Abstract
The purpose of the present investigation was to prepare an intranasal in situ gel with increased nasal residence time in order to improve bioavailability of metoprolol tartrate. The in situ gel systems containing carbopol, hydroxypropyl methylcellulose K4M and K15M in different concentrations were prepared. The samples were characterized for viscosity, rheological behavior, gelation behavior, gel strength, and mucoadhesion. The formulations F10 (0.4% w/v carbopol, 1% w/v hydroxylpropyl methylcellulose K15M) and F13 (0.3% w/v carbopol, 1% w/v hydroxypropyl methylcellulose K15M) showed gel strength of 40.33±0.47 and 43.00±1.41, respectively, and mucoadhesion strength 31.48±0.14×103 and 32.12±0.05×103 dyne/cm2, respectively. In vitro release profiles showed initial burst followed by slow release. F10 and F13 released 88.08±0.98 and 91.18±1.09% drug in 8 h. R2 value for F10 (0.9953) and F13 (0.9942) was maximum for Higuchi, showing mixed order kinetics while n value obtained on treatment with Korsemayer Pappas equation were near to 0.5, suggesting release by fickian diffusion mechanism. The nasal permeability of formulations F10 and F13 were found to be 0.057 and 0.063 cm/s, respectively. Histopathological examination revealed slight degeneration of nasal epithelium with increased vascularity by F10 but no inflammation by formulation F13. Thus, a pH triggered in situ gel system containing low concentration (0.3% w/v) of carbopol demonstrated sustained release of metoprolol tartrate without any destructive effect on the mucosa.
Keywords: Carbopol, hydroxypropyl methylcellulose, in situ gel, metoprolol tartrate, mucoadhesion
Nasal drug delivery is now being considered as a valuable alternative to the parenteral routes for administering drugs that show poor oral bioavailability[1]. Nasal route is currently having considerable attention for several reasons, including rapid absorption into the systemic circulation, elimination of first-pass hepatic metabolism, and low proteolytic activity in the nasal mucosa. There are, however, limitations for the drugs of very short biological half-life, rapid absorption and the large mucociliary clearance of the nasal mucosa are unfavorable to sustain the drug level in the systemic circulation[2,3].
A significant increase in the nasal residence time of drugs and consequently bioavailability can be achieved by using in situ gel systems. Polymers employed in such delivery system may demonstrate transition from sol to gel state due to change in a specific physicochemical parameter (pH, temp or ionic conc.) in their environment[4]. Murthy et al.[5], reported that sumatriptatin in situ gel system is promising for prolonging nasal residence time and thereby nasal absorption. Jian et al.[6], studied the intranasal in situ gel system of scopolamine hydrobromide for antimotion sickness. The symptom of motion sickness was significantly decreased from intranasal in situ gel system in comparison with subcutaneous and oral administration of scopolamine hydrobromide (P<0.01).
Metoprolol tartrate (MT), ((RS)-isopropylamino-3-p(2-methoxyethyl))phenoxypropen-2-ol(2R,3R)-tartrate) a selective β-blocker, has been used widely for the treatment of hypertension[7]. It is completely absorbed from GIT, (95%) but is subjected to considerable first-pass metabolism. Thus, its oral absolute bioavailability is only 40%[7,8]. Several strategies have been used to avoid its first pass metabolism like buccal tablet[9], intranasal microspheres[10] to mention a few. In the present research work, nasal in situ gel of MT was formulated, which can be easily administered in the sol form and convert to gel at the site of administration. Thus, prolonged residence at the nasal mucosa is anticipated, which may provide sustained release of the drug, improve the permeation of the drug, and avoid first-pass metabolism.
MATERIALS AND METHODS
Metoprolol tartrate (MT), Carbopol 934P (CP), HPMC K4M and K15M were obtained from Polydrug labs,Thane, India, Ruger chemical Co. Inc., Irvington, NJ and Colorcon Asia Pvt. Ltd., Singapore, respectively. Sodium taurocholate (Loba chemicals, Mumbai, India) was added as penetration-enhancer, benzalkonium chloride (Merck Chemicals, Mumbai, India) as preservative, sodium bisulfate (Loba Chemicals, Mumbai, India) was added as antioxidant, and sodium chloride (Loba chemicals, Mumbai, India) was added for maintaining tonicity. All the chemicals used were of analytical grade.
Preparation of formulations:
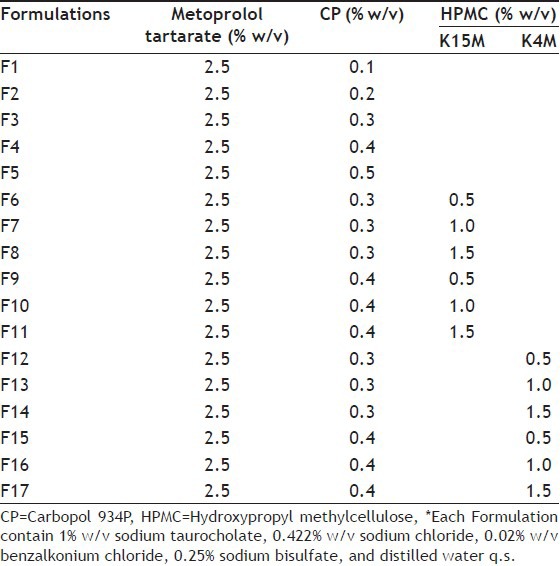
The formulations containing CP (Table 1) were prepared by dispersing CP in distilled water with continuous stirring until completely dissolved and allowed to hydrate overnight. For the preparation of formulation containing CP and HPMC of different grades, HPMC was added in distilled water and allowed to hydrate, and then CP was sprinkled over the solution and allowed to hydrate overnight. After complete hydration of polymers, a separate solution of drug and sodium chloride was added to the polymeric solution. To the above solution, sodium bisulfate was then added with continuous mixing until clear solution. All the formulations were adjusted to pH 4.5 by 0.5 M sodium hydrochloride solution and terminally sterilized by autoclaving at 121° and 15 psig for 20 min[11,12,13].
TABLE 1.
COMPOSITION OF FORMULATIONS
Drug content:
The formulations (400 μl) were dissolved in phosphate buffer saline (PBS) pH 7.4 in 100 ml volumetric flasks, and volume was made up to 100 ml. The solution was filtered and analyzed at 274 nm spectrophotometrically (Beckman, DU 64 spectrophotometer, USA)[11,14].
Gelation behavior studies:
The gelation behavior of formulations was determined by using gelation cells fabricated with Teflon. The gelation cells were cylindrical reservoirs holding 3 ml of gelation solution (0.5 M NaOH). Within the cells at the bottom, a 400 μl transparent plastic cup was located to hold the gel after its formation. The formulation (250 μl) was carefully placed into the cavity of the cup using a micropipette, and 2 ml of gelation solution was added slowly. Gelation was assessed by visual examination[14].
Viscosity measurement and rheological behavior:
Viscosity was determined in the liquid and gel state by the Brookfield viscometer (CAP 2000, Brookfield Engineering lab, Stoughton, USA). Cone no. 1 was used for formulation in the liquid state while cone no. 3 was used for gelled formulations. Viscosity was measured at 10 rpm over the period of 30 s. For studying rheological behavior, small amount of the gel was placed on the plate, cone no.3 was held on the plate, and shear stress was noted under increasing shear rate. Each measurement was done for 30 s[11,15,16].
Measurement of gel strength:
The gel strength was determined using device shown in fig. 1. The sample was placed in a 100 ml graduated cylinder and gelled by neutralization with 0.5M NaOH. The apparatus for measuring gel strength (35 g) was placed on the gel, and time in seconds required to sink 0.5 cm down through the polymer gel was determined[17,18,19].
Fig. 1.
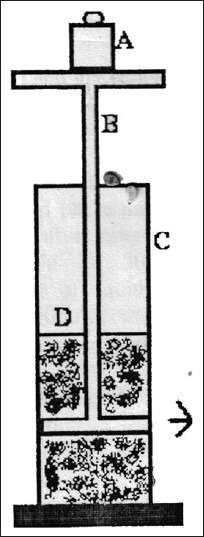
Gel strength measuring device. A-Weights, B-device, C-measuring cylinder, D-polymer gel
Determination of the mucoadhesive force:
Mucoadhesive force was determined according to the method adopted by Yong et al.[18] and Elhady et al.[20], using porcine nasal mucosa and phosphate buffer saline (PBS) pH 7.4 as the moistening fluid. At the time of testing, a section of tissue (E) was secured, keeping the mucosal side out, onto each glass (C) vial using a rubber band and an aluminum cap. The diameter of each exposed mucosal membrane was 1.1 cm. One vial with a section of tissue was connected to the balance (A), and the other was fixed on a height-adjustable pan (F). To the exposed tissue on this vial, 0.5 g gel was applied. The height of the vial was adjusted so that the gel could adhere to the mucosal tissues of both vials. A constant weight was placed on the upper vial and applied for 2 min, after which it was removed, and the upper vial was connected to the balance. Weights (B) were added at a constant rate to the pan on the other side of the modified balance of the used device until the two vials were separated (fig. 2). The muco-adhesive force, expressed as the detachment stress in dynes/cm2, was determined from the minimal weights that detached the two vials using the equation: Detachment stress (dyne/cm2)=mg/A...(1), where, m is the weight added to the balance, g is the acceleration due to gravity taken as 980 cm/sec2, and A is then area of tissue exposed and is equal to πr2 (r-radius of the exposed membrane).
Fig. 2.
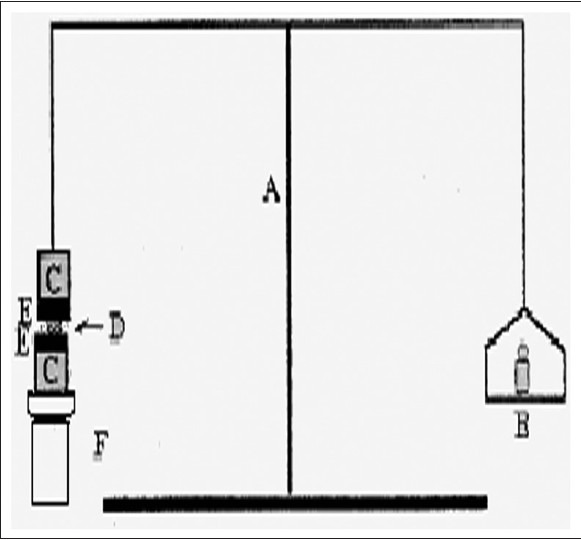
Mucoadhesive force measuring device.
A-Modified Balance, B-weights, C-glass vial, D-polymer gel, E-porcine nasal mucosa, F-supports to vial to adjust height.
In vitro release study:
The in vitro release of MT from the gels was measured through a cellulose acetate dialysis membrane employing Franz diffusion cells with a diffusional area 0.785 cm2 and 16 ml volume of the receptor compartment. Four hundred microliter formulation (containing 10 mg MT) was placed in the donor compartment while PBS, pH 7.4 was filled in the receptor compartment. During the study, temperature was maintained at 37±0.5°. At the predetermined time intervals, aliquot of 1 ml was withdrawn from the receiver compartment and replaced with same amount of the drug-free buffer[11,14,15,21,22,23]. The samples were suitably diluted, and amount of MT was determined by UV spectrophotometer at 274 nm. The study was done for 8 h.
In vitro permeation study:
Porcine nasal mucosa obtained from the local slaughterhouse was used as model membrane. The mucosa covering the ventral nasal conchae was carefully removed using forceps and a scalpel. After being rinsed in saline solution and then distilled water, a piece of nasal mucosa was mounted as flat sheet in a two chamber Franz diffusion cell (area, 0.785 cm2, volume of the donor compartment 16 ml) maintained at 37±0.5° with the mucosal side facing the donor compartment. Within 1.5 h of slaughter, the mucosa was separated and mounted on the diffusion cell. Four hundred microliter formulation (contained 10 mg MT) was placed on the mucosal surface in the donor while PBS pH 7.4 was placed in the receiver compartment. An aliquot of 1 ml was withdrawn after a fixed time interval and replaced with same amount of drug-free buffer. Aliquots so withdrawn were suitably diluted and analyzed spectrophotometrically at 274 nm. The study was continued for 8 h.
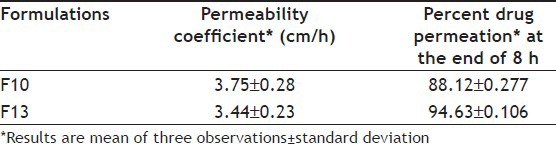
The permeability coefficient was calculated using the following equation, and values are shown in Table 2[18,19,20,22,23,24,25]. P=dQ/dt Co.A...(2), where, dQ/dt represents the permeability rate, Co represents the initial concentration in the donor chamber, while A is the effective surface area of the mucosa.
TABLE 2.
PERMEABILITY COEFFICIENTS AND PERCENT DRUG PERMEATION OF OPTIMIZED FORMULATIONS
Histological studies:
Formulations were applied for 8 h on the porcine nasal mucosa mounted on the diffusion cell, thereafter the nasal mucosa was fixed in 10% neutral carbonate-buffered formalin for at least 24 h and then cut vertically against the nasal mucosa at the central region in 4 mm widths. Each section was dehydrated using a graded series of ethanol solutions and was then embedded in paraffin wax. Tissues were divided into small pieces (about 3 μm in thickness) and stained with hematoxylin and eosin. All sections were examined by Nikon optiphoto microscope (Nikon Fx-35A, Nikon Instruments Inc., Japan)[25,26].
Stability study:
Formulations F13 and F10 were stored at 40±2° at 75±5% RH for 3 months and evaluated for drug content, viscosity, gelation behavior, gel strength, and in vitro release study.
RESULTS AND DISCUSSION
The in situ gels were made using gel-forming and mucoadhesive polymers such as CP 934P, HPMC K4M and HPMC K15M. They are known to be beneficial in improving the residence time and drug release characteristics. The physico-chemical properties of the prepared formulations are shown in Table 3. From the gelation behavior, it was observed that formulations containing CP with concentrations equal or less than 0.2% w/v could not form strong gel and at concentrations equal to or greater than 0.5% w/v formed very stiff gel at physiological pH. As the concentration of CP was increased, the solution became highly acidic. The highly acidic solution would not be neutralized by the buffering action of the nasal pH or fluid, may irritate nasal mucosa. The CP solution with 0.3-0.4% w/v concentration retained liquid state (free flowing) at pH 4.5 and gelled at physiologic pH. Reduction in CP concentration without compromising its gelling property but improved consistency and strength is possible by adding viscosity-enhancing agent HPMC. Thus, from the results of gelation behavior, the optimum concentration of CP was selected as 0.3 and 0.4% w/v for the preparation of in situ gel containing HPMC K4M and K15M.
TABLE 3.
EVALUATION OF FORMULATIONS
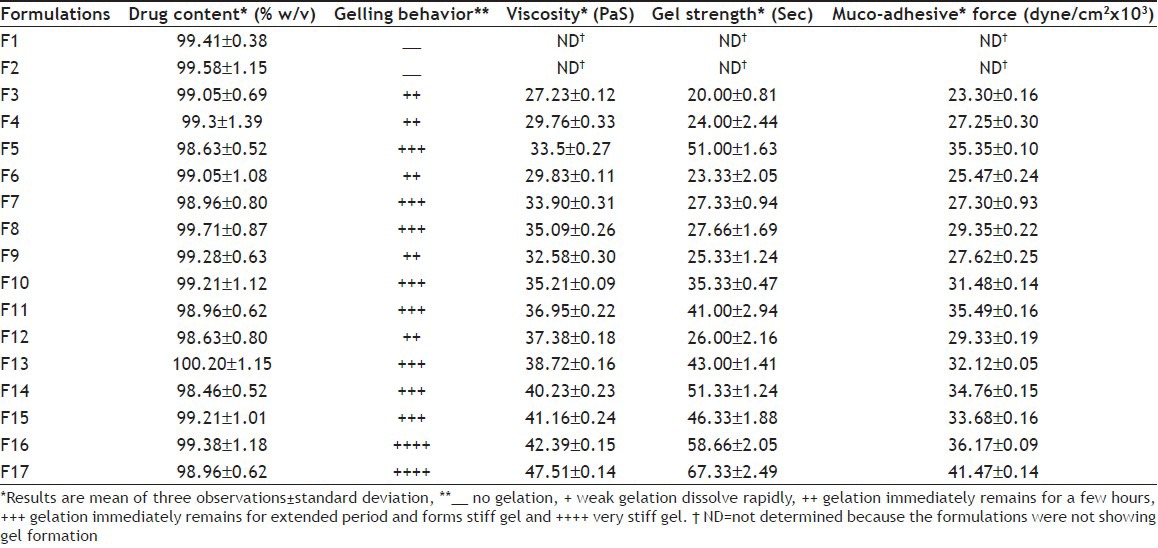
The rise in viscosity of all formulations at nasal pH was due to sol to gel conversion. CP has the ability to gel with the rise in pH. In the development of nasal in situ gel, appropriate gel strength of the formulation is important. The formulation must be administered easily as drops and must have sufficient strength to prevent post-nasal drip or anterior leakage. When the gel strength and gelation capacity of formulations were compared, it was observed that formulations having gel strength less than 25 s formed weak gel structure, which lasted for short time. Thus, rapid erosion drug leakage in the nasal cavity may happen, while formulations having gel strength greater than 50 s formed very stiff gel, which may cause discomfort. Thus, gel strength between 25 and 50 s was considered optimum for easy administration and prolonged retention without leakage of dose. The formulation F3 due to lower gel strength (20.00±81 s) may not retain integrity while formulations F5, F11, F14, F16, and F17 with higher gel strength may cause discomfort due to stiffness.
All formulations exhibited pseudoplastic flow as evidenced by shear thinning with the increase in rate of shear and absence of yield value (fig. 3). All formulations demonstrated thixotropic behavior as indicated by time-dependent change in viscosity at constant rate of shear and recovery of consistency after removal of stress (fig. 4). The area under the hysterises loop was found to be 146.12 and 123.61 dynes/cm2/sec2 for formulations F13 and F10, respectively.
Fig. 3.
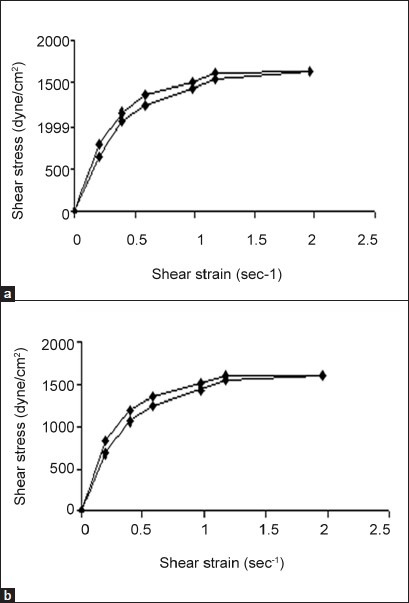
Rheogram of in situ gels.
The values are mean of three determinations. a. F13 and b. F10
Fig. 4.
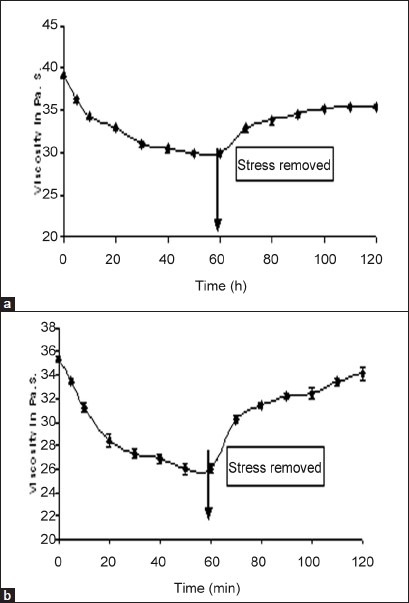
Thixogram of in situ gels.
The values are mean of three determinations. a. F13 and b. F10
The mucoadhesive forces of formulations F13 and F10 were found between 30-32×103 dyne/cm2. The mucoadhesive force increased with addition of HPMC. The increase may be due to greater hydrogen bonding between gel and the mucin. The secondary bond forming groups (e.g., hydroxyl, ether, oxygen, and amine), which are the principle source of mucoadhesion, were responsible for the greater mucoadhesion[20].
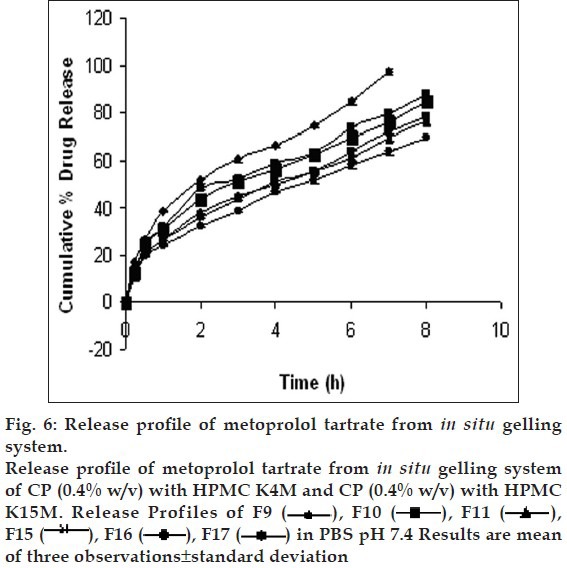
Drug release profiles shows that the initial rates of release were very rapid due to incomplete gel formation, but the release become slow after complete gelling and remained so. The release profiles exhibited an inflection point, which indicated gel formation on the diffusion membrane in the donor compartment of the diffusion cell. Formulations containing CP alone below 0.5% w/v concentration could not sustain the release, whereas formulations having HPMC in CP formulation were highly effective in sustaining the drug release. (figs. 5 and 6). Formulations F13 and F10 showed 88.08±0.98 and 91.18±1.09% drug release in 8 h, respectively.. The treatment with Peppas equation gave n value less than or near to 0.5; F10 (0.49) and F13 (0.45). Drug release was probably by usual molecular diffusion due to a chemical potential gradient. The R2 values of formulations F10 (0.9953) and F13 (0.9942) were highest for the Higuchi plot. Therefore, it can be concluded that the Higuchi release kinetics was followed by the gels.
Fig. 5.
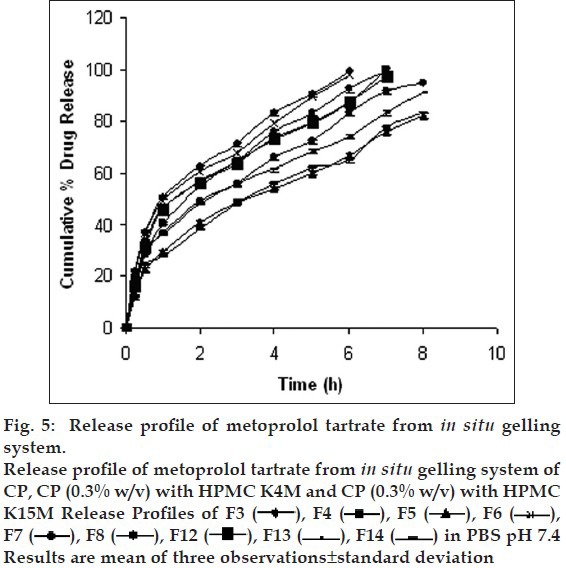
Fig. 6.
From the results of gelation behavior, viscosity, gel strength, mucoadhesion, and in vitro release studies, formulations F10 and F13 were selected for in vitro permeation study. The permeability coefficient of formulation F10 and F13 were found to be 0.057 and 0.063 cm/sec, respectively (Table 3). There was slow permeation because of the slow drug release. Formulation F10 showed 88.12±0.277% drug permeation while F13 showed 94.63±0.106% drug permeation in 8 h. Histological study indicated that (figs. 7a, b, and c), F13 did not cause degeneration or vascularity of nasal epithelium after 8 h, while F10 was found to cause increased degeneration of nasal epithelium coupled with ulceration of nasal epithelium with inflammation and increased vascularity. It is speculated that CP with low concentration (0.3% w/v) can be safe for nasal administration. No significant change (P>0.05) was found in drug content, gelling capacity, viscosity, and in vitro release after 3 months stability study.
Fig. 7.
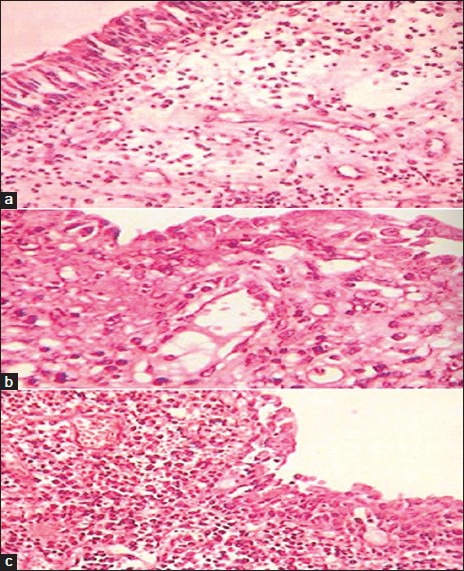
Histology of porcine nasal mucosa.
Histology of porcine nasal mucosa after treatment with (a) control (b) formulation F13 (c) formulation F10
The formulation F13 was found capable of controlling the drug release for 8 h without any destructive effect on the nasal mucosa. It also showed good mucoadhesive strength, which may result to the longer retention in the nasal cavity, avoid first-pass effect, and improve bioavailability. Therefore, if these findings translate to the in vivo conditions, then nasal administration of MT can be viewed as a viable alternative to its oral administration.
Footnotes
Khan, et al.: In situ Gel of Metoprolol Tartrate
REFERENCES
- 1.Zhou M, Donovan MD. Intranasal mucociliary clearance of putative bioadhesive polymer gels. Int J Pharm. 1996;135:115–25. [Google Scholar]
- 2.Ahn BN, Kim SK, Shim CK. Proliposomes as an intranasal dosage form for the sustained delivery of Propranolol. J Control Rel. 1995;34:203–10. [Google Scholar]
- 3.Schipper NG, Verhoef JC, Merkus FW. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm Res. 1991;8:807–14. doi: 10.1023/a:1015830907632. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Lohei E, Tregoa A, Pelea Y. A novel in situ forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Rel. 1997;44:201–8. [Google Scholar]
- 5.Majithiya RJ, Ghosh PK, Umrethia ML, Murthy RS. Thermoreversible mucoadhesive gel for nasal delivery of Sumatriptan. AAPS PharmaSciTech. 2006;7:67. doi: 10.1208/pt070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao S, Zhang Q, Jian X. Preperation of ion activated in situ gel systems of Scopolamine hydrobromide and evaluation of its antimotion sickness efficacy. Acta Pharmacol Sin. 2007;28:201–8. doi: 10.1111/j.1745-7254.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 7.Brian HB, Lefkcowitz RJ. The Pharmacological Basis of Therapeutics. In: Gilman AG, Thodore RW, Alan NS, editors. Adrenergic receptor antagonist. New York: Pergamon Press; 1991. pp. 237–95. [Google Scholar]
- 8.Narendra C, Srinath MS, Babu G. Optimization of bilayer floating tablet containing Metoprolol tartrate as a model drug for gastric retention. AAPS PharmaSciTech. 2006;7:34. doi: 10.1208/pt070234. [DOI] [PubMed] [Google Scholar]
- 9.Nakhat PD, Kondawar AA, Rathi LG, Yeole PG. Development and in vitro evaluation of buccoadhesive tablets of Metoprolol tartrate. Indian J Pharm Sci. 2008;70:121–4. doi: 10.4103/0250-474X.40349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajinikanth PS, Sankar C, Mishra B. Sodium alginate microspheres of Metoprolol tartrate for intranasal systemic delivery: Development and evaluation. Drug Deliv. 2003;10:21–8. doi: 10.1080/713840323. [DOI] [PubMed] [Google Scholar]
- 11.Charoo A, Kohli K, Ali A. Preparation of in situ forming ophthalmic gels of ciprofloxacin hydrochloride for the treatment of bacterial conjunctivitis; in vitro and in vivo studies. J Pharm Sci. 2003;92:407–13. doi: 10.1002/jps.10290. [DOI] [PubMed] [Google Scholar]
- 12.Lin HR, Sung KC. Carbopol/Pluronic phase change solutions for ophthalmic drug delivery. J Control Rel. 2000;69:379–88. doi: 10.1016/s0168-3659(00)00329-1. [DOI] [PubMed] [Google Scholar]
- 13.Nakamuri K, Maitani Y, Lowman AM, Takayamak, Peppas NA, Nagai T. Uptake and release of Budesonide from mucoadhesive pH sensitive copolymers and their application to nasal delivery. J Control Rel. 1999;61:329–35. doi: 10.1016/s0168-3659(99)00150-9. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramanium J, Kant S, Pandit JK. In vitro and in vivo evaluation of the gelrite gellan gum based ocular delivery system for Indomethacin. Acta Pharm. 2003;53:251–61. [PubMed] [Google Scholar]
- 15.Srividya B, Amin PD, Cardoza RM. Sustained ophthalmic delivery of Ofloxacin from a pH triggered in situ gelling system. J Control Rel. 2001;73:205–11. doi: 10.1016/s0168-3659(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 16.Deem DE. Rheology of dispersed system, In Pharmaceutical Dosage Forms: Disperse Systems. In: Rieger MM, Banker GS, editors. Vol. 1. New York: Marcel Dekker, Inc; 1988. pp. 367–425. [Google Scholar]
- 17.Choi HG, Jung TH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in situ gelling and mucoadhesive Acetaminophen liquid suppository. Int J Pharm. 1998;185:33–44. [Google Scholar]
- 18.Yong CS, Choi JS, Quan QI, Rhee JD, Kim CK, Lim SJ, et al. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing Diclofenac sodium. Int J Pharm. 2001;26:195–205. doi: 10.1016/s0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]
- 19.Han RY, Fang JY, Sung KC. Mucoadhesive buccal disc novel Nalbupine prodrug controlled delivery: Effect of formulation variables on drug release and mucoadhesive performance. Int J Pharm. 1999;177:201–7. doi: 10.1016/s0378-5173(98)00343-3. [DOI] [PubMed] [Google Scholar]
- 20.Elhady SS, Mortada ND, Gehanne AS, Zaki NM, Taha RA. Development of in situ gelling and mucoadhesive Mebeverine hydrochloride solution for rectal administration. Saudi Pharm J. 2003;11:159–71. [Google Scholar]
- 21.Maiteni Y, Uchida M, Takahashi S, Nakagaki NM, Nagai T. Effect of bile salts on the nasal mucosa: Membrane potential measurement. Int J Pharm. 1991;69:21–7. [Google Scholar]
- 22.Maitani Y, Asano S, Takahashi S, Nakagaki M, Agai T. Permeability of Insulin entrapped in liposomes through the nasal mucosa of rabbits. Chem Pharm Bull. 1992;40:1569–72. doi: 10.1248/cpb.40.1569. [DOI] [PubMed] [Google Scholar]
- 23.Chavanpatil MD, Vavia PR. The influence of absorption enhancers on nasal absorption of Acyclovir. Eur J Pharm Biol. 2004;57:483–7. doi: 10.1016/j.ejpb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Zhao Y. Nasal insulin delivery in the chitosan solution in vitro and in vivo studies. Int J Pharm. 2004;281:11–23. doi: 10.1016/j.ijpharm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Wadell C, Bjork E, Camber O. Nasal drug delivery-evaluation of an in vitro model using porcine nasal mucosa. Eur J Pharm Sci. 1999;7:197–206. doi: 10.1016/s0928-0987(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 26.Ugwoke MI, Agu RO, Jorissen M, Augustijns P, Sciot R, Verbeke N, et al. Toxicological investigations of the effects of carboxymethyl cellulose on ciliary beat frequency of human epithelial dells in primary suspension culture and in vivo on rabbit nasal mucosa. Int J Pharm. 2000;201:33–51. doi: 10.1016/s0378-5173(00)00484-1. [DOI] [PubMed] [Google Scholar]


