Abstract
Background
The prognosis for patients with recurrent glioblastoma remains poor. The purpose of this study was to assess the potential role of MR spectroscopy as an early indicator of response to anti-angiogenic therapy.
Methods
Thirteen patients with recurrent glioblastoma were enrolled in RTOG 0625/ACRIN 6677, a prospective multicenter trial in which bevacizumab was used in combination with either temozolomide or irinotecan. Patients were scanned prior to treatment and at specific timepoints during the treatment regimen. Postcontrast T1-weighted MRI was used to assess 6-month progression-free survival. Spectra from the enhancing tumor and peritumoral regions were defined on the postcontrast T1-weighted images. Changes in the concentration ratios of n-acetylaspartate/creatine (NAA/Cr), choline-containing compounds (Cho)/Cr, and NAA/Cho were quantified in comparison with pretreatment values.
Results
NAA/Cho levels increased and Cho/Cr levels decreased within enhancing tumor at 2 weeks relative to pretreatment levels (P = .048 and P = .016, respectively), suggesting a possible antitumor effect of bevacizumab with cytotoxic chemotherapy. Nine of the 13 patients were alive and progression free at 6 months. Analysis of receiver operating characteristic curves for NAA/Cho changes in tumor at 8 weeks revealed higher levels in patients progression free at 6 months (area under the curve = 0.85), suggesting that NAA/Cho is associated with treatment response. Similar results were observed for receiver operating characteristic curve analyses against 1-year survival. In addition, decreased Cho/Cr and increased NAA/Cr and NAA/Cho in tumor periphery at 16 weeks posttreatment were associated with both 6-month progression-free survival and 1-year survival.
Conclusion
Changes in NAA and Cho by MR spectroscopy may potentially be useful as imaging biomarkers in assessing response to anti-angiogenic treatment.
Keywords: anti-angiogenic therapy, bevacizumab, Cho, glioblastoma, magnetic resonance spectroscopy, NAA
Patients with recurrent glioblastoma multiforme (GBM) have a median survival time of 3–6 months, despite attempts at treatment using a variety of chemotherapy regimens.1 Available treatments have been limited by problems with delivery to the tumor because of widespread tumor infiltration, the blood–brain barrier, and the rapid development of resistance to conventional cytotoxic agents.2,3 Recently, significant interest has been generated in targeting angiogenesis, a prominent feature of malignant gliomas. Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) approved by the FDA in 2009 as monotherapy for recurrent GBM.4 In recent studies, patient cohorts treated with bevacizumab alone or in combination with cytotoxic agents have demonstrated higher progression-free survival (PFS) and overall survival (OS) benefits compared with historical controls.5–8 Treatment with bevacizumab is thought to normalize tumor vasculature and is associated with remarkable reduction in contrast enhancement on T1-weighted MRI.9
Although structural MRI remains the standard for assessing GBM recurrence, it provides little information about tumor biology. In assessing tumor response using the Macdonald criteria,10 contrast enhancement is the primary surrogate marker for tumor using MRI. Distinguishing actual tumor response to therapy from blood–tumor barrier improvement with decreased contrast leakage in GBM patients treated with bevacizumab or other VEGF-targeting agents can be challenging because diminished contrast enhancement commonly seen on standard posttreatment MRI may not always correspond with reduction of viable tumor, a phenomenon called pseudo-response.11 Furthermore, the mechanism by which bevacizumab provides therapeutic benefit remains unclear. Although patients symptomatically benefit greatly from reduced cerebral edema and intracranial pressure, it remains unclear whether anti-angiogenic agents have a direct cytotoxic effect.12
Advanced MRI techniques have been developed to provide information about underlying tumor biology and vascular permeability. Specifically, proton MR spectroscopic imaging (MRSI) provides biochemical information about proliferative tumor activity.13 In GBM, choline is significantly elevated due to increased cellular turnover and accelerated membrane synthesis that occur in rapidly dividing cancer cells.14 Levels of n-acetylaspartate (NAA) serve as a marker for neuronal health and viability,15 which are compromised in tumor progression.16–18 Thus MRSI is commonly used for diagnosing and grading brain tumors as well as evaluating response to therapy.16
In this study, we sought to (i) assess the feasibility of serial MRSI in a multicenter tumor-related trial, (ii) identify metabolic changes in recurrent GBM induced by bevacizumab in combination with cytotoxic agents, (iii) correlate metabolic changes with clinical performance status, and (iv) determine whether MRS markers correlate with patient outcomes. To the best of our knowledge, this is the first multicenter clinical trial to investigate metabolic changes by serial MRSI in recurrent GBM treated with bevacizumab and other chemical agents.
Materials and Methods
The Radiation Therapy Oncology Group (RTOG), in collaboration with the American College of Radiology Imaging Network (ACRIN), both cooperative groups funded by the National Cancer Institute, conducted a randomized, prospective multi-institutional study of bevacizumab used in combination with either temozolomide or irinotecan in patients with recurrent GBM (RTOG 0625/ACRIN 6677). Twenty-three institutions participated in the study. Each participating site obtained institutional review board approval before subject accrual and conducted the trial in compliance with the Health Insurance Portability and Accountability Act. Informed consent was obtained for all subjects.
A subset of 6 institutions conducted a substudy of advanced MRI, including dynamic contrast enhanced imaging, dynamic susceptibility contrast imaging, and MRSI. Sites were required to qualify for participation in the advanced MRI substudy by submitting test cases to the ACRIN core lab, including MRS data on phantoms and healthy subjects. Separate consent was obtained for substudy participation. Results of the advanced MRI substudy specific to MRS are reported in this manuscript. Of the 6 institutions that were accredited for advanced MRI, only 3 sites contributed analyzable MRS data. The rationale for excluding data is discussed in the results.
Study Subjects
Study accrual commenced in May 2007 and was completed in May 2009. Patients with GBM who failed external beam radiotherapy plus temozolomide were included. All patients had recurrent histologically proven glioblastoma or gliosarcoma, with indisputable MRI evidence for tumor progression or regrowth within 14 days prior to registration. Other inclusion criteria included: age ≥18 years; KPS ≥70; at least 42 days since completion of mandated prior radiation + temozolomide; at least 28 days since any prior surgical resection of GBM or treatment with an investigational or cytotoxic agent; baseline MRI within 96 h or more than 4 weeks after resection of recurrent or progressive tumor, respectively; and confirmation (with PET, MRS, or biopsy) of true progressive disease rather than radiation necrosis following prior interstitial brachytherapy, Gliadel wafer placement, or stereotactic radiosurgery. Exclusion criteria included acute intratumoral hemorrhage on MRI and severe active comorbidities. Detailed inclusion and exclusion criteria can be viewed online at http://www.acrin.org/Portals/0/Protocols/6677/RTOG062-ACRIN6677.pdf (section 3.0).
Treatments
Bevacizumab was used in combination with cytotoxic agents, either temozolomide (first arm) or irinotecan (second arm). Bevacizumab was administered every 2 weeks at a dose of 10 mg/kg i.v. Details on the treatment regimens can be viewed online at http://www.acrin.org/Portals/0/Protocols/6677/RTOG062-ACRIN6677.pdf (section 7.0).
MRI/MRSI
Patients in the advanced MRI substudy had serial MRI and MRS exams prior to treatment (baseline), 2 and 8 weeks posttreatment, and every 2 months until the endpoint of the study (96 wk). Standard MRI protocols used conventional sequences (T1, T2, fluid attenuated inversion recovery [FLAIR], post-gadolinium T1, and volumetric post-gadolinium T1). Analyzable MRS studies were performed at 3 different sites: Washington University, St Louis (Siemens Avanto 1.5 T scanner); Medical College of Wisconsin (GE 1.5 T Signa Excite scanner); and Tel Aviv Medical Center (3.0 T GE Signa Excite scanner). All data were acquired using a 2D hybrid MRSI sequence with point-resolved spectroscopy excitation pulse sequence for signal localization. Water suppression was achieved with a modified chemical selective saturation method known as water suppression enhanced through T1 effects (WET). Table 1 summarizes the acquisition parameters from the 3 sites. Sites were instructed to manually shim before the acquisition to obtain the best magnetic field homogeneity. The imaging time for MRSI was <10 min at all 3 sites.
Table 1.
Acquisition parameters
| Site | Field Strength | Vendor | TE (ms) | TR (ms) | FOV (mm2) | Phase Encoding | Slice Thickness (cm) | Number of Patients |
|---|---|---|---|---|---|---|---|---|
| Wash U | 1.5 T | Siemens | 144 | 1700 | 160 | 16 × 16 | 1.5 | 5 |
| MCW | 1.5 T | GE | 144 | 1500 | 160 | 16 × 16 | 1 | 1 |
| Tel Aviv | 3.0 T | GE | 144 | 1500 | 240 | 16 × 16 | 1.5 | 7 |
Abbreviations: Wash U, Washington University, St Louis; MCW, Medical College of Wisconsin; Tel Aviv, Tel Aviv Medical Center; TE, echo time; TR, repetition time; FOV, field of view.
MRS Data Analysis
The spectroscopic raw data were analyzed using LC Model 6.1 software19 to determine the quantities of the metabolites NAA at 2 ppm, creatine (Cr) at 3 ppm, choline-containing compounds (Cho) at 3.2 ppm, and lactate, a doublet at 1.3 ppm. In addition, the presence of lipids (0.5–2 ppm) was recorded. Only fitted spectra with estimated standard deviations (Cramer–Rao lower bounds, automatically provided by the LC Model software) expressed in percent of the estimated concentrations lower than 25% were accepted.20
The spectra were analyzed in 3 regions of interest at baseline and all subsequent timepoints. Voxels were classified by visual inspection into predominantly (i) enhancing tumor, defined by the corresponding T1-weighted postcontrast images, (ii) non-enhancing peritumoral parenchyma (periphery), and (iii) normal brain contralateral to tumor containing white matter (Fig. 1A).21 MRSI data were overlaid on the T1-weighted postcontrast images for spectra classification. These images were either generated using SAGE 7 (GE Healthcare) or provided in the form of screenshots from the individual sites. The same voxel locations have been used in follow-up studies regardless of presence or absence of subsequent enhancement. Region-of-interest sizes of enhancing tumor and periphery varied from patient to patient. An MRSI voxel was classified as enhancing tumor even if only partial enhancement of subvoxels was observed. Peritumoral MRSI voxels were unenhanced on T1-weighted postcontrast images but typically had FLAIR hyperintensity. We examined the changes from baseline in the metabolic ratios NAA/Cho, NAA/Cr, and Cho/Cr during treatment. Typical spectra (Fig. 1B) revealed the NAA peak to exceed the Cho peak in normal tissue, whereas this relationship is reversed in tumor.
Fig. 1.
(A) The 3 regions of interest were defined on the corresponding T1-weighted postcontrast images: (grey voxels) enhancing tumor, (black voxels) peritumoral tissue, and (white voxels) normal tissue on the contralateral side of tumor. (B) Typical MRS spectra were obtained from (left) the enhancing tumor and from (right) contralateral normal tissue. Tumor tissue is characterized by elevated Cho and decreased NAA.
Statistical Data Analysis
Patients from both study arms were pooled for analysis. We sought to examine trends in the MRS metabolic ratios (NAA/Cho, NAA/Cr, and Cho/Cr) over time and to determine whether changes in these ratios at either 2, 8, or 16 weeks posttreatment were predictive of either 6-month PFS (PFS-6) or 1-year OS.
For each of the metabolic ratios, data through 6 months (wk 24) were examined graphically and modeled longitudinally to examine trends over time. To account for correlation due to multiple longitudinal measurements obtained on each subject, a repeated-measures ANOVA was used with generalized estimating equations. To assess the ability of changes in MRS metabolic ratios to predict PFS-6 and 1-year OS, area under the receiver operating characteristic curve (ROC AUC) was estimated empirically with a lower bound of the 95% confidence interval (CI) of at least 0.5. Finally, changes in metabolic ratios at specific timepoints (2 wk, 8 wk, and 16 wk) were correlated with changes in KPS by Spearman rank correlation coefficient, with values of 0.5 to <0.8 considered moderate and values of ≥0.8 considered substantial. All statistical data analyses were performed with SAS 9.2.
Results
Study Cohort
A total of 123 subjects from 23 institutions were enrolled into RTOG 0625/ACRIN 6677. Although 6 institutions were accredited for advanced MRI, only 3 contributed analyzable MRS data. The other 3 institutions failed for the following reasons: (i) site 1 scanned only 1 subject but did not submit the MRS raw data as instructed by ACRIN; (ii) site 2 scanned only 1 patient, and although the data met the quality assurance requirements, it was later discovered that this patient was ineligible because tissue had not been submitted for central pathology review as mandated per protocol (http://www.acrin.org/Portals/0/Protocols/6677/RTOG062-ACRIN6677.pdf, section 3.1.2); and (iii) site 3 scanned only 1 patient but did not perform a baseline scan. From the 3 institutions that contributed analyzable data, 4 subjects were excluded for no postbaseline imaging (n = 1), suboptimal spectra quality (n = 1), and missing baseline scans (n = 2).
In summary, of the 123 subjects enrolled into RTOG 0625/ACRIN 6677, a total of 20 consented to the advanced MRSI substudy, of whom 13 (9 men; mean age, 54.8 ± 12.9 y) had analyzable MRS datasets, including a baseline scan. Seven subjects were excluded for no postbaseline imaging (n = 1), ineligibility (n = 1), missing raw data (n = 1), suboptimal spectra quality (n = 1), and missing baseline scans (n = 3). An average of 6 timepoints (range, 3–15) were obtained for the 13 subjects analyzed.
Longitudinal Changes of the MRS Metabolic Ratios
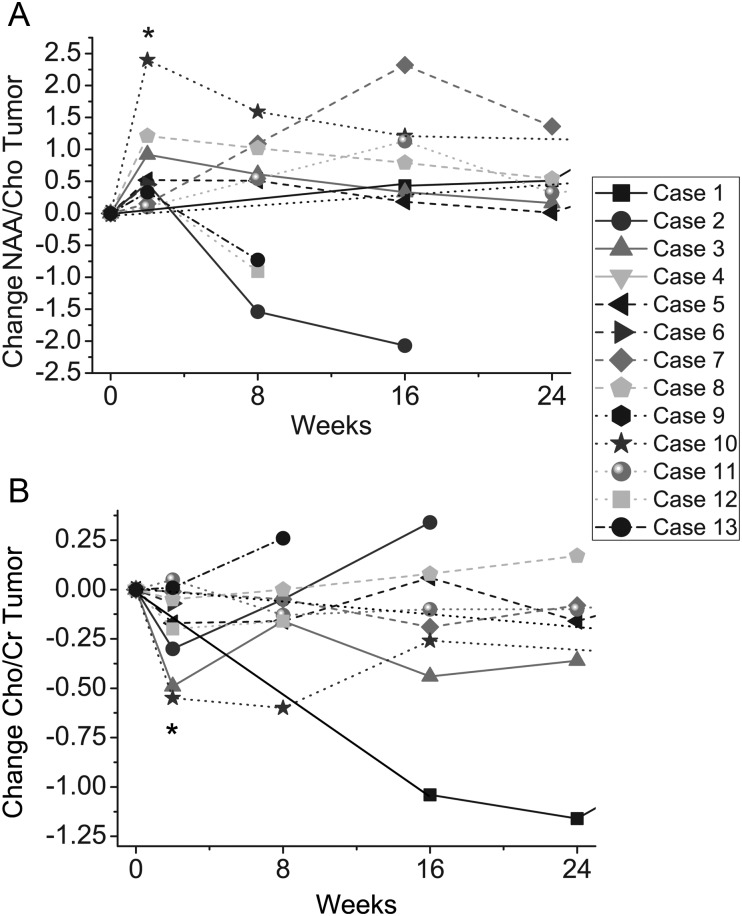
Intratumoral changes in NAA/Cho and Cho/Cr relative to pretreatment values in each patient within the first 6 months of the study are shown in Fig. 2A and B. At 2 weeks posttreatment, bevacizumab in combination with cytotoxic agents resulted in a significant increase on average in NAA/Cho levels within the enhancing tumor (P = .048; Fig. 2A) and in a decrease on average in Cho/Cr levels (P = .016; Fig. 2B). No further significant changes were observed in NAA/Cho levels or Cho/Cr levels after 2 weeks of treatment (at 8, 16, or 24 wk). No significant changes in levels of NAA/Cr in the enhancing tumor were observed at any timepoint (results not shown). In addition, no significant changes were observed over time in any of the metabolic ratios as measured in the periphery of the tumor (results not shown).
Fig. 2.
Changes in (A) NAA/Cho levels and (B) Cho/Cr levels in enhancing tumor relative to baseline levels. NAA/Cho levels significantly increase at 2 wk posttreatment (P = .048), and Cho/Cr significantly decreases at 2 wk posttreatment (P = .016) indicated by asterisks.
MRS Metabolic Ratios as Predictor of PFS-6
Tumor Metabolic Ratios
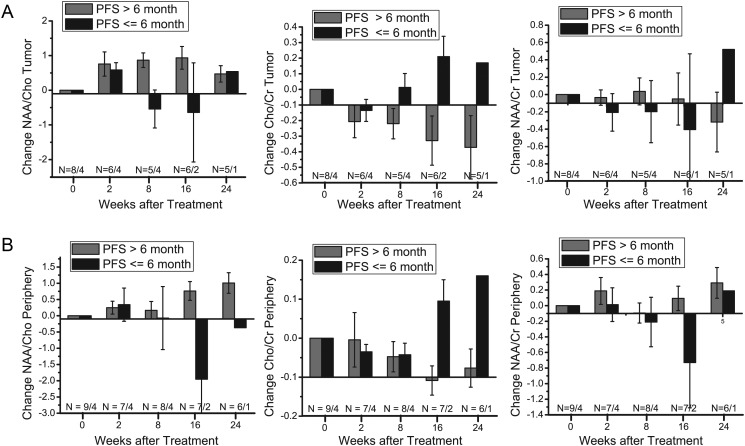
Nine of the 13 patients (69%) were alive and progression free at 6 months. Summarized in Table 2 are empirical estimates of the AUC for prediction of PFS-6 and the corresponding 95% CI for change in each metabolic ratio at 2, 8, and 16 weeks. Figure 3A displays the changes in metabolic ratios from baseline in the enhancing tumor with subjects grouped by PFS-6 status.
Table 2.
ROC analysis of MRS changes in relation to PFS-6 and relative to 12-mo survival
| Change From Baseline | Wk 2 | Wk 8 | Wk 16 | |
|---|---|---|---|---|
| ROC analysis of MRS changes in relation to PFS-6 | ||||
| Tumor | NAA/Cho | 0.50 (0.09, 0.91) | 0.85 (0.53, 1) | 0.75 (0.21, 1) |
| Cho/Cr | 0.54 (0.14, 0.95) | 0.83 (0.47, 1) | 1.00 (1, 1) | |
| NAA/Cr | 0.46 (0, 0.99) | 0.60 (0.11, 1) | 0.46 (0, 1) | |
| Periphery | NAA/Cho | 0.39 (0, 0.88) | 0.47 (0.02, 0.92) | 1.00 (1, 1) |
| Cho/Cr | 0.52 (0.13, 0.91) | 0.41 (0.01, 0.80) | 1.00 (1, 1) | |
| NAA/Cr | 0.54 (0.06, 1) | 0.63 (0.22, 1) | 0.93 (0.73, 1) | |
| ROC analysis of MRS changes in relation to 12-mo survival | ||||
| Tumor | NAA/Cho | 0.42 (0, 0.92) | 0.80 (0.47, 1) | 0.80 (0.45, 1) |
| Cho/Cr | 0.42 (0, 0.88) | 0.78 (0.44, 1) | 0.73 (0.19, 1) | |
| NAA/Cr | 0.67 (0.25, 1) | 0.60 (0.17, 1) | 0.57 (0.03, 1) | |
| Periphery | NAA/Cho | 0.60 (0.20,1) | 0.58 (0.22, 0.95) | 1.00 (1, 1) |
| Cho/Cr | 0.72 (0.36, 1) | 0.50 (0.13, 0.87) | 0.89 (0.63, 1) | |
| NAA/Cr | 0.50 (0.06, 0.94) | 0.78 (0.47, 1) | 1.00 (1, 1) | |
ROC analysis with empirical estimates of AUC (95% CI).
AUC ≥ 0.85 and lower bound of the 95% CI > 0.5 are highlighted in bold non-italics.
AUC > 0.75 and lower bound of the 95% CI > 0.4 are highlighted in bold italics.
Fig. 3.
(A) Changes in NAA/Cho, Cho/Cr, and NAA/Cr from baseline in tumor voxels grouped by PFS-6 survivors (PFS >6 mo) and non–PFS-6 survivors (PFS ≤ 6 mo). (B) Changes in NAA/Cho, Cho/Cr, and NAA/Cr from baseline in peritumoral voxels grouped by PFS-6 survivors (PFS >6 mo) and non–PFS-6 survivors (PFS ≤ 6 mo). Error bars represent SEs of the mean. Numbers of patients are noted on the bottom.
Changes at 2 weeks posttreatment from baseline for all 3 ratios had poor performance for prediction of PFS-6; in particular, the data demonstrate a uniform increase in NAA/Cho levels and a uniform decrease in Cho/Cr levels in all subjects regardless of PFS-6 status.
Changes at 8 and 16 weeks posttreatment were predictive of PFS-6 for 1 or more metabolic ratios. In particular, changes posttreatment for NAA/Cho had AUCs of 0.85 (95% CI = 0.53–1.00) at 8 weeks and 0.75 (95% CI = 0.21–1) at 16 weeks. At 8 weeks posttreatment there was a trend toward lower Cho/Cr compared with baseline in subjects who were alive and progression free (AUC = 0.83, 95% CI = 0.47–1.00), with changes at 16 weeks demonstrating perfect discriminatory ability for this patient set (AUC = 1). NAA/Cr changes in the tumor were not predictive of PFS-6 at any timepoint.
Periphery Metabolic Ratios
We also performed ROC analysis for the peritumoral tissue voxels. Before 16 weeks posttreatment, we were unable to distinguish patients who were alive and progression free at 6 months from those who were not (Fig. 3B). However, changes in all 3 metabolic ratios demonstrated good discriminatory ability at 16 weeks (Table 2), with perfect discrimination observed for NAA/Cho and Cho/Cr for this patient set (AUC = 1).
MRS Metabolic Ratios as Predictor of 1-Year OS
Tumor Metabolic Ratios
In addition to establishing whether early changes in metabolic ratios were predictive of PFS-6, we investigated whether these metabolic changes were predictive of 1-year survival. Seven of the 13 patients (54%) were alive at 12 months. For each metabolic ratio, Table 2 summarizes the empirical estimates of the AUC and the corresponding 95% CI. Our findings were similar to those for prediction of PFS-6. Changes in the enhancing tumor in both NAA/Cho and Cho/Cr at 8 weeks showed reasonably good discriminatory ability (AUC = 0.80, 95% CI = 0.47–1 and AUC = 0.78, 95% CI = 0.44–1, respectively), and similar results extended to 16 weeks for NAA/Cho. Changes in NAA/Cr were not predictive of 1-year OS.
Periphery Metabolic Ratios
Similar to findings for PFS-6, changes at 16 weeks for all 3 metabolic ratios also demonstrated good prediction of 1-year survival (Table 2), with perfect discrimination observed for NAA/Cho and NAA/Cr (AUC = 1). Interestingly, we also noted that changes in NAA/Cr in the periphery after 8 weeks were suggestive of survival (AUC = 0.78, 95% CI = 0.47–1.00).
Correlations Between Changes in KPS and Metabolic Ratios
Changes in metabolic ratios at specific timepoints were correlated with changes in KPS using Spearman rank correlation coefficient. The mean time interval between the 8-week scan and the corresponding assessment of KPS was 7 days (range, 0–13 d), and the mean time interval between the 16-week scan and the corresponding assessment of KPS was 5 days (range, 0–13 d). Table 3 summarizes the correlations between the change in KPS and the corresponding change in each MRS marker. There is a moderate correlation between change in NAA/Cr and change in KPS at 8 weeks (P = .045, ρ = 0.59), as well as between change in NAA/Cho and change in KPS at 8 weeks (P = .066, ρ = 0.55).
Table 3.
Spearman rank correlations between change in KPS and MRS marker
| Region | Marker | Wk 8 Spearman | Wk 16 Spearman |
|---|---|---|---|
| Tumor | NAA/Cho | 0.426 (n = 9, P = .253) | 0.209 (n = 8, P = .620) |
| Cho/Cr | −0.005 (n = 9, P = .990) | −0.061 (n = 8, P = .885) | |
| NAA/Cr | 0.218 (n = 9, P = .573) | 0.360 (n = 8, P = .381) | |
| Periphery | NAA/Cho | 0.546 (n = 12, P = .066) | 0.451 (n = 9, P = .223) |
| Cho/Cr | −0.312 (n = 12, P = .324) | −0.316 (n = 9, P = .407) | |
| NAA/Cr | 0.586 (n = 12, P = .045) | 0.341 (n = 9, P = .370) |
Moderate correlations with P > 0.5 are highlighted in bold.
Discussion
Our preliminary MRS data suggest that treatment with bevacizumab in combination with irinotecan or temozolomide results in a transient increase in NAA/Cho at 2 weeks. We believe that these changes are mainly due to decreases in choline, because we observed decreases in Cho/Cr at 2 weeks, while no changes in NAA/Cr were observed. The reduction in choline may be attributed to either a reduction of tumor size or recovery of normal function within tumor cells. As previously mentioned, the same voxel locations from baseline were used in later timepoints regardless of enhancement.
Our study confirms that treatment with bevacizumab in combination with cytotoxic agents has a direct metabolic effect on the microenvironment of recurrent GBM, indicated by a transient decrease in tumor malignancy at 2 weeks in all patients regardless of survival. However, at 8 weeks after the initial normalization period, only progression-free survivors and 1-year overall survivors showed improvement in NAA/Cho. This finding is in keeping with reports of other MRS studies in patients with brain tumors undergoing anti-angiogenic therapy. A previous multimodal study22 of bevacizumab in recurrent GBM utilizing 1H MRS and 31P MRS showed pH decreases 6 to 8 weeks after treatment. Of the 16 patients enrolled in that study, 7 received irinotecan in addition to bevacizumab. A study of cediranib anti-angiogenic treatment without additional cytoxic agents by Kim et al21 showed significant increases in NAA/Cho after 28 days posttreatment. These findings suggest a direct antitumor effect with anti-angiogenic treatment.
One of the major challenges for imaging recurrent GBM is to distinguish pseudo-response from actual tumor response in the presence of anti-angiogenic treatment. We used MRS to investigate whether early metabolic changes (eg, NAA/Cho) are predictive of patient outcome. At 8 weeks, patients who were alive and progression free at 6 months had higher levels of NAA/Cho, indicating that an increase in NAA/Cho is associated with treatment response. Also, lower levels of Cho/Cr at 8 weeks were predictive of PFS-6. Similar results were observed when we performed ROC analysis relative to 12-month OS. These findings suggest that changes in Cho and NAA may potentially be useful as an imaging biomarker in assessing response to anti-angiogenic treatment. Furthermore, increases in peritumoral NAA/Cho and NAA/Cr and decreases in Cho/Cr 16 weeks following anti-VEGF therapy were associated with both PFS-6 and 12-month OS. A significant correlation between NAA recovery in the periphery and improving KPS was observed. This raises the possibility that clinical deficits in these patients are at least in part related to tumor replacement within and/or sustained injury to the tumor periphery.
These findings, although very encouraging, need to be confirmed in a larger study. Previously, Kim et al21 reported that increases in NAA/Cho levels in enhancing tumor at 56 days (8 wk) after administration of cediranib showed a high correlation with 6-month OS. This confirms our finding that changes in NAA/Cho may potentially be useful as an imaging biomarker in assessing response to anti-angiogenic treatment.
Other studies have used alternative MRI techniques23 to predict treatment response of anti-VEGF therapy, including diffusion-weighted imaging,22,24–27perfusion-weighted imaging,28–30 and T2-weighted imaging/FLAIR.31,32 For example, smaller apparent diffusion coefficient values suggest higher cell densities—thus, decreases in ADC after treatment were predictive of disease progression, while increases in apparent diffusion coefficient correlated with treatment response.25 In addition, changes in ktrans, a measure of vascular permeability, correlated with duration of OS and PFS.29
Our clinical trial also included other advanced imaging components, including dynamic contrast enhanced and dynamic susceptibility contrast perfusion MRI and diffusion-weighted imaging. Future studies will include the combined analysis of MRS and other advanced MRI data to determine whether MRS adds predictive value for outcome in patients with GBM.
Our current MRS study focused on only Cho and NAA changes. Kim et al21 reported significant decreases in lactate and lipid concentrations at 56 days (8 wk) after cediranib treatment in recurrent GBM: the same timepoint when NAA/Cho improved. Increases in lactate and lipids in gliomas are associated with anaerobic metabolism and necrosis, respectively. Our study did not identify significant lactate or lipid changes, perhaps due to the small number of patients.
We acknowledge that this study is limited by its small sample size. Multicenter 1H MRS studies in brain tumor patients are challenging, although some studies have been previously successfully performed.33,34 Typical challenges involve the spectral quality of the MRS data, including low signal-to-noise ratios, insufficient separation of metabolite peaks due to suboptimal shimming, inadequate water suppression, and discrepancy of MRS voxel location in subsequent exams. In addition, proximity to skull can result in a contaminating lipid signal.
In this trial, 7/ 20 patients had to be excluded from the data analysis: 1 was ineligible, 1 opted out of the study after the first baseline scan, and 5 were excluded for technical reasons, the most common reason (n = 3) being that patients had missing baseline scans. While the variability among MRS data of different GBM patients may be quite large, the metabolic changes within individual patients by repeat MRS studies are more compelling due to less variability. Therefore, we believe it is necessary to evaluate the changes in metabolites from baseline. Thus, to evaluate the metabolic response to treatment, it is best to compare spectra from the same region of interest over time. Therefore, it is essential to obtain good-quality MRS data at baseline before treatment is initiated. In our clinical trial, the most common reason datasets were excluded from the analysis was that baseline exams were missing or inadequately acquired.
Nevertheless, 1 patient needed to be excluded because all of the MRS data were poor, and 1 site was not able to submit the needed raw data. We conclude from our experience with this trial that MRS in a multi-institutional setting is very complex and requires better quality control for future studies. Moving forward to other multi-institutional trials in which MRS is part of the evaluation, we suggest the following for improvement: (i) improved protocol standardization (consistent echo time, repetition time, etc), (ii) better education of MR technologists or physicists at the sites, and (iii) multiple submissions of MRS data from phantoms and healthy volunteers to obtain intra- and intersubject variability for each of the sites, as well as intersite variability before the trial begins.35
Another limitation of this study pertains to possible partial volume effects. Voxels were assigned as either tumoral, peritumoral, or normal based on visual inspection of the MRSI grid overlaid with the baseline T1-weighted postcontrast MRIs. Thus, each voxel may contain partial volume fractions of more than 1 tissue type. However, despite this limitation, we believe that spectroscopy may add clinical value to distinguish real response from pseudo-response after anti-angiogenic treatment. To date, most imaging studies have revealed that a decrease in enhancing tumor based on T1-weighted images may not be a good predictor of patient outcome. Spectroscopy may help distinguish a true response based on the tumor biology.
Finally, the MRS scans in our study came from 3 different institutions, with different magnetic field strengths and slightly different parameters. Of the 9 participants who were PFS-6 survivors and the 4 participants who were not, Washington University (1.5 T Siemens scanner) had 4 and 1, respectively; Tel Aviv Medical Center (3.0 T GE scanner) had 4 and 3; and the Medical College of Wisconsin (1.5 T GE scanner) had 1 PFS-6 survivor. The distribution of PFS-6 and non–PFS-6 survivors in our analysis makes a systematic error very unlikely; however, due to the small sample size, formal hypothesis testing is not feasible. In addition, the fact that we are evaluating the change between the individual subjects' baseline scan and their follow-up scan and that each subject had the same scan parameters across all timepoints means that subjects acted as their own controls. This allowed us to compare these changes across different scanner platforms and diminished the effects of individual site setups.
Despite these limitations, our study showed that improvements in metabolic markers NAA/Cho and Cho/Cr in the enhancing tumor 8 weeks post anti-angiogenic treatment with cytotoxic agents were predictive of patient outcome. Improvements in NAA/Cho, Cho/Cr, and NAA/Cr 16 weeks posttreatment were also predictive of patient outcome. Therefore, despite all of its associated challenges in multicenter trials, MRS may be worth implementing, as it may add clinical value to distinguish real response from pseudo-response after anti-angiogenic treatment. In conclusion, changes in Cho and NAA may potentially be useful as an imaging biomarker in assessing response to anti-angiogenic treatment; however, these findings need to be confirmed in a larger study.
Funding
This work was supported by the National Cancer Institute to ACRIN (grants U01 CA079778 and U01 CA080098).
Acknowledgments
We thank the ACRIN team, specifically Bernadine Dunning, Jamie Downs, Kesha Smith, Sandy Toland-Cary, Cyndi Price, Maxine Crooks, and James Gimpel, and Dr Charles Apgar for supporting this study. We thank Darryl L'Heureux for editing the manuscript and Melanie Yeh for statistical analysis. We thank ACRIN and RTOG for supporting this study. Special thanks to the ACRIN/RTOG principal investigators and supporters of the study Drs. Orna Eizenstein, Ben Corn, Deborah Blumenthal from Tel Aviv Sourasky Medical Center, Dr. Joseph Simpson from Washington University Medical School and Drs. Scott Rand and Linda Grossheim of the Medical College of Wisconsin. In addition, we thank the MRI sites for the data acquisition; and finally we thank the patients and their families for participating in this study.
Conflict of interest statement. M.R.G. has consultancies with Genentech, Abbott, and Merck and grants from EMD Serono, Merck, and Glaxo SmithKline. G.A.S. is president/CEO of Siemens Healthcare; Siemens manufactures and sells medical imaging equipment. G.A.S. served as a consultant to Genentech in 2009 (total compensation <$10,000). D.P.B., through Duke University: (i) receives imaging core lab support from Eisai Pharmaceuticals to provide image analysis for an unrelated project; (ii) received pulse sequence support for his research scanner from Siemens Healthcare as part of an unrelated project for which D.P.B. was principal investigator; (iii) received imaging core lab support from Adnexus Therapeutics/Bristol Myers Squibb to provide image analysis for an unrelated project. D.B. is a member of the GE Medical Systems Neuro MRI advisory board; he is currently president (an uncompensated position) of the American Society of Functional Neuroradiology (an affiliate of the American Society of Neuroradiology) and has been on the executive committee for the last 3 years. R.C.M. has been paid honoraria, travel, lodging, meals, acting and modeling fees by Siemens Healthcare related to the BioGraph mMR (PET/MR) imaging system. None of the studies in the investigation were performed on that system.
References
- 1.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 2.Thaker NG, McDonald PR, Zhang F, et al. Designing, optimizing, and implementing high-throughput siRNA genomic screening with glioma cells for the discovery of survival genes and novel drug targets. J Neurosci Methods. 2010;185(2):204–212. doi: 10.1016/j.jneumeth.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17(6):301–312. doi: 10.1016/j.molmed.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 5.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Reardon DA, Turner S, Peters KB, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw. 2011;9(4):414–427. doi: 10.6004/jnccn.2011.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakobsen JN, Hasselbalch B, Stockhausen MT, Lassen U, Poulsen HS. Irinotecan and bevacizumab in recurrent glioblastoma multiforme. Expert Opin Pharmacother. 2011;12(5):825–833. doi: 10.1517/14656566.2011.566558. [DOI] [PubMed] [Google Scholar]
- 9.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12(3):233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 11.Gerstner ER, Frosch MP, Batchelor TT. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol. 2010;28(6):e91–93. doi: 10.1200/JCO.2009.25.0233. [DOI] [PubMed] [Google Scholar]
- 12.Verhoeff JJ, van Tellingen O, Claes A, et al. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson SJ, Cha S. Imaging glioblastoma multiforme. Cancer J. 2003;9(2):134–145. doi: 10.1097/00130404-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Bendszus M, Warmuth-Metz M, Klein R, et al. MR spectroscopy in gliomatosis cerebri. AJNR Am J Neuroradiol. 2000;21(2):375–380. [PMC free article] [PubMed] [Google Scholar]
- 15.Moffett JR, Namboodiri MA, Cangro CB, Neale JH. Immunohistochemical localization of N-acetylaspartate in rat brain. Neuroreport. 1991;2(3):131–134. doi: 10.1097/00001756-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Nelson SJ. Multivoxel magnetic resonance spectroscopy of brain tumors. Mol Cancer Ther. 2003;2(5):497–507. [PubMed] [Google Scholar]
- 17.Oh J, Henry RG, Pirzkall A, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging. 2004;19(5):546–554. doi: 10.1002/jmri.20039. [DOI] [PubMed] [Google Scholar]
- 18.Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2003;49(2):223–232. doi: 10.1002/mrm.10367. [DOI] [PubMed] [Google Scholar]
- 19.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 20.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Catana C, Ratai EM, et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71(11):3745–3752. doi: 10.1158/0008-5472.CAN-10-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattingen E, Jurcoane A, Bahr O, et al. Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro Oncol. 2011;13(12):1349–1363. doi: 10.1093/neuonc/nor132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6(11):648–657. doi: 10.1038/nrclinonc.2009.150. [DOI] [PubMed] [Google Scholar]
- 24.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252(1):182–189. doi: 10.1148/radiol.2521081534. [DOI] [PubMed] [Google Scholar]
- 25.Ringelstein A, Turowski B, Gizewski ER, et al. [Evaluation of ADC mapping as an early predictor for tumor response to chemotherapy in recurrent glioma treated with bevacizumab/irinotecan: proof of principle] Rofo. 2010;182(10):868–872. doi: 10.1055/s-0029-1245570. [DOI] [PubMed] [Google Scholar]
- 26.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Pope WB. Nonlinear registration of diffusion-weighted images improves clinical sensitivity of functional diffusion maps in recurrent glioblastoma treated with bevacizumab. Magn Reson Med. 2012;67(1):237–245. doi: 10.1002/mrm.23003. [DOI] [PubMed] [Google Scholar]
- 27.Ellingson BM, Cloughesy TF, Lai A, et al. Graded functional diffusion map-defined characteristics of apparent diffusion coefficients predict overall survival in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(10):1151–1161. doi: 10.1093/neuonc/nor079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorensen AG, Emblem KE, Polaskova P, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2011;72(2):402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essock-Burns E, Lupo JM, Cha S, et al. Assessment of perfusion MRI-derived parameters in evaluating and predicting response to antiangiogenic therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(1):119–131. doi: 10.1093/neuonc/noq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radbruch A, Lutz K, Wiestler B, et al. Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro Oncol. 2012;14(2):222–229. doi: 10.1093/neuonc/nor200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellingson BM, Cloughesy TF, Lai A, et al. Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2012;106(1):111–119. doi: 10.1007/s11060-011-0638-x. [DOI] [PubMed] [Google Scholar]
- 33.Negendank WG, Sauter R, Brown TR, et al. Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg. 1996;84(3):449–458. doi: 10.3171/jns.1996.84.3.0449. [DOI] [PubMed] [Google Scholar]
- 34.Sijens PE, Knopp MV, Brunetti A, et al. 1H MR spectroscopy in patients with metastatic brain tumors: a multicenter study. Magn Reson Med. 1995;33(6):818–826. doi: 10.1002/mrm.1910330612. [DOI] [PubMed] [Google Scholar]
- 35.Lee PL, Yiannoutsos CT, Ernst T, et al. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging. 2003;17(6):625–633. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]