Abstract
Purpose.
To develop new therapies against ocular neovascularization (NV), we tested the effect of peroxisome proliferator-activated receptor-β/δ (PPAR-β/δ) agonism and antagonism on angiogenic behaviors and in human retinal microvascular endothelial cells (HRMEC) and on preretinal NV in rat oxygen-induced retinopathy (OIR).
Methods.
HRMECs were treated with the PPAR-β/δ agonist GW0742 and the antagonist GSK0660. Messenger RNA levels of a PPAR-β/δ target gene, angiopoietin-like-4 (angptl4) were assayed by qRT-PCR. HRMEC proliferation and tube formation were assayed according to standard protocols. OIR was induced in newborn rats by exposing them to alternating 24-hour episodes of 50% and 10% oxygen for 14 days. OIR rats were treated with GW0742 or GSK0660. Angptl4 protein levels were assessed by ELISA and preretinal NV was quantified by adenosine diphosphatase staining.
Results.
GW0742 significantly increased angptl4 mRNA, and GSK0660 significantly decreased angptl4 mRNA. GW0742 had no effect on HRMEC proliferation, but caused a significant and dose-responsive increase in tube formation. GSK0660 significantly reduced serum-induced HRMEC proliferation and tube formation in a dose-dependent manner. Intravitreal injection of GW0742 significantly increased total retinal Angptl4 protein, but intravitreal injection of GSK0660 had no effect. Intravitreal injection of GW0742 significantly increased retinal NV, as did GW0742 administered by oral gavage. Conversely, both intravitreal injection and intraperitoneal injection of GSK0660 significantly reduced retinal NV.
Conclusions.
PPAR-β/δ activation exacerbates, and its inhibition reduces, preretinal NV. PPAR-β/δ may regulate preretinal NV through a prodifferentiation/maturation mechanism that depends on Angptl4. Pharmacologic inhibition of PPAR-β/δ may provide a rational basis for therapeutic targeting of ocular NV.
Keywords: retinopathy of prematurity, nuclear transcription factor, angiogenesis, vascular endothelial growth factor, peroxisome proliferator-activated receptor
The goal of this study was to determine the effect of pharmacologic manipulation of PPAR-β/δ on retinal angiogenesis. We found that inhibition of PPAR-β/δ reduces neovascularization in the rat model of oxygen-induced retinopathy.
Introduction
Angiogenesis is the formation of new capillaries from preexisting vessels. Physiologic angiogenesis occurs during embryonic development, as a part of the wound healing response, and during the menstrual cycle.1–6 However, angiogenesis can become dysregulated and contribute to morbidity in diseased tissues. This pathologic angiogenesis, also known as neovascularization (NV), occurs in tumor progression, rheumatoid arthritis, psoriasis, and many other conditions. Ocular NV is a defining feature of retinopathy of prematurity (ROP), diabetic retinopathy (DR), and age-related macular degeneration (ARMD), some of the leading causes of irreversible blindness in the developed world.6–11 To more effectively prevent and treat ocular NV, it is necessary to gain a thorough understanding of the cellular and molecular mechanisms regulating angiogenesis in the eye.
Peroxisome proliferator-activated receptors (PPARs) are transcription factors and members of the steroid nuclear hormone receptor family.12 Three PPAR isoforms have been characterized: PPAR-α (NR1C1), PPAR-β/δ (NR1C2), and PPAR-γ (NR1C3).13,14 PPARs bind to genomic DNA at loci called peroxisome proliferator response elements (PPREs). A PPRE is a consensus sequence that is functionally linked to, and usually located within, the promoter sequence of the PPAR's target gene.13,15 PPARs regulate transcription through a classical mechanism that involves: ligand binding to the PPAR, followed by dissociation of PPAR corepressors; heterodimerization between the PPAR and retinoid acid receptor (RXR); association of the heterodimer with coactivators; binding of the ligand-bound PPAR/RXR-containing protein complex to a PPRE; and, finally, transcriptional activation of the target gene.13 Other biologically relevant mechanistic variants have been described, suggesting that PPAR-β/δ is a ligand-dependent or -independent transcriptional suppressor.13,15
PPARs are widely known for their roles in lipid metabolism, insulin sensitivity, inflammation, and cell proliferation and/or differentiation.15–19 PPAR-α regulates fatty acid uptake and oxidation in a wide variety of tissues.20 PPAR-γ regulates adipocyte differentiation and glucose transport.21,22 PPAR-β/δ is expressed ubiquitously and has metabolic regulatory activities that overlap with the other two isoforms.23 Substantial evidence supports its role in the regulation of cell proliferation and differentiation.18,24
PPAR-β/δ activation induces differentiation of keratinocytes, colonocytes, and trophoblast giant cells in a ligand-dependent manner.24–26 Studies from various laboratories have indicated a direct role for PPAR-β/δ in angiogenic cell behaviors and in the angiogenic component of tumor growth.27,28 For example, activation of PPAR-β/δ by a chemically synthesized, high-affinity ligand induces cultured endothelial cells (ECs) to proliferate and form tubes.27 Müller-Brüsselbach et al.28 demonstrated defective tumor angiogenesis in PPAR-β/δ−/− mice transplanted with wild-type tumor cells. Whether PPAR-β/δ activation induces or inhibits cell proliferation appears to be cell type– and tissue-specific.18 Therefore, it remains to be determined whether PPAR-β/δ plays a similar role in ocular NV.
In this study, in vitro experiments were performed to investigate the influence of PPAR-β/δ on discrete aspects of retinal angiogenesis. First, the effects of PPAR-β/δ on endothelial cell proliferation and tube formation were investigated using human retinal microvascular endothelial cells (HRMECs). Second, to further investigate the therapeutic potential of PPAR-β/δ manipulation, the rat model of oxygen-induced retinopathy (OIR) was used to assess the effects of pharmacologic agonists and antagonists of PPAR-β/δ on the retinal neovascular response. These studies will help to define the role of PPAR-β/δ in mediating pathologic ocular angiogenesis.
Methods
HRMEC Culture
Primary cultures of HRMECs (Cell Systems, Kirkland, WA) were seeded into tissue culture flasks coated with attachment factor (Cell Signaling, Danvers, MA). HRMECs were grown and cultured in phenol red–free endothelial basal medium (EBM; Lonza, Walkersville, MD) supplemented with 10% FBS, 1× antibiotic/antimycotic solution, and endothelial cell growth supplements (EGM SingleQuots; Lonza), hereafter referred to as growth medium. When experimental conditions required serum-free (SF) medium, EBM with no FBS or growth supplements was used. All cultures were incubated at 37°C, 5% CO2, and 95% relative humidity (20.9% oxygen). Passages 6 to 8 were used for these experiments.
Quantitative Real-Time RT-PCR (qRT-PCR) of Angiopoietin-Like Protein-4 (angptl4) mRNA in HRMEC
HRMECs were seeded in six-well plates at 2 × 105 cells/well and maintained under standard tissue culture conditions. At 80% confluency, the cells were serum starved for 12 hours, then treated on a background of 0.5% serum-containing vehicle (0.1% DMSO) or PPAR-β/δ agonist GW0742 (0.01, 0.1, or 1.0 μM; Tocris Bioscience, Ellisville, MO), or on a background of 2% serum-containing vehicle or PPAR-β/δ antagonist GSK0660 (0.01, 0.1, or 1.0 μM; Tocris Bioscience) for 6 hours. Cells were washed twice with cold PBS and total RNA was collected using a commercial kit (RNeasy Kit; Qiagen, Valencia, CA), according to the manufacturer's instructions. Total RNA isolated from the culture wells was reverse transcribed using a high-capacity commercial kit (cDNA Archive Kit; Applied Biosystems, Foster City, CA), according to the manufacturer's instructions. Quantitative RT-PCR was performed in duplicate by coamplification of human angptl4 cDNAs versus β-actin (normalization control) in separate wells, using gene-specific gene expression assays (TaqMan; Applied Biosystems), according to the manufacturer's instructions.
HRMEC Proliferation
HRMECs were seeded at 3 × 103 cells/well in a 96-well plate in growth medium for 8 hours to allow them to settle and attach. Cells were serum-starved for 12 hours and then treated with SF medium containing vehicle (0.1% DMSO) or increasing concentrations of GW0742 (0.01–1.0 μM), or 2% serum medium or 25 ng/mL vascular endothelial growth factor (VEGF) medium containing vehicle (0.1% DMSO) or increasing concentrations of GSK0660 (0.01–1.0 μM) for 24 hours. Cells were then labeled with bromodeoxyuridine (BrdU) for 12 hours, and BrdU incorporation was quantified using a colorimetric BrdU ELISA (Roche, Indianapolis, IN), according to the manufacturer's instructions. The experiment was replicated four times.
HRMEC Tube Formation
Twenty-four–well tissue culture plates were coated with 400 μL of growth factor–reduced basement membrane matrix (Matrigel; Becton Dickenson, Franklin Lakes, NJ). HRMECs were seeded at 2.5 × 104 cells/well and treated with SF medium containing vehicle (0.1% DMSO) or increasing concentrations of GW0742 (0.01–1.0 μM) or 2% serum medium containing vehicle (0.1% DMSO) or GSK0660 (0.01–1.0 μM) for 24 hours. In another experiment, HRMECs were treated with 0.5% medium containing 25 ng/mL VEGF and vehicle (0.1% DMSO) or GSK0660 (0.01–1.0 μM) for 12 hours. Tubes were observed with an inverted microscope (IMT-2; Olympus, Melville, NY) and six images/well were captured in a systematic pattern with a digitizing camera (DMC Camera; Polaroid, Cambridge, MA) at ×10 magnification. Capillary-like structures were measured to determine the mean tube length per field using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsbweb.nih.gov/ij/index.html), and these values were normalized. The relative tube length per field of each treatment group is reported. The experiments were replicated three times.
Rat OIR
All animal procedures used in this study were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Within 8 hours after birth, litters of Sprague–Dawley rat pups and their mothers (Charles River Laboratories; Wilmington, MA) were transferred to oxygen exposure chambers in which they were subjected to alternating 24-hour periods of 50% and 10% oxygen for 14 days. On postnatal day 14, referred to as day 14(0), the oxygen-exposed rats were removed to room air. They remained at room air for an additional 6 days, hereafter described as day 14(1) through day 14(6).
Intravitreal Injections
Rats were anesthetized by isoflurane (Butler Animal Health Supply, Dublin, OH) inhalation and a drop of 0.5% proparacaine (Allergan, Hormigueros, PR) was topically applied to the cornea before intravitreal injection. The globe was penetrated approximately 0.5 mm posterior to the ora ciliaris, using a 30-gauge needle with a 19° bevel and 10-μL syringe (Hamilton Co., Reno, NV). The needle was advanced to the posterior vitreous at a steep angle to avoid contact with the lens. The injection bolus (5 μL) was delivered near the trunk of the hyaloid artery proximal to the posterior pole of the retina. After injection, a topical antibiotic suspension (Vigamox; Alcon Laboratories, Fort Worth, TX) was applied. Noninjected eyes were also treated with topical proparacaine and antibiotic to control for the potential of these agents to influence retinal vessel growth.
Treatment Groups
A subset of oxygen-exposed rats was administered vehicle (0.1% DMSO in PBS), GW0742 or GSK0660 (20, 100, or 500 nM) by intravitreal injection on days 14(0) and 14(3). Noninjected animals were used as controls. The dose regime administered by intravitreal injection was determined by preliminary dose/response experiments in OIR rats and yielded an optimal local dose/response profile (see Fig. 4 later in text). We found that GW072 and GSK0660 regulated preretinal NV at lower concentrations than those required to produce responses in our EC proliferation and tubulogenesis assays. These differences may be attributed to the action of these drugs on other retinal cell types and their potential regulation of the angiogenic cascade at multiple points. Another subset of oxygen-exposed rats was administered vehicle (10% ethanol in corn oil) or GW0742 (1.0 or 10 mg/kg) by oral gavage once daily for 6 days [14(0)–14(5)]. The last subset was administered vehicle (1% DMSO in PBS) or GSK0660 (0.2 or 1.0 mg/kg) by intraperitoneal injection on days 14(0), 14(2), and 14(4). Administration routes were chosen based on formulation differences and anticipated systemic bioavailability for the two drugs.
Figure 4.
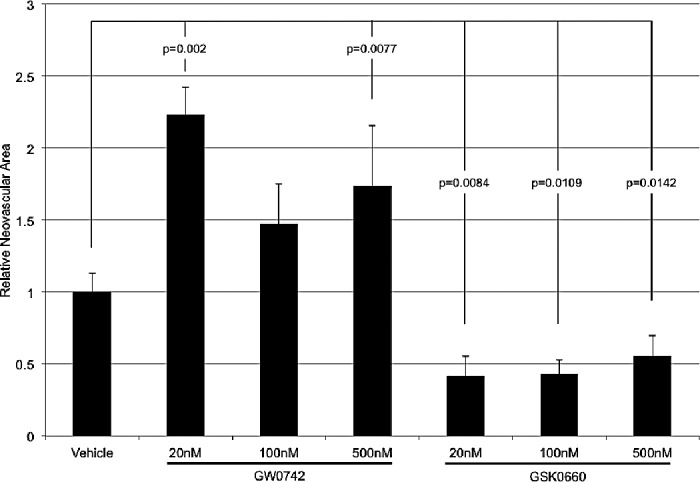
The effect of local administration of GW0742 and GSK0660 on OIR-induced NV. GW0742 and GSK0660 were intravitreally injected. GW0742 significantly increased OIR-induced NV at the lowest and highest concentrations. At all concentrations, GSK0660 significantly decreased retinal NV. Each bar represents the mean ± SEM.
Quantification of Retinal NV
On day 14(6), all rats were euthanized and their retinas dissected. After dissection, the retinal vasculature was stained for adenosine diphosphatase (ADPase) activity, according to well-established procedures.29 Images of ADPase-stained retinas were digitized, captured, and displayed at ×20 magnification. For each retinal image, preretinal vessel tufts were outlined with an irregular polygon, the pixels within the polygon were counted, and the total number of pixels from each polygon in a retina were pooled and converted to square millimeters. The data shown are normalized to NV values from vehicle-treated eyes.
Angptl4 Production in the OIR Rat Retina
Following OIR, rats were intravitreally injected on day 14(1) with vehicle (0.1% DMSO in PBS), 500 nM GW0742, or 500 nM GSK0600. Retinas were collected on day 14(2), sonicated in lysis buffer, and assayed for Angptl4 protein concentration with a colorimetric sandwich ELISA kit (US Biologicals, Salem, MA). The amount of Angptl4 (pg/mL) in retinas was normalized to total protein concentration (mg/mL) of retinal lysates using a bicinchoninic acid assay (Pierce, Rockford, IL).
Statistical Analyses
Data were analyzed with commercial software (JMP; SAS Institute, Cary, NC) using ANOVA with Student's post hoc analysis. P < 0.05 was considered statistically significant.
Results
Effect of PPAR-β/δ Agonism and Antagonism on angptl4 mRNA
HRMECs treated with 0.5% serum medium plus 0.01, 0.1, and 1.0 μM GW0742 for 6 hours exhibited a significant increase in angptl4 mRNA expression at every dose (P < 0.0001; Fig. 1A). Treatment with 2% serum plus GSK0660 significantly decreased angptl4 mRNA expression in a dose-responsive fashion (P = 0.0035, P = 0.0092, and P = 0.0002, respectively; Fig. 1B).
Figure 1.

The effect of GW0742 and GSK0660 on angptl4 expression in HRMEC. (A) Quantitative RT-PCR analysis of angptl4 mRNA revealed significant activation of PPAR-β/δ with GW0742 treatment. (B) GSK0660 treatment led to a significant reduction in angptl4 expression. Each bar represents the mean ± SEM.
Effect of PPAR-β/δ Agonism and Antagonism on HRMEC Proliferation
Treatment of HRMECs with SF medium plus 0.01, 0.1, or 1.0 μM GW0742 for 24 hours had no effect on proliferation. HRMECs treated with 2% serum medium plus 0.01, 0.1, or 1.0 μM GSK0660 for 24 hours exhibited dose-dependent decreases in HRMEC proliferation. There was a 39% (P = 0.0034) reduction in serum-induced proliferation at the highest concentration tested (Fig. 2A). GSK0660 inhibited VEGF-induced proliferation by 33.1% (P < 0.0001) at the 1.0 μM concentration (Fig. 2B).
Figure 2.
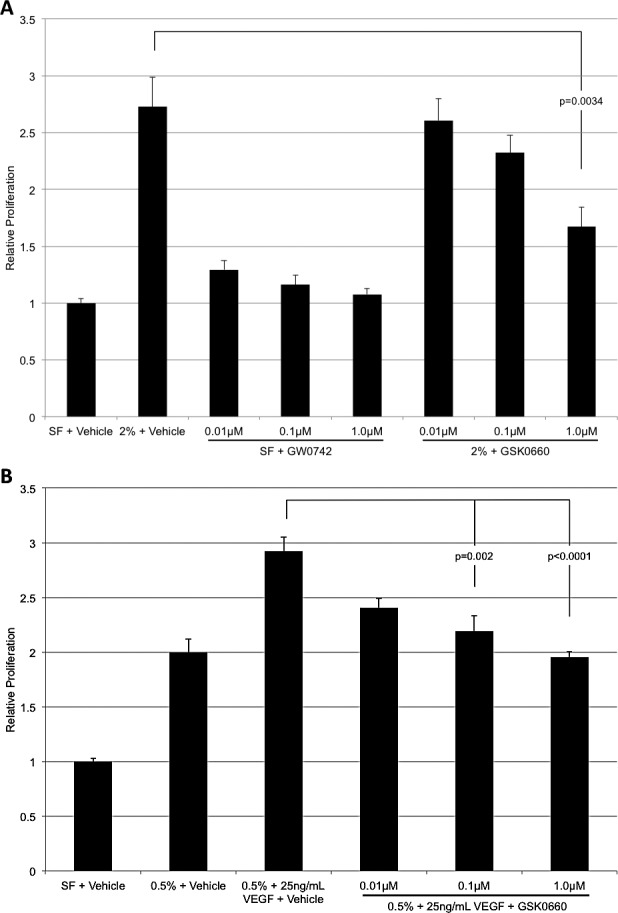
The effect of GW0742 and GSK0660 on HRMEC proliferation. (A) GW0742 exhibited no effect on HRMEC proliferation. GSK0660 treatment led to a dose-dependent reduction of serum-induced proliferation. (B) GSK0660 treatment results in a dose-dependent reduction of VEGF-induced proliferation. Each bar represents the mean ± SEM.
Effect of PPAR-β/δ Agonism and Antagonism on HRMEC Tube Formation
Increasing concentrations of GW0742 in SF medium increased the average HRMEC tube length in a dose-dependent manner. Increases of 89.5% (P < 0.0001) and 118% (P = 0.0028) were observed at the 0.1 and 1.0 μM GW0742 concentrations, respectively. Increasing concentrations of GSK0660 in 2% serum medium reduced the average serum-induced HRMEC tube length in a dose-dependent manner. We observed percentage decreases of 18.7% (P = 0.04) and 40% (P = 0.0067) at the 0.1 and 1.0 μM concentrations, respectively. These data and representative images are shown in Figures 3A, 3B. GSK0660 inhibited VEGF-induced tube formation by 50.6% (P = 0.0016) at the 1.0 μM concentration (Fig. 3C).
Figure 3.
The effect of GW0742 and GSK0660 on HRMEC tube formation. (A) Representative images of tube formation in HRMECs treated with SF medium with vehicle, 2% serum with vehicle, 1.0 μM GW0742 in SF media, and 1.0 μM GSK0660 in 2% serum media. (B) GW0742 treatment induced tube formation in a dose-dependent manner. HRMEC tube formation was induced by 2% serum, and this induction was significantly inhibited with increasing concentrations of GSK0660. (C) GSK0660 inhibits VEGF-induced tube formation. Each bar represents the mean ± SEM.
Effect of Local Administration of PPAR-β/δ Agonists and Antagonists on the Severity of OIR
Intravitreal administration of GW0742 increased the retinal NV response in OIR rats. Injecting GW0742 at 20 nM, 100 nM, and 500 nM increased NV by 123% (P = 0.0001), 47.5% (P = 0.0578), and 73% (P = 0.0077), respectively. Treatment with GSK0660 decreased NV by 58.5% (P = 0.0084) at 20 nM, 56.9% (P = 0.0109) at 100 nM, and 44.4% (P = 0.0142) at 500 nM. These data are shown in Figure 4.
Effect of Systemic Administration of PPAR-β/δ Agonists and Antagonists on the Severity of OIR
A similar trend was observed when GW0742 was administered by oral gavage for 6 consecutive days. As shown in Figure 5, GW0742 increased retinal NV by 109.1% (P = 0.0067; 10 mg/kg). Conversely, intraperitoneal administration of GSK0660 on days 14(0), 14(2), and 14(4) significantly reduced retinal NV. Retinal NV was decreased by 50.3% (P = 0.0062) at the low concentration and 59.4% (P = 0.0017) at the high concentration. These data and representative images are shown in Figure 5.
Figure 5.
The effect of systemic administration of GW0742 and GSK0660 on OIR-induced NV. (A) Representative images of retinal quadrants in rats treated with vehicle by oral gavage, 10 mg/kg GW0742 by oral gavage, and 1.0 mg/kg GSK0660 by intraperitoneal injection. (B) GW0742 was administered by oral gavage (1.0 and 10 mg/kg) and GSK0660 by intraperitoneal injection (0.2 and 1.0 mg/kg). GW0742 significantly increased the NV area at the highest concentration tested, whereas GSK0660 significantly decreased the NV area at both concentrations tested. Each bar represents the mean ± SEM.
Effect of PPAR-β/δ Agonists and Antagonists on Angptl4 Production in OIR Retinas
Intravitreal administration of GW0742 on day 14(1) significantly induced retinal Angptl4 production by 89.8% (P = 0.0246) when assayed 24 hours later. Intravitreal injection of GSK0660 had no effect on retinal Angptl4 production 1 day following injection. These data are shown in Figure 6.
Figure 6.
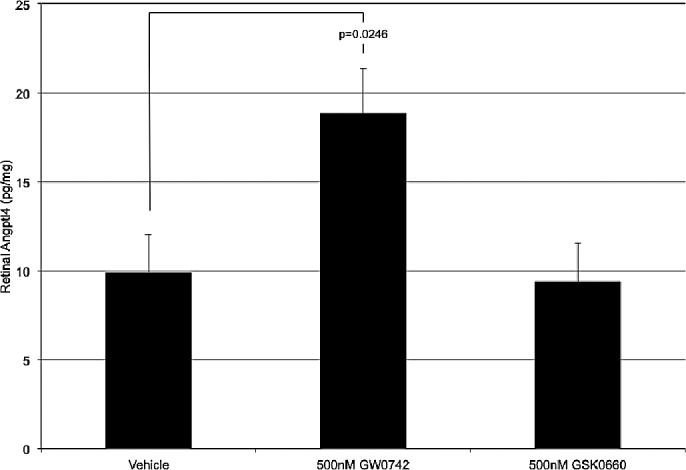
The effect of intravitreally injected GW0742 and GSK0660 on total retinal Angptl4 production. GW0742 and GSK0660 were injected at 500 nM concentration and retinas were collected 1 day later. Following GW0742 injection, Angptl4 was significantly increased. GSK0660 had no effect on Angptl4 production in the retina. Each bar represents the mean ± SEM.
Discussion
This study is the first to examine the role of PPAR-β/δ in retinal angiogenesis, using in vitro models of angiogenic cell behaviors and an in vivo model of preretinal NV. Our experiments were performed using GW0742, a chemically synthesized, highly selective PPAR-β/δ agonist, and GSK0660, a potent competitive antagonist that also demonstrates inverse agonist activity.30,31 These two compounds permitted a clear demonstration of a regulatory role for PPAR-β/δ in ocular NV.
Preliminary studies conducted in our laboratory have shown that PPAR-β/δ is expressed in HRMECs and in rat retina (Penn JS, et al. IOVS 2009;50:ARVO E-Abstract 2958). PPAR-β/δ activity level is tightly correlated with the induced expression of angptl4 mRNA, providing a convenient surrogate marker of PPAR-β/δ activation.31,32 GW0742 treatment significantly increased angptl4 mRNA in quiescent HRMECs at all concentrations (Fig. 1A). GW0742 is approximately 2000- and 1000-fold more selective for PPAR-β/δ (EC50 = 0.001 μM) over PPAR-γ (EC50 = 2.0 μM) and PPAR-α (EC50 = 1.1 μM), respectively (human forms).30 Conversely, GSK0660 treatment decreased angptl4 mRNA expression in serum-stimulated HRMEC (Fig. 1B). These findings demonstrate the effect of activation or deactivation of PPAR-β/δ on expression of PPAR-β/δ target genes, and confirm the reliability of our pharmacologic reagents in a relevant experimental setting.
In this study, treatment with PPAR-β/δ agonists GW0742 or GW501516 (data not shown) did not affect HRMEC proliferation at concentrations known to activate PPAR-β/δ (Fig. 2). Ligand activation of PPAR-β/δ has been reported to decrease or increase EC proliferation.27,33 Piqueras et al.27 reported increased proliferation of human umbilical endothelial vein cells (HUVECs), human aortic endothelial cells (HAECs), and a fused human endothelial/A549 cell line (EAHy926), but only after 72 hours of treatment with the PPAR-β/δ agonist, GW501516. Since VEGF is a principal growth factor involved in the proliferative response of ECs,8 these authors performed experiments to determine whether PPAR-β/δ agonism increased VEGF expression and if blockade of the VEGF receptors, VEGFR1 and VEGFR2, influenced the observed GW501516-induced EC proliferation. GW501516 treatment induced VEGF mRNA and protein in the conditioned medium of EAHy926, and expression of dominant negative PPAR-β/δ abrogated this effect. Furthermore, pretreatment with the VEGF receptor 1/2 antagonist, cyclo-VEGFI, reduced GW501516-induced proliferation.27 Stephen et al.34 reported similar findings: 14 days of treatment with GW501516 induced HUVEC proliferation, VEGF-A expression, and Flt-1 (VEGFR1) expression. We assessed HRMEC proliferation after 1 day, and up to 14 days, of exposure to GW0742 or GW501516, but did not observe any effect (data not shown). We conclude that HRMEC proliferation may be independent of GW0742 and GW501516 treatment. Comparison of our data with those of other studies supports the notion that pharmacologic activation of PPAR-β/δ has mixed effects on EC proliferation. Similar observations have been reported for cancer cell proliferation.18
GSK0660 is a specific PPAR-β/δ antagonist/inverse agonist; we found that it decreases serum-induced HRMEC proliferation and, to our knowledge, this is a novel finding. The GSK0660 IC50 values (human PPARs) for PPAR-β/δ are, respectively, 0.155 μM and >10 μM for -α and -γ.31 Consequently, minimal inhibition, if any, of PPAR-α and -γ occurred in our proliferation experiments. Therefore, GSK0660 inhibition of PPAR-β/δ reduces microvascular EC proliferation, which is an important component of retinal NV.
In addition to EC proliferation, retinal NV also depends on cell migration and vascular remodeling: two EC angiogenic behaviors that are modeled by in vitro VEGF-dependent tubulogenesis assays.35 In normal retinal vascular development, tubulogenesis allows for the eventual maturation of new, patent vessels.8 The literature is replete with studies suggesting that PPAR-β/δ plays a role in cellular differentiation, an important component of tubulogenesis.25,27,36–40 Accordingly, we tested GW0742 in a tube formation assay and observed a significant, dose-dependent induction of HRMEC tube formation. Although PPAR-β/δ activation is likely to be largely responsible for the tube induction at all the concentrations tested, PPARs-α and -γ also may have been activated at the 1.0 μM GW7042 concentration in these experiments. That activation of these other PPAR isoforms contributed to increased tube formation is unlikely, because ligand activation of these receptors has been reported to be antiangiogenic.27 We also found that GSK0660 inhibited tube formation in a dose-dependent manner, and this effect is solely PPAR-β/δ–dependent at the concentrations tested (Fig. 3). Piqueras et al.27 demonstrated that GW501516 induced EAHy926 cells to form microvascular tubes and increased VEGF mRNA in these cells. Additionally, the observed GW501516-induced tube formation was blocked with the VEGFR1/2 receptor antagonist cyclo-VEGFI, indicating that a VEGF signaling component exists downstream from PPAR-β/δ activation in these cells.27 In preliminary experiments, we found no evidence for GW0742-induced expression of VEGF or VEGFR1 and VEGFR2 in HRMEC; however, we did observe a significant increase of proangiogenic angptl4 mRNA by qRT-PCR at all concentrations (Fig. 1A). Experimental evidence suggests that Angptl4 has a prodifferentiation biologic function, an important component of tube formation.36,40,41 Although PPAR-β/δ activation is likely responsible for the majority of the induction of angptl4 mRNA, PPAR-γ also induces it,41 and at the 1.0 μM GW0742 concentration, PPAR-γ may contribute to the observed increase. Interestingly, PPAR-β/δ siRNA-mediated knockdown blocked GW501516-induced human keratinocyte differentiation and additional evidence suggested that Angptl4 signaled prodifferentiation events downstream from PPAR-β/δ activation.36 These data suggest, and we hypothesize, that increased PPAR-β/δ–dependent transcription of angptl4 promotes HRMEC tubulogenesis by mediating HRMEC differentiation/maturation. Conversely, GSK0660 decreased angptl4 mRNA expression in our qRT-PCR experiments (Fig. 1B). Given the PPAR-α, -β/δ, and -γ IC50 values for GSK0660 cited above, it is highly probable that the GSK0660-dependent decrease in angptl4 mRNA we observed is solely attributed to inhibition of PPAR-β/δ, perhaps explaining the observed GSK0660-dependent decrease in HRMEC proliferation and tube formation.
To explore the therapeutic potential of targeting PPAR-β/δ, we assessed the efficacy of PPAR-β/δ–directed compounds using the rat model of OIR. This model consistently produces preretinal NV that mimics human ROP.42,43 Following oxygen exposure to induce OIR, local (intravitreal) and systemic (oral gavage) administration of GW0742 led to a significant increase in NV response (Figs. 4, 5). In the case of systemic administration, we did not determine GW0742 serum concentrations. However, GW0742 serum concentrations in mice that were fed rodent chow supplemented with GW0742 at 1 and 10 mg/kg were 440.4 and 2270 nmol/L, respectively.44,45 The EC50 values for murine PPARs are: -β/δ, 50 nM; -α, 8900 nM; and -γ, >10,000 nM.45,46 Therefore, the murine serum concentrations were well below those expected to activate PPARs-α and -γ. Relatively few studies have explored the effect of GW0742 on angiogenesis. Gaudal et al.47 reported that GW0742 induced angiogenesis in mouse skeletal muscle. Furthermore, Wagner et al.48 reported similar findings in heart muscle. It is well established that the neovascular response in the rat model of OIR has a significant endothelial cell proliferation component, and our cell culture experiments suggest that GW0742 does not initiate EC proliferation. This discrepancy may reflect differences in the cytokine/growth factors available in our in vitro and in vivo model systems, differences between the species (human RMEC versus rat OIR), differences in stimulation of PPAR-β/δ target genes in diverse cell types within the eye, or because in vivo GW0742 may exert its influence by cell proliferation–independent mechanisms, all of which have the potential to affect the NV response of the retina. It is well established in the rat OIR model that retinal VEGF increases postoxygen exposure.8,49 We administered GW0742 to OIR rats 1 day following removal to room air [day 14(1)] and assayed retinal VEGF 24 hours later, when secreted VEGF is at its highest concentration in the retina. We found no effect of this drug on OIR-induced retinal VEGF (data not shown). Our in vitro results suggest that GW0742 agonism of PPAR-β/δ drives tube formation, an angiogenic cell behavior that is dominated by maturation/differentiation, rather than proliferation.40 Additionally, GW0742-induced PPAR-β/δ activation in HRMECs increased proangiogenic angptl4 mRNA, and the results of other studies suggest that Angptl4 may mediate prodifferentiation signaling events downstream from PPAR-β/δ.36 These data are supported by our findings that retinal Angptl4 protein increased significantly following intravitreal injection of GW0742 (Fig. 6). Therefore, we propose that GW0742 agonism of PPAR-β/δ promotes preretinal NV via an Angptl4-dependent prodifferentiation/maturation mechanism in the rat model of OIR.
Conversely, GSK0660 treatment reduced preretinal NV in rat OIR. The drug was administered by local (intravitreal [IVIT]) injection or by systemic (intraperitoneal [IP]) injection. GSK0660 is rapidly cleared from the blood.31 Our doses of GSK0660 administered by IP injection were based on a report describing the efficacy of GSK0660 against copper-induced liver damage in mice. The authors report that doses as high as 10 mg/kg/day had no toxicity.50 Our in vivo data are in agreement with our in vitro data because GSK0660 inhibited HRMEC proliferation and differentiation, suggesting that pharmacologic inhibition of PPAR-β/δ may represent a reasonable therapy against retinal NV. We also injected OIR rats with GSK0660, as we did for GW0742, to test for any effects on retinal VEGF and we found none (data not shown). Any blockade of the differentiation/maturation component of the neovascular response may be related to reduced HRMEC angptl4 mRNA expression, because we observed a GSK0660-dependent decrease of angptl4 in HRMEC (Fig. 1), although we did not observe a decrease in Angptl4 protein in GSK0660 OIR retinas (Fig. 6). We hypothesize that local expression and secretion of Angptl4 protein by retinal endothelial cells induces their maturation and differentiation via an autocrine mechanism that is critical to the neovascular response. However, the Angptl4 levels produced by EC may be relatively small compared with cumulative contributions of other retinal cells, such that we were unable to detect any GSK0660-dependent reduction in total retinal Angptl4. Additionally, although we observed a substantial PPAR-β/δ activation-dependent increase in retinal Angptl4 protein, there is no reason to assume that GSK0660 should produce the opposite results since PPAR-β/δ transcriptional mechanisms and their inhibition may vary according to the cell type. Consistent with the notion that Angptl4 is critical, in the mouse model of OIR angptl4-null mice demonstrated delayed normal development of the retinal vasculature and decreased retinal NV compared with wild-type controls.51
Currently, there is little information regarding the mechanistic details of Angptl4-dependent proangiogenic signaling in the retina. It is possible that GW0742-induced PPAR-β/δ–dependent angptl4 expression promotes angiogenic EC behaviors via an autocrine loop in OIR rats. As discussed above, GSK0660 could block an Angptl4-dependent autocrine loop operating in retinal endothelial cells. Paracrine mechanisms may also operate. For example, retinal pigment epithelium cells (ARPE-19) cultured in high glucose express and secrete higher Angptl4 levels compared with normal glucose controls. Conditioned medium from the high-glucose cultures induces retinal EC-tubulogenesis, and this effect was blocked by Angptl4-targeted RNAi knockdown.52 It follows that PPAR-β/δ drugs could act by either blocking or activating angptl4 expression in non-EC retinal cell types and regulate any putative Angptl4-dependent paracrine signaling mechanisms in the neovascular retina. Angptl4 is an orphan ligand and, consequently, the angiogenic signaling mechanisms downstream of Angptl4 in EC are poorly understood. Thus, it is not known whether PPAR-β/δ drugs directly target Angptl4, its downstream signaling intermediates, and/or regulate the expression of these signaling intermediates in EC. These questions raise important considerations for future studies.
Even though our data suggest that PPAR-β/δ inhibition may warrant continued investigation as a therapeutic modality against retinal NV, we also realize that caution must be exercised due to the many potentially beneficial PPAR-β/δ–related biologic functions. PPAR-β/δ has been shown to be antiapoptotic, anti-inflammatory, neuroprotective, and to suppress tumorigenesis.53–60 Additionally, pharmacologic activation of PPAR-β/δ protects against EC dysfunction.45 Consequently, any studies that are designed to explore pharmacologic inhibition of PPAR-β/δ in the retina should carefully address potential deleterious side effects related to retinal inflammation and retinal cell death. These studies should also carefully monitor the overall health of the experimental subjects since PPAR-β/δ has several known positive effects on metabolic homeostasis and cardiovascular disease, and given its controversial role in carcinogenesis.53,61 As a crude indicator of potential toxicity, we administered 20 nM GW0742 or GSK0660 via IVIT injection, to 7-day-old room-air rats, and assessed the retinal avascular area 3 days later. We found no difference between vehicle and the drug-injected groups, indicating that these drugs have no effect on the normal development of the retinal vasculature and no gross retinal vascular toxicity. Additionally, there were no differences between the body weights of drug- versus vehicle-injected OIR rats in this study (data not shown). Although we propose an Angptl4-dependent mechanism, our normal vascular development data after pharmacologic manipulation contrast with data describing vascular development in the Angptl4−/− mouse.51 There are a number of possible explanations for this difference: (1) species differences between rat and mouse vascular development, (2) the difference caused by deleting a gene as opposed to reducing its activity pharmacologically, (3) differences in the bioavailability of the pharmacologic agents to different layers of the retina and vasculature, and (4) compensatory changes in response to life-long absence of a gene product as opposed to the temporary (3 days) reduction of its activity in this model.
The method of drug administration is a critical parameter that must be addressed. In our studies, GSK0660 was efficacious against retinal NV when administered by IVIT or IP injection. Intravitreal injection has the advantages of producing high levels of drug at active sites of neovascular disease, but deleterious side effects are associated with this route of drug administration, including endophthalmitis, cataractogenesis, and glaucoma.62–72 Systemic administration could avoid these side effects, but it is hampered by the need for repeated dosing to obtain target concentrations of active drug in diseased tissues. It also needlessly exposes disease-free organs and tissues to active drug. These factors must be carefully considered since PPAR-β/δ is ubiquitously expressed and has multiple beneficial physiologic functions. Given these considerations, optimization of drug delivery is of particular importance for future studies.
Notably, PPAR-β/δ regulates the transcription of numerous genes, and it is of critical importance that future studies precisely define the PPAR-β/δ–dependent components of NV in retinal tissue. This is necessary to develop more precisely targeted therapeutic strategies. New therapies for retinal NV remain desirable, because many patients receive limited benefit from the currently available VEGF-directed drugs, which, although widely successful, nonetheless have drawbacks (potential toxicity to neurons, elevated intraocular pressure, refractory responses, and so forth).73,74
This study is the first to demonstrate that PPAR-β/δ may play a key role in mediating retinal NV. Pharmacologic manipulation of PPAR-β/δ affects angiogenic endothelial cell behaviors that contribute to the retinal neovascular response. These findings are significant because they suggest that PPAR-β/δ may be a novel therapeutic target for the treatment of retinal neovascular disorders, such as ROP.
Acknowledgments
Supported by National Eye Institute/National Institutes of Health Grants EY007533 and EY007533-S27; the Carl Marshall Reeves and Mildred Almen Reeves Foundation, Inc.; and Research to Prevent Blindness.
Disclosure: M.E. Capozzi, None; G.W. McCollum, None; S.R. Savage, None; J.S. Penn, PanOptica, Inc. (F, C), Janssen (F, C, R), Alcon (F, C, R)
References
- 1. Klagsbrun M. Regulators of angiogenesis: stimulators, inhibitors, and extracellular matrix. J Cell Biochem. 1991; 47: 199–200 [DOI] [PubMed] [Google Scholar]
- 2. Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992; 267: 10931–10934 [PubMed] [Google Scholar]
- 3. Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996; 87: 1153–1155 [DOI] [PubMed] [Google Scholar]
- 4. Risau W. Mechanisms of angiogenesis. Nature. 1997; 386: 671–674 [DOI] [PubMed] [Google Scholar]
- 5. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003; 60: 107–114 [DOI] [PubMed] [Google Scholar]
- 6. Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost. 2001; 86: 23–33 [PubMed] [Google Scholar]
- 7. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1: 27–31 [DOI] [PubMed] [Google Scholar]
- 8. Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008; 27: 331–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bressler NM, Bressler SB. Preventative ophthalmology. Age-related macular degeneration. Ophthalmology. 1995; 102: 1206–1211 [DOI] [PubMed] [Google Scholar]
- 10. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998; 43: 245–269 [DOI] [PubMed] [Google Scholar]
- 11. Ashton N. Retinal vascularization in health and disease: Proctor Award Lecture of the Association for Research in Ophthalmology. Am J Ophthalmol. 1957; 44: 7–17 [DOI] [PubMed] [Google Scholar]
- 12. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999; 20: 649–688 [DOI] [PubMed] [Google Scholar]
- 13. Tan NS, Michalik L, Desvergne B, Wahli W. Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J Steroid Biochem Mol Biol. 2005; 93: 99–105 [DOI] [PubMed] [Google Scholar]
- 14. Bishop-Bailey D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol Ther. 2009; 124: 141–150 [DOI] [PubMed] [Google Scholar]
- 15. Adhikary T, Kaddatz K, Finkernagel F, et al. Genomewide analyses define different modes of transcriptional regulation by peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). PLoS One. 2011; 6: e16344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006; 86: 465–514 [DOI] [PubMed] [Google Scholar]
- 17. Kilgore KS, Billin AN. PPARbeta/delta ligands as modulators of the inflammatory response. Curr Opin Investig Drugs. 2008; 9: 463–469 [PubMed] [Google Scholar]
- 18. Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) in cell proliferation and cancer. Biochim Biophys Acta. 2009; 1796: 230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimaldi PA. Metabolic and nonmetabolic regulatory functions of peroxisome proliferator-activated receptor beta. Curr Opin Lipidol. 2010; 21: 186–191 [DOI] [PubMed] [Google Scholar]
- 20. Muoio DM, Way JM, Tanner CJ, et al. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes. 2002; 51: 901–909 [DOI] [PubMed] [Google Scholar]
- 21. Vidal-Puig AJ, Considine RV, Jimenez-Linan M, et al. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest. 1997; 99: 2416–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimaya A, Kurosaki E, Shioduka K, Nakano R, Shibasaki M, Shikama H. YM268 increases the glucose uptake, cell differentiation, and mRNA expression of glucose transporter in 3T3-L1 adipocytes. Horm Metab Res. 1998; 30: 543–548 [DOI] [PubMed] [Google Scholar]
- 23. Bishop-Bailey D, Swales KE. The role of PPARs in the endothelium: implications for cancer therapy. PPAR Res. 2008; 2008: 904251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-beta/delta in epithelial cell growth and differentiation. Cell Signal. 2006; 18: 9–20 [DOI] [PubMed] [Google Scholar]
- 25. Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor beta/delta (PPARbeta/delta) in gastrointestinal tract function and disease. Clin Sci (Lond). 2008; 115: 107–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Müller R, Körnhoff M, Peters JM, Müller-Brüsselbach S. A role for PPARβ/δ in tumor stroma and tumorigenesis. PPAR Res. 2008; 2008: 534294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piqueras L, Reynolds AR, Hodivala-Dilke KM, et al. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol. 2007; 27: 63–69 [DOI] [PubMed] [Google Scholar]
- 28. Müller-Brüsselbach S, Körnhoff M, Rieck M, et al. Deregulation of tumor angiogenesis and blockade of tumor growth in PPARbeta-deficient mice. EMBO J. 2007; 26: 3686–3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Penn JS, Tolman BL, Henry MM. Oxygen-induced retinopathy in the rat: relationship of retinal nonperfusion to subsequent neovascularization. Invest Ophthalmol Vis Sci. 1994; 35: 3429–3435 [PubMed] [Google Scholar]
- 30. Sznaidman ML, Haffner CD, Maloney PR, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)—synthesis and biological activity. Bioorg Med Chem Lett. 2003; 13: 1517–1521 [DOI] [PubMed] [Google Scholar]
- 31. Shearer BG, Steger DJ, Way JM, et al. Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocrinol. 2008; 22: 523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robciuc MR, Skrobuk P, Anisimov A, et al. Angiopoietin-like 4 mediates PPAR delta effect on lipoprotein lipase-dependent fatty acid uptake but not on beta-oxidation in myotubes. PLoS One. 2012; 7: e46212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shirotani M, Yui Y, Hattori R, Kawai C. U-61431F, a stable prostacyclin analogue, inhibits the proliferation of bovine vascular smooth muscle cells with little antiproliferative effect on endothelial cells. Prostaglandins. 1991; 41: 97–110 [DOI] [PubMed] [Google Scholar]
- 34. Stephen RL, Gustafsson MC, Jarvis M, et al. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004; 64: 3162–3170 [DOI] [PubMed] [Google Scholar]
- 35. Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2003; 44: 1722–1731 [DOI] [PubMed] [Google Scholar]
- 36. Pal M, Tan MJ, Huang RL, et al. Angiopoietin-like 4 regulates epidermal differentiation. PLoS One. 2011; 6: e25377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borland MG, Foreman JE, Girroir EE, et al. Ligand activation of peroxisome proliferator-activated receptor-beta/delta inhibits cell proliferation in human HaCaT keratinocytes. Mol Pharmacol. 2008; 74: 1429–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burdick AD, Bility MT, Girroir EE, et al. Ligand activation of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007; 19: 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARbeta/delta selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006; 13: 53–60 [DOI] [PubMed] [Google Scholar]
- 40. Auerbach R, Akhtar N, Lewis RL, Shinners BL. Angiogenesis assays: problems and pitfalls. Cancer Metastasis Rev. 2000; 19: 167–172 [DOI] [PubMed] [Google Scholar]
- 41. Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008; 295: E1056–E1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularization in the newborn rat. Invest Ophthalmol Vis Sci. 1993; 34: 576–585 [PubMed] [Google Scholar]
- 43. Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995; 36: 2063–2070 [PubMed] [Google Scholar]
- 44. Takata Y, Liu J, Yin F, et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008; 105: 4277–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quintela AM, Jimenez R, Gomez-Guzman M, et al. Activation of peroxisome proliferator-activated receptor-beta/-delta (PPARbeta/delta) prevents endothelial dysfunction in type 1 diabetic rats. Free Radic Biol Med. 2012; 53: 730–741 [DOI] [PubMed] [Google Scholar]
- 46. Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(–/–) mice. Atherosclerosis. 2005; 181: 29–37 [DOI] [PubMed] [Google Scholar]
- 47. Gaudel C, Schwartz C, Giordano C, Abumrad NA, Grimaldi PA. Pharmacological activation of PPARbeta promotes rapid and calcineurin-dependent fiber remodeling and angiogenesis in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008; 295: E297–E 304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wagner N, Jehl-Pietri C, Lopez P, et al. Peroxisome proliferator-activated receptor beta stimulation induces rapid cardiac growth and angiogenesis via direct activation of calcineurin. Cardiovasc Res. 2009; 83: 61–71 [DOI] [PubMed] [Google Scholar]
- 49. Robbins SG, Conaway JR, Ford BL, Roberto KA, Penn JS. Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors. 1997; 14: 229–241 [DOI] [PubMed] [Google Scholar]
- 50. Sanchez-Siles AA, Ishimura N, Rumi MA, et al. Administration of PPARbeta/delta agonist reduces copper-induced liver damage in mice: possible implications in clinical practice. J Clin Biochem Nutr. 2011; 49: 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perdiguero EG, Galaup A, Durand M, et al. Alteration of developmental and pathological retinal angiogenesis in angptl4-deficient mice. J Biol Chem. 2011; 286: 36841–36851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yokouchi H, Eto K, Nishimura W, et al. Angiopoietin-like protein 4 (ANGPTL4) is induced by high glucose in retinal pigment epithelial cells and exhibits potent angiogenic activity on retinal endothelial cells. [published online ahead of print February 7, 2013] Acta Ophthalmol. doi:10.1111/aos.12097 [DOI] [PubMed] [Google Scholar]
- 53. Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012; 12: 181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Di Paola R, Esposito E, Mazzon E, Paterniti I, Galuppo M, Cuzzocrea S. GW0742, a selective PPAR-beta/delta agonist, contributes to the resolution of inflammation after gut ischemia/reperfusion injury. J Leukoc Biol. 2010; 88: 291–301 [DOI] [PubMed] [Google Scholar]
- 55. Tan NS, Michalik L, Noy N, et al. Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev. 2001; 15: 3263–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang X, Kume S, Tanaka Y, et al. GW501516, a PPARdelta agonist, ameliorates tubulointerstitial inflammation in proteinuric kidney disease via inhibition of TAK1-NFkappaB pathway in mice. PLoS One. 2011; 6: e25271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Galuppo M, Di Paola R, Mazzon E, et al. GW0742, a high affinity PPAR-beta/delta agonist reduces lung inflammation induced by bleomycin instillation in mice. Int J Immunopathol Pharmacol. 2010; 23: 1033–1046 [DOI] [PubMed] [Google Scholar]
- 58. Wang N. PPAR-delta in vascular pathophysiology. PPAR Res. 2008; 2008: 164163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu KK. Peroxisome proliferator-activated receptors protect against apoptosis via 14-3-3. PPAR Res. 2010; 2010: 417646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hall MG, Quignodon L, Desvergne B. Peroxisome proliferator-activated receptor beta/delta in the brain: facts and hypothesis. PPAR Res. 2008; 2008: 780452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benetti E, Patel NS, Collino M. The role of PPARbeta/delta in the management of metabolic syndrome and its associated cardiovascular complications. Endocr Metab Immune Disord Drug Targets. 2011; 11: 273–284 [DOI] [PubMed] [Google Scholar]
- 62. Bonini-Filho MA, Jorge R, Barbosa JC, Calucci D, Cardillo JA, Costa RA. Intravitreal injection versus sub-Tenon's infusion of triamcinolone acetonide for refractory diabetic macular edema: a randomized clinical trial. Invest Ophthalmol Vis Sci. 2005; 46: 3845–3849 [DOI] [PubMed] [Google Scholar]
- 63. Chieh JJ, Roth DB, Liu M, et al. Intravitreal triamcinolone acetonide for diabetic macular edema. Retina. 2005; 25: 828–834 [DOI] [PubMed] [Google Scholar]
- 64. Jonas J, Heatley G, Spaide R, Varma R. Intravitreal triamcinolone acetonide and secondary ocular hypertension. J Glaucoma. 2005; 14: 168–171 [DOI] [PubMed] [Google Scholar]
- 65. Jonas JB, Martus P, Degenring RF, Kreissig I, Akkoyun I. Predictive factors for visual acuity after intravitreal triamcinolone treatment for diabetic macular edema. Arch Ophthalmol. 2005; 123: 1338–1343 [DOI] [PubMed] [Google Scholar]
- 66. Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002; 109: 920–927 [DOI] [PubMed] [Google Scholar]
- 67. Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004; 111: 218–224; discussion 224–225 [DOI] [PubMed] [Google Scholar]
- 68. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004; 111: 2044–2049 [DOI] [PubMed] [Google Scholar]
- 69. Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008; 28: 1395–1399 [DOI] [PubMed] [Google Scholar]
- 70. Jonas JB, Kamppeter BA, Harder B, Vossmerbaeumer U, Sauder G, Spandau UH. Intravitreal triamcinolone acetonide for diabetic macular edema: a prospective, randomized study. J Ocul Pharmacol Ther. 2006; 22: 200–207 [DOI] [PubMed] [Google Scholar]
- 71. Moshfeghi DM, Kaiser PK, Bakri SJ, et al. Presumed sterile endophthalmitis following intravitreal triamcinolone acetonide injection. Ophthalmic Surg Lasers Imaging. 2005; 36: 24–29 [PubMed] [Google Scholar]
- 72. Moshfeghi DM, Kaiser PK, Scott IU, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136: 791–796 [DOI] [PubMed] [Google Scholar]
- 73. Sang DN, D'Amore PA. Is blockade of vascular endothelial growth factor beneficial for all types of diabetic retinopathy? Diabetologia. 2008; 51: 1570–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wirostko B, Wong TY, Simo R. Vascular endothelial growth factor and diabetic complications. Prog Retin Eye Res. 2008; 27: 608–621 [DOI] [PubMed] [Google Scholar]




