Abstract
Systemic inflammation is associated with impaired wound healing in diabetic patients. Using immunohistochemistry techniques, the authors investigated changes in skin inflammation and skin blood vessels in human and experimental diabetes. Comparing to the non-DM human subjects, the total number of inflammatory cells per biopsy and the number of inflammatory cells around blood vessels, a strong indication of inflammation, were higher in DM subjects irrespective of their risk for developing diabetic foot ulcer. Inflammatory cell infiltration was robustly increased in all diabetic animal models compared to their non-diabetic controls. The number and density of blood vessels and CD31 positive proliferating endothelial cells around pre-existing skin vessels was also higher in the DM patients. However, there were no differences in the skin blood flow between the non-DM and DM subjects. The number of skin blood vessels was also increased in the DM animals; however, these differences were less obvious than the ones observed for inflammatory cells. We conclude that skin inflammation and skin blood vessel density is increased in diabetic human subjects and in rodent and rabbit models of diabetes.
Keywords: Diabetes, Wound Healing, Skin Changes, Skin Inflammation, Skin Blood Vessels
INTRODUCTION
Foot ulcers and impaired wound healing are common problems in diabetes as they can affect 15% of all diabetic (DM) patients during their lifespan resulting in more than 70,000 lower extremity amputations per year in the USA alone1,2. As the diabetes pandemic continues unabated and diabetic patients live longer, it should be expected that the incidence of diabetic foot problem would increase over the next few decades.
Recent studies by our group have shown that systemic inflammation is associated with impaired wound healing in DM patients3. In addition, we have shown that skin biopsies from both the forearm and dorsum of the foot of DM patients have increased inflammatory cell infiltration. However, there is little information regarding the blood vessel density in the skin of DM patients. Furthermore, it is not known whether the skin changes observed in DM patients are also present in various experimental animal models of diabetes commonly used in wound healing studies. Given the lack of satisfactory animal models, the possible similarities and differences between human and experimental diabetes may prove very helpful in designing mechanistic studies in the future.
In the present study we used immunohistochemistry techniques to compare the inflammatory cell infiltration and blood vessel density changes between non-diabetic (non-DM) and diabetic (DM) rabbits, rats and mice. We also compared the animal study results with the results obtained from the forearm and dorsal foot skin biopsies of non-DM and DM human subjects.
MATERIALS AND METHODS
Human Subjects
Forearm skin biopsies
In the present study we evaluated 2mm forearm skin biopsies from human subjects. These biopsies were obtained from a well -characterized cohort of healthy control (non-DM) subjects and diabetic (DM) patients that had participated in a prospective study involving investigation of the mechanisms of wound healing impairment in diabetes. Detailed information regarding this group is provided elsewhere3
Foot Skin Biopsies
For the present study we used discarded skin specimens that were collected for the same study as the forearm skin biopsies. Hematoxylin and Eosin (H&E) and CD31 immunohistochemistry staining was performed in all biopsies. In our previous study round cells were confirmed as being inflammatory cells by staining for CD45RO, a marker of lymphocytes and Factor XIIIa, a marker of dermal dendrocytes3. Skin blood flow was monitored using a Laser Doppler Perfusion Imager (Lisca PIM 2.0, Lisca Development AB, Linkoping, Sweden) as previously described4. In brief, a 2x2 cm skin area was scanned after all subjects were acclimatized in room with controlled temperature for 30 minutes. The skin area was the same that skin biopsies were performed after skin blood flow measurements were completed. The protocol was approved by the Institutional Review Board (IRB) of the Beth Israel Deaconess Medical Center. All participants gave written informed consent.
Animals
Rabbit model
New Zealand White male rabbits weighing 3.0 to 3.2 kg were obtained from Millbrook Farms (Amherst, MA). Six rabbits were made diabetic by injecting two doses of 50 mg/kg of alloxan monohydrate via the marginal ear vein, 48 hours apart. Seven rabbits in the non-diabetic group received vehicle alone (saline). Rabbits with fasting blood glucose over 250 mg/dL were considered diabetic. Ten days after alloxan administration, rabbits were anesthetized using ketamine (25 mg/kg i.m.) and xylazine (3 mg/kg i.m.) and skin biopsies from the ear were obtained using a 6-mm punch biopsy.
Rat model
Eight-week old male Sprauge-Dawley rats were obtained from Charles River Laboratories. Ten rats were made diabetic by administering a single dose of Streptozotocin (STZ) (60mg/Kg, i.p.) in citrate buffer (0.1M). The non-diabetic group (9 rats) received equal volume of vehicle alone. Rats with fasting blood glucose over 250 mg/dL were considered diabetic. 6–8 weeks after STZ treatment, rats were anesthetized using ketamine (35 mg/kg i.m.) and xylazine (2.5 mg/kg i.m.) and 6mm full thickness skin punch biopsies were obtained from the shaved dorsum of the rat.
Mouse model
C57BL6 and WBB6F1 male mice were obtained from Jackson Laboratories. Nine 8-weeks-old mice were made diabetic by administering 50 mg/kg STZ (i.p q.i.d for 5 consecutive days) in citrate buffer (0.1M). In the non-diabetic group, 9 mice were treated with vehicle alone. Fasting blood glucose was monitored a week after the last injection and mice with blood glucose over 250 mg/dL were considered diabetic. 6–8 weeks after STZ treatment, mice were anesthetized using ketamine (100 mg/kg i.p.) and xylazine (5 mg/kg i.p.) and 6mm full thickness skin punch biopsies were obtained from the shaved dorsum of the mice. All animal studies were conducted in accordance with Institutional Animal Care and Use Committee (IACUC) approved protocols.
Tissue analysis
Human specimens
Biopsies were embedded in optimal cutting temperature compound (OCT) and frozen in liquid nitrogen immediately after collection, stored at − 80°C and were analyzed by a pathologist with expertise in dermatopathology (AK). 5-μm sections were cut and fixed in acetone immediately before staining. To study skin inflammation, sections were stained with H&E according to standard protocols for cryosections. The number of inflammatory round cells was counted in each biopsy. To evaluate skin blood vessel density, immunohistochemistry for CD31 was performed using a mouse monoclonal antibody (JC70A, Abcam, Cambridge, MA, USA), as described elsewhere (3). CD31 serves as a marker for endothelial cells; therefore immunohistochemistry for CD31 was performed with the purpose of identifying both proliferating endothelial cells and skin blood vessels. Endothelial cells were characterized as proliferating when they were observed as single, CD31 positive cells that were in the proximity of pre-existing blood vessels but did not have a lumen5.
Animal samples
Skin samples were fixed in 10% formalin immediately after collection and embedded in paraffin blocks. 6-μm sections were cut and stained with H&E according to standard techniques for paraffin sections. Analysis was performed by an observer (AT) who was unaware of the group each biopsy belonged under the supervision of the pathologist (AK).Round inflammatory cells and blood vessels were counted in four different visual fields per section (400x). In order to avoid the risk of misclassifying other cell types as inflammatory, which in most cases are transversely cut fibroblasts/fibrocytes that may appear round, we mainly counted round cells with a diameter of 6–12 microns in the proximity of blood vessels where inflammatory cells are first observed during the inflammatory process. We also took into consideration additional subtle morphological details and we excluded cells with characteristics that are compatible with fibroblasts/fibrocytes (diameter 12–15 microns, bland looking nucleus). CD31 (a marker of endothelial cells) staining was performed using a purified rat monoclonal antibody (BD 550274, BD Biosciences, San Jose, CA, USA) for mouse, a rabbit polyclonal antibody (250590, ABBiotech San Diego, CA) for rat and a rat monoclonal antibody (AB 56299, Abcam, Cambridge, MA, USA) for rabbit specimens. Briefly, sections were deparaffinized, hydrated and subjected to proteinase K treatment for antigen retrieval. Overnight incubation with the primary antibody at 4°C was followed by biotinylated secondary antibody (Vector laboratories, Burlingame, CA,USA) and avidin-biotin-peroxidase complex by using the Vectastain elite ABC rat kit and diaminobenzidine (DAB) as chromogen development (Vector laboratories, Burlingame, CA, USA). The number of CD31 positive endothelial cells and/or the number of blood vessels were counted in four different high power fields per section (400×). Results are expressed as average number/visual field.
Statistical analysis
Data analysis was performed using Minitab (Minitab, State College, PA). Analysis of variance (ANOVA) and the t-test were employed for the comparisons among the groups for normally distributed data. Non-parametrical data were analyzed through Kruskal-Wallis analysis of variance. The Pearson test was used to for the calculation of the correlation coefficient. Data are presented as mean ± sd.
RESULTS
Human Forearm Biopsies
We examined the forearm skin biopsies from 12 healthy non-DM subjects (age 60 ± 6 years, 4 males, group C). DM subjects were divided into two groups, 10 diabetic subjects with neuropathy not severe enough to be at risk of developing foot ulceration (low-risk) (age 56 ± 9 yrs, 4 males, 3 Type 1 diabetes, diabetes duration 12 ± 13 yrs, group D), and 50 neuropathic diabetic subjects at risk of foot ulceration (high-risk) (age 56 ± 8 yrs, 37 males, 21 Type 1 diabetes, diabetes duration 22 ± 14 yrs, group DN). Diabetes duration was 32 ± 13 years in Type 1 DM and 13 ± 9 in type 2 DM patients (p<0.001). There were no other major differences in the clinical characteristics among these three groups.
H&E Analysis
Comparing to the non-DM subjects, the total number of inflammatory cells per biopsy was higher in both DM groups (low-risk and high -risk) (C: 83 ± 44, D: 143 ± 51, DN: 110 ± 39, p<0.01) (Fig. 1). Similar results were observed for the number of inflammatory cells around vessels, a strong indication of inflammation (C: 8 ± 7, D: 17 ± 9, DN 14± 11, p<0.05). Further, There were no differences between Type 1 and Type 2 DM subjects in both the total number of inflammatory cells in the dermis (118 ± 47 vs. 112 ± 40 respectively, p=NS) and the number of inflammatory cells around vessels (12 ± 9 vs. 15 ± 11, p=NS).
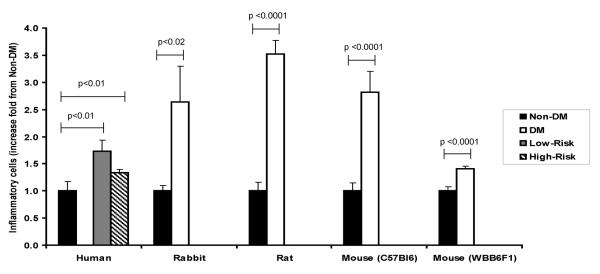
Figure 1.
Fold change in the skin inflammatory cells comparing non-diabetic (non-DM) and diabetic (DM) within respective species. Skin inflammatory cells were increased in DM patients with low or high risk for foot problems when compared to non-DM subjects. Similar results were observed in DM rabbits, rats and mice when compared to their non-DM counterparts. Data are presented as mean ± SE.
The number of skin vessels also trended to be higher in the two diabetic groups when compared to the non-DM subjects but failed to reach statistical significance (C: 5.9 ± 2.9, D: 7.2 ± 2.2, DN: 6.8 ± 2.2, p=NS).
CD31 Staining Analysis
The number of CD31 positive proliferating endothelial cells around pre-existing skin vessels was higher in both low-risk and high-risk DM subjects (C: 0 ± 0, D: 0.5 ± 0.5, DN 0.5± 0.7, p<0.05). The number of skin blood vessels per mm2 was marginally higher in the two DM groups C: 21 ± 9, D: 23 ± 7, DN 28± 12, p=0.057). However, when the two DM groups were merged in one group, the number of vessels per mm2 was higher in the DM group when compared to the non-diabetic subjects (27 ± 11 vs. 21 ± 9, p<0.05). There were no differences between Type 1 and Type 2 DM subjects in both proliferating cells (0.5 ± 0.6 vs. 0.5 ± 0.7 respectively, p=NS) and the number of vessels per mm2 (26 ± 12 vs. 28 ± 11, p=NS).
There were no differences in the skin blood flow between the DM and non-DM subjects [C: 1.01 ± 0.23 (arbitrary units), D: 1.27 ± 0.41, DN 1.05 ± 0.37, p=NS]. Strong correlation was observed between the number of inflammatory cells around the vessels and the number of CD31 positive proliferating cells around pre-existing skin vessels (r=0.44, p <0.0001) and the number of skin blood vessels per mm2 (r=0.24, p <0.05). No correlation was observed between the number of vessels and skin blood flow.
Human Foot Biopsies
We have also investigated discarded skin specimens from the foot that were obtained during foot surgery from 7 non-DM healthy subjects (age 55±18, 3 males) and 5 DM subjects (age 54±16, 3 males). The results were similar to the ones observed in the forearm biopsies although they failed to reach statistical significance due to the small number of participants. Thus, H&E staining showed that DM patients trended to have higher number of skin blood vessels per biopsy when compared to the non-DM (45 ± 57 vs. 27 ± 20, p=NS) while the number of CD45RO expressing cells around blood vessels, a marker of lymphocytes, also trended to be higher in the DM patients (88 ± 26 vs. 77 ± 34, p=NS).
Rabbits
H&E Analysis
The number of inflammatory cells per visual field from ear skin biopsies was higher in the DM animals when compared to the non-DM ones (2.2 ± 1.2 vs. 0.8 ± 0.2, p<0.02) (Fig. 1, 2a and 2b). The number of blood vessels per visual field was also higher in the diabetic rabbits (5.5 ± 2.3 vs. 2.6 ± 0.8, p<0.01). When non-DM and DM animals were considered as one group, a strong correlation was observed between the number of inflammatory cells and skin vessels, r=0.83, p<0.0001.
Figure 2.
Images a–f: Representative images of H&E staining in non-DM and DM rabbit ear skin (a and b respectively), non-DM and DM rat skin (c and d) and non-DM and DM mouse skin (e and f). Black arrows indicate round inflammatory cells while green arrows indicate blood vessels. The number of infiltrating inflammatory cells were increased in all diabetic animal models when compared to non-diabetic animals (p<0.05) as well as the number of blood vessels (p<0.05). Images g, f: Representative images of H&E and CD31 staining in the same mouse skin biopsy. H&E staining (g) identified fully formed (mature) skin blood vessels. CD31 staining (h) confirmed that the identified structures in the H&E stained biopsy were blood vessels (green arrows) while it also identified additional endothelial cells that represent newly regenerating blood vessels (red arrows).
CD31 Staining Analysis
The number of skin blood vessels trended to be higher in the DM rabbits compared to non-DM rabbits, although no statistical difference was observed, probably due to small number of tested animals (7.8 ± 4.0 vs. 5.7 ± 3.1, p=NS).
Rats
H&E Analysis
Inflammation, assessed as the number of inflammatory round cells, was higher in the skin of DM rats when compared to the non-DM rats (3.5 ± 0.8 vs. 1.0 ± 0.3, p <0.0001) (Fig. 1, 2c and 2d). The number of skin blood vessels was also higher in the DM rats compared to the non-DM rats (3.5 ± 0.9 vs. 2.7 ± 0.6, p<0.05).
CD31 Staining Analysis
The number of skin blood vessels identified by CD31 staining was increased in the DM rats compared to the respective non-DM controls (5.3 ± 1.3 vs 3.8 ± 0.7, p<0.01).
Mice
H&E Analysis
DM C57Bl6 mice had higher inflammatory cells per visual field than the non-DM C57Bl6 mice (3.8 ± 1.4 vs 1.3 ± 0.6, p <0.0001) (Fig. 1). The number of blood vessels was also higher in the DM mice (5.3 ± 1.4 vs. 3.2 ± 1.1, p<0.01). A strong correlation was observed between the number of inflammatory cells and vessels when non-DM and DM animals were grouped together, r=0.79, p<0.0001.
Compared to non-DM WBB6F1 mice, DM WBB6F1 mice also had higher number of inflammatory cells (12.1 ± 1.4 vs. 8.6 ± 1.7, p<0.0001) and higher number of skin blood vessels (3.8 ± 1.6 vs. 1.4 ± 0.8, p<0.001) (Fig. 1, 2e and 2f). In addition, a correlation existed between these two parameters (p=0.54, p<0.05).
Finally, we compared differences between the two mice strains. Within the non-DM mice, WBB6F1 mice had higher number of inflammatory cells (8.6 ± 1.7 vs. 1.3 ± 0.6, p <0.0001) compared to C57Bl6 mice, while similar differences existed between the DM mice (12.1 ± 1.4 vs. 3.2 ± 1.1, p<0.0001). However, the number of skin blood vessels was lower in both the non-DM WBB6F1 mice when compared to the non-DM C57Bl6 mice (1.4 ± 0.8 vs. 3.2 ± 1.1, p<0.001) and the DM WBB6F1 compared to the DM C57Bl6 mice (3.8 ± 1.6 vs. 5.3 ± 1.4, p=0.05).
CD31 Staining Analysis
The number of CD31 positive endothelial cells per visual field was higher in the skin of the DM WBB6F1 mice when compared to their respective non-DM mice (6.8 ± 1.5 vs 3.8 ± 1.0, p<0.01). The number of skin blood vessels was also increased in the DM group when compared to the non-DM group (3.7 ± 0.8 vs 1.8 ± 0.5, p<0.01) (Fig. 2g and 2h).
CONCLUSIONS
The main finding of the present study is that there is increased skin inflammation in both human and animal models of diabetes. This is, to the best of our knowledge, the first study to report increased inflammation at the skin level in both human and experimental diabetes. In addition, we have observed an increase in the number of skin blood vessels in various animal models of experimental diabetes that is similar to the one observed in human diabetes. Finally, strong correlation was observed between the blood vessel density and inflammatory cell infiltration in both human and animal models of diabetes.
Diabetes, especially type 2, and obesity are associated with increased systemic inflammation, as described by the elevated circulating inflammatory cytokines6,7. In addition, inflammation in the adipose tissue has been proposed as one of the main factors that lead to the development of insulin resistance and type 2 diabetes8,9. However, there is limited information regarding inflammation at the skin level. A previous study from our unit was one of the first to describe increased number of inflammatory cells in the dermis, especially around hair follicles and blood vessels constituting a specific sign of inflammation, in diabetes3. In the present paper, we have expanded these observations and have shown that these changes are present in both nonneuropathic and neuropathic patients. Of interest, the present findings are in contrast with previous studies from our group that have indicated that systemic inflammation, assessed by the measurement of serum inflammatory cytokines, is present only in diabetic patients with neuropathy but is absent in diabetic patients without neuropathy10. These results indicate that in diabetes, skin inflammation probably manifests before the development of systemic inflammation and need further investigation.
An increase was also noticed in the endothelial cells proliferating around pre-existing skin blood vessels and the density of skin blood vessels in the diabetic patients but there were no changes in the skin blood flow. These results suggest that although there is increased endothelial cell proliferation and new vessel formation, this does not result in an increase of functional vessels that can increase skin blood flow. To the best of our knowledge, there are no previous reports regarding changes in skin blood vessels in diabetes. Previous studies have reported either reduced density of functional capillaries in subjects with metabolic syndrome11,12 or no changes in subjects with impaired glucose tolerance and type 2 diabetes13. Furthermore, our previous studies have shown impaired endothelial and smooth muscle function in the microcirculation resulting in impaired vasodilation in subjects with pre-diabetes, and diabetic subjects with or without complications4,14. These data indicate that although blood vessel density is increased, the functional capacity of these vessels, especially under conditions of stress remains limited and more studies are required to further evaluate this finding.
The possible mechanisms that are involved in the observed increased skin blood vessel density are not well understood. Inflammation is known to promote angiogenesis and may play a major role in the observed results15,16. Previous studies in obese subjects have also shown an increase in serum Vascular Endothelial Growth Factor (VEGF) and have lead to the hypothesis that adipocytes produce angiogenic factors that stimulate neovascularization which in turn plays an important role in allowing fat mass expansion17–19. Further studies will be required to investigate whether similar mechanisms can also affect angiogenesis at the skin level.
No differences were observed in both skin inflammation and skin vessel changes between Type 1 and 2 diabetic subjects. As expected, the duration of diabetes was longer in the type 1 patients although it was considerably long in both groups. Nonetheless, these results indicate that skin changes are similar in both types of diabetes of long duration.
Increased inflammatory cell infiltration and increased blood vessel density were also observed in the skin of various experimental animal models of diabetes. We opted to study all these animal models because there is a lack of a single model that satisfactorily represents the human condition representing skin and wound healing changes. Current consensus is that findings should be confirmed in more than one animal model. The rabbit ear model has the advantages that similar to human wounds, rabbit wounds heal mainly by re-epithelialization, and allows the introduction of ischemia and neuropathy by ligating the central and the rostral ear artery and the central and rostral nerves respectively20,21. On the other hand, rodents heal mainly by contraction but are easier to manipulate and also allow the study of various genetically engineered models. Despite the above differences, similar results were observed for both, skin inflammation and skin blood vessel density in these various models regarding. The consistency of these findings in different species provides strong validation of the observed results. This can enable the conduction of future mechanistic studies to further understand the underlying mechanisms leading to the observed results in more than one animal model.
The number of inflammatory cells was clearly increased in all studied diabetic animal models, indicating that skin inflammation is prominent in these diabetic models. On the other hand, the changes in the skin vessel density, although heading in the same direction did not achieve statistical significance in all models. Thus, no statistical significance was reached in the H&E analysis in humans despite significant differences in CD31 analysis. No differences were observed in the CD31 analysis in the rabbit ear, despite the existence of such difference when H&E analysis was employed. The main reason for these findings was that the skin vessel changes were less prominent than the inflammatory cell changes and larger numbers of human and large animals would be required to reach statistical significance in all measurements. Despite these limitations, the specificity of CD31 analysis and the confirmation it provided that that the structures observed by H&E analysis are blood vessels, clearly indicate an increase in the skin blood vessel numbers in diabetic humans, rats and mice and a similar, but less prominent, increase in diabetic rabbits. Given the logistic limitations such as the number of animals that can be studied, mainly due to costs and availability of animal facilities, our results suggest that mice may be the preferred animal model to study diabetes-related changes in the skin vessels.
The study has its limitations. Only type 1 diabetes animal models were studied. However, there are no major differences in the wound healing processes between type 1 and 2 animal models and the models presented in this study are the commonly used models of diabetic wound healing. Also, no differences were observed regarding inflammation or vascular density at the skin level between type 1 and type 2 diabetic subjects. Therefore, we believe that the choice of animal models should not influence the observed results. Moreover, no statistical differences were reached in the human foot skin specimens. The main reason for this was the small subject numbers. It should be noted that due to obvious risks, no biopsies were obtained from the feet of diabetic subjects. Instead, the feet skin samples from diabetic subjects came from discarded tissues during various operations. However, the fact that the observed results from the discarded skin of the foot were similar to those from the skin biopsies of the forearm, clearly indicate that changes at both sites in diabetic subjects are similar and supports the hypothesis of a generalized effect of diabetes. In addition to the above, the inflammatory cells were identified by H & E staining, a commonly used technique that lacks specificity. However, our previous studies using CD45RO staining have clearly indicated that our technique specifically evaluates inflammatory cells in the human biopsies3. Furthermore, specific precautions, described in the methods section, were taken in the animal biopsies to exclude other types of cells, mainly transversely cut fibroblasts and/or fibrocytes that appear round, despite the fact that they are ovoid or spindle shaped. We therefore strongly believe that the observed cells were inflammatory and that there were no biases that could have affected our results.
In summary, our results indicate that there is increased number of skin inflammatory cells and blood vessels in human diabetes and also in rodent and the rabbit ear models of experimental diabetes.
Acknowledgements
This work was supported by National Institutes of Health Grants R01-DK076937 and R01 NS066205 to AV. The project described was supported by the Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institute of Health. AT was supported by the SFRH/BD/48624/2008 grant and EL by the SFRH/BPD/46341/2008 grant from the Portuguese Foundation for Science and Technology (FCT). We also want to thank Dr. Mauricio Contreras for providing us with rat skin specimens.
Footnotes
Duality of Interests: None
Author Contributions: AV was responsible for the study concept, design and initial data analysis. AV and AT wrote the manuscript. AT, ECL, FT, SK, MEA, IK, JP, EC and LPN obtained all the data. AK supervised the skin biopsy analysis. All authors participated in the data analysis and interpretation processes and reviewed and approved the final report.
BIBLIOGRAPHY
- 1.Vital Statistics. Alexandria, VA: 1996. [Google Scholar]
- 2.Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999 Mar;22(3):382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 3.Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012 Nov;61(11):2937–2947. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veves A, Akbari CM, Primavera J, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes. 1998 Mar;47(3):457–463. doi: 10.2337/diabetes.47.3.457. [DOI] [PubMed] [Google Scholar]
- 5.Wagner N, Morrison H, Pagnotta S, et al. The podocyte protein nephrin is required for cardiac vessel formation. Hum Mol Genet. 2011 Jun 1;20(11):2182–2194. doi: 10.1093/hmg/ddr106. [DOI] [PubMed] [Google Scholar]
- 6.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of clinical investigation. 2006 Jul;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001 Jul 18;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007 Jan;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003 Dec;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009 Jun;94(6):2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czernichow S, Greenfield JR, Galan P, et al. Macrovascular and microvascular dysfunction in the metabolic syndrome. Hypertens Res. 2010 Apr;33(4):293–297. doi: 10.1038/hr.2009.228. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer-Aguiar LG, Laflor CM, Bouskela E. Skin microcirculatory dysfunction is already present in normoglycemic subjects with metabolic syndrome. Metabolism. 2008 Dec;57(12):1740–1746. doi: 10.1016/j.metabol.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Jaap AJ, Shore AC, Stockman AJ, Tooke JE. Skin capillary density in subjects with impaired glucose tolerance and patients with type 2 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 1996 Feb;13(2):160–164. doi: 10.1002/(SICI)1096-9136(199602)13:2<160::AID-DIA36>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Caballero AE, Arora S, Saouaf R, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999 Sep;48(9):1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 15.Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011;24(1):18–27. doi: 10.3109/08941939.2010.521232. [DOI] [PubMed] [Google Scholar]
- 16.Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor necrosis factor-alpha-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011 Jul;131(7):1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 17.Garcia de la Torre N, Rubio MA, Bordiu E, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008 Nov;93(11):4276–4281. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 18.Rega G, Kaun C, Demyanets S, et al. Vascular endothelial growth factor is induced by the inflammatory cytokines interleukin-6 and oncostatin m in human adipose tissue in vitro and in murine adipose tissue in vivo. Arterioscler Thromb Vasc Biol. 2007 Jul;27(7):1587–1595. doi: 10.1161/ATVBAHA.107.143081. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y. Angiogenesis modulates adipogenesis and obesity. The Journal of clinical investigation. 2007 Sep;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn ST, Mustoe TA. Effects of ischemia on ulcer wound healing: a new model in the rabbit ear. Ann Plast Surg. 1990 Jan;24(1):17–23. doi: 10.1097/00000637-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan L, Cai X, Wu S, et al. Gene expression of pro-inflammatory cytokines and neuropeptides in diabetic wound healing. J Surg Res. 2011 May 15;167(2):336–342. doi: 10.1016/j.jss.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]