Abstract
Individual meals are products of a complex interaction of signals related to both short-term and long-term availability of energy stores. In addition to maintaining the metabolic demands of the individual in the short term, levels of energy intake must also maintain and defend body weight over longer periods. To accomplish this, satiety pathways are regulated by a sophisticated network of endocrine and neuroendocrine pathways. Higher brain centers modulate meal-size through descending inputs to caudal brainstem regions responsible for the motor pattern generators associated with ingestion. Gastric and intestinal signals interact with central nervous system pathways to terminate food intake. These inputs can be modified as a function of internal metabolic signals, external environmental influences, and learning to regulate meal-size.
INTRODUCTION
While we often think that when we put the fork down is a conscious decision, the reality is that ingestive behavior is orchestrated by a wide range of biological signals that greatly impact how much we eat. Assuring sufficient calories is vital to life and so it is not surprising that evolution has selected for organisms with the ability to carefully regulate their food intake to match their energy needs. Other than some “filter feeders” that do not have to seek out their food supply, most organisms organize their feeding into discrete bouts we call meals. Meal initiation is largely opportunistic. Food availability, time of day and learning all play important roles in determining when meals begin.
However, determining when meals end is largely a product of biological signals that are generated by the physical, biochemical and signaling qualities of ingested food. The purpose of this review is to detail what we know about these so-called “satiety” signals and how they are used to make it possible for organisms to match their caloric intake to their caloric needs and maintain a stable body weight. Among these pathways are adaptations that enable us to eat more than is necessary in the short term to guard against uncertainty and scarcity over longer periods. Understanding these processes has practical implications for the development of weight loss strategies.
Currently, the most effective treatment for obesity is bariatric surgery [1]. Of the various options available Roux-en-y gastric bypass (RYGB) is considered to be the gold standard[2]. The surgery involves the formation of a small gastric pouch directly beneath the esophagus. An area of space that is roughly the volume of an egg prevents nutrients from entering the greater stomach, and upper intestine. The stomach empties through a surgical anastomosis with the jejunum, vastly reducing the transit time of nutrients through the gastrointestinal tract and enhancing the release of a number of intestinal satiety signals. It is unclear if, or to what degree gastric restriction, or, increases in intestinal hormones is responsible for reductions in meal-size after this surgery. However, the weight loss produced by such surgeries is much larger in magnitude and far more durable than weight loss achieved by the traditional approaches of diet and exercise[1]. The success of these surgeries points to the important nature of the signals generated by the gastrointestinal tract and has implications for the development of less invasive weight loss strategies.
SATIETY
Gastric Distention
For most people satiety is tantamount to the psychological sensation of fullness. Gastric mechanoreceptors are activated by distention both during and after meals[3]. Maximal distention occurs when inhibitory signals from the intestine slow gastric emptying rates to asymptotic levels. It has been postulated that meals end primarily, or even solely, as a function of volume-effects[4-7]. There is no question that gastric volume is a rate-limiting factor in meal-size. In rats with an inflatable cuff surgically implanted around the pylorus to prevent gastric emptying, infusing a gastric preload reduces meal-size in proportion to the volume of infusate[7]. Under these conditions meal-size is unaffected by the caloric-density of the preload. Thus, in isolation the stomach does not respond to differences in caloric-density or macronutrient composition crucial for normal sequences of meal-termination and the regulation of meal size. Rather, caloric composition must be sensed post-gastrically.
Post-gastric Signals
Infusing nutrients into the intestine reduces meal-size in proportion to the number of calories infused[8]. Figures 1-2 show how cells located in the intestinal epithelium sense and respond to nutrients. Under normal conditions, roughly a third of all calories consumed exit the stomach prior to the end of the meal[9-11]. What remains is gradually metered out over time via negative feedback from the intestine. Gastric emptying is thus characterized by an initial rapid phase followed by a slower linear phase in which emptying rates are determined by caloric content rather than volume-effects[12]. Given that no absorption occurs in the stomach regulating the appearance of nutrients into circulation and to critical organs such as the liver is primarily a function of gastric emptying.
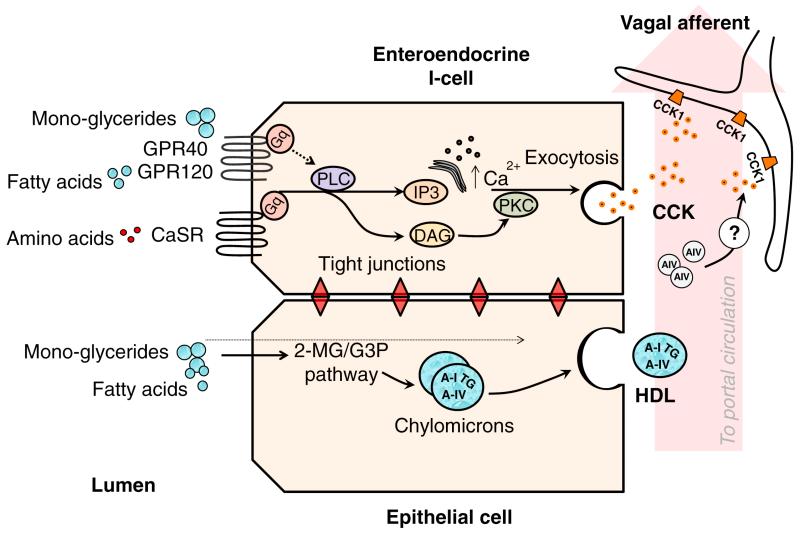
Figure 1. Nutrient-sensing in intestinal I-cells.
Luminal nutrients activate specialized receptors coupled to Gq proteins. The activation of PLC, and downstream effector pathways triggers membrane depolarization and the release of CCK which activates CCK1 receptors expressed on vagal afferent fibers. Following uptake into epithelial cells monoglycerides (MG) and free fatty acids (FA) can either diffuse across the cell and exit into circulation from the basolateral membrane, or be re-synthesized into triglycerides (TG) by the 2-monoglyceride (2MG) or α-glycerol-3 phosphate (G3P) pathways. The re-synthesized TG’s are assembled into chylomicrons before undergoing exocytosis by the golgi apparatus. Once in circulation, a portion of the Apo A-IV dissociates from the chylomicron and acts on CCK1 receptors via an unknown mechanism.
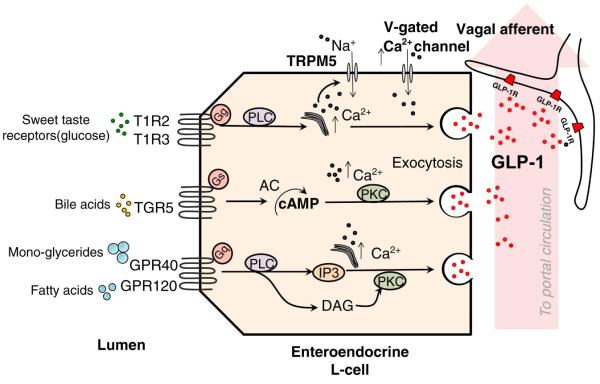
Figure 2. Nutrient-sensing in intestinal L-cells.
G-protein coupled receptors and their effector pathways that have been identified as potential nutrient sensors on intestinal L-cells. Activation of these receptors triggers elevations in intracellular Ca2+ stores and the Ca2+ sensitive transient receptor potential channel M5 (TRPM5)[136, 137]. The resulting depolarization triggers the release of GLP-1 and the subsequent activation of GLP-1 receptors expressed on vagal afferent fibers.
As food exits the stomach, coordinated contractions of the gallbladder release bile and pancreatic juices that, among other things, contain high levels of bicarbonate. This neutralizes the acidity of the exiting chyme. In addition, these secretions intersperse pancreatic amylase, trypsinogen, and lipases that are necessary for digestion. Carbohydrates and proteins are broken down into simple sugars and amino acids, respectively, before being taken up by the intestinal epithelium through active transport[13, 14]. Lipids, however, must be emulsified by bile before being cleaved into fatty acids and monoglycerides by lipases and enter the enterocyte via passive diffusion, or active transport[15]. If nutrients exit the stomach too quickly malabsorption may occur. By regulating the appearance of chyme into the intestine gastric emptying optimizes the digestion of these macronutrients within the proximal bowel.
For these processes to be effective, the gut must be able to signal to multiple sites of action simultaneously, i.e. the liver, gallbladder, pancreas, and stomach, in order to coordinate these diverse responses required for efficient digestion. Therefore, it is essential that the macronutrient makeup and caloric intensity of the chyme be read with a high degree of accuracy. Blood-borne factors are ideally suited to this task in that they are able to reach multiple tissues and organ systems simultaneously. Together, with the enteric nervous system, these signals control gastric emptying in such a way that approximately the same amount of calories per minute are emptied into the duodenum.
Intestinal Cholecystokinin (CCK)
By the late 1960s and early 1970’s improved extraction and purification techniques made it possible to begin studying the exogenous administration of relatively pure formulations of peptide hormones in a much more pointed, and focused way than in previous decades[16]. A landmark study by Gibbs et al. reported the effects of semi-purified CCK derived from porcine intestine, and synthetically derived CCK-8 on food intake in rats[17]. These authors found that meal-size was significantly reduced in rats after IP administration to both compounds.
Today, we know that CCK is released from specialized intestinal cells called I-cells in the duodenum in response to dietary nutrients, and especially lipids and fatty acids[18-20]. Figure 1 highlights how fatty acids and dietary amino acids stimulate CCK release in the intestine. These nutrients stimulate specialized G-protein-coupled receptors (GPR40/GPR120)[18, 20] located on the apical surface of I-cells and the extracellular calcium-sensing receptor (CaSR)[19] that responds to aromatic amino acids. When activated, these receptors evoke CCK release into portal circulation, stimulating gall bladder contractions and the release of bile[21]. In addition to its effect on food intake CCK has direct and indirect inhibitory effects on gastric emptying, including the relaxation of the proximal stomach, suppression of gastric and duodenal pressure waves, and contraction of the pylorus[22, 23]. In each case, these effects are mediated through actions at the CCK1 receptor. The effect of CCK on food intake is believed to be mediated through the vagus nerve, as vagotomy prevents the anorectic effect of CCK in rodents[24, 25]. It has not been determined if CCK’s effect in humans is also mediated entirely through the vagus. It may well be that there is also an endocrine component to this effect particularly in humans.
It is important to point out that to be considered a satiety signal a candidate hormone, or peptide must display features consistent with the physiological regulation of energy intake that go beyond simply reducing food intake[26]. It is generally agreed that a satiety factor should display a short duration of action so as not to interfere with the duration between meals. The signal should reduce food intake at doses that reflect prandial levels, but not as a consequence of visceral illness. Finally, blocking the endogenous signal should increase meal-size relative to baseline values. There is now a broad range of evidence demonstrating that CCK fulfills each of the above requirements[12, 27-29].
Apolipoprotein A-IV (Apo A-IV)
Another satiety factor released in response to lipids is Apo A-IV. Apo A-IV is a 46-kDa protein secreted from the small intestine in response to lipid absorption and chylomicron formation (Figure 1). Inside the enterocyte, fatty acids are reesterified and assembled along with cholesterol and apolipoproteins into chylomicron particles. Chylomicrons are composed of a TG and cholesterol core, coated with apolipoproteins. Apolipoproteins serve three main functions: to emulsify the lipid particles in the aqueous environment of the lymph and blood, to maintain the structural integrity of the chylomicron particle, and to direct the metabolism of the particles at the peripheral tissues by binding to cell surface receptors or through enzymatic activities. Once in the plasma, ~25% of the chylomicron associated Apo A-IV diffuses from the chylomicron and can be found in the lipoprotein-free fraction of the plasma as well as in the HDL pool. Therefore, the presence of A-IV in the periphery is uniquely linked to the intestinal absorption and secretion of dietary lipid[26].
Circulating levels of Apo A-IV peak within 15 min of a meal and remain elevated for approximately 30 additional minutes[30]. Like CCK, Apo A-IV has inhibitory effects on gastric emptying[31], and given peripherally, Apo A-IV reduces food intake in rats in a dose-dependent manner at doses that mimic prandial levels[32]. Also like CCK, Apo A-IV is expressed in the hypothalamus and central administration reduces food intake[33].
Tso and colleagues recently reported that CCK deficient mice are unresponsive to the anorectic effect of peripherally administered Apo A-IV[34], indicating that reductions in food by this peptide are mediated via a CCK-dependent mechanism. These authors also found that the anorectic effect of peripherally administered Apo A-IV was abolished in rats after subdiaphragmatic vagotomy, and greatly attenuated in rats pretreated with the CCK1 receptor antagonist lorglumide.
Intestinal Glucagon like peptide-1 (GLP-1)
While both CCK and Apo A-IV are preferentially released in response to dietary fats, other gastrointestinal hormones, such as the incretin hormone GLP-1, appear more strongly regulated by carbohydrates. While not universal, at least some work has made the case that GLP-1 is secreted more strongly in the presence of glucose than isocaloric amounts of fat[35]. GLP-1 is a 36 amino acid peptide derived from the post-translational processing of preproglucagon made in intestinal L-cells, the expression of which increases distally along the gastrointestinal tract[36]. The nutrient-sensing properties of these cells have yet to be fully elucidated (Figure 2), but at least part of the secretory response of GLP-1 is mediated by the glucose sensor/taste receptor T1R2 + T1R3 and the G-protein, gustducin[37], that are expressed on the apical region of GLP-1 containing cells. Fats may be sensed directly or indirectly through the stimulation of Gpr120 by fatty acids[38, 39] or the actions of bile acids on TGR5 receptors [40] [41]. In humans and rodents GLP-1 and GLP-1 analogues decrease food intake in a dose-dependent manner [42, 43] and inhibit gastric motility[44, 45].
Since under normal feeding conditions GLP-1(7-36) is released rapidly within minutes of a meal before most of the glucose reaches the lower intestine, it seems likely that glucose activates the release of GLP-1(7-36) through means other than direct contact with L-cells[46]. Consistent with this hypothesis Rocca and Brubaker demonstrated that the secretion of GLP-1(7-36) from the ileum is regulated by a complex neuroendocrine loop, in which nutrients in the duodenum induce GLP-1(7-36) release from ileal L-cells via vagal innervation[47].
Once in circulation GLP-1 undergoes rapid degradation by the enzyme dipetidel-petidase (DPP) IV. After passing through the liver, only 25% of the intestinally secreted GLP-1 enters general circulation where the half-life is less than 2 min[48, 49]. It remains uncertain whether sufficient amounts of intestinal GLP-1 reach posthepatic sites. Thus, the precise sites of action for this effect are not well defined. Subdiaphragmatic vagotomy substantially reduces the satiating effect of intraperitonal infusion of GLP-1 on spontaneous meals, but not the satiating of hepatic-portal vein infusions in rat[50]. Taken together these data imply that it is likely that GLP-1 acts at multiple sites of action to inhibit food intake. Like the other satiety factors we have discussed so far GLP-1 also confers inhibitory effects on gastric emptying mediated via the vagus[51].
While GLP-1 is regarded as a putative satiation factor there are two aspects of GLP-1 signaling that deviate from the criteria initially put forth by Gibbs and Smith. First, of two studies that have examined the ability of GLP-1 antagonists to increase meal-size[52, 53], only one found that it did so[53], and in that study this effect was only observed at times when food intake was otherwise low. The rather limited conditions of this effect imply that further work is needed before meaningful conclusions can be drawn regarding the role of endogenously released GLP-1 on meal-size. Second, there is evidence that the pharmacological activation of the GLP-1 system increases the duration of satiety. Peripheral or icv chronic treatment with long-acting pharmacological GLP-1 analogues such as exendin-4 and liraglutide increase intermeal intervals[53], and produce significant weight loss in rodents[42] and humans[54]. It should also be noted, however, that these compounds are designed specifically to have much longer half-lives than the native peptide[55]. Consequently, it is difficult to know whether such effects are reflective of the endogenous biological actions of GLP-1 or are a product of pharmacological activation of GLP-1 receptors for longer periods and in more distal locations than would occur for endogenous GLP-1 that is secreted after meals[3].
Peptide YY (PYY)
PYY is a 36 amino acid peptide that belongs to the pancreatic poly peptide family that is cosecreted from L-cells with GLP-1. Two endogenous forms of PYY are released into circulation, PYY1-36 and PYY3-36. Additional PYY3-36 is created by cleavage of the Tyr-Pro amino acid residues of PYY1-36 by the enzyme DPP-IV, the same enzyme responsible for the degradation of GLP-1. Under fasting conditions circulating levels of PYY1-36 are much higher than levels of PYY3-36. After a meal, however, this relationship is reversed, and, PYY3-36 becomes the dominant form of the peptide in blood[56].
The secretion of PYY3-36 may be under the control by the same nutrient-sensing receptors and pathways as GLP-1. Unlike GLP-1, however, PYY is preferentially secreted in response to fat[57] and has no incretin effect, i.e. its release does not potentiate glucose stimulate insulin secretion[58]. Like GLP-1, PYY inhibits gastric emptying and intestinal transit times. Actions that help lower prandial glucose excursions and facilitate the digestion of fats and other nutrients in the proximal bowel.
The mechanism and routes through which PYY exerts its anorectic effects are distributed across multiple regions of the central nervous system. Baraboi and coworkers showed that PYY3-36 reduces food intake through both hormonal actions on circumventricular organs and direct effects on Y2 receptors on vagal afferent fibers[59]. In addition, there is evidence that PYY crosses the blood brain barrier where it may exert actions on Y2 receptors expressed on neuropeptide Y (NPY) neurons in the hypothalamus resulting in the inhibition of inhibitory feedback onto pro-opiomelaninconcentrating hormone (POMC) neurons (Figure 3)[60]. Functional magnetic resonance imaging studies have shown that in addition to affecting hypothalamic and brainstem regions PYY3-36 alters activity in midbrain regions in the mesoaccumbens area associated with reward[61]. Hence, PYY3-36 is thought to affect multiple aspects food intake to exert its anorectic effect.
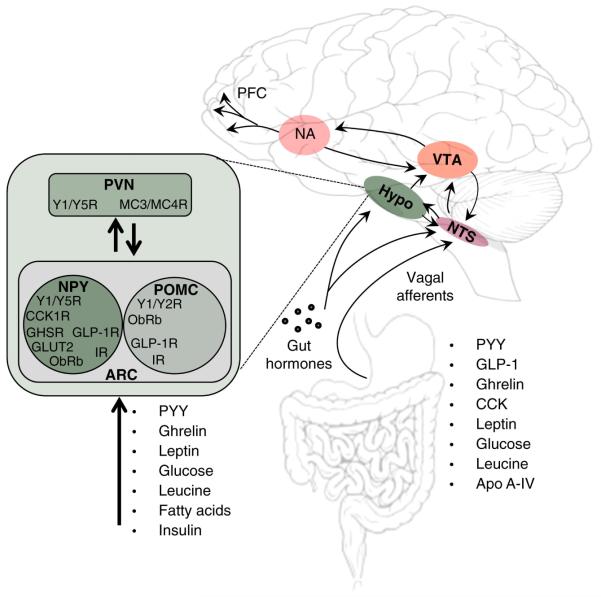
Figure 3. Central nervous system integration of satiety signals.
Examples of peripherally derived signals that act on central nervous system pathways to affect food intake. Nutrient-sensing is distributed across multiple regions of the central nervous system. Circuits in the hypothalamus (hypo) and brainstem interact with higher centers to initiate and terminate meals.
Amylin
Amylin is a 37-residue peptide from the calcitonin gene co-secreted with insulin from β-cells in the pancreas[62]. In rats, amylin is secreted into circulation within minutes of a meal where it has a half-life of approximately 15 min[63]. Peripherally administered amylin reduces meal size at doses that match prandial circulating levels without producing signs of visceral illness[64-66]. Further, antagonists of the amylin receptor increase meal-size at doses that block the actions of exogenously administered amylin[67, 68]. For these reasons it is widely held that amylin fulfills the criteria of a physiological signal of satiation. Like CCK and the other satiety factors described so far, amylin has inhibitory effects on gastric emptying that are vagally mediated[69]. Unlike CCK, the amylin’s effect on food intake results from actions in the area postrema, a circumventricular organ that lacks a normal blood-brain-barrier as a result of high concentration of fenestrated capillaries. In rodents, electrolytic lesions to the area postrema prevent amylin induced reductions in food-intake[70, 71].
Special Considerations of knock-out-models
Despite the above pharmacological evidence, amylin knock out (KO) mice have normal food intake and growth curves compared with wild type mice[72, 73]. However, it must be recognized that genetically modified mice are subject to developmental compensation that limit the interpretations of negative data as a true absence of a function. Apo A-IV[74] and GLP-1 receptor KO[75] mice also eat normally and have normal growth patterns relative to wild-type mice.
CCK receptor deficient rats eat larger meal-sizes than wild type rats[76]. However, CCK is also expressed in the hypothalamus where it negatively regulates the expression of NPY. Thus, it is not clear if the CCK deficiency affects meal-size through central or peripheral sites of actions. The same can be said of many of the metabolic cells in the gastrointestinal tract. The key point is that physiological conclusions from animals with targeted gene disruption of these factors must be made with caution.
CENTRAL NERVOUS SYSTEM INTEGRATION
These are just several of a number of satiety factors produced in the gut. When considered as a whole the number and breadth of these signals is impressive. These signals converge at multiple levels of central nervous system. This convergence occurs as a function of both integrated signaling systems within individual neurons and communication between sets of neurons that receive separate peripheral inputs. Signals from the periphery act at vagal afferent-fibers that connect to higher centers via the brainstem, or enter the brain from circulation through active transport, or passive transport at circumventricular organs such as the area postrema (Figure 3).
Vagal Afferent Signaling
At the cellular level, CCK is one of a number of inputs onto vagal afferent fibers innervating the viscera and gastrointestinal tract. Rather than responding to a single modality, or peptide. Vagal afferent fibers integrate signals from multiple sources. For example, many neurons in the nodose ganglion bearing CCK1 receptors also express OB-Rb, and, respond to mechanically-induced distention of the stomach [77]. These inputs can simultaneously be affected by other gastric and intestinally derived factors to regulate overall activity[78]. Hence, it is the balance of these inputs that is considered to be important in determining meal-size.
In many cases, the convergence of these signals is often complex, and counterintuitive. For example, rather than being secreted from adipose tissue, the leptin that acts upon vagal afferent fibers is secreted from the gastric mucosa, before traveling to the duodenum where it gains access to vagal afferent fibers expressing CCK receptors[79]. The reason for this is unclear. What is known is that gastric leptin is secreted with its soluble receptor in a complex that protects it from the hydrolytic conditions of the stomach[80]. This complex then moves to the duodenum, where it interacts with transmembrane leptin receptors expressed on the apical surface of CCK expressing I-cells[81]. Once inside the cell, leptin is transferred to the Golgi apparatus where it forms a new leptin-leptin receptor complex before being secreted into lymph[82]. In addition to CCK, leptin potentiates neuronal activity caused by gastric distention, demonstrating that different processes contribute additively to the overall activity in these neurons[77].
Importantly, these inputs can also be opposed by other factors such as ghrelin. Ghrelin levels peak just prior to a meal and fall precipitously soon afterwards. Pharmacologically, ghrelin increases food intake and palatable ratings for food in humans and increases food intake and adiposity in rodents[83, 84]. The peptide is a 28 amino acid sequence released from the oxyntic cells of the gastric mucosa[85]. The active form of ghrelin is produced when preproghrelin undergoes a post-translational modification by the ezyme ghrelin O-acyltransferase (GOAT), resulting in the esterfication of a medium chain fatty-acid to a serine 3 residue that is necessary for binding to and activating the growth hormone secretagogue receptor (GHSR) [86]. Circulating levels of ghrelin are inversely related to fat mass[87], and are regulated in the short term by the time of day[88], duration of intermeal interval, and anticipatory meal-related cues[89]. In addition to increasing food intake, ghrelin opposes the actions of CCK and leptin on vagal afferent fibers directly, and by inhibiting activity caused by mechanical distention[90].
The convergence of multiple inputs onto the same afferent neuron allows the vagus to integrate large volumes of information. As a consequence, these neurons are able to respond rapidly to changes in the internal milieu brought about by feeding. Moreover, releasing multiple signals in response to a single stimulus results in greater sensitivity and detection thresholds. For example, the presence of lipids in the intestine results in the release of multiple satiety factors, including CCK, Apo A-IV, GLP-1 and others that result in a high degree of accuracy in nutrient-sensing networks spread throughout the periphery and central nervous system pathways. This organization protects the organism from relying too heavily on any one input since it is the activity of these areas as a whole that is important to overall response. Thus, the removal of any one peptide or signal in isolation, either pharmacologically, or through targeted-deletion produces only modest effects on meal-size. A fact that is all too apparent in many pharmacologically-based weight loss strategies that generally result in weight loss of only 5% or less compared to placebo-treated individuals[91]. While even small amounts of weight loss can lead to metabolic improvements[92], it falls well short of helping individuals reach an ideal body weight.
One reason for the success of bariatric approaches to obesity may be related to its actions on multiple pathways in the periphery. RYGB profoundly increases prandial CCK[93] APO A-IV[94], GLP-1[93, 95, 96], PYY[93, 96], amylin[97], insulin[95, 96], glucagon[93] and other factors as part of an integrated response to surgically-induced changes in nutrient-handling. These changes may incur and maintain weight loss independent of gastric restriction also produced by this surgery. Total weight loss after RYGB can be in excess of 25% of preoperative values[1]. Thus, targeting multiple pathways may be one way to overcome the 5% ceiling effect seen in traditional weight loss approaches.
The Brainstem
In addition to relying on multiple signals, nutrient-sensing is distributed across multiple regions of the central nervous system. Most of the vagal afferent fibers that innervate the viscera and gastrointestinal tract project to the nucleus of the solitary tract (NTS) rostral to obex (Figure 3)[98]. Within this region, a vast number of neuronal phenotypes are responsive to meal-related stimuli, including adrenergic neurons that project to the paraventricular nucleus of the hypothalamus (PVN)[99], and GLP-1 expressing cells that project to the PVN, dorsal medial hypothalamus (DMH) 22401907 [100], and ventral tegmental area (VTA) [101].
In the NTS neurons respond to changes in circulating glucose[102] and the amino acid leucine[103] demonstrating direct sensitivity of this region to ambient levels of energy yielding substrates. It remains to be established whether ascending projections to the forebrain are necessary for nutrient-sensing pathways in the brainstem to effect changes in meal-size or frequency.
At one time, nutrients were thought to be sensed exclusively in the gut and specific regions of the hypothalamus[104]. Today, we know that this nutrient-sensing network is also distributed in brainstem. Likewise, we now know that many neurons in the hypothalamus[105, 106] also sense and respond to the secretion of satiety factors that at one time were thought to act exclusively upon metabolic pathways in the brainstem. Early lesioning studies also implied that the brainstem lacked direct access to long-term metabolic signals such as leptin[107].
More recent evidence, however, demonstrates that a subpoplulation of neurons in the NTS expresses the long form of the leptin receptor (ObRb)[108]. These neurons project to the VTA and lateral hypothalamic areas, and may affect food intake through actions on dopamine signaling in motivation and reward-related areas. Targeted disruption of ObRb signaling exclusively in NTS neurons results in increased food intake and early-onset obesity in mice[108], albeit to a lesser degree than that produced in whole-animal leptin receptor disruption. The key point is that metabolic pathways in the brainstem integrate and responds to acute and long-term changes in energy homeostasis as part of a broader network.
Today, the hypothalamus continues to be recognized for its role in the integration of metabolically relevant information from the hindbrain and elsewhere. The difference is in our awareness of how the same information is processed simultaneously in other areas before being integrated in the hypothalamus and other centers. As stated earlier, this distribution protects the organism from relying too heavily on any one input and increases the sensitivity with which it is able to detect and respond to change.
The Hypothalamus
Among the most prominent centers for the integration of such signals is the basomedial region of the hypothalamus. Orexigenic NPY and anorectic POMC neurons respond to acute and long term metabolic inputs in opposing ways. POMC-expressing neurons cleave the parent molecule into α-melanocytestimulating hormone (α-MSH), which acts upon melanocortin-3 and melanocortin-4 receptors (MC3 and MC4) located in the PVN, and elsewhere[105, 109]. Activity at these receptors elicits powerful reductions in food intake and increased energy expenditure[110-112]. NPY expressing neurons in arcuate (ARC) and dorsomedial hypothalamic nuclei project to the PVN, ventral medial (VMH), and lateral hypothalamic (LH) areas, and increases food intake through actions at Y1, Y2, and Y5 receptor subtypes[113].
Activity in both NPY and POMC neurons is regulated by changes in circulating leptin. In NPY expressing neurons, decreases in leptin reduce inhibition of a slow depolarizing current[114], resulting in greater NPY release and larger meal-sizes, whereas reduced leptin signaling in POMC neurons results in the opposite effect. These compensatory increases in food intake result in the restoration of weight loss and defend body energy stores.
To regulate meal-size, both cell types send dense projections to the PVN[115], which in turn projects to adrenergic neurons in the NTS that regulate meal-size[116-119]. By putting on weight, consequent increases in leptin increase the output from POMC neurons that ultimately render an individual more sensitive to the anorectic effect of CCK and other satiety factors[16]. Increased leptin action on NPY neurons produces the opposite effect. In this way, adiposity signals establish thresholds for the actions of satiety factors by modulating the sensitivity of the NTS to signals that terminate meals. Hence, it is the balance of NPY-induced anabolic and α-MSH induced catabolic responses that is considered to be important.
Like pathways in the brainstem, neurons in the basomedial hypothalamus also respond to acute changes in nutrient flux, including circulating levels of all three macronutrients[120-123], numerous satiety signals[105], and other factors[124]. For example, circulating ghrelin stimulates activity in NPY neurons in the ARC[105], and ghrelin-induced increases in food intake are prevented by NPY receptor antagonists[125, 126]. CCK, GLP-1, PYY and other signals of acute changes in energy balance produce similar effects, either through circulation or through ascending inputs from the brainstem. These inputs influence our decisions about when, and how much to eat that is, in part, reflected by activity in motivation and reward-related pathways and in areas of the brain involved in executive decision making such as the prefrontal cortex (PFC).
Reward Pathways
Among the regions affected by various metabolic inputs are the dopaminergic pathways of the mesoaccumbens pathway. Palatable foods, sexual activity, or drugs of abuse all result in an increase in dopamine (DA) release from neurons in the VTA and the adjacent substantia nigra into forebrain structures such as the nucleus accumbens (NA)[127-129]. Activity in these regions increases when we anticipate having access to palatable foods[130] as well as when we consume them[130, 131]. Neuroimaging studies performed in humans demonstrate that, generally speaking, the hungrier one is, the greater the activity in the PFC will be[130, 131]. These examples link endocrine activity with motivational and reward-related processes in the forebrain, and provide a neural correlate for what most of already know through experience, which is that the hungrier you are the more attractive food can be.
The mechanisms by which endocrine and neuroendocrine factors affect these regions are becoming clearer. Leptin can affect DA release in the NA by engaging pathways in NTS and lateral hypothalamus[132], or through actions on ObRb receptors expressed in the VTA directly[133]. Leptin-induced activity in these cells restores DA content in leptin-deficient animals and is associated with decreased food intake and weight loss in rats[132]. Likewise, long-term RNAi-mediated knockdown of ObRb in the VTA leads to increased food intake and weight gain in rodents. The presumption is that by inhibiting dopaminergic neurons in these pathways leptin reduces the reward-incentive for food.
GLP-1-producing cells in the NTS project directly to the VTA and the NA, and pharmacological data shows that GLP-1R activation in the these areas decreases food intake, especially of highly-palatable foods[134]. Antagonism at GLP-1 receptors in the VTA significantly increases food intake, establishing a physiological relevance for centrally derived GLP-1 signaling in this region and the influence of ascending pathways in the brainstem on reward-related processes in higher centers[134].
As in other areas of the brain, the actions of leptin and GLP-1 in the VTA are opposed by the orexigenic hormone ghrelin and the GHSR receptor. Ghrelin administered directly into the VTA simulates accumbal DA release[135], VTA -opioid receptor expression, and the consumption of sweetened beverages in rats[126]. Ghrelin administered intravenously to healthy volunteers during functional magnetic resonance imaging increases the neural response to food pictures in the orbitofrontal cortex and striatum, and increases subjective ratings for palatable foods.
Concluding remarks
Over short periods of time, constraints on meal-size reflect the capacity of the gastrointestinal tract and viscera to cope with increases in nutrient-flux. This information is provided in the form of satiety factors that reflect the macronutrient and caloric characteristics of the chyme. As gastric emptying slows, distention-related signals from the stomach act upon neuronal networks in the brainstem that combine with CCK and other satiety signals to terminate food intake.
The thresholds for meal termination can be modulated through descending projections to the brainstem and viscera. Inhibitory projections from the PVN attenuate meal-induced activity in the NTS, while descending inputs from the dorsal motor nucleus mobilize insulin and other factors through innervation to the pancreas, liver and gallbladder to accommodate greater increases in nutrient-flux to alter meal-size when necessary. These networks are regulated by internal and external cues that converge at multiple levels of the peripheral and central nervous systems to affect food-related behaviors, such as the initiation and termination of meals. Activity in these networks is regulated by multiple and redundant inputs limiting the influence of any one signal in isolation on these behaviors.
Eating remains a critical biological function and its complex regulation reflects its importance to the survival of the organism. Our increasing knowledge of how this system is organized opens up new possibilities to understand why we gain weight in our modern environment. Maybe more importantly, our growing knowledge of these systems makes it possible to make this biology work for us in the service of new therapies that can reduce food intake and body weight. The success of bariatric surgical procedures points to the ability of altered signals to achieve substantial and sustained weight loss. Harnessing those signals without the need for surgical rearrangement of the GI tract remains an elusive but important research goal.
Acknowledgments
Grant Support: This work was supported by a grant from Ethicon Endo-Surgery, and NIH Grant 1R01DK093848. APC is supported by a CIHR fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 2.Stefater MA, Wilson-Perez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33:595–622. doi: 10.1210/er.2011-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhans W, Geary N. Overview of the physiological control of eating. Forum Nutr. 2009;63:9–53. doi: 10.1159/000264392. [DOI] [PubMed] [Google Scholar]
- 4.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. American Journal of Physiology. 1996;271:R766–R769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan JM, Moran TH. Gastrointestinal signaling in the control of food intake. In: Stricker EM, Woods SC, editors. Handbook of Behavioral Neurobiology. Neurobiology of Food and Fluid Intake. no. 2. vol. 4. Kluwer Academic/Plenum Publishing; New York: 2004. pp. 273–303. [Google Scholar]
- 6.Eisen S, Davis JD, Rauhofer E, Smith GP. Gastric negative feedback produced by volume and nutrient during a meal in rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1201–1214. doi: 10.1152/ajpregu.2001.281.4.R1201. [DOI] [PubMed] [Google Scholar]
- 7.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 8.Canbeyli RS, Koopmans HS. Comparison of gastric, duodenal and jejunal contributions to the inhibition of food intake in the rat. Physiol Behav. 1984;33:951–957. doi: 10.1016/0031-9384(84)90235-x. [DOI] [PubMed] [Google Scholar]
- 9.van der Velde P, Koslowsky I, Koopmans HS. Measurement of gastric emptying during and between meal intake in free-feeding Lewis rats. Am J Physiol. 1999;276:R597–605. doi: 10.1152/ajpregu.1999.276.2.R597. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan JM, Siemers WH, Smedh U, Schwartz GJ, Grill HJ. Gastric branch vagotomy and gastric emptying during and after intragastric infusion of glucose. Am J Physiol. 1997;273:R1786–1792. doi: 10.1152/ajpregu.1997.273.5.R1786. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan JM, Spector AC, Grill HJ. Dynamics of gastric emptying during and after stomach fill. Am J Physiol. 1992;263:R813–819. doi: 10.1152/ajpregu.1992.263.4.R813. [DOI] [PubMed] [Google Scholar]
- 12.Moran TH. Gut peptide signaling in the controls of food intake. Obesity (Silver Spring) 2006;14(Suppl 5):250S–253S. doi: 10.1038/oby.2006.318. [DOI] [PubMed] [Google Scholar]
- 13.Levin RJ. Digestion and absorption of carbohydrates--from molecules and membranes to humans. Am J Clin Nutr. 1994;59:690S–698S. doi: 10.1093/ajcn/59.3.690S. [DOI] [PubMed] [Google Scholar]
- 14.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 15.Maldonado-Valderrama J, Wilde P, Macierzanka A, Mackie A. The role of bile salts in digestion. Adv Colloid Interface Sci. 2011;165:36–46. doi: 10.1016/j.cis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Woods SC, Langhans W. Inconsistencies in the assessment of food intake. Am J Physiol Endocrinol Metab. 2012;303:E1408–1418. doi: 10.1152/ajpendo.00415.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. Journal of Comparative and Physiological Psychology. 1973;84:488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 18.Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou AP, Sei Y, Zhao X, Feng J, Lu X, Thomas C, Pechhold S, Raybould HE, Wank SA. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka T, Katsuma S, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- 21.Malagelada JR, Go VL, DiMagno EP, Summerskill WH. Interactions between intraluminal bile acids and digestive products on pancreatic and gallbladder function. J Clin Invest. 1973;52:2160–2165. doi: 10.1172/JCI107400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser R, Fone D, Horowitz M, Dent J. Cholecystokinin octapeptide stimulates phasic and tonic pyloric motility in healthy humans. Gut. 1993;34:33–37. doi: 10.1136/gut.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedenberg FK, Desipio J, Korimilli A, Bohning M, Sum E, Parkman HP, Richter JE, Fisher RS. Tonic and phasic pyloric activity in response to CCK-octapeptide. Dig Dis Sci. 2008;53:905–911. doi: 10.1007/s10620-008-0214-1. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz DN, Goldman SA. Vagal mediation of the cholecystokinin satiety effect in rats. Physiol Behav. 1982;29:599–604. doi: 10.1016/0031-9384(82)90226-8. [DOI] [PubMed] [Google Scholar]
- 25.Smith GP, Gibbs J, Jerome C, Pi-Sunyer FX, Kissileff HR, Thornton J. The satiety effect of cholecystokinin: a progress report. Peptides. 1981;2(Suppl 2):57–59. doi: 10.1016/0196-9781(81)90011-5. [DOI] [PubMed] [Google Scholar]
- 26.Smith GP. Introduction to the reviews on peptides and the control of food intake and body weight. Neuropeptides. 1999;33:323–328. doi: 10.1054/npep.1999.0056. [DOI] [PubMed] [Google Scholar]
- 27.Geary N. Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav. 2004;81:719–733. doi: 10.1016/j.physbeh.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. American Journal of Physiology. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- 29.Smith GP, Gibbs J. Satiating effect of cholecystokinin. Ann N Y Acad Sci. 1994;713:236–241. doi: 10.1111/j.1749-6632.1994.tb44071.x. [DOI] [PubMed] [Google Scholar]
- 30.Kohan AB, Wang F, Li X, Bradshaw S, Yang Q, Caldwell JL, Bullock TM, Tso P. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am J Physiol Gastrointest Liver Physiol. 2012;302:G628–636. doi: 10.1152/ajpgi.00225.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Apolipoprotein A-IV acts in the brain to inhibit gastric emptying in the rat. Am J Physiol. 1996;270:G49–53. doi: 10.1152/ajpgi.1996.270.1.G49. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–1006. doi: 10.1152/ajpgi.1992.262.6.G1002. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest. 1993;91:1830–1833. doi: 10.1172/JCI116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimichi G, Lo CC, Tamashiro KL, Ma L, Lee DM, Begg DP, Liu M, Sakai RR, Woods SC, Yoshimatsu H, et al. Effect of peripheral administration of cholecystokinin on food intake in apolipoprotein AIV knockout mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1336–1342. doi: 10.1152/ajpgi.00325.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 36.Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept. 2003;114:189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 37.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci. 2009;1170:91–94. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 38.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 39.Burns RN, Moniri NH. Agonism with the omega-3 fatty acids alpha-linolenic acid and docosahexaenoic acid mediates phosphorylation of both the short and long isoforms of the human GPR120 receptor. Biochem Biophys Res Commun. 2010;396:1030–1035. doi: 10.1016/j.bbrc.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 40.Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, et al. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol Pharmacol. 2010;78:617–630. doi: 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jessen L, Aulinger BA, Hassel JL, Roy KJ, Smith EP, Greer TM, Woods SC, Seeley RJ, D’Alessio DA. Suppression of food intake by glucagon-like Peptide-1 receptor agonists: relative potencies and role of dipeptidyl peptidase-4. Endocrinology. 2012;153:5735–5745. doi: 10.1210/en.2012-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 44.Schirra J, Kuwert P, Wank U, Leicht P, Arnold R, Goke B, Katschinski M. Differential effects of subcutaneous GLP-1 on gastric emptying, antroduodenal motility, and pancreatic function in men. Proc Assoc Am Physicians. 1997;109:84–97. [PubMed] [Google Scholar]
- 45.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hermann C, Goke R, Richter G, Fehman HC, Arnbold R, Goke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 47.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–1694. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 48.Hui H, Farilla L, Merkel P, Perfetti R. The short half-life of glucagon-like peptide-1 in plasma does not reflect its long-lasting beneficial effects. Eur J Endocrinol. 2002;146:863–869. doi: 10.1530/eje.0.1460863. [DOI] [PubMed] [Google Scholar]
- 49.Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol. 1996;271:E458–464. doi: 10.1152/ajpendo.1996.271.3.E458. [DOI] [PubMed] [Google Scholar]
- 50.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 52.Ruttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9-39) fails to increase spontaneous meal size in rats. Physiol Behav. 2010;100:291–296. doi: 10.1016/j.physbeh.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal GLP-1 plays a physiological role in satiety. Endocrinology. 2008 doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jespersen MJ, Knop FK, Christensen M. GLP-1 agonists for type 2 diabetes: pharmacokinetic and toxicological considerations. Expert Opin Drug Metab Toxicol. 2013;9:17–29. doi: 10.1517/17425255.2013.731394. [DOI] [PubMed] [Google Scholar]
- 56.Grandt D, Schimiczek M, Beglinger C, Layer P, Goebell H, Eysselein VE, Reeve JR., Jr. Two molecular forms of peptide YY (PYY) are abundant in human blood: characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regul Pept. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 57.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab. 2007;92:4052–4055. doi: 10.1210/jc.2006-2273. [DOI] [PubMed] [Google Scholar]
- 58.Adrian TE, Sagor GR, Savage AP, Bacarese-Hamilton AJ, Hall GM, Bloom SR. Peptide YY kinetics and effects on blood pressure and circulating pancreatic and gastrointestinal hormones and metabolites in man. J Clin Endocrinol Metab. 1986;63:803–807. doi: 10.1210/jcem-63-4-803. [DOI] [PubMed] [Google Scholar]
- 59.Baraboi ED, Michel C, Smith P, Thibaudeau K, Ferguson AV, Richard D. Effects of albumin-conjugated PYY on food intake: the respective roles of the circumventricular organs and vagus nerve. Eur J Neurosci. 2010;32:826–839. doi: 10.1111/j.1460-9568.2010.07318.x. [DOI] [PubMed] [Google Scholar]
- 60.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 61.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 62.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, Chang JK, Rizza RA. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- 64.Lutz TA, Geary N, Szabady MM, Del Prete E, Scharrer E. Amylin decreases meal size in rats. Physiol Behav. 1995;58:1197–1202. doi: 10.1016/0031-9384(95)02067-5. [DOI] [PubMed] [Google Scholar]
- 65.Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, Vu C, Roth J, Parkes D. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1855–1863. doi: 10.1152/ajpregu.00297.2007. [DOI] [PubMed] [Google Scholar]
- 66.Lutz TA, Del Prete E, Scharrer E. Reduction of food intake in rats by intraperitoneal injection of low doses of amylin. Physiol Behav. 1994;55:891–895. doi: 10.1016/0031-9384(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 67.Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J. Amylin receptor blockade stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R568–574. doi: 10.1152/ajpregu.00213.2004. [DOI] [PubMed] [Google Scholar]
- 68.Mollet A, Gilg S, Riediger T, Lutz TA. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol Behav. 2004;81:149–155. doi: 10.1016/j.physbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Jodka C, Green D, Young A, Gedulin B. Amylin modulation of gastric emptying in rats depends upon an intact vagus nerve. Diabetes. 1996;45(Supp. 2):235A. [Google Scholar]
- 70.Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord. 2001;25:1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- 71.Potes CS, Lutz TA. Brainstem mechanisms of amylin-induced anorexia. Physiol Behav. 2010;100:511–518. doi: 10.1016/j.physbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 72.Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, Gebre-Medhin S, Galson DL, Zajac JD, Karsenty G. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol. 2004;164:509–514. doi: 10.1083/jcb.200312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mollet A, Meier S, Grabler V, Gilg S, Scharrer E, Lutz TA. Endogenous amylin contributes to the anorectic effects of cholecystokinin and bombesin. Peptides. 2003;24:91–98. doi: 10.1016/s0196-9781(02)00280-2. [DOI] [PubMed] [Google Scholar]
- 74.Weinstock PH, Bisgaier CL, Hayek T, Aalto-Setala K, Sehayek E, Wu L, Sheiffele P, Merkel M, Essenburg AD, Breslow JL. Decreased HDL cholesterol levels but normal lipid absorption, growth, and feeding behavior in apolipoprotein A-IV knockout mice. J Lipid Res. 1997;38:1782–1794. [PubMed] [Google Scholar]
- 75.Seeley RJ, Woods SC, D’Alessio D. Targeted gene disruption in endocrine research--the case of glucagon-like peptide-1 and neuroendocrine function. Endocrinology. 2000;141:473–475. doi: 10.1210/endo.141.2.7372. [DOI] [PubMed] [Google Scholar]
- 76.Bi S, Moran TH. Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Neuropeptides. 2002;36:171–181. doi: 10.1054/npep.2002.0895. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Wu X, Zhou S, Owyang C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: implications for leptin as a regulator of short-term satiety. Am J Physiol Gastrointest Liver Physiol. 2011;300:G217–227. doi: 10.1152/ajpgi.00356.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Dockray GJ. The versatility of the vagus. Physiol Behav. 2009;97:531–536. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol. 45:1–16. doi: 10.5115/acb.2012.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M. Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006;290:G242–249. doi: 10.1152/ajpgi.00334.2005. [DOI] [PubMed] [Google Scholar]
- 81.Cammisotto PG, Gingras D, Bendayan M. Transcytosis of gastric leptin through the rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol. 2007;293:G773–779. doi: 10.1152/ajpgi.00260.2007. [DOI] [PubMed] [Google Scholar]
- 82.Cammisotto PG, Bendayan M, Sane A, Dominguez M, Garofalo C, Levy E. Receptor-Mediated Transcytosis of Leptin through Human Intestinal Cells In Vitro. Int J Cell Biol. 2010;2010:928169. doi: 10.1155/2010/928169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 84.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 85.Pfluger PT, Kirchner H, Gunnel S, Schrott B, Perez-Tilve D, Fu S, Benoit SC, Horvath T, Joost HG, Wortley KE, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G610–618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- 86.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 88.Sanchez J, Oliver P, Pico C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflugers Arch. 2004;448:500–506. doi: 10.1007/s00424-004-1283-4. [DOI] [PubMed] [Google Scholar]
- 89.Zizzari P, Hassouna R, Longchamps R, Epelbaum J, Tolle V. Meal anticipatory rise in acylated ghrelin at dark onset is blunted after long-term fasting in rats. J Neuroendocrinol. 2011;23:804–814. doi: 10.1111/j.1365-2826.2011.02183.x. [DOI] [PubMed] [Google Scholar]
- 90.Page AJ, Slattery JA, Milte C, Laker R, O’Donnell T, Dorian C, Brierley SM, Blackshaw LA. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1376–1384. doi: 10.1152/ajpgi.00536.2006. [DOI] [PubMed] [Google Scholar]
- 91.Powell AG, Apovian CM, Aronne LJ. New drug targets for the treatment of obesity. Clin Pharmacol Ther. 2011;90:40–51. doi: 10.1038/clpt.2011.82. [DOI] [PubMed] [Google Scholar]
- 92.Magkos F, Yannakoulia M, Chan JL, Mantzoros CS. Management of the metabolic syndrome and type 2 diabetes through lifestyle modification. Annu Rev Nutr. 2009;29:223–256. doi: 10.1146/annurev-nutr-080508-141200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Worm D, Almdal T, Naver LS, Hvolris LE, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22:1084–1096. doi: 10.1007/s11695-012-0621-4. [DOI] [PubMed] [Google Scholar]
- 94.Culnan DM, Cooney RN, Stanley B, Lynch CJ. Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring) 2009;17:46–52. doi: 10.1038/oby.2008.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, et al. Weight-Independent Changes in Blood Glucose Homeostasis After Gastric Bypass or Vertical Sleeve Gastrectomy in Rats. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, Drewe J, von Flue M, Beglinger C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 97.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151:1588–1597. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sumal KK, Blessing WW, Joh TH, Reis DJ, Pickel VM. Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- 99.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 100.Renner E, Puskas N, Dobolyi A, Palkovits M. Glucagon-like peptide-1 of brainstem origin activates dorsomedial hypothalamic neurons in satiated rats. Peptides. 2012;35:14–22. doi: 10.1016/j.peptides.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 101.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 2000;856:37–47. doi: 10.1016/s0006-8993(99)02327-6. [DOI] [PubMed] [Google Scholar]
- 103.Blouet C, Schwartz GJ. Brainstem Nutrient Sensing in the Nucleus of the Solitary Tract Inhibits Feeding. Cell Metab. 2012 doi: 10.1016/j.cmet.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woods SC, Seeley RJ, Porte D, Jr., Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 105.Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE. Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann N Y Acad Sci. 2003;994:175–186. doi: 10.1111/j.1749-6632.2003.tb03178.x. [DOI] [PubMed] [Google Scholar]
- 106.Merino B, Cano V, Guzman R, Somoza B, Ruiz-Gayo M. Leptin-mediated hypothalamic pathway of cholecystokinin (CCK-8) to regulate body weight in free-feeding rats. Endocrinology. 2008;149:1994–2000. doi: 10.1210/en.2007-1286. [DOI] [PubMed] [Google Scholar]
- 107.Kaplan JM, Seeley RJ, Grill HJ. Daily caloric intake in intact and chronic decerebrate rats. Behav Neurosci. 1993;107:876–881. [PubMed] [Google Scholar]
- 108.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2012;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 110.Thiele T, van DG, Yagaloff K, Fisher S, Schwartz M, Burn P, Seeley R. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. American Journal of Physiology. 1998;274:R248–254. doi: 10.1152/ajpregu.1998.274.1.R248. [DOI] [PubMed] [Google Scholar]
- 111.Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, et al. Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes. 2000;49:177–182. doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- 112.Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Research. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- 113.Chambers AP, Woods SC. The role of neuropeptide Y in energy homeostasis. Handb Exp Pharmacol. 2012:23–45. doi: 10.1007/978-3-642-24716-3_2. [DOI] [PubMed] [Google Scholar]
- 114.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 115.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 116.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. American Journal of Physiology. 1999;276:R1545–R1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 117.Moran TH, Aja S, Ladenheim EE. Leptin modulation of peripheral controls of meal size. Physiol Behav. 2006;89:511–516. doi: 10.1016/j.physbeh.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 118.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiol Behav. 1995;58:755–760. doi: 10.1016/0031-9384(95)00108-u. [DOI] [PubMed] [Google Scholar]
- 120.Blouet C, Jo YH, Li X, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 122.Jo YH, Su Y, Gutierrez-Juarez R, Chua S., Jr. Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J Neurophysiol. 2009;101:2305–2316. doi: 10.1152/jn.91294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 124.Ryan KK, Kohli R, Gutierrez-Aguilar R, Gaitonde SG, Woods SC, Seeley RJ. Fibroblast Growth Factor-19 Action in the Brain Reduces Food Intake and Body Weight and Improves Glucose Tolerance in Male Rats. Endocrinology. 2013 doi: 10.1210/en.2012-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1728–1737. doi: 10.1152/ajpregu.00597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Skibicka KP, Shirazi RH, Hansson C, Dickson SL. Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology. 2012;153:1194–1205. doi: 10.1210/en.2011-1606. [DOI] [PubMed] [Google Scholar]
- 127.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 128.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 129.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 130.Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 132.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 134.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jerlhag E, Janson AC, Waters S, Engel JA. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS One. 2012;7:e49557. doi: 10.1371/journal.pone.0049557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 137.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15:421–431. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]