Abstract
Background
Vascular endothelial growth factor (VEGF) that is secreted by tumor cells plays a key role in angiogenesis. Matrix metalloproteinase 9 (MMP-9) is produced by inflammatory cells, such as stromal granulocytes (PMN), remodels the extracellular matrix and is known to promote angiogenesis indirectly by interacting with VEGF. The aim of this study was to determine the role of PMN-derived MMP-9, its interaction with VEGF, and the efficacy of anti-angiogenic therapy targeting MMP-9 with oral Doxycycline and VEGF with Bevacizumab in pancreatic cancer (PDAC).
Methodology/principal findings
Inhibitors to MMP-9 (Doxycycline) and VEGF (Bevacizumab) were used alone or in combination in an in vitro angiogenesis assay to test their effect on angiogenesis caused by MMP-9, VEGF, PMN and PDAC cells. In an in vivo model of xenografted PDAC, treatment effects after 14 days under monotherapy with oral Doxycycline or Bevacizumab and a combination of both were evaluated.
In vitro, PMN-derived MMP-9 had a direct and strong proangiogenic effect that was independent and additive to PDAC-derived VEGF. Complete inhibition of angiogenesis required the inhibition of VEGF and MMP-9. In vivo, co-localization of MMP-9, PMN and vasculature was observed. MMP inhibition with oral Doxycycline alone resulted in a significant decrease in PDAC growth and mean vascular density comparable to VEGF inhibition alone.
Conclusions/significance
PMN derived MMP-9 acts as a potent, direct and VEGF independent angiogenic factor in the context of PDAC. MMP-9 inhibition is as effective as VEGF inhibition. Targeting MMP-9 in addition to VEGF is therefore likely to be important for successful anti-angiogenic treatment in pancreatic cancer.
Keywords: VEGF, MMP-9, Neutrophil granulocyte, Pancreatic cancer
Introduction
The acquisition of an angiogenic phenotype is essential for tumor progression beyond a microscopic size [1, 2]. Tumor angiogenesis is a complex process involving many pro- and antiangiogenic signalling molecules as well as proteinases. Vascular endothelial growth factor (VEGF) is the first and best characterized proangiogenic factor. VEGF is required for endothelial cell proliferation, migration and survival. It plays a key role in tumor angiogenesis and is commonly produced and secreted by tumor cells [3–7].
Extracellular proteinases of the matrix metalloproteinase (MMP) class are needed for extracellular matrix remodeling during angiogenesis, but also play an important role during tumor invasion and metastasis [8]. One MMP, MMP-9, was identified as an essential indirect factor during the acquisition of an angiogenic phenotype [9–11]. It facilitates VEGF liberation from the extracellular matrix and is involved in the VEGF-VEGF-receptor interaction [9, 12–15]. MMP-9 is predominantly secreted by inflammatory cells, such as tumor associated macrophages (TAM) and stromal polymorphonuclear neutrophil granulocytes (PMN). TAM are frequently found at the sites of pathological angiogenesis and important for tumor angiogenesis [16, 17]. PMN promote tumor progression by releasing their secretory granules that contain prestored proteases, in particular MMP-9 [9–11]. Consequently, ablation of neutrophils or reconstitution experiments with MMP-9 null mice demonstrated that MMP-9 produced by bone marrow-derived inflammatory cells, such as TAM and PMN, is important for tumor growth and angiogenesis [9, 15, 18].
The aggressive local growth of pancreatic ductal adenocarcinoma (PDAC) is supported by peritumoral inflammation and a pronounced desmoplastic reaction. During angiogenesis in PDAC, PMN derived MMPs may therefore play a particularly important role in addition to VEGF [10, 19–22]. Consequently, both MMP and VEGF inhibition have been predicted to affect tumor growth, but in recent clinical trials the efficacy of monotherapy with either MMP or VEGF inhibitors was limited and inconsistent in advanced and metastatic PDAC [23–28]. This may in part be due to frequent musculoskeletal side effects caused by the MMP inhibitor Marimastat which may have prevented sufficient dosage [23–25]. However, a subgroup of patients with low volume disease benefited from MMP inhibition [23, 25]. Tetracyclines, such as Doxycycline, also act as MMP inhibitors and may be a promising alternative to Marimastat [29, 30], since they have been used for decades as antimicrobial drugs with relatively few side effects.
Given the important role of PMN derived MMP-9 in tumor angiogenesis, we speculated that the protease may also directly promote angiogenesis, independent of tumor cell derived VEGF. The aim of this study was therefore to determine whether PMN derived MMP-9 is indeed a direct and VEGF independent proangiogenic factor. Furthermore, we evaluated the efficacy of anti-angiogenic treatment with Doxycycline alone and in combination with VEGF inhibition in vitro and in vivo.
Methods
Cell culture
Cells were grown as monolayer cultures in humidified 5% CO2 and 95% air at 37°C. Human umbilical vein endothelial cells (HUVEC) were freshly isolated from human umbilical veins by collagenase digestion as described previously [31]. HUVEC were grown in endothelial cell growth medium (ECGM, Promocell, Heidelberg, Germany), containing 10% heat-inactivated fetal bovine serum (FBS) and 1% Penicillin–Streptomycin (Gibco Invitrogen, Karlsruhe, Germany).
HUVEC were frozen in liquid nitrogen and cultured for up to 7 passages. Only HUVEC from passage 1–5 were used in experiments. CAPAN-1 cells (ATCC, Manassas, USA) were grown in Dulbecco’s modified Eagle’s medium—high glucose (Gibco Invitrogen, Karlsruhe, Germany) containing 10% FBS and 1% Penicillin–Streptomycin. CAPAN-1 from passage 10–30 were used for experiments.
Human PMN were isolated from heparinized venous blood using Histopaque-1077 and -1119 kits (Sigma–Aldrich, St. Louis, USA) using a double density gradient in a polysuccrose and sodium diatrizoate solution as previously described [32]. Only freshly prepared PMN were used for experiments. All cells were routinely verified by morphology, growth curve analysis, and tested for Mycoplasma.
In vitro angiogenesis assay
Spheroids were generated as described previously [33, 34]. Briefly, HUVEC were suspended in ECBM + 10% FBS and 20% Carboxymethylcellulose (Sigma–Aldrich, St. Louis, USA) and seeded in hanging drops overnight (Karl Roth GmbH, Karlsruhe, Germany).
Spheroids were harvested within 24 h and embedded into Bovine collagen-I (R&D-Systems Inc., Minneapolis, USA) gels. To assay angiogenesis, 100 μl ECBM containing 10% FBS and the corresponding substances VEGF (BD Pharmingen, San Diego, USA), activated MMP-9 (Chemicon International, Temecula, USA), anti-VEGF antibody (Biozol Diagnostica, Eching, Germany), anti-MMP-9 antibody (Chemicon International, Temecula, USA), TIMP-1 (Chemicon International, Temeluca, USA), Batimastat (BB-94, British Biotech Inc., Oxford, UK) and Doxycycline (Sigma–Aldrich, St. Louis, USA) and/or cells were added on top of the gels followed by overnight incubation (37°C, 5% CO2, 100% humidity). The optimal concentration for each reagent was determined in preliminary experiments (data not shown).
In-gel angiogenesis was quantified by measuring the cumulative length of all capillary like sprouts originating from the central plain of an individual spheroid (CSL) after 24 h using a digitized imaging system (AxioVison, Zeiss, Jena, Germany) connected to an inverted microscope (Axiovert 200, Zeiss, Jena, Germany). At least 10 spheroids per experimental group were analyzed. This analysis takes into consideration that the angiogenic response induced by a specific substance is best reflected by the length of individual capillary-like sprouts as well as the number of capillary like sprouts. All experiments were performed in duplicate or triplicate.
Quantitative Western blot analysis
VEGF and MMP-9 expression in HUVEC, PMN and CAPAN-1 supernatants was quantified by Western blot analysis. Cells were cultured for 72 h in FCS free medium. A fraction of the supernatant was assayed for VEGF and MMP-9 content.
For SDS polyacrylamide gel electrophoresis, Vertical Minigel Units G42 (Biometra, Göttingen) were used together with 12% polyacrylamide gels (acrylamide: bis-acrylamide 30:0.8). Protein concentration was equilibrated to 200 μg using a Coomassie protein assay (Pierce, Rockford, USA).
For VEGF detection, rabbit anti-human VEGF polyclonal antibody (Abcam, Cambridge, UK) was used. MMP-9 was detected by rabbit anti-human MMP-9 polyclonal antibody (Affinity BioReagents, Golden, USA). The secondary antibody was a HRP-coupled goat anti-rabbit MMP-9 polyclonal antibody (Sigma–Aldrich, St. Louis, USA).
Mice
All animal procedures were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Athymic female mice (nu/nu) were obtained from the breeding facility at the Massachusetts General Hospital’s Research Animal Facility. Mice were maintained in a germ-free environment and had access to food and water available ad libitum.
In vivo treatment
Eight- to ten-week-old mice (nu/nu) were used as tumor cell recipients. 1 × 106 CAPAN-1 cells/mouse were injected subcutaneously on the back of the mouse approximately 1 cm distal to the base of the neck. 14 days after tumor cell injection, tumor volume (V) was calculated (V = width × length × depth) and mice were randomized (n = 6/group). Treatment with Bevacizumab (Genentech, San Francisco, CA), Doxycycline (Sigma–Aldrich, St. Louis, MO), a combination of Bevacizumab and Doxycycline or the vehicle (PBS) was initiated for 14 days. Bevacizumab was injected twice weekly intraperitoneally (5 mg/kg BW). Doxycycline was administered orally via drinking water (8 mg/d, based on average water intake of a 30 g mouse). The animals were monitored during the treatment for their bodyweight to assess side effects and did not show any significant loss in weight (less than 10% of bodyweight).
Tumor volume was also measured weekly. After 14 days, tumors were removed, a section was preserved with 4% paraformaldehyde in phosphate-buffered saline (PBS; Invitrogen) and paraffin embedded for immunostaining. The remaining tissue was snap frozen and cryo-preserved at −80°C for further analysis.
Immunostaining
Primary antibodies for immunohistochemical staining were a rat polyclonal mouse neutrophil antibody (Angio-Proteomie, Boston, MA), a rat monoclonal CD34 antibody (Abcam, Cambridge, MA) and a goat polyclonal MMP-9 (R&D Systems, Minneapolis, MN). Primary antibodies were detected by using anti-goat biotinylated horse antibody (Vector Laboratories, Burlingame, CA) and anti-rat biotinylated rabbit antibody (Vector Laboratories, Burlingame, CA). Paraffin embedded sections of tumors deparaffinized, hydrated with TBS and blocked with H2O2. Antigen retrieval was achieved by boiling tissue in Retrievit (BioGenex, San Ramon, CA). After blocking with Avidin/Biotin (Vector Laboratories, Burlingame, CA) and 5% goat or rabbit serum in TBS, slides were incubated with primary antibodies overnight at 4°C. Sections were washed three times in TBST, followed by secondary antibody for 1 h at RT. After washing in TBS, sections were incubated with Vectastain ABC (Vector Laboratories, Burlingame, CA) for 30 min and developed with DAB (Invitrogen, Carlsbad, CA). Slides were counterstained with hematoxylin and viewed with a Y-FL microscope (Nikon, Japan). Images were acquired with a DP25 camera and acquisition software (Olympus, Japan).
Histological assessment
All histological measurements were performed on composite images of whole slides. Those were generated with Autostitch® software from overlapping single images of the whole slides. Mean vascular density was calculated by counting CD-34 positive blood vessels in the composite images of each slide and dividing the number obtained by the non-necrotic tumor area on the slide for six animals per group.
Quantitative analysis for MMP-9 and PMN positive cells was performed by counting the number of cells in the composite images of each slide and dividing the number obtained by the non-necrotic tumor area on the slide in six animals per group.
VEGF and MMP-9 concentration
MMP-9 and VEGF concentration in the tumor tissue was determined by a MMP-9 ELISA (MMP-9 Biotrak, Amersham Biosciences, NJ) and VEGF ELISA (Quantikine, R&D Systems, MN). Briefly, 50 mg of snap frozen tumor tissue were lysed in RIPA buffer and the lysate was cleared by centrifugation. Protein concentration was determined (2-D Quant Kit, Amersham Biosciences, NJ) and equal amounts of protein were used in the respective ELISAs. ELISAs were performed according to the manufacturer’s instructions.
Statistical analysis
Statistical comparisons of in vitro and in vivo data sets were performed by a two-tailed Student’s t test or one-way ANOVA with Tukey post test. The data were considered to be significantly different when P < 0.05 and are presented as mean ± standard error of the mean.
Results
In vitro, the angiogenic activity of MMP-9 and VEGF is additive and independent
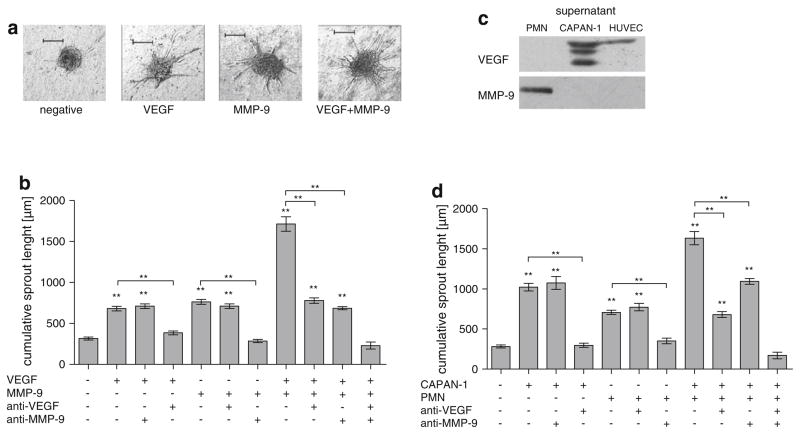
To determine the role of MMP-9 in angiogenesis in relation to VEGF, a 3-dimensional in vitro sprouting angiogenesis assay was used (Fig. 1a). Unstimulated HUVEC had a very low baseline mean cumulative sprout length (CSL) below 500 μm (Fig. 1). The addition of exogenous MMP-9 alone to the angiogenesis assay resulted in a more than twofold increase of the CSL compared to the negative control (682 μm vs. 317 μm, P < 0.001; Fig. 1b). VEGF had a similar effect (764 μm vs. 317 μm, P < 0.001; Fig. 1b). The combined addition of MMP-9 and VEGF resulted in an additive effect with a more than twofold increase in sprouting compared to the effect of each protein alone (1,714 μm vs. VEGF: 764 μm and MMP-9: 682 μm; P < 0.001 Fig. 1b). MMP-9 is therefore a potent stimulant of angiogenesis and acts additive to VEGF.
Fig. 1.
Comparison of the angiogenic effect of PDAC tumor cells, VEGF, granulocytes and MMP-9. Quantitative three-dimensional in vitro angiogenesis assay. Capillary sprouting originating from the spheroids was quantified. Human umbilical vein endothelial cells (HUVEC) have a low baseline level of capillary sprouting. Representatives are shown in a from left to right: control HUVEC, HUVEC + VEGF (10 ng/ml), HUVEC + MMP-9 (0.1 ng/ml), HU-VEC + VEGF (10 ng/ml) + MMP-9 (0.1 ng/ml). b The effect of VEGF and MMP-9 is additive (P < 0.001, compared to VEGF or MMP-9 alone). Antibodies to VEGF or MMP-9 (10 μg/ml each) inhibit the specific stimulus induced by VEGF or MMP-9 but not vice versa (P < 0.001 compared to corresponding control). c Quantitative Western blot analysis of CAPAN-1, PMN and HUVEC culture supernatants for VEGF and MMP-9. After 72 h of culture in FCS free medium, HUVEC, PMN and CAPAN-1 supernatants were quantified by SDS–PAGE and Western Blot. MMP-9 was only found only in the PMN supernatant whereas VEGF was identified in the CAPAN-1 and HUVEC supernatants. d The effect of PMN and CAPAN-1 cells is additive (P < 0.001, compared to CAPAN-1 or PMN alone). Antibodies to VEGF or MMP-9 (10 μg/ml each) inhibit the specific stimulus induced by CAPAN-1 or PMN but not vice versa (P < 0.001 compared to corresponding control). Error bars: SEM; **: P < 0.001. To avoid overcrowding of the graphs, not all P values are denoted, please see Results section
Antibodies against VEGF had no effect on MMP-9 stimulated spheroids (764 μm vs. 711 μm; Fig. 1b) but completely inhibited VEGF induced sprouting (385 μm vs. 682 μm, P < 0.001; Fig. 1b). Antibodies against MMP-9 likewise completely inhibited MMP-9 induced angiogenesis (285 μm vs. 764 μm, P < 0.001; Fig. 1b) but had no effect on VEGF induced sprouting (682 μm vs. 712 μm; Fig. 1b). The angiogenic effect of MMP-9 and VEGF together is only completely blocked if antibodies against both VEGF and MMP-9 are used (231 μm vs. 1,714 μm, control: 317 μm; Fig. 1b). Antibodies against VEGF (780 μm vs. 1,714 μm, control: 317 μm; Fig. 1b) or antibodies against MMP-9 (686 μm vs. 1,714 μm, control: 317 μm; Fig. 1b) alone only partially inhibited angiogenesis in spheroids stimulated with both MMP-9 and VEGF. MMP-9 thus induces angiogenesis independent of VEGF, implying a direct angiogenic effect of the protease.
In vitro PMN and PDAC cells are additive and independent angiogenic factors
To determine the source of MMP-9 and VEGF in vitro, quantitative Western blot analysis of supernatants from CAPAN-1, PMN and HUVEC was performed. CAPAN-1 cells were the major source of secreted VEGF. HUVEC demonstrated minimal secretion of VEGF, while PMN did not produce the protein. MMP-9 was only secreted by PMN (Fig. 1c).
To determine whether VEGF secreted by CAPAN-1 tumor cells and MMP-9 secreted by PMN play a functional role in angiogenesis in vitro, the 3-dimensional in vitro sprouting angiogenesis assay was used. The addition of PMN to the angiogenesis assay resulted in a more than 2.5-fold increase in sprouting (708 μm vs. 283 μm, P < 0.001; Fig. 1d). CAPAN-1 cells had a similar effect (1,022 μm vs. 283 μm, P < 0.001; Fig. 1d). Similar to MMP-9 and VEGF, there is an additive angiogenic effect of PMN and CAPAN-1 cells if both are added to the assay together (1,632 μm vs. 708 μm and 1,022 μm, P < 0.001; Fig. 1d).
PMN and CAPAN-1 cells also act as independent sources of angiogenic factors. Antibodies to VEGF completely inhibited the effect caused by CAPAN-1 cells (298 μm vs. 1,022 μm, control: 283 μm, P < 0.001; Fig. 1d) but did not affect stimulation by PMN (708 μm vs. 772 μm; Fig. 1d). PMN induced sprouting was abolished by antibodies to MMP-9 (708 μm vs. 350 μm, control: 283 μm, P < 0.001; Fig. 1d), which did not inhibit CA-PAN-1 induced sprouting (1,022 μm vs. 1,074 μm; Fig. 1d). The angiogenic effect of both PMN and CAPAN-1 to the assay was only completely blocked if antibodies against both VEGF and MMP-9 are used (171 μm vs. 1,632 μm, control: 283 μm; Fig. 1d). Antibodies against VEGF (679 μm vs. 1,632 μm, control: 283 μm; Fig. 1d) or antibodies against MMP-9 (1,094 μm vs. 1,632 μm, control: 283 μm; Fig. 1d) alone only partially inhibited angiogenesis in spheroids stimulated by PMN and CAPAN-1 together.
In vitro, Doxycycline inhibits MMP-9 and PMN angiogenic activity as effectively as endogenous and exogenous MMP inhibitors
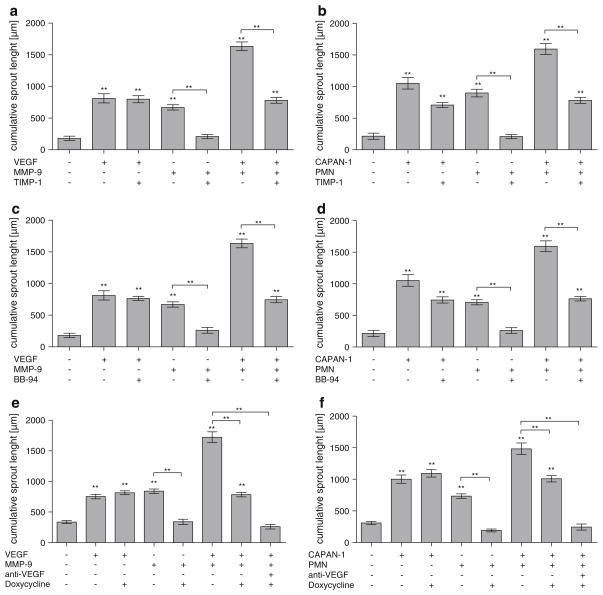
To determine if the antibiotic Doxycycline inhibits angiogenesis by blocking MMP-9, its efficacy was compared in vitro to the endogenous MMP-9 inhibitor TIMP-1 and the exogenous MMP inhibitor Batimastat. The endogenous MMP inhibitor TIMP-1 (40 ng/ml) specifically blocked MMP-9 (669 μm vs. 207 μm; control: 180 μm, P < 0.001; Fig. 2a) and PMN activity (897 μm vs. 207 μm; control: 215 μm, P < 0.001; Fig. 2b). TIMP-1 did not affect VEGF induced angiogenesis (812 μm vs. 798 μm; Fig. 2a). The artificial MMP inhibitor Batimastat (BB-94, 1 μg/ml) prevented all MMP-9 (669 μm vs. 261 μm, control: 180 μm, P < 0.001; Fig. 2c) and PMN (707 μm vs. 261 μm; control: 215 μm; P < 0.001; Fig. 2d) angiogenic activity. VEGF (812 μm vs. 763 μm; Fig. 2c) or CAPAN-1 cells (1,053 μm vs. 744 μm; Fig. 2d) retained their angiogenic effect in the presence of BB-94.
Fig. 2.
Characterization of the antiangiogenic effect of TIMP-1, BB-94 and Doxycycline. Quantitative three-dimensional in vitro angiogenesis assay. Capillary sprouting originating from the spheroids was quantified. Human umbilical vein endothelial cells (HUVEC) have a low baseline level of capillary sprouting. Using VEGF and MMP-9 or CAPAN-1 and PMN, antibodies to MMP-9 can be replaced by the endogenous MMP inhibitor TIMP-1 (40 ng/ml; a, b), the synthetic MMP inhibitor BB-94 (1 μg/ml; c, d) or by Doxycycline (50 μM/ml; e, f). Error bars: SEM; **: P < 0.001. To avoid overcrowding of the graphs, not all P values are denoted, please see Sec Results
Doxycycline (50 μM/ml = 0.052 mg/ml) inhibited angiogenesis as effectively as TIMP-1 or Batimastat. It completely blocked sprouting by MMP-9 (841 μm vs. 341 μm; control: 336 μm, P < 0.001; Fig. 2e) and PMN (734 μm vs. 191 μm; control: 308 μm, P < 0.001; Fig. 2f). VEGF or CAPAN-1 induced stimulation was not affected by Doxycycline (VEGF: 752 μm vs. 816 μm; CAPAN-1: 1,002 μm vs. 1,092 μm; Fig. 2e, f). A complete inhibition of angiogenesis induced by MMP-9 and VEGF together was only achieved if Doxycycline was used in conjunction with antibodies to VEGF (262 μm vs. control: 336 μm; Fig. 2e). Doxycycline alone only partially inhibited angiogenesis in spheroids stimulated by both MMP-9 and VEGF (784 μm vs. control: 336 μm; Fig. 2e). The same effects are observed if spheroids were stimulated with both CAPAN-1 and PMN (Fig. 2f).
MMP inhibition effectively affects tumor growth and vascular density in vivo
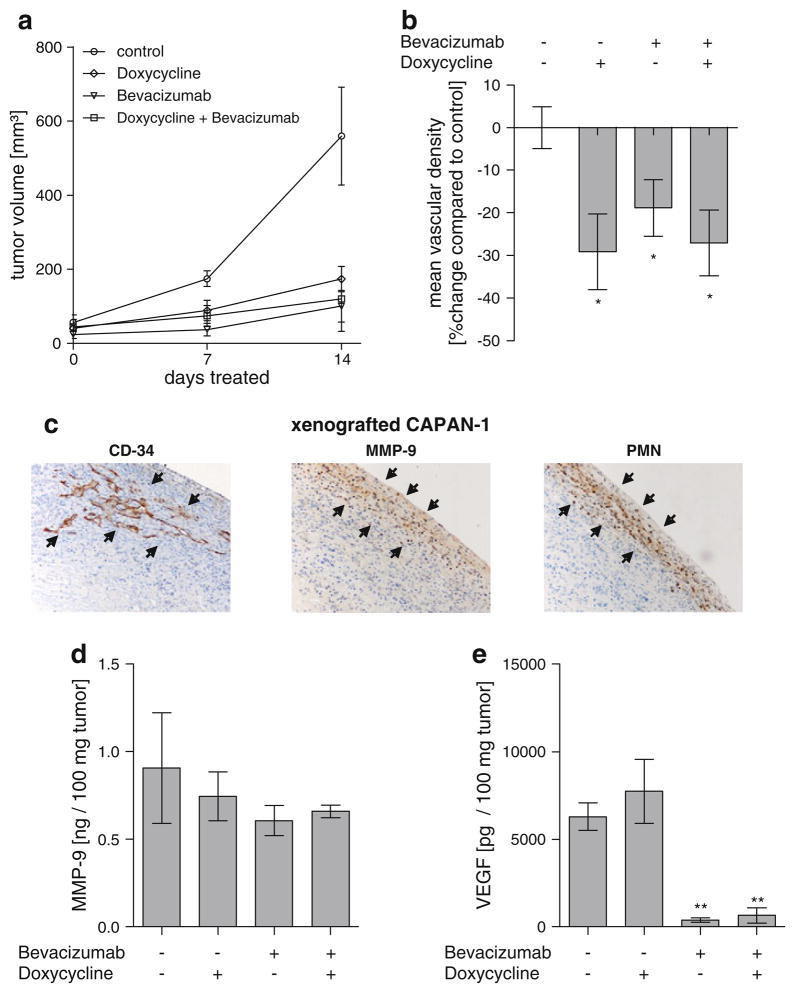
To determine the relevance of the in vitro findings in vivo, mice bearing CAPAN-1 PDAC cell derived tumors (n = 6/group) were treated for 14 days either with antibodies to VEGF (Bevacizumab), Doxycycline or the combination of both. Treatment with orally administered Doxycycline alone was sufficient to significantly reduce the tumor growth rate and mean vascular density and was comparable to treatment with Bevacizumab. Tumor volume after 14 days of treatment was 559.5 mm3 in the control group, 173.5 mm3 for Doxycycline (P < 0.05), 100.4 mm3 for Bevacizumab (P < 0.01) and 120.2 mm3 for the combination of both (P < 0.01; Fig. 3a).
Fig. 3.
Treatment of xenografted human PDAC with the MMP-9 inhibitor Doxycycline, the VEGF inhibitor Bevacizumab or a combination of both for 14 d. a Tumor volume during treatment. Monotherapy with Bevacizumab and Doxycycline and the combination of both significantly reduced tumor growth. b Mean vascular density was significantly lower in all treated animals if compared to control. c The majority of the vasculature present in the xenografted CAPAN-1 PDAC tumors was found in the periphery, adjacent to PMN and MMP-9 positive cells. Vasculature was less frequently found towards the center of the tumor, PMN- and MMP-9 positive cells were almost absent here. d Total MMP-9 in the tumor was not different in the different groups, whereas Bevacizumab caused a significant reduction in VEGF (e). Error bars: SEM; *:P < 0.05
The reduction of tumor growth correlated with a significant reduction of mean vascular density (MVD) in the treatment groups. The MVD was 48.51 vessels/1,000 pixels2 viable tumor in the control group. It was reduced to 34.39 for Doxycycline (P < 0.05), 39.37 for Bevacizumab (P < 0.05) and 35.39 for the combined treatment with Doxycycline and Bevacizumab (P < 0.05 Fig. 3b).
The vast majority of MMP-9 positive cells and PMN co-localized immediately adjacent to areas with the highest density of tumor vasculature in all groups. The majority of MMP-9 positive cells, PMN and vasculature were found in the periphery of the tumors in all groups (Fig. 3c). Treatment with Doxycycline did not affect the number of MMP-9 positive cells and PMN within the tumor tissue (data not shown). The concentration of total MMP-9 within the tumor tissue was also not reduced by the presence of Doxycycline, indicating that the drug acts through the inhibition of activated MMP-9. It was 0.90 ng/100 mg tumor in the control group, 0.75 ng/100 mg tumor for Doxycycline (P > 0.05), 0.61 ng/100 mg tumor for Bevacizumab (P > 0.05) and 0.66 ng/100 mg tumor for the combination of both (P > 0.05; Fig. 3d). In contrast, treatment with Bevacizumab resulted in a significant reduction of VEGF within the tumor tissue. The concentration of VEGF was 6,291 pg/100 mg tumor in the control group. It was reduced to 391 pg/100 mg tumor by monotherapy with Bevacizumab (P < 0.001) and to 651 pg/100 mg tumor by combined treatment with Bevacizumab and Doxycycline (P < 0.01). Treatment with Doxycycline alone did not cause a significant reduction of VEGF within tumor tissue (3,058 pg/100 mg tumor, P > 0.05; Fig. 3e).
Discussion
MMP-9 was originally thought to be involved in tumor angiogenesis indirectly by remodeling of the extracellular matrix [8] and interacting with VEGF [9, 12].
In this study, we show that MMP-9 derived from PMN also is a direct and potent factor in tumor angiogenesis. MMP-9 and PMN alone induced robust in vitro angiogenesis, even in the absence of VEGF and in the presence of antibodies to VEGF. MMP-9 thus has a direct proangiogenic effect in addition to its interaction with VEGF and the VEGF receptor [9, 12]. Interestingly, MMP-9 and VEGF together consistently caused about 15% more sprouting in the in vitro angiogenesis assay than expected based on the sprouting induced by VEGF or MMP-9 alone. This may in part be due to the interaction of MMP-9 with VEGF [9, 12].
Remodeling of the extracellular matrix by MMP-9 [35, 36] cannot account for the direct proangiogenic effect of MMP-9 since collagen I, which is no substrate for the protease [37, 38], was used exclusively in all in vitro experiments. However, MMP-9 is known to directly promote cell migration by altering cell–cell adhesions and MMP-9 induced cell migration is abolished by TIMP-1, its specific endogenous inhibitor [39, 40]. We also observed that angiogenesis induced by MMP-9 was abolished by TIMP-1, which did not affect CAPAN-1 and VEGF mediated angiogenic activity. MMP-9 may therefore exert its direct proangiogenic effect by promoting endothelial cell migration.
In vivo, MMP-9 positive cells predominantly co-localized with PMN. TAM, which are another important source of MMP-9 in cancer [16, 17], were therefore not a major source of stromal MMP-9 in our model system. Our findings thus suggest that MMP-9 derived from PMN may be as important as VEGF in tumor angiogenesis in the context of pancreatic cancer. Its potential to induce sprouting in the vitro assay equalled that of VEGF and its action was additive to VEGF. Consequently, angiogenesis induced by both VEGF and MMP-9 required the inhibition of both proteins to completely abolish all sprouting activity in vitro. In vivo, oral treatment with the MMP-9 inhibitor Doxycycline alone was as effective as VEGF inhibition with Bevacizumab. MMP-9 is therefore likely an important potential target for anti-angiogenic therapy. Clinical trials targeting either VEGF or MMP in advanced and metastatic pancreatic cancer have been of limited success [23–28]. This may in part be due to insufficient MMP inhibition caused dosage limitations necessary to prevent the musculoskeletal side effects of the synthetic MMP inhibitor Marimastat [23–25]. Doxycycline, used in this study, may be a viable alternate MMP inhibitor. It effectively inhibited MMP-9 activity in vitro. In vivo, its oral administration alone was sufficient to effectively inhibit angiogenesis and reduce tumor growth comparable to VEGF inhibition. The concentration used is easily achievable and has demonstrated few severe side effects in its decade long use as antimicrobial agent in humans [30].
Incomplete inhibition of angiogenesis by targeting VEGF or MMP alone, as observed in vitro in this study, may also account for the limited efficacy in clinical trials. Moreover, malignant progression and increased metastasis formation has been described in animal models under anti-angiogenic monotherapy with VEGF inhibitors [41, 42]. Therefore, anti-angiogenic therapy that targets several independent angiogenic factors may be favourable. Combination therapy targeting both VEGF and MMP-9 did not show a clear advantage in vivo in this study. We attribute this observation to the marked effects of each mono-therapy. Additionally, angiogenic factors in a tumor may change and increase with malignant progression. A clear advantage of antiangiogenic therapy with both MMP and VEGF inhibitors may thus only become apparent in advanced or metastatic cancers, whereas monotherapy is sufficient in the small cancers that were treated in this study. Since combined MMP and VEGF inhibition was required to abolish all sprouting angiogenesis in vitro, further evaluation of the benefits of combination therapy in vivo seems warranted.
Taken together, PMN derived MMP-9 is a potent, direct and VEGF independent angiogenic factor. Therapeutic strategies targeting MMP-9 in addition to or together with VEGF may thus be important for successful anti-angiogenic treatment in human pancreatic cancer and offer a viable alternate treatment for this devastating disease.
Acknowledgments
The authors would like to thank Nancy Neyhard, Amy Stirman, Silke Hempel and Bettina Waldvogel for their technical assistance and Andrew Liss for his assistance composing the manuscript.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Dirk Bausch, Department of General and Visceral Surgery, University of Freiburg, Hugstetter Str. 55, 79106 Freiburg, Germany.
Thomas Pausch, Klinik für Allgemein, Viszeral- und Transplantationschirurgie, Universität Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany.
Tobias Krauss, Radiologische Universitätsklinik Freiburg i. Br., Hugstetter Str. 55, 79106 Freiburg i. Br., Germany.
Ulrich Theodor Hopt, Department of General and Visceral Surgery, University of Freiburg, Hugstetter Str. 55, 79106 Freiburg, Germany.
Carlos Fernandez-del-Castillo, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, 15 Parkman St., WAC 460, Boston, MA 02114-2622, USA.
Andrew L. Warshaw, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, 15 Parkman St., WAC 460, Boston, MA 02114-2622, USA
Sarah P. Thayer, Department of Surgery, Massachusetts General Hospital and Harvard Medical School, 15 Parkman St., WAC 460, Boston, MA 02114-2622, USA
Tobias Keck, Email: Tobias.Keck@uniklinik-freiburg.de, Department of General and Visceral Surgery, University of Freiburg, Hugstetter Str. 55, 79106 Freiburg, Germany.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 4.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Manseau EJ, Dvorak AM, Dvorak HF. VEGF-A induces angiogenesis, arteriogenesis, lymphangiogenesis, and vascular malformations. Cold Spring Harb Symp Quant Biol. 2002;67:227–237. doi: 10.1101/sqb.2002.67.227. [DOI] [PubMed] [Google Scholar]
- 5.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 6.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108(Pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 7.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 9.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103(33):12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104(51):20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277(39):36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 13.Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G, Hommes DW, Lamers CB, Sier CF. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 2008;44(13):1904–1913. doi: 10.1016/j.ejca.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Kuwai T, Kim JS, Fan D, Kim SJ, Fidler IJ. Stromal metalloproteinase-9 is essential to angiogenesis and progressive growth of orthotopic human pancreatic cancer in parabiont nude mice. Neoplasia. 2007;9(11):979–986. doi: 10.1593/neo.07742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94(15):1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 18.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandol S, Edderkaoui M, Gukovsky I, Lugea A, Gukovskaya A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7(11 Suppl):S44–S47. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roland CL, Dineen SP, Toombs JE, Carbon JG, Smith CW, Brekken RA, Barnett CC., Jr Tumor-derived intercellular adhesion molecule-1 mediates tumor-associated leukocyte infiltration in orthotopic pancreatic xenografts. Exp Biol Med(Maywood) 2010;235(2):263–270. doi: 10.1258/ebm.2009.009215. [DOI] [PubMed] [Google Scholar]
- 21.Bloomston M, Zervos EE, Rosemurgy AS., 2nd Matrix metalloproteinases and their role in pancreatic cancer: a review of preclinical studies and clinical trials. Ann Surg Oncol. 2002;9(7):668–674. doi: 10.1007/BF02574483. [DOI] [PubMed] [Google Scholar]
- 22.Hotz HG, Hines OJ, Hotz B, Foitzik T, Buhr HJ, Reber HA. Evaluation of vascular endothelial growth factor blockade and matrix metalloproteinase inhibition as a combination therapy for experimental human pancreatic cancer. J Gastrointest Surg. 2003;7 (2):220–227. doi: 10.1016/s1091-255x(02)00157-9. discussion 227–228. S1091255X02001579. [DOI] [PubMed] [Google Scholar]
- 23.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87(2):161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans JD, Stark A, Johnson CD, Daniel F, Carmichael J, Buckels J, Imrie CW, Brown P, Neoptolemos JP. A phase II trial of marimastat in advanced pancreatic cancer. Br J Cancer. 2001;85(12):1865–1870. doi: 10.1054/bjoc.2001.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19(15):3447–3455. doi: 10.1200/JCO.2001.19.15.3447. [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27(13):2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 27.Ko AH, Dito E, Schillinger B, Venook AP, Xu Z, Bergsland EK, Wong D, Scott J, Hwang J, Tempero MA. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest New Drugs. 2008;26(5):463–471. doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 28.Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, Vokes EE. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23(31):8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 29.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12(2):12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 30.Hanemaaijer R, Visser H, Koolwijk P, Sorsa T, Salo T, Golub LM, van Hinsbergh VW. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res. 1998;12(2):114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7. [PubMed] [Google Scholar]
- 33.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143(5):1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112(Pt 19):3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 35.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270(11):5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 38.Patterson ML, Atkinson SJ, Knauper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503(2–3):158–162. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- 39.Akahane T, Akahane M, Shah A, Connor CM, Thorgeirsson UP. TIMP-1 inhibits microvascular endothelial cell migration by MMP-dependent and MMP-independent mechanisms. Exp Cell Res. 2004;301(2):158–167. doi: 10.1016/j.yexcr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008;217(3):643–651. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Anti-angiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]