Abstract
Background
We sought to determine the surgical treatment and functional outcome and identify the predictors of survival in a retrospective cohort of patients with spinal cord ependymoma using data collected from the Korea Spinal Oncology Research Group database.
Methods
The data regarding 88 patients who had been surgically treated for histologically confirmed spinal cord intramedullary and extramedullary ependymoma from January 1989 to December 2009 were retrospectively reviewed.
Results
Histopathological examination revealed myxopapillary ependymoma in 24 patients, ependymoma in 61 patients, and anaplastic ependymoma in 3 patients. Gross total removal was achieved in 72 patients, subtotal removal in 15 patients, and partial removal in 1 patient. Twenty patients were treated with postoperative radiation. Fifty-two patients had stable or improved postoperative neurological function, while 36 experienced neurological deterioration. A permanent decrease in McCormick classification grade was seen in 17 patients. The progression-free survival rate was 87% for all patients at 5 years and 80% at 10 years. During follow-up, local recurrence/progression was seen in 13 patients. Diffuse meningeal spread developed in 2 anaplastic ependymoma patients. Postoperative radiotherapy after incomplete resection did not significantly correlate with longer times to recurrence. Multivariate analysis revealed histology and surgical extent of resection as independent predictors of longer progression-free survival.
Conclusions
Gross total removal alone is a good treatment strategy for spinal ependymomas. Early diagnosis and surgery, before severe paralysis, are important to obtain good functional outcomes. Subtotal resection with radiation therapy for intramedullary lesions appears to offer no advantages over gross total removal.
Keywords: myxopapillary ependymoma, radiation, spinal ependymoma, survival
In the spinal cord, ependymomas are the most common neuroepithelial tumors, accounting for 50%–60% of adult spinal cord tumors. Ependymomas are unencapsulated lesions but are usually well circumscribed with smooth, regular margins.1 The ultimate goal in treatment of spinal cord ependymomas is progression-free survival (PFS) with a good functional outcome.2 Most patients with spinal cord ependymomas are good candidates for surgery, particularly those who show initially mild or moderate neurological deficits.3–5 However, the role of adjuvant radiotherapy and chemotherapy is still debatable.2,6–9
Previously reported adverse prognosis factors for spinal cord ependymomas include age, poor performance status/neurological function, higher grade, less extensive resection, and subtherapeutic radiation dose.1,4,7,10 However, many spinal cord ependymoma series reported in the literature have a relatively small number of patients; there are also differences in patient populations among different studies, and sometimes a very long recruitment period. These factors complicate the interpretation of previous findings.
The Korea Spinal Oncology Research Group (KSORG) was founded to promote clinical research on the management of spinal tumors and develop educational programs for improving neuro-oncological care. The KSORG database allows a descriptive analysis of patients with spinal cord tumors. The aim of this study was to determine functional outcome and survival predictors in a retrospective cohort of spinal cord ependymomas based on the KSORG database.
Materials and Methods
Patient Population
The KSORG was established in 2009 by spine surgeons from the Seoul National University Hospital (SNUH), Seoul National University Bundang Hospital (SNUBH), Samsung Medical Center (SMC), and several other affiliated institutions. The principal goal of the KSORG was to evaluate the surgical treatment of spinal neoplasms in a prospective/retrospective multicenter clinical series. The KSORG database includes 442 cases of spinal cord tumor, including 176 intramedullary spinal cord tumors and 266 extramedullary spinal cord tumors, spanning January 1989 to December 2009. Of these patients, 103 with histologically confirmed spinal cord intramedullary and extramedullary ependymomas were identified at SNUH, SNUBH, and SMC and retrospectively reviewed. Children younger than 18 years were excluded from the study, as were adults with spinal cord ependymomas who had received previous surgery or radiation therapy. This resulted in a final study population of 88 patients. The records of all patients were retrieved and demographic data were collected, including age, sex, affected period, and surgical outcome. Relevant clinical data were obtained through a review of the patients' charts and operative reports. Telephonic interviews were conducted as necessary. The extent of surgical resection, use of any adjuvant therapy, length of follow-up, evidence of recurrence, and complications were also noted. The length of follow-up was defined as the period from the date of surgery to the patient's most recent clinic visit.
Histopathological grading of all tumors was performed according to World Health Organization (WHO) criteria. The study was independently reviewed and approved by the institutional review boards at all participating institutions.
Surgery and Adjuvant Therapy
In our study, surgery was the initial treatment; 4 senior surgeons (C.K.C., E-S.K., W.E., and H-J.K.) carried out the surgery in all patients by using standard dorsal approaches, as indicated by the location of the lesion. Our surgical techniques did not differ from those previously published in the literature. All patients underwent posterior approach surgery and tumor resection using a surgical microscope and intraoperative spinal cord monitoring. Piecemeal resections were performed, given the difficulty in removing these tumors in an en bloc fashion. The extent of surgery was determined from surgical reports and postoperative imaging studies. The extent of surgery was classified as follows: (i) gross total removal (GTR), (ii) subtotal removal (STR), or (iii) partial removal.
GTR was defined if the surgeon had described a complete removal of tumor or if there was no evidence of tumor from postoperative MRI. If a small piece of tumor was left in place by the surgeon's impression or obvious retained fragment in postoperative MRI (80%–99% resection), the procedure was considered to be an STR, based on documented intraoperative ultrasonography of 80%–99%. In the same manner, <80% resection was defined as a partial resection. Mere open biopsy was not performed for histological confirmation in our study.
Postoperative radiotherapy was given to patients with a diagnosis of anaplastic tumor or residual/recurred tumor and to some patients in whom GTR was performed. All patients were treated with megavoltage photon beams. Treatment was given to the tumor volume based on the imaging results and operative findings. The radiotherapy volume was the gross tumor plus 1.5–2 cm margin above and below the tumor bed based on the imaging result and the treating physician's preferences. The total tumor dose ranged from 45 to 50 Gy. All patients received 1.5 or 2 Gy per fraction per day.
Additional chemotherapy including etoposide and carboplatin was also considered for patients with tumor recurrence/progression who were unsuitable for surgical retreatment.
Assessment of Clinical and Radiological Outcome
We applied a modified McCormick classification (MCC) (Table 1) for the assessment of neurological function in our patients.11 This assessment was performed before surgery, at discharge, at 1 and 6 months after surgery, and annually thereafter. MRI was conducted in all patients before surgery. MRI findings were assessed in detail, including location (the cord, conus, and/or cauda) and involved level. Neurological deterioration after surgery was evaluated in association with the intraoperative findings of the tumor, as well as with the preoperative MRI and histological findings. Tumor recurrence and progression were defined as clinical and/or radiographical progression on follow-up MRI. Progression was defined as tumor regrowth on follow-up imaging studies after an incomplete resection; these studies were either done as part of routine postoperative assessment or initiated by a change in the patient's clinical status. Tumor regrowth following complete resection was considered as recurrence.
Table 1.
Modified McCormick classification (MCC) grade for spinal cord tumors
| MCC Grade | Definition |
|---|---|
| 1 | Neurologically normal |
| Gait normal | |
| Normal professional activity | |
| 1b | Tired after walking several kilometers |
| Running is impossible, or moderate sensorimotor deficit does not significantly affect the involved limb | |
| Moderate discomfort in professional activity | |
| 2 | Presence of sensorimotor deficit affecting function of involved limb |
| Mild to moderate gait difficulty | |
| Severe pain or dysesthetic syndrome impairs quality of life | |
| Independent function and ambulation maintained | |
| 3 | More severe neurological deficit |
| Requires cane and/or brace for ambulation or maintains significant bilateral upper-extremity impairment | |
| May or may not function independently | |
| 4 | Severe neurological deficit |
| Requires wheelchair or cane and/or brace with bilateral upper-extremity impairment | |
| Usually not independent |
Statistical Analysis
For comparison of 2 groups, Student's t-test was used for continuous and parametric values, and a chi-square test and Fisher's exact test were used for categorical dates and values, respectively. PFS was estimated using the Kaplan–Meier technique. Log-rank tests and Cox proportional hazards models were performed to determine factor-influenced progression. Statistical analysis was supported by commercially available software (SPSS version 20.0). P < .05 was regarded as statistically significant.
Results
Patient Demographics
Table 2 summarizes the patient characteristics. Of the 88 patients, 60 were men, with age at surgery ranging from 18 to 71 years (median, 40.2 y). Histopathological examination revealed myxopapillary ependymoma (MP; WHO grade I) in 24 patients, ependymoma (EP; WHO grade II) in 61 patients, and anaplastic ependymoma (AE; WHO grade III) in 3 patients. Most of the cases of EP/AE involved the cervical cord (75%), and all but 1 MP (96%) involved the thoracolumbar junction and lumbosacral area. There were 59 patients with exclusively intramedullary growth (58 EP/AEs and 1 MP). Completely extramedullary growth was seen in 17 patients (1 EP and 16 MPs), and combined extramedullary/intramedullary growth was observed in 12 patients (5 EPs and 7 MPs). The most common symptom was pain, which occurred in 78 of 88 patients (97%), followed by numbness/sensory change in 65 patients (74%), motor weakness in 40 patients (45%), and bowel/bladder symptoms in 22 patients. Symptoms were present for a mean of 22.2 months before diagnosis (>1 y in 62% of patients). The follow-up period ranged from 2 to 22 years (median, 72.9 ± 40.1 mo).
Table 2.
Demographic data and treatment characteristics in 88 patients with spinal cord ependymoma
| Parameter | No. of Patients |
||
|---|---|---|---|
| Ependymoma (n = 64) | Myxopapillary (n = 24) | Total (N = 88) | |
| Age, y | 41.55 ± 12.61 | 36.38 ± 13.98 | |
| Sex | |||
| male:female | 46:18 | 14:10 | 60:28 |
| Involved level | |||
| Cervical | 48 | 0 | 48 |
| Thoracic | 10 | 1 | 11 |
| Thoracolumbar | 5 | 11 | 16 |
| Lumbosacral | 1 | 12 | 13 |
| Location | |||
| Intramedullary | 58 | 1 | 59 |
| Conus medullaris | 5 | 7 | 12 |
| Cauda equina | 1 | 16 | 17 |
| Symptom | |||
| Pain | 54 | 24 | 78 |
| Sensory change | 47 | 18 | 65 |
| Motor weakness | 34 | 6 | 40 |
| Bladder/bowel | 13 | 9 | 22 |
| Duration of symptoms, mo | 19.9 | 29 | 23.1 |
Primary Treatment and Perioperative Complications
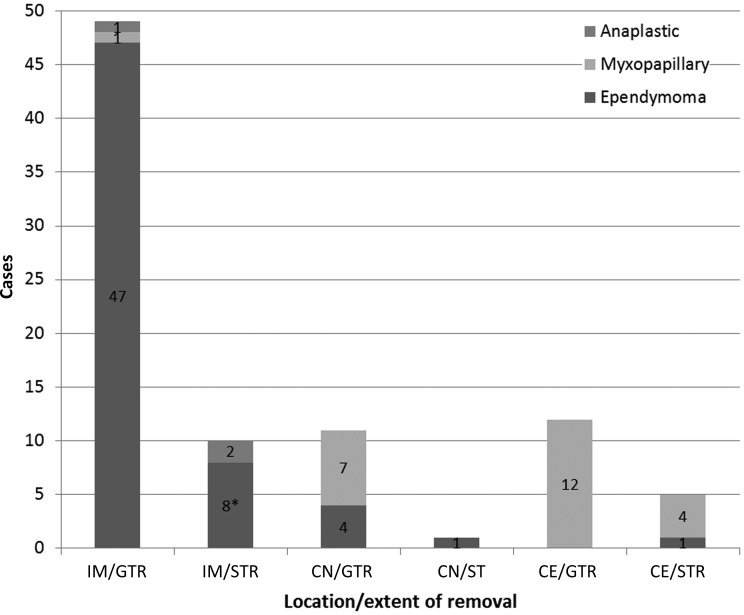
Outcomes and complications of treatment for the 88 patients are presented in Table 3. Surgery was performed only once in 84 patients, twice in 2 patients, and thrice in 2 patients. Overall, GTR was achieved in 72 patients (81.8%), STR in 15 patients (14.8%), and partial removal in 1 patient (1.3%). In the 59 cases of intramedullary lesion, GTR was achieved in 49 cases; STR or partial removal was preferred in 10 patients to avoid induction of new neurological deficits, since the cleavage plane was hard to define. Of the 29 cases of extramedullary lesion, the tumor was completely encapsulated with absence of connections to the cauda equina and/or conus medullaris in 23 cases, and GTR was achieved; STR was performed because of the involvement of the nerve root and conus medullaris in 6 cases (Fig. 1). Of the 88 patients who underwent surgery, 20 (22.7%) were treated with postoperative radiotherapy (10 patients with EP, 3 with AE, and 7 with MP). Chemotherapy was also administered to 1 patient with diffuse meningeal seeding.
Table 3.
Surgery and adjuvant treatment for 88 patients with ependymoma
| Parameter | No. of Patients |
||
|---|---|---|---|
| Ependymoma (n = 64) | Myxopapillary (n = 24) | Total | |
| Type of surgery | |||
| GTR | 52 (EP: 51, AE: 1) | 20 | 72 |
| STR | 11 (EP: 9, AE: 2) | 4 | 15 |
| Partial | 1 (EP: 1) | 0 | 1 |
| Adjuvant therapy | |||
| Radiotherapy | 13 (AE: 3) | 7 | 20 |
| Chemotherapy | 1 (AE: 1) | 0 | 1 |
| Complication | |||
| Motor weakness | 6 | 0 | 6 |
| Bladder/bowel | 3 | 5 | 8 |
| CSF leakage | 2 | 1 | 3 |
| Wound problem | 5 | 2 | 7 |
| Postop kyphosis | 3 | 0 | 3 |
Fig. 1.
The extent of surgical removal in 88 cases according to histology and location. * = 1 patient who had undergone partial resection. Abbreviations: IM, intramedullary; CN, conus medullary; CE, cauda equina.
The postsurgical mortality rate within 30 days was 0%. However, 2 AE patients died 12 and 22 months after surgery, respectively. In terms of postoperative complications, 3 patients had a cerebrospinal fluid leak within 30 days after surgery; these patients were treated successfully with CSF-leak closure surgery and/or lumbar drainage. Seven patients developed wound problems and were treated successfully with antibiotics. Three patients developed postoperative kyphosis.
Functional Outcome During Follow-up
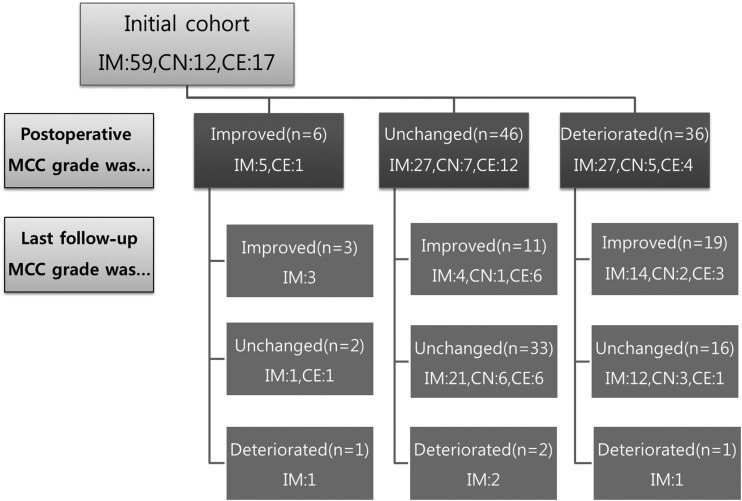
An overview of the functional status of the patients before and after surgery and at last follow-up is shown in Fig. 2. Of the 88 patients, 6 (as assessed by MCC) had improved neurological function after surgery. Forty-six had unchanged neurological function, and 36 experienced a deterioration of neurological function postoperatively. Of the 36 patients who experienced deterioration of neurological function, 14 experienced paralyses and/or bladder/bowel dysfunction; 20 experienced proprioceptive deficits; and 11 experienced dysesthesia and/or paraesthesia. Seventeen of the 36 patients had recovered by more than 1 MCC grade during the follow-up period. During the follow-up period, we encountered only 3 cases of late deterioration. In 2 cases, the cause was recurrence of AE. In the third case, neurological deterioration was caused by the progression of a residual tumor.
Fig. 2.
Functional status of 88 patients with spinal cord tumor before and after surgery and at final follow-up. Abbreviations: IM, intramedullary; CN, conus medullary; CE, cauda equina.
Patients with good preoperative MCC (grade 1 vs all other grades) tended to maintain functional status postoperatively and during follow-up (Tables 4 and 5). The last functional outcome in cases of EP was significantly favorable if the preoperative neurological symptom was mild (P = .000). On the other hand, functional outcome in cases of MP did not reach statistical significance (P = .061).
Table 4.
Summary of outcomes at the last assessment in 61 patients with ependymoma
| Group | Postop Modified MCC Grade |
Follow-up Modified MCC Grade |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1b | 2 | 3 | 4 | 1 | 1b | 2 | 3 | 4 | |
| Preop modified MCC grade | ||||||||||
| 1 (n = 22) | 9 | 10 | 3 | 15 | 7 | |||||
| 1b (n = 22) | 10 | 9 | 3 | 2 | 13 | 4 | 3 | |||
| 2 (n = 10) | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | |
| 3 (n = 4) | 2 | 1 | 1 | 2 | 1 | 1 | ||||
| 4 (n = 3) | 3 | 3 | ||||||||
| Total | 9 | 22 | 17 | 6 | 7 | 19 | 24 | 7 | 6 | 5 |
Table 5.
Summary of outcomes at the last assessment in 24 patients with myxopapillary ependymoma
| Group | Postop Modified MCC Grade |
Follow-up Modified MCC Grade |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1b | 2 | 3 | 4 | 1 | 1b | 2 | 3 | 4 | |
| Preop modified MCC grade | ||||||||||
| 1 (n = 7) | 4 | 1 | 2 | 5 | 2 | |||||
| 1b (n = 11) | 1 | 8 | 2 | 4 | 5 | 2 | ||||
| 2 (n = 3) | 3 | 1 | 1 | 1 | ||||||
| 3 (n = 2) | 1 | 1 | 1 | 1 | ||||||
| 4 (n = 1) | 1 | 1 | ||||||||
| Total | 5 | 9 | 8 | 1 | 1 | 9 | 9 | 4 | 1 | 1 |
Tumor Recurrence and Progression
Overall, out of 88 patients, 15 (17%) presented with recurrent or progressive disease. Local recurrence/progression was seen in 13 of these 15 patients (71%). Diffuse meningeal spread developed in 2 AE patients. No distant recurrences (2 spinal levels below or above the primary tumor site) were observed in our series. The 13 patients with local recurrence included 2 out of 51 EP patients with GTR who experienced recurrence; tumor progression was seen in 7 out of 10 cases of incomplete resection. Two out of 20 MP cases with GTR experienced recurrence, whereas tumor progression was seen in 2 of 4 MP patients after incomplete resection.
Ten recurrences were seen in 59 patients with intramedullary lesion (17.0%), 2 recurrences in 12 patients with combined extramedullary/intramedullary growth (16.7%), and 3 recurrences in 17 patients with extramedullary lesion (17.7%). In 68 patients initially treated with surgery only, there were 6 patients with recurrent or progressive disease. Nine of 20 patients initially treated with surgery and radiation developed tumor recurrence/progression. Tumor recurrence/progression resulted in a decrease of MCC in 3 of 15 patients.
Prognosis-Related Factors
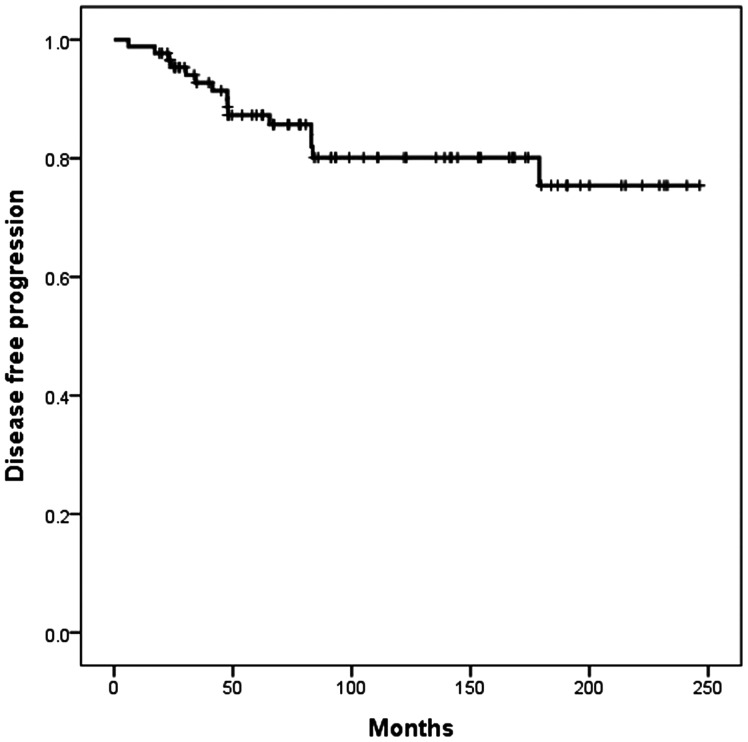
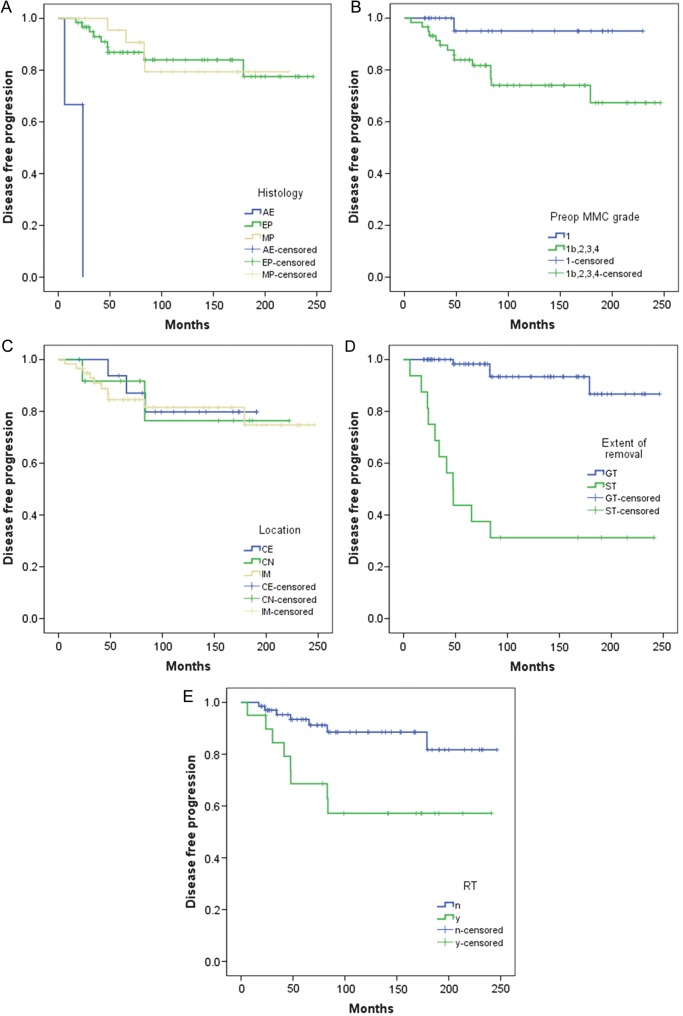
For all patients, PFS rates were 87% at 5 years and 80% at 10 years (Fig. 3). On univariate analysis, a comparison of PFS among different histological types of tumor showed a difference in the AE group (P = .000; Fig. 4A). There was significant difference in PFS between preoperative MCC grade 1 and all other grades (P = .039; Fig. 4B). However, there was no significant difference in PFS to compare with the locations of tumor among intramedullary, conus, and cauda equina (P = .975; Fig. 4C). The extent of removal significantly affected the PFS rate (P = .000; Fig. 4D). The use of radiation as adjuvant therapy after incomplete resection did not correlate with longer times to recurrence. On the contrary, the mean PFS time to recurrence for those who received radiotherapy was 157 months, compared with 219 months for those who did not receive radiotherapy (P = .006; Fig. 4E).
Fig. 3.
PFS of 88 patients with spinal cord tumor.
Fig. 4.
(A) PFS of 88 patients with spinal cord tumor according to EP histology (log-rank test; P = .000). (B) PFS of 88 patients with spinal cord tumor according to preoperative MCC (log-rank test; P = .039). (C) PFS of 88 patients with spinal cord tumor according to location of tumor (log-rank test; P = .975). IM, intramedullary; CN, conus medullary; CE, cauda equina. (D) PFS of 88 patients with spinal cord tumor according to extent of tumor removal (log-rank test; P = .000). (E) PFS of 88 patients with spinal cord tumor according to initial treatment (surgery alone vs surgery and adjuvant radiotherapy [RT], log-rank test; P = .006). Abbreviations: n, no (RT was not done); Y, yes (RT was done).
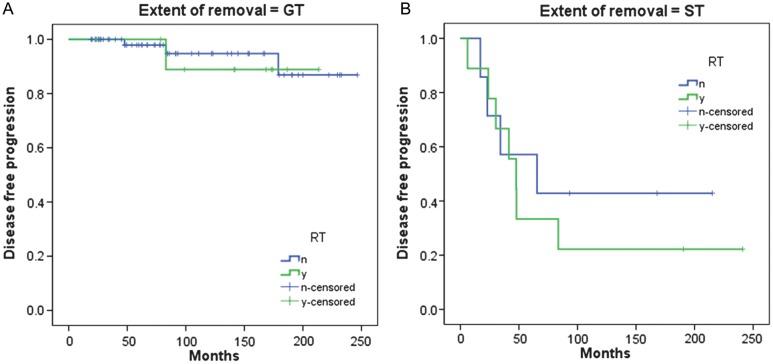
Regarding the extent of removal, the PFS rates in patients who had GTR with or without adjuvant radiotherapy were 89% and 95%, respectively, at 10 years (P = .771; Fig. 5A). The PFS rates for patients who underwent STR with or without adjuvant radiotherapy were 22% and 43%, respectively, at 10 years (P = .541; Fig. 5B). According to a multivariate Cox regression analysis of variance including histology, preoperative MCC grade, the use of radiotherapy, and degree of resection, histology and extent of resection were independent predictors of shorter PFS; we did not identify any other predictive factors (Table 6).
Fig. 5.
(A) PFS of 72 patients who had undergone GTR according to initial treatment (surgery alone vs surgery and adjuvant radiotherapy [RT], P = .771). (B) PFS of 16 patients who had undergone STR according to initial treatment (surgery alone vs surgery and adjuvant RT, P = .541). Abbreviations: n, no (RT was not done); Y, yes (RT was done).
Table 6.
Multivariate analysis for factors that influenced PFS
| Factor | No. of Patients (N= 88) | Analysis |
||
|---|---|---|---|---|
| HR | 95% CI | P | ||
| Preoperative MMC grade | 6.39 | 0.84–48.62 | .145 | |
| 1 | 29 | |||
| 1b, 2, 3, 4 | 59 | |||
| Histology | 14.80 | 1.86–117.69 | .011 | |
| EP, MP | 85 | |||
| AE | 3 | |||
| Extent of removal | 13.88 | 3.92–49.20 | .000 | |
| GTR | 72 | |||
| STR | 16 | |||
| Radiation | 0.75 | 0.22–2.51 | .638 | |
| No | 68 | |||
| Yes | 20 | |||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Discussion
Role of Surgical Management
Although it is widely accepted that the standard treatment for spinal cord EPs consists of surgical resection, with the goal being GTR, the general consensus has been to treat patients with conservative STR rather than with aggressive GTR, especially if neurological compromise is a possibility.5,12 Nevertheless, GTR has been associated with improved prognosis for EPs.3,13,14 With the introduction of microsurgical technique and the use of monitoring techniques such as somatosensory evoked potential and motor evoked potential, the removal of intramedullary spinal cord tumors has become associated with even lower morbidity and mortality rates, making GTR the primary goal in the treatment of spinal cord EPs.3,5,15 The present is the largest series incorporating contemporary microsurgical techniques and MRI. In our series, 80% of tumors underwent GTR. The rate of GTR in other series varies considerably from 50% to 92%.16
Functional outcome after surgery was good in most of our patients. Fifty-two of 88 patients (70.5%) had stable or improved MCC grades directly after surgery. Thirty-six patients in this study (29.5%) experienced acute neurological decline after surgery, but nearly half of these patients returned to baseline within 1 month of surgery. The neurological decline found in this study is similar to that reported in other large EP series.1,15,17,18 Patients who had only minor deficits at presentation had a significantly better chance of having only minor deficits at long-term follow-up, whereas patients with major deficits at presentation rarely made a meaningful recovery. Therefore, it is important to use a high index of suspicion when evaluating a patient for neurological complaints so that lesions can be found and resected before major neurological deficits develop.1
Role of Radiotherapy
Although radiation has been used as adjuvant therapy after GTR and STR of intramedullary EPs, the role of adjuvant radiotherapy after spinal EP surgery remains unclear.6,7,8,18 Some studies advocate adjuvant radiotherapy in cases of GTR and STR alike. Most authors, however, agree that radiotherapy is unnecessary if complete removal has been accomplished and recommend adjuvant radiotherapy only for patients treated with STR. In addition, patients with AEs or severe metastatic disease may also be good candidates for adjuvant radiotherapy. In a retrospective cohort of 26 patients, Gavin Quigley and colleagues2 could not demonstrate a statistical difference in PFS in the portion of their cohort who underwent STR with radiotherapy. This was despite excellent results regarding improved functional outcome, which compared favorably with patients in whom GTR was achieved (79% vs 75%, respectively).
The necessity for radiotherapy is closely related to the extent of tumor resection. However, no definitive study has shown a benefit of radiation over clinical follow-up and reoperation for residual tumor. In our series, 20 patients, including 4 who had total tumor removal, received postoperative radiation. Our data did not support a significant reduction in tumor recurrence/progression following postoperative radiation. In recent times, the probability of complete removal has increased because of improved surgical techniques, and reoperation has been recommended for regrowth of a residual tumor.1 Radiation therapy is reserved for cases of residual tumor even after reoperation. When technical reasons prevent complete excision, or in cases with metastatic spread, radiotherapy is advocated.6,7 However, radiotherapy may cause reactive gliosis and fibrosis, making reoperation for recurrence technically difficult, and long-term radiation myelopathy can lead to severe neurological dysfunction.19
Survival and Prognostic Factors
The degree of surgical resection has been shown to significantly alter survival in spinal EP. In our study, consistent with previous literature, the survival rate of patients following complete excision was statistically better than that of patients after incomplete resection and radiation therapy. The 5-year PFS rate of spinal EPs has been reported in the literature to be between 57% and 90%. In our study, the 5-year PFS rate was 87%, and the 10-year PFS rate was 80%. However, progression rates after partial removal or STR range from 20% to 50% at 5 years. Our results also showed that the use of postoperative adjuvant radiotherapy is not independently associated with survival in the setting of other clinical variables, as suggested by previous authors. This may be partially because of selection bias, caused by more aggressive tumors receiving radiotherapy while those with a more benign course did not generally receive radiotherapy; patients did not routinely receive radiotherapy unless there was evidence of significant residual disease.13
Chang and colleagues6 reported that patients with GTR have a significantly longer PFS than those who undergo incomplete excision and adjuvant radiotherapy. Gomez et al.14 reviewed the records of 37 patients who had spinal cord EP, 7 of whom had MP. These investigators reported that only the extent of surgery was significantly correlated with PFS (P = .006) and that radiotherapy was not correlated with PFS (P = .75). On the other hand, some reports did not find that the extent of initial resection significantly affected either PFS or overall survival for EP patients.18
Our study could not shed light on the prognostic significance of location or tumor grade except for AE. Despite significant poor prognosis in AE, the small number of patients with high-grade EPs limited the power to detect the effect of tumor grade on prognosis. Similarly, neither MP nor cellular histological subtypes predicted clinical outcome. Published studies also debate the prognostic significance of tumor grade and histology in spinal EPs.14 However, there appears to be a consensus in the published literature that early diagnosis and treatment as well as good preoperative functional status are important factors for producing good long-term, postoperative functional results.6,17,20
Study Limitations
Our study is limited by its retrospective design. Postoperative MRI was not in routine use during the first part of the study period, and resection grade was not clearly established. In addition, EP is a low-grade tumor with slow progression. Although most patients in our study had a long follow-up period, there were some patients with a relatively short follow-up.
Conclusion
In cases of spinal ependymoma, early diagnosis and surgery, before severe paralysis, are important to obtain good functional outcomes. Although patients may be worse during the initial postoperative phase, they often recover. Patients with a good neurological status at presentation have the best chance for a good neurological outcome after treatment. GTR alone is a good treatment strategy for spinal EPs, even for recurred tumors. STR with radiation therapy for intramedullary lesions appears to offer no advantages over GTR; therefore, radiation therapy should be reserved for anaplastic-type tumors or residual mass after reoperation.
Funding
This work was not supported by any grant or research funding.
Conflict of interest statement. None declared.
References
- 1.Kucia EJ, Bambakidis NC, Chang SW, et al. Surgical technique and outcomes in the treatment of spinal cord ependymomas, part 1: intramedullary ependymomas. Neurosurgery. 2011;68(1 Suppl Operative):57–63. doi: 10.1227/NEU.0b013e318208f181. [DOI] [PubMed] [Google Scholar]
- 2.Gavin Quigley D, Farooqi N, Pigott TJD, et al. Outcome predictors in the management of spinal cord ependymoma. Eur Spine J. 2007;16(3):399–404. doi: 10.1007/s00586-006-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boström A, von Lehe M, Hartmann W, et al. Surgery for spinal cord ependymomas: outcome and prognostic factors. Neurosurgery. 2011;68(2):302–308. doi: 10.1227/NEU.0b013e3182004c1e. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz TH, McCormick PC. Intramedullary ependymomas: clinical presentation, surgical treatment strategies and prognosis. J Neurooncol. 2000;47(3):211–218. doi: 10.1023/a:1006414405305. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Wang Z, Li Z, Liu B. Microsurgical treatment and functional outcomes of multi-segment intramedullary spinal cord tumors. J Clin Neurosci. 2009;16(5):666–671. doi: 10.1016/j.jocn.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Chang UK, Choe WJ, Chung SK, et al. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57:133–139. doi: 10.1023/a:1015789009058. [DOI] [PubMed] [Google Scholar]
- 7.Wahab SH, Simpson JR, Michalski JM, et al. Long term outcome with post-operative radiation therapy for spinal canal ependymoma. J Neurosurg. 2007;83:85–89. doi: 10.1007/s11060-006-9310-2. [DOI] [PubMed] [Google Scholar]
- 8.Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005;71:205–210. doi: 10.1007/s11060-004-1386-y. [DOI] [PubMed] [Google Scholar]
- 9.Lee TT, Gromelski EB, Green BA. Surgical treatment of spinal ependymoma and post-operative radiotherapy. J Neurosurg. 1998;4:309–313. doi: 10.1007/s007010050103. [DOI] [PubMed] [Google Scholar]
- 10.Milano MT, Johnson MD, Sul J, et al. Primary spinal cord glioma: a Surveillance, Epidemiology, and End Results database study. J Neurooncol. 2010;98:83–92. doi: 10.1007/s11060-009-0054-7. [DOI] [PubMed] [Google Scholar]
- 11.McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72(4):523–532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 12.Halvorsen CM, Kolstad F, Hald J, et al. Long-term outcome after resection of intraspinal ependymomas: report of 86 consecutive cases. Neurosurgery. 2010;67(6):1622–1631. doi: 10.1227/NEU.0b013e3181f96d41. [DOI] [PubMed] [Google Scholar]
- 13.Bagley CA, Wilson S, Kothbauer KF, et al. Long term outcomes following surgical resection of myxopapillary ependymomas. Neurosurg Rev. 2009;32(3):321–334. doi: 10.1007/s10143-009-0190-8. [DOI] [PubMed] [Google Scholar]
- 14.Gomez DR, Missett BT, Wara WM, et al. High failure rate in spinal ependymomas with long-term follow-up. Neuro Oncol. 2005;7:254–259. doi: 10.1215/S1152851704001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagasawa DT, Smith ZA, Cremer N, et al. Complications associated with the treatment for spinal ependymomas. Neurosurg Focus. 2011;31(4):E13. doi: 10.3171/2011.7.FOCUS11158. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura M, Ishii K, Watanabe K, et al. Surgical treatment of intramedullary spinal cord tumors: prognosis and complications. Spinal Cord. 2008;46(4):282–286. doi: 10.1038/sj.sc.3102130. [DOI] [PubMed] [Google Scholar]
- 17.Aghakhani N, David P, Lacroix C. Intramedullary spinal ependymomas: analysis with particular attention to patients with no preoperative neurological deficit. Neurosurgery. 2008;62(6):1279–1285. doi: 10.1227/01.neu.0000333299.26566.15. [DOI] [PubMed] [Google Scholar]
- 18.Akyurek S, Chang EL, Yu T-K, et al. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncol. 2006;80(2):177–183. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 19.Hanbali F, Fourney DR, Marmor E, et al. Spinal cord ependymoma: radical surgical resection and outcome. Neurosurgery. 2002;51(5):1162–1174. doi: 10.1097/00006123-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Harrop JS, Ganju A, Groff M, Bilsky M. Primary intramedullary tumors of the spinal cord. Spine. 2009;34(22 Suppl):S69–S77. doi: 10.1097/BRS.0b013e3181b95c6f. [DOI] [PubMed] [Google Scholar]