular biology of NO and its signalling responses can usher targeted therapies for NO modulation, which can further broaden the horizons of medicine by enriching therapeutic armamentarium.
Footnotes
Deshpande, et al.: Nitric Oxide Modulators as Medicinal Agents
REFERENCES
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.Sessa WC. The nitric oxide synthase family of proteins. J Vasc Res. 1994;31:131–43. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- 4.Marletta MA. Nitric oxide synthase: Function and mechanism. Adv Exp Med Biol. 1993;338:281–4. [PubMed] [Google Scholar]
- 5.Schönafinger K. Heterocyclic NO prodrugs. Farmaco. 1999;54:316–20. doi: 10.1016/s0014-827x(99)00031-2. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Higgs EA. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995;9:1319–30. [PubMed] [Google Scholar]
- 7.Moilanen E, Whittle B, Moncada S. Nitric oxide as a factor in inflammation. In: Gallin JI, Snyderman R, Fearon DT, Hayes BF, Nathan C, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 787–800. [Google Scholar]
- 8.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 10.Clancy RM, Amin AR, Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998;41:1141–51. doi: 10.1002/1529-0131(199807)41:7<1141::AID-ART2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Bauer JA, Booth BP, Fung HL. Nitric oxide donors: Biochemical pharmacology and therapeutics. Adv Pharmacol. 1995;34:361–81. doi: 10.1016/s1054-3589(08)61098-4. [DOI] [PubMed] [Google Scholar]
- 12.Loskove JA, Frishman WH. Nitric oxide donors in the treatment of cardiovascular and pulmonary diseases. Am Heart J. 1995;129:604–13. doi: 10.1016/0002-8703(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 13.Murad F, Mittal CK, Arnold WP, Katsuki S, Kimura H. Guanylate cyclase: Activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–58. [PubMed] [Google Scholar]
- 14.Feelisch M. The use of nitric oxide donors in pharmacological studies. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:113–22. doi: 10.1007/pl00005231. [DOI] [PubMed] [Google Scholar]
- 15.Feelisch M. Biotransformation to nitric oxide of organic nitrates in comparison to other nitrovasodilators. Eur Heart J. 1993;14:123–32. [PubMed] [Google Scholar]
- 16.Feelisch M. The biochemical pathways of nitric oxide formation from nitrovasodilators: appropriate choice of exogenous NO donors and aspects of preparation and handling of aqueous NO solutions. J Cardiovasc Pharmacol. 1991;17:S25–33. [Google Scholar]
- 17.Münzel T, Harrison DG. Evidence for a role of oxygen-derived free radicals and protein kinase C in nitrate tolerance. J Mol Med (Berl) 1997;75:891–900. doi: 10.1007/s001090050181. [DOI] [PubMed] [Google Scholar]
- 18.Butler AR, Rhodes P. Chemistry, analysis, and biological roles of S-nitrosothiols. Anal Biochem. 1997;249:1–9. doi: 10.1006/abio.1997.2129. [DOI] [PubMed] [Google Scholar]
- 19.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem. 1996;271:18596–603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 20.Bates JN, Baker MT, Guerra R, Jr, Harrison DG. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991;42:S157–65. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- 21.Kowaluk EA, Seth P, Fung HL. Metabolic activation of sodium nitroprusside to nitric oxide in vascular smooth muscle. J Pharmacol Exp Ther. 1992;262:916–22. [PubMed] [Google Scholar]
- 22.Morley D, Keefer LK. Nitric oxide/nucleophile complexes: A unique class of nitric oxide-based vasodilators. J Cardiovasc Pharmacol. 1993;22:S3–9. [PubMed] [Google Scholar]
- 23.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, et al. Complexes of. NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991;34:3242–7. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 24.Morley D, Maragos CM, Zhang XY, Boignon M, Wink DA, Keefer LK. Mechanism of vascular relaxation induced by the nitric oxide (NO)/nucleophile complexes, a new class of NO-based vasodilators. J Cardiovasc Pharmacol. 1993;21:670–6. doi: 10.1097/00005344-199304000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Asahi Y, Shinozaki K, Nagaoka M. Chemical and kinetic studies on stabilities of 3-morpholinosydnonimine and its N-ethoxy-carbonyl derivative. Chem Pharm Bull. 1971;19:1079–88. [Google Scholar]
- 26.Bohn H, Schönafinger K. Oxygen and oxidation promote the release of nitric oxide from sydnonimines. J Cardiovasc Pharmacol. 1989;14:S6–12. [PubMed] [Google Scholar]
- 27.Feelisch M, Ostrowski J, Noack E. On the mechanism of NO release from sydnonimines. J Cardiovasc Pharmacol. 1989;14:S13–22. [PubMed] [Google Scholar]
- 28.Singh RJ, Hogg N, Joseph J, Konorev E, Kalyanaraman B. The peroxynitrite generator, SIN-1, becomes a nitric oxide donor in the presence of electron acceptors. Arch Biochem Biophys. 1999;361:331–9. doi: 10.1006/abbi.1998.1007. [DOI] [PubMed] [Google Scholar]
- 29.Bohn H, Martorana PA, Schönafinger K. Cardiovascular effects of the new nitric oxide donor, pirsidomine. Hemodynamic profile and tolerance studies in anesthetized and conscious dogs. Eur J Pharmacol. 1992;220:71–8. doi: 10.1016/0014-2999(92)90013-t. [DOI] [PubMed] [Google Scholar]
- 30.Mülsch A, Hecker M, Mordvintcev PI, Vanin AF, Busse R. Enzymic and nonenzymic release of NO accounts for the vasodilator activity of the metabolites of CAS 936, a novel long-acting sydnonimine derivative. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:92–100. doi: 10.1007/BF00168778. [DOI] [PubMed] [Google Scholar]
- 31.Kier LB, Al-Shamma A, Hahn R, Tye A. Mesoionic pseudo-oxatriazoles as hypotensive agents. J Pharm Sci. 1966;55:1467–8. [Google Scholar]
- 32.Corell T, Pedersen SB, Lissau B, Moilanen E, Metsä-Ketelä T, Kankaanranta H, et al. Pharmacology of mesoionic oxatriazole derivatives in blood, cardiovascular and respiratory systems. Pol J Pharmacol. 1994;46:553–66. [PubMed] [Google Scholar]
- 33.Virta M, Karp M, Vuorinen P. Nitric oxide donor-mediated killing of bioluminescent Escherichia coli. Antimicrob Agents Chemother. 1994;38:2775–9. doi: 10.1128/aac.38.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanikiat P, Woodward DF, Armstrong RA. Investigation of the role of nitric oxide and cyclic GMP in both the activation and inhibition of human neutrophils. Br J Pharmacol. 1997;122:1135–45. doi: 10.1038/sj.bjp.0701477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vilpo JA, Vilpo LM, Vuorinen P, Moilanen E, Metsä-Ketelä T. Mode of cytostatic action of mesoionic oxatriazole nitric oxide donors in proliferating human hematopoietic cells. Anticancer Drug Des. 1997;12:75–89. [PubMed] [Google Scholar]
- 36.Järvinen TA, Moilanen T, Järvinen TL, Moilanen E. Nitric oxide mediates interleukin-1 induced inhibition of glycosaminoglycan synthesis in rat articular cartilage. Mediators Inflamm. 1995;4:107–11. doi: 10.1155/S0962935195000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malo-Ranta U, Ylä-Herttuala S, Metsä-Ketelä T, Jaakkola O, Moilanen E, Vuorinen P, et al. Nitric oxide donor GEA 3162 inhibits endothelial cell-mediated oxidation of low density lipoprotein. FEBS Lett. 1994;337:179–83. doi: 10.1016/0014-5793(94)80269-6. [DOI] [PubMed] [Google Scholar]
- 38.Hernández M, Elmedal B, Mulvany MJ, Simonsen U. Mechanisms of relaxations of bovine isolated bronchioles by the nitric oxide donor, GEA 3175. Br J Pharmacol. 1998;123:895–905. doi: 10.1038/sj.bjp.0701684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaali K, Li L, Paakkari I, Vapaatalo H. Relaxing effects of NO donors on guinea pig trachea in vitro are mediated by calcium-sensitive potassium channels. J Pharmacol Exp Ther. 1998;286:110–4. [PubMed] [Google Scholar]
- 40.Karup G, Preikschat H, Wilhelmsen ES, Pedersen SB, Marcinkiewicz E, Cieślik K. Mesoionic oxatriazole derivatives: A new group of NO-donors? Pol J Pharmacol. 1994;46:541–52. [PubMed] [Google Scholar]
- 41.Holm P, Kankaanranta H, Metsä-Ketelä T, Moilanen E. Radical releasing properties of nitric oxide donors GEA 3162, SIN-1 and S-nitroso-N-acetylpenicillamine. Eur J Pharmacol. 1998;346:97–102. doi: 10.1016/s0014-2999(98)00009-0. [DOI] [PubMed] [Google Scholar]
- 42.Kankaanranta H, Rydell E, Petersson AS, Holm P, Moilanen E, Corell T, et al. Nitric oxide-donating properties of mesoionic 3-aryl substituted oxatriazole-5-imine derivatives. Br J Pharmacol. 1996;117:401–6. doi: 10.1111/j.1476-5381.1996.tb15204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gasco A, Boulton AJ. Furoxans and benzofuroxans. Adv Heterocycl Chem. 1981;29:251–340. [Google Scholar]
- 44.Calvino R, Fruttero R, Ghigo D, Bosia A, Pescarmona GP, Gasco A. 4-Methyl-3-(arylsulfonyl) furoxans: A new class of potent inhibitors of platelet aggregation. J Med Chem. 1992;35:3296–300. doi: 10.1021/jm00095a028. [DOI] [PubMed] [Google Scholar]
- 45.Stilo AD, Sorba G, Medana C, Fruttero R, Gasco A. Furoxan derivatives as NO donors. Farmaco. 1997;52:339–41. [Google Scholar]
- 46.Heller R, Bussolino F, Calvino R, Ghigo D, Alessio P, Todde R, et al. S35b, a new phenylsulfonylfuroxan compound, inhibits thrombin-induced synthesis of platelet-activating factor and prostacyclin in human endothelial cells. Agents Actions. 1993;40:157–65. doi: 10.1007/BF01984055. [DOI] [PubMed] [Google Scholar]
- 47.Feelisch M, Schönafinger K, Noack E. Thiol-mediated generation of nitric oxide accounts for the vasodilator action of furoxans. Biochem Pharmacol. 1992;44:1149–57. doi: 10.1016/0006-2952(92)90379-w. [DOI] [PubMed] [Google Scholar]
- 48.Medana C, Ermondi G, Fruttero R, Di Stilo A, Ferretti C, Gasco A. Furoxans as nitric oxide donors. 4-Phenyl-3-furoxancarbonitrile: Thiol-mediated nitric oxide release and biological evaluation. J Med Chem. 1994;37:4412–6. doi: 10.1021/jm00051a020. [DOI] [PubMed] [Google Scholar]
- 49.Hecker M, Vorhoff W, Bara AT, Mordvintcev PI, Busse R. Characterization of furoxans as a new class of tolerance-resistant nitrovasodilators. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:426–32. doi: 10.1007/BF00169084. [DOI] [PubMed] [Google Scholar]
- 50.Fry NL, Mascharak PK. Photoactive ruthenium nitrosyls as NO donors: How to sensitize them toward visible light. Acc Chem Res. 2011;44:289–98. doi: 10.1021/ar100155t. [DOI] [PubMed] [Google Scholar]
- 51.Rose MJ, Mascharak PK. Photoactive ruthenium nitrosyls: Effects of light and potential application as NO donors. Coord Chem Rev. 2008;252:2093–2114. doi: 10.1016/j.ccr.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hishikawa K, Nakagawa H, Furuta T, Fukuhara K, Tsumoto H, Suzuki T, et al. Multiple bond-conjugated photoinduced nitric oxide releaser working with two-photon excitation. Bioorg Med Chem Lett. 2010;20:302–5. doi: 10.1016/j.bmcl.2009.10.120. [DOI] [PubMed] [Google Scholar]
- 53.Nandurdikar RS, Maciag AE, Cao Z, Keefer LK, Saavedra JE. Diazeniumdiolated carbamates: A novel class of nitric oxide donors. Bioorg Med Chem. 2012;20:2025–9. doi: 10.1016/j.bmc.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satyanarayana K, Deshpande SR, Subbarao B, Rao MN. Synthesis and nitric oxide donor activity of phenylsydnones. Indian Drugs. 2002;39:578–82. [Google Scholar]
- 55.Deshpande SR, Ghongade AM, Pai VK. Synthesis and biological evaluation of 2-(N-substituted)-3H-phthalazin-1,4-diones and 1-(N-substituted) 2,4,5-trihydropyridazin-3,6-diones as potent vasodilators. Indian J Pharm Educ Res. 2010;44:1–7. [Google Scholar]
- 56.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muscará MN, Wallace JL. Nitric oxide. V. therapeutic potential of nitric oxide donors and inhibitors. Am J Physiol. 1999;276:G1313–6. doi: 10.1152/ajpgi.1999.276.6.G1313. [DOI] [PubMed] [Google Scholar]
- 58.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–52. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCall TB, Feelisch M, Palmer RM, Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991;102:234–8. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore WM, Webber RK, Jerome GM, Tjoeng FS, Misko TP, Currie MG. L-N6-(1-iminoethyl) lysine: A selective inhibitor of inducible nitric oxide synthase. J Med Chem. 1994;37:3886–8. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- 61.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–63. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 62.Young RJ, Beams RM, Carter K, Clark HA, Coe DM, Chambers CL, et al. Inhibition of inducible nitric oxide synthase by acetamidine derivatives of hetero-substituted lysine and homolysine. Bioorg Med Chem Lett. 2000;10:597–600. doi: 10.1016/s0960-894x(00)00055-x. [DOI] [PubMed] [Google Scholar]
- 63.Southan GJ, Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–94. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 64.Zhang ZG, Reif D, Macdonald J, Tang WX, Kamp DK, Gentile RJ, et al. ARL 17477, a potent and selective neuronal NOS inhibitor decreases infarct volume after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1996;16:599–604. doi: 10.1097/00004647-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 65.Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA. 1990;87:10043–7. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleinert H, Euchenhofer C, Ihrig-Biedert I, Förstermann U. Glucocorticoids inhibit the induction of nitric oxide synthase II by down-regulating cytokine-induced activity of transcription factor nuclear factor-kappa B. Mol Pharmacol. 1996;49:15–21. [PubMed] [Google Scholar]
- 67.Kunz D, Walker G, Eberhardt W, Pfeilschifter J. Molecular mechanisms of dexamethasone inhibition of nitric oxide synthase expression in interleukin 1 beta-stimulated mesangial cells: Evidence for the involvement of transcriptional and posttranscriptional regulation. Proc Natl Acad Sci USA. 1996;93:255–9. doi: 10.1073/pnas.93.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simmons WW, Ungureanu-Longrois D, Smith GK, Smith TW, Kelly RA. Glucocorticoids regulate inducible nitric oxide synthase by inhibiting tetrahydrobiopterin synthesis and l-arginine transport. J Biol Chem. 1996;271:23928–37. doi: 10.1074/jbc.271.39.23928. [DOI] [PubMed] [Google Scholar]
- 69.Mühl H, Kunz D, Rob P, Pfeilschifter J. Cyclosporin derivatives inhibit interleukin 1 beta induction of nitric oxide synthase in renal mesangial cells. Eur J Pharmacol. 1993;249:95–100. doi: 10.1016/0014-2999(93)90666-6. [DOI] [PubMed] [Google Scholar]
- 70.Saura M, Pérez-Sala D, Cañada FJ, Lamas S. Role of tetrahydrobiopterin availability in the regulation of nitric-oxide synthase expression in human mesangial cells. J Biol Chem. 1996;271:14290–5. doi: 10.1074/jbc.271.24.14290. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi K, Yasuhiro T, Asada Y, Sugawa Y. Role of nitric oxide in pathogenesis of aspirin-induced gastric mucosal damage in rats. Digestion. 1998;59:298–307. doi: 10.1159/000007506. [DOI] [PubMed] [Google Scholar]
- 72.Wallace JL, Cirino G. The development of gastrointestinal-sparing nonsteroidal antiinflammatory drugs. Trends Pharmacol Sci. 1994;15:405–6. doi: 10.1016/0165-6147(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 73.Minuz P, Lechi C, Zuliani V. NO aspirins: Antithrombotic activity of derivatives of acetylsalicylic acid releasing nitric oxide. Cardiovasc Drug Rev. 1998;16:31–47. [Google Scholar]
- 74.del Soldato P, Sorrentino R, Pinto A. NO-aspirins: A class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol Sci. 1999;20:319–23. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- 75.Bandarage UK, Chen L, Fang X, Garvey DS, Glavin A, Janero DR, et al. Nitrosothiol esters of diclofenac: Synthesis and pharmacological characterization as gastrointestinal-sparing prodrugs. J Med Chem. 2000;43:4005–16. doi: 10.1021/jm000178w. [DOI] [PubMed] [Google Scholar]
- 76.El-Demerdash E, El-Mesallamy HO, Abu-Zaid NM, Gad MZ. The potential therapeutic effect of nitric oxide modulators in experimentally-induced gastric ulcers. Drug Discov Ther. 2010;4:276–84. [PubMed] [Google Scholar]
- 77.Liu T, Li J, Liu Y, Xiao N, Suo H, Xie K, et al. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation. 2012;35:1676–84. doi: 10.1007/s10753-012-9484-z. [DOI] [PubMed] [Google Scholar]
- 78.Gourgiotis I, Kampouri NG, Koulouri V, Lempesis IG, Prasinou MD, Georgiadou G, et al. Nitric oxide modulates apomorphine-induced recognition memory deficits in rats. Pharmacol Biochem Behav. 2012;102:507–14. doi: 10.1016/j.pbb.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 79.Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, Ellis CL, et al. Cardioprotective effect of beta-3 adrenergic receptor agonism: Role of neuronal nitric oxide synthase. J Am Coll Cardiol. 2012;59:1979–87. doi: 10.1016/j.jacc.2011.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheung A, Newland PL, Zaben M, Attard GS, Gray WP. Intracellular nitric oxide mediates neuroproliferative effect of neuropeptide y on postnatal hippocampal precursor cells. J Biol Chem. 2012;287:20187–96. doi: 10.1074/jbc.M112.346783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishima Y, Chen D, Fang J, Maeda H, Minomo A, Kragh-Hansen U, et al. S-Nitrosated human serum albumin dimer is not only a novel anti-tumor drug but also a potentiator for antitumor drugs with augmented EPR effects. Bioconjug Chem. 2012;23:264–71. doi: 10.1021/bc2005363. [DOI] [PubMed] [Google Scholar]
- 82.Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem Biophys Res Commun. 2012;419:523–8. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 83.Kodela R, Chattopadhyay M, Kashfi K. NOSH-Aspirin: A novel nitric oxide-hydrogen sulfide-releasing hybrid: A new class of antiinflammatory pharmaceuticals. ACS Med Chem Lett. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma M, Rai SK, Tiwari RK, Tiwari M, Chandra R. Effects of nitric oxide modulators on cardiovascular risk factors in mild hyperhomocysteinaemic rat model. Basic Clin Pharmacol Toxicol. 2008;103:25–30. doi: 10.1111/j.1742-7843.2008.00215.x. [DOI] [PubMed] [Google Scholar]


