Abstract
Background
Among patients with glioblastoma (GBM) who progress on standard temozolomide, the optimal therapy is unknown. Resistance to temozolomide is partially mediated by O6-methylguanine-DNA methyltransferase (MGMT). Because MGMT may be depleted by prolonged temozolomide administration, dose-intense schedules may overcome resistance.
Methods
This was a multicenter, phase 2, single-arm study of temozolomide (75–100 mg/m2/day) for 21 days of each 28-day cycle. Patients had GBM in first recurrence after standard therapy. The primary end point was 6-month progression-free survival (PFS6).
Results
Fifty-eight participants were accrued, 3 of whom were ineligible for analysis; one withdrew before response assessment. There were 33 men (61%), with a median age of 57 years (range, 25–79 years) and a median Karnofsky performance score of 90 (range, 60–100). Of 47 patients with MGMT methylation results, 36 (65%) had methylated tumors. There were 7 (13%) partial responses, and PFS6 was only 11%. Response and PFS did not depend on MGMT status; MSH2, MLH1, or ERCC1 expression; the number of prior temozolomide cycles; or the time off temozolomide. Treatment was well tolerated, with limited grade 3 neutropenia (n = 2) or thrombocytopenia (n = 2).
Conclusions
Dose-intense temozolomide on this schedule is safe in recurrent GBM. However, efficacy is marginal and predictive biomarkers are needed.
Keywords: dose-intense temozolomide, MGMT promoter methylation, recurrent glioblastoma
Patients with glioblastoma (GBM) have a poor prognosis, with a median survival of approximately 15 months.1,2 After standard therapy, which includes surgical resection, radiotherapy, and concurrent and adjuvant temozolomide (TMZ), tumors invariably recur. Treatment options for patients with recurrent GBM are limited, consisting mainly of cytotoxic chemotherapy regimens with marginal efficacy and substantial toxicity3 and anti-angiogenic therapy with bevacizumab.4,5 The standard 5-day TMZ regimen is well-tolerated in patients with recurrent GBM and achieved a response rate of 19% and 6-month progression-free survival (PFS6) of 21% in the era before TMZ was used in first-line therapy.6
Resistance to TMZ is mediated in part by O6-methylguanine-DNA methyltransferase (MGMT), a DNA repair enzyme in which activity can be curtailed by promoter methylation.7 Alternative TMZ dosing schedules that provide prolonged exposure and higher cumulative doses than the standard 5-day regimen may deplete MGMT and overcome resistance.8 A variety of regimens have been evaluated in recurrent GBM, including 7 days on/7 days off at 150 mg/m2/day (7/7 regimen),9 21 days on/7 days off at 75 mg/m2/day (21/7 regimen),10 50 mg/m2/day continuously,11 and one dose at 200 mg/m2 followed 12 h later by 9 doses of 90–100 mg/m2 every 12 h.12 In the phase 2 RESCUE study, 91 patients with recurrent GBM after at least 3 cycles of standard TMZ received TMZ 50 mg/m2/day continuously,11 a regimen that intensifies dose from 750–1000 mg/m2 per 28-day cycle to 1400 mg/m2 per 28-day cycle. PFS6 was 23.9%, which is better than the 15% reported for historical controls.13 The regimen appeared to be particularly effective in patients whose tumors recurred at least 2 months after completion of standard TMZ (PFS6, 35.7%) or during the first 3–6 cycles of standard TMZ (PFS6, 27.3%). Patients whose tumors recurred after 6 cycles but before completing standard TMZ had the least favorable outcomes (PFS6, 7.4%). The 21/7 regimen was more recently tested in 833 patients with newly diagnosed GBM who were randomly assigned to receive standard adjuvant TMZ or the 21/7 regimen after radiotherapy.14 Median overall survival (OS) and PFS were similar in both groups, even when stratified by MGMT methylation status.
MGMT is only one potential mechanism of resistance to alkylating agents. The mismatch repair (MMR) system comprises a group of proteins (mutL homolog 1 [MLH1], PMS2, mutS homolog 2 [MSH2], MSH3, and MSH6) involved in correcting errors in DNA base pairing that may arise during replication. Similar to increased MGMT activity, MMR deficiency may cause resistance to TMZ.15 Mutations in the genes that encode MLH1 and MSH2 explain the limited cytotoxicity of TMZ in colon cancer and other cell lines.16 In addition, MMR deficiency may result from MLH1 promoter methylation, which is present in nearly half of colon cancer specimens.17 There is also evidence that MSH6 mutations in GBM mediate resistance to TMZ.18,19
In the current study, patients with GBM in first recurrence after RT and TMZ were treated with the 21/7 regimen at 75–100 mg/m2/day. In addition to response, survival, and safety, we evaluated MGMT promoter methylation status and MMR status with use of MSH2 and MLH1 expression as biomarkers.
Materials and Methods
The study was approved by the local institutional review boards at the 6 participating sites. Informed consent was obtained from all participants. Eligible patients were ≥18 years of age with life expectancy of ≥8 weeks, Karnofsky performance status (KPS) ≥60, and adequate bone marrow and organ function. Patients were required to have histologically confirmed GBM or gliosarcoma with unequivocal evidence of progression during or after standard combined modality therapy (consisting of radiation therapy plus concomitant daily TMZ, followed by ≥2 cycles of adjuvant TMZ at 150–200 mg/m2/day for 5 days per 28-day cycle). Patients who had received any other anti-tumor therapies were excluded. A period of 21 days from previous TMZ was required before study therapy could begin. At the time of data analysis, histology was centrally reviewed for all subjects by a neuropathologist (K.L.L.).
Participants were treated with TMZ (100 mg/m2/day) by mouth for 21 consecutive days of a 28-day cycle for 12 cycles or until second progression. Patients with a history of myelosuppression during TMZ therapy received a dose of 75 mg/m2/day. Prophylaxis against Pneumocystis jirovecii pneumonitis was required for all participants. Follow-up included weekly complete blood counts, physical and neurologic examinations every 4 weeks, and brain imaging with MRI every 8 weeks. Treatment was interrupted for absolute neutrophil count <1.0 × 109/L, platelet count <75 × 109/L, or Common Terminology Criteria for Adverse Events, version 3.0, grade 3 drug-related nonhematologic toxicity (except alopecia, nausea, vomiting, and fatigue). Treatment was resumed when toxicity recovered to grade ≤1, and dose reduction was permitted to 75 and 50 mg/m2/day.
Imaging was evaluated at each time point with use of the modified Macdonald criteria.20 Subjects who were classified by site investigators as achieving complete response, partial response, or PFS6 had their scans centrally reviewed by 2 blinded physicians (A.D.N. and D.R.R.). The primary study objective was PFS6; secondary objectives OS, radiographic response rate, and safety; and exploratory objective to correlate MGMT promoter methylation status with response and survival. The binomial test was used to compare the observed PFS6 to 15%, the value observed in previous studies. The study had 84% power to detect an improvement from 15% to 30%. Assuming a 10% drop-out rate, 61 subjects were required.
We performed immunohistochemical analysis to assess the expression of MSH2, MLH1, and excision repair cross-complementing rodent repair deficiency, complementation group 1 (ERCCI), in tumor cells. In brief, 5 µm formalin-fixed, paraffin-embedded tissue sections were deparaffinized in xylene, followed by a graded alcohol rehydration. Antigen retrieval was performed by microwaving tissues in citrate buffer (for ERCC1) or EDTA buffer (for MSH2 and MLH1) for 5 min. Primary antibody incubation with MSH2 antisera (1:200; NA27, Calbiochem, EMD Millipore, Billerica, MA), MLH1 antisera (1:200; NCL-L-MLH1, Novacastra, Leica, Butler Grove, IL), or ERCC1 (1:100; sc-10785, Santa Cruz Biotechnology, Santa Cruz, CA) was performed for 1 h at room temperature with use of a BioGenex i6000 automated staining platform (BioGenex Laboratories Inc., Fremont, CA). Detection of each primary antibody was performed using the BioGenex SS Multilink secondary, followed by HRP conjugation to the secondary with use of the Biogenex SS HRP Labeling kit. Visualization of each target was accomplished using the DAB substrate kit (Vector Laboratories Inc., Burlingame, CA). The sections were subsequently counterstained with hematoxylin and dehydrated in a graded series of alcohol before coverslip application.
Tumor specimens from GBM cases were analyzed for MSH2, MLH1, and ERCC1 expression with use of brightfield image analysis coupled with CRi image spectroscopy. Before slide scanning, each case was reviewed by 2 independent pathologists (R.L. and K.L.L.), and an area of GBM was circled, with the percentage of tumor cell content recorded. Of importance, staining for each target was noted to be present at significant levels only in GBM tumor cells (not expressed in normal), as confirmed by trained pathologists (R.L. and K.L.L.). Slides were then scanned using a Vectra multispectral imaging system (Caliper Life Sciences, Hopkinton, MA) attached to an Olympus BX51 fluorescent microscope. After image acquisition, analysis was performed using the Caliper InForm in each identifiable nucleus in the circled tumor area. The DAB and hematoxylin spectra were unmixed, and the resulting DAB intensity (optical density [OD]) for each cell of the tumor, in both the cytoplasmic and the nuclear compartments, was obtained. To calculate the mean nuclear DAB OD, the cytoplasmic OD for each cell was subtracted from the paired nuclear OD to remove background staining. The tumor cells from each case were then averaged by the relative tumor content per case (e.g., the top 70% of cells in a sample with 70% tumor noted). This resulted in mean nuclear OD expression values for each stain in each case.
The methylation status of the MGMT promoter was determined using methylation-specific polymerase chain reaction (PCR) testing on formalin-fixed paraffin-embedded material (MDxHealth, Liege, Belgium). Tissue sections (15 × 10 um; ∼150 µm total thickness) were xylene treated for paraffin removal and DNA extracted, and PCR was performed on bisulfite-treated DNA. Samples were then scored as methylated or unmethylated with use of previously described methods.21
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. Of the 58 patients accrued, 3 later proved to be ineligible after central pathology review. Alternative diagnoses in these subjects were anaplastic astrocytoma (WHO grade III), post-treatment changes only, and poorly differentiated metastatic tumor. These patients were excluded from response, survival, and correlative analyses. One patient withdrew consent shortly after starting study therapy and, therefore, could not be evaluated for response or PFS. All patients were evaluable for toxicity. In general, the population was representative of that typically accrued to clinical trials for recurrent GBM, with the exception that the proportion of tumors with unmethylated MGMT promoters was less than typically reported (see below).
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Median age, years (range) | 57 (25–79) |
| Male:female ratio | 33 (61) : 22 (39) |
| Median KPS score (range) | 90 (60–100) |
| MGMT promoter methylation | |
| Methylated | 36 (65) |
| Unmethylated | 11 (20) |
| Missing/insufficient tissue | 8 (15) |
| Progression during initial TMZ therapy | 28 (52) |
| Progression after initial TMZ therapy | 26 (48) |
| Median number of TMZ cycles given as part of initial therapy (range) | 6 (2–16) |
Abbreviations: KPS, Karnofsky performance status; TMZ, temozolomide.
Response and Survival
Central imaging review did not change response category for any of the subjects. However, in a small number of cases, central review resulted in an earlier diagnosis of progression. Of 54 evaluable patients, none achieved complete response. There were 7 (13%) partial responses and 19 (35%) patients for whom stable disease was the best response. Twenty-six subjects (48%) experienced progressive disease. Several factors proved not to be predictors of response, including MGMT methylation status, duration of TMZ therapy before initial progression, and use of TMZ at the time of initial progression.
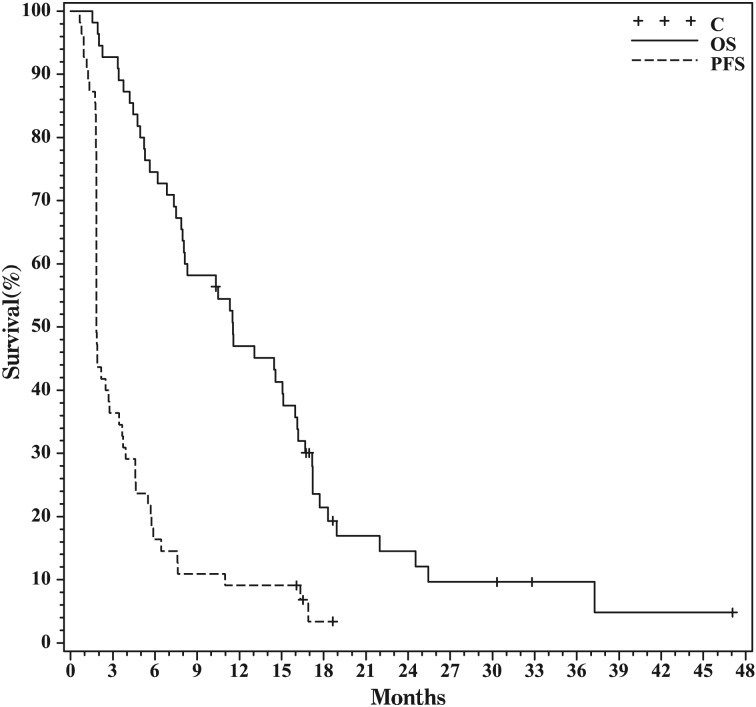
Overall, median PFS was 56 days (95% confidence interval [CI], 56–84 days), PFS6 was 11%, and median OS was 11.7 months (95% CI, 8.1–16.2 months) (Fig. 1). In the subset of tumors with methylated MGMT promoters, median PFS was 66 days (95% CI, 53–335 days), PFS6 was 33%, and median OS was 22.3 months (95% CI, 4.3–37.9 months). In the subset of tumors with unmethylated MGMT promoters, median PFS was 57 days (95% CI, 56–112 days), PFS6 was 9%, and median OS was 11.7 months (95% CI, 8.0–15.3 months). Although MGMT methylation status did not influence PFS (P = .11), patients with methylated MGMT had longer OS (P = .01). Median PFS and OS were also stratified on the basis of whether patients were on TMZ at the time of initial progression and on the duration of adjuvant TMZ that they had received at the time of initial progression. There were no significant differences in either PFS or OS across groups (Table 2; P = .61 and .25, respectively).
Fig. 1.
Kaplan-Meier curve showing progression-free and overall survival for the full cohort.
Table 2.
Survival data stratified by use and duration of use of temozolomide (TMZ)
| Median PFS, days (95% CI) | Median OS, months (95% CI) | |
|---|---|---|
| On TMZ* | 57.5 (56, 119) | 11.8 (6.3, 17.5) |
| Off TMZ* | 56 (56, 84) | 13.3 (7.5, 16.5) |
| ≤6 mos of TMZ** | 56 (55, 114) | 11.5 (8.0, 17.5) |
| >6 mos of TMZ** | 57.5 (56, 105) | 14.0 (7.0, 16.5) |
Abbreviations: OS, overall survival; PFS, progression-free survival.
Toxicity
None of the subjects ended treatment as a result of toxicity. There were no cases of P. jirovecii pneumonitis or toxic deaths. Common toxicities (grades 1 and 2) included fatigue, nausea, liver function test abnormalities, leukopenia, lymphopenia, anemia, and thrombocytopenia. Grade 3 and 4 toxicities are summarized in Table 3.
Table 3.
Grade 3 and 4 toxicities
| Toxicity | Grade 3 | Grade 4 |
|---|---|---|
| Fatigue | 3 | 0 |
| Anemia | 1 | 0 |
| Leukopenia | 3 | 0 |
| Lymphopenia | 18 | 3 |
| Neutropenia | 2 | 0 |
| Thrombocytopenia | 2 | 0 |
| Vomiting | 1 | 0 |
Correlative Studies
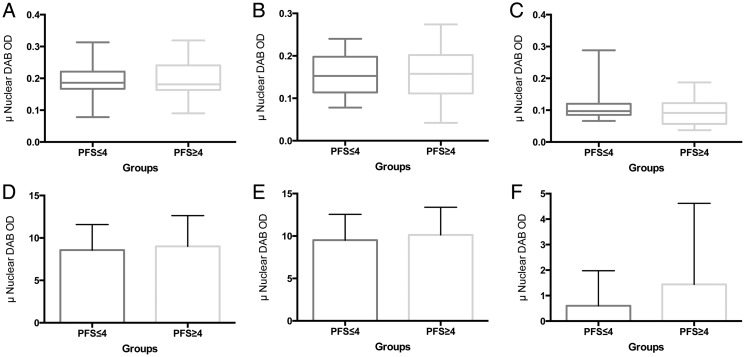
Because of the small number of subjects who achieved PFS6, samples were dichotomized on the basis of PFS of ≥4 or <4 months. To examine whether global differences in staining intensity were evident between the 2 PFS conditions, we compared the mean intensity of stain in PFS ≥4 and PFS <4. For each stain (MSH2, MLH1, and ERCC1), there were no significant differences observed (Fig. 2A–C). Extending the analyses to H-scores, calculated from both the intensity in tumor cells and the percentage of tumor cells with positive staining for each case, analyses of the PFS ≥ 4 and PFS <4 groups also failed to show any significant differences (Fig. 2D–F).
Fig. 2.
Expression of DNA repair enzymes stratified by progression-free survival (PFS). MSH2 (A), MLH1 (B), and ERCC1 (C) intensities did not differ between PFS <4 and ≥4. H-scores for MSH2 (D), MLH1 (E), and ERCC1 (F) revealed no significant differences between PFS <4 and ≥4.
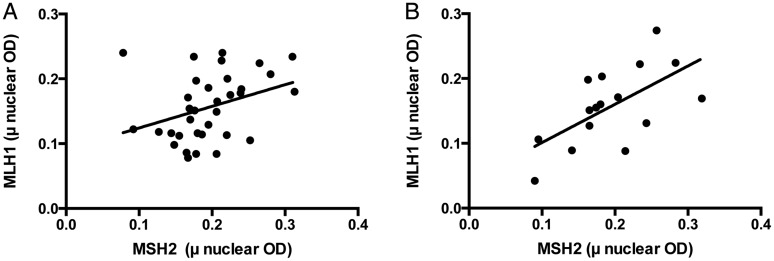
To examine the relationship between MSH2 and MLH1 in each of the PFS conditions (PFS ≥ 4 and PFS < 4), correlational analyses were performed. For PFS ≤4, an examination of the relationship between MSH2 and MLH1 revealed a significant correlation (r = 0.3432, P = .0469), and subsequent analysis of linear regression yielded an R2 of 0.1178 (Fig. 3A). Analysis of the relationship between MSH2 and MLH1 intensities in PFS ≥ 4 also demonstrated a significant correlation (r = 0.6202, P = .0104), and linear regression analysis yielded an R2 of 0.3847 (Fig. 3B). Further examination of the degree and direction of regression in PFS ≥ 4 (F = 8.752, P = .0104) and PFS < 4 (F = 4.274, P = .0469) showed that each condition demonstrated a significant positive deviation, although the degree of positive deviation in the PFS ≥ 4 appeared to be more linear, with a 1/slope = 1.693.
Fig. 3.
Correlations between MSH2 and MLH1. In cases with progression-free survival (PFS) < 4, the correlation between MSH2 and MLH1 (A) demonstrated an R2 of 0.1178. In contrast, a more positively inflected correlation between MSH2 and MLH1 was seen in cases with PFS ≥ 4 had an R2 of 0.3847.
Discussion
In this phase 2 study, patients with GBM in first recurrence were treated with TMZ using a 21/7 dosing regimen. Although the regimen proved to be safe, the efficacy results were disappointing, with PFS6 of only 11% and a 13% partial response rate. We found no evidence that time since prior TMZ or duration of the initial TMZ regimen impacted efficacy. We also failed to identify a predictive role for MGMT methylation status; however, as has been shown consistently in other studies,14 patients whose tumors had methylated MGMT promoters achieved significantly longer OS. The correlative data presented confirm the known correlation between MSH2 and MLH1 expression, but neither marker nor ERCC1 was predictive of PFS. Ideally, tissue samples for analysis of MMR protein status would be obtained after progression during the initial TMZ regimen, before enrollment into this study, but this was not feasible logistically.
In a prior study of a similar 21/7 TMZ dosing regimen in 33 chemotherapy-naive patients with GBM, there was an overall response rate of 9% and PFS6 of 30.3%.10 Our study in TMZ-pretreated patients showed that this regimen has significantly less activity. These findings also differ from those reported in the RESCUE study,22 in which a larger proportion of patients achieved PFS6 and there was a tendency toward particular efficacy in patients whose tumors recurred at least 2 months after completion of standard TMZ or during the first 3–6 cycles of standard TMZ therapy. In comparison with RESCUE, our study involved a smaller number of patients and a different regimen. Although we are not aware of supporting pharmacokinetic or pharmacodynamic data, continuous low-dose TMZ therapy used in the RESCUE study could be more effective than the 21/7 regimen. Finally, the results reported here are inferior to those reported in 64 patients with recurrent GBM using the 7/7 regimen; the difference likely reflects that >35% of subjects in that study were chemotherapy naive, an issue that the authors attempted to address analytically.9
Our results appear to be most similar to the RESCUE subgroup of patients whose tumors recurred after >6 months of adjuvant TMZ therapy but before completion of adjuvant TMZ therapy. In this extended cohort, PFS6 was 7.4%.22 For reasons that are challenging to explain, in our study, there was no suggestion that TMZ use or number of prior TMZ cycles at the time of recurrence influenced likelihood of response, PFS, or OS. Our findings also appear to be consistent with the recently reported results of RTOG 0525, in which there was no difference in PFS or OS between patients with newly diagnosed GBM who were treated with standard 5-day TMZ or a 21/7 regimen in the adjuvant context.14 In that study, MGMT status was not predictive of response but did provide meaningful prognostic information. It is worth acknowledging that, in an intracranial model of GBM in immunocompromised rodents, dose-intense TMZ successfully depleted tumor MGMT activity without impacting survival,23 suggesting that other resistance mechanisms must be overcome to achieve clinical benefit.
An important question for clinicians who treat patients with GBM is what role, if any, the 21/7 TMZ regimen should play in treating recurrent GBM. The data presented here suggest that the regimen has very limited activity. The current study was not adequately powered to assess the role of 21/7 TMZ in specific patient subsets, such as IDH1 (R132H) mutant GBM, for whom this regimen may have a role. However, on the basis of these data, it appears that our ongoing search for effective therapies in recurrent GBM must continue.
Funding
This work was supported by Merck.
Acknowledgments
These data were presented in part at annual meetings of the Society for Neuro-Oncology (2010), American Academy of Neurology (2011), and American Society of Clinical Oncology (2011).
Conflict of interest statement. P.Y.W. discloses research support and speaker fees from Merck. R.B. discloses grant funding and consulting fees from Novartis. J.-J.Z. discloses clinical trials funding from Novocure, Myrexis, Inc. and Immuno-Cellular Therapeutics, Ltd. All other authors: no conflicts.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 4.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 5.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 8.Tolcher AW, Gerson SL, Denis L, et al. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88(7):1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25(22):3357–3361. doi: 10.1200/JCO.2007.10.7722. [DOI] [PubMed] [Google Scholar]
- 10.Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO) Br J Cancer. 2006;95(9):1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 12.Balmaceda C, Peereboom D, Pannullo S, et al. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas. Cancer. 2008;112(5):1139–1146. doi: 10.1002/cncr.23167. [DOI] [PubMed] [Google Scholar]
- 13.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert MR, Wang M, Aldape KD, et al. RTOG 0525: A randomized phase III trial comparing standard adjuvant temozolomide with a dose-dense schedule in newly diagnosed glioblastoma [abstract] J Clin Oncol. 2011;29S:2006. [Google Scholar]
- 15.Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin Cancer Res. 1998;4(1):1–6. [PubMed] [Google Scholar]
- 16.Taverna P, Liu L, Hanson AJ, Monks A, Gerson SL. Characterization of MLH1 and MSH2 DNA mismatch repair proteins in cell lines of the NCI anticancer drug screen. Cancer Chemother Pharmacol. 2000;46(6):507–516. doi: 10.1007/s002800000186. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21(35):5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 18.Cahill DP, Levine KK, Betensky RA, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13(7):2038–2045. doi: 10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15(14):4622–4629. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 21.Vlassenbroeck I, Califice S, Diserens AC, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. The Journal of Molecular Diagnostics: JMD. 2008;10(4):332–337. doi: 10.2353/jmoldx.2008.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry JR, Mason WP, Belanger K, et al. The temozolomide RESCUE study: A phase II trial of continuous (28/28) dose-intense temozolomide (TMZ) after progression on conventional 5/28 day TMZ in patients with recurrent malignant glioma. J Clin Oncol. 2008;26:2010. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 23.Robinson CG, Palomo JM, Rahmathulla G, et al. Effect of alternative temozolomide schedules on glioblastoma O(6)-methylguanine-DNA methyltransferase activity and survival. Br J Cancer. 2010;103(4):498–504. doi: 10.1038/sj.bjc.6605792. [DOI] [PMC free article] [PubMed] [Google Scholar]