Abstract
Objectives
To evaluate the cost-effectiveness of statins for primary prevention of myocardial infarction (MI) and stroke in patients with chronic kidney disease (CKD).
Background
Patients with CKD have an elevated risk of MI and stroke. Although HMG Co-A reductase inhibitors (“statins”) may prevent cardiovascular events in patients with non-dialysis-requiring CKD, adverse drug effects and competing risks could materially influence net effects and clinical decision-making.
Methods
We developed a decision-analytic model of CKD and cardiovascular disease (CVD) to determine the cost-effectiveness of low-cost generic statins for primary CVD prevention in men and women with hypertension and mild-to-moderate CKD. Outcomes included MI and stroke rates, discounted quality adjusted life years (QALYs) and lifetime costs (2010 USD), and incremental cost-effectiveness ratios.
Results
For 65 year-old men with moderate hypertension and mild-to-moderate CKD, statins reduced the combined rate of MI and stroke, yielded 0.10 QALYs, and increased costs by $1,800 ($18,000 per QALY gained). For patients with lower baseline cardiovascular risks, health and economic benefits were smaller; for 65 year-old women, statins yielded 0.06 QALYs and increased costs by $1,900 ($33,400 per QALY gained). Results were sensitive to rates of rhabdomyolysis and drug costs. Statins are less cost-effective when obtained at average retail prices, particularly in patients at lower CVD risk.
Conclusions
While statins reduce absolute CVD risk in patients with CKD, increased risk of rhabdomyolysis, and competing risks associated with progressive CKD, partly offset these gains. Low-cost generic statins appear cost-effective for primary prevention of CVD in patients with mild-to-moderate CKD and hypertension.
Keywords: chronic kidney disease, statins, cardiovascular disease, cost-effectiveness, guidelines
Introduction
Approximately 8% of U.S. adults are believed to have chronic kidney disease (CKD)(1). Because CKD is an independent risk factor for cardiovascular disease (CVD), patients with CKD face increased risks of cardiovascular (CV) morbidity and mortality(2–4). HMG-CoA reductase inhibitors (“statins”) are widely used to prevent CVD in the general population. Although clinical trials failed to demonstrate a benefit from statins in end-stage renal disease (ESRD) (5,6), subgroup analyses of several large trials (7–10) and three meta-analyses (11–13) demonstrate that statins reduce the relative risk of myocardial infarction (MI) and stroke in patients with mild-to-moderate CKD at a magnitude similar to reduction for non-CKD patients(7,14). Results from the Study of Heart and Renal Protection (SHARP) trial also suggest that statins prevent CV events in patients with advanced, non-dialysis requiring CKD(15). Given the high CV risk for patients with non-dialysis-requiring CKD and the relatively low cost of generic statins, these drugs have the potential to improve health at an acceptable cost. Balanced against the potential benefits is the fact that patients with CKD experience decreased life-expectancy, higher healthcare costs, and reduced quality of life. Additionally, statin-associated rhabdomyolysis (i.e., life-threatening muscle toxicity) is more common in persons with CKD than in the general population(16).
Although clinical trials support the efficacy of statins for patients with non-dialysis-requiring CKD, no consensus recommendations exist for their use in primary prevention. Several guidelines recommend that CKD be considered a “coronary heart disease (CHD) risk equivalent” similar to diabetes when deciding whether to initiate statins(17,18). However, the Adult Treatment Panel (ATP) III guidelines do not include CKD as an independent risk factor for CV events(19). Treating CKD as a “CHD risk equivalent” could lead to statin treatment for up to 2 million additional persons with CKD in the U.S.(20).
We developed a decision-analytic model to assess the cost-effectiveness of statins for primary CVD prevention in non-dialysis requiring CKD. The analysis focuses on the sizable subset of the CKD population with hypertension but with no other traditional CV risk factors for whom the ATP III guidelines would not recommend statins.
Methods
Decision-analytic model
We developed a Markov model of CVD and CKD to evaluate statin therapy for primary CV prevention in the following cohorts (Appendix Figures S1a–S1c):
-
1)
The base case – 65 year-old men and women with mild-to-moderate CKD and moderate hypertension (SBP 130–140 mmHg on treatment).
-
2)
Additional cohorts of men and women defined by: a) age at initiation of statins; b) presence (and degree) of hypertension; c) CKD stage; d) progression of CKD.
The model follows simulated patients over their lifetimes in 3-month intervals. Individuals can have an acute MI or stroke, die during or after surviving an MI or stroke, or die from non-CV causes. Those with prior non-fatal CV events have further elevated risks of mortality from both repeat CV events and CVD-associated complications. (Appendix Figure S1a)
Patients progress through CKD stages 3a (estimated GFR (eGFR) 45–59 ml/min/1.73m2), 3b (eGFR 30–44 ml/min/1.73m2), 4 (eGFR 15–29 ml/min/1.73m2) and 5 (eGFR <15 ml/min/1.73m2). Costs, quality of life, mortality, and rates of CV events vary by CKD stages until patients progress to stage 5 CKD, after which they experience mortality rates and costs equal to the averages of similarly-aged U.S. patients with ESRD. (Appendix; Mortality in ESRD; Figure S1b) Outcomes included quality-adjusted life years (QALYs), life years, direct healthcare costs in 2010 US dollars and incremental cost-effectiveness ratios (ICERs) discounted at 3% annually(21). We report costs and ICERs rounded to the nearest $100.
Data
Model inputs were derived from published literature. (Table 1) Rates and variability of CKD progression were derived from large observational cohorts of comparable patient populations via model calibration. (Appendix; Modeling CKD Progression) Baseline probabilities of MI and stroke were derived from age- and sex-based Framingham risk scores. (Appendix; Determining Baseline Cardiovascular Risk) Baseline hazards of CV events estimated from the Framingham equations were multiplied by CKD stage-specific hazard ratios reflecting the increased risk of CV events independently associated with CKD stages 3a, 3b, and 4 (3,4). All-cause mortality rates for patients in each CKD stage were calculated by multiplying CKD stage-specific all-cause mortality hazards by age- and sex-specific mortality in the general population obtained from U.S. life tables(4,22) For each CKD stage, rates of non-cardiovascular death were imputed from all-cause mortality rates by adjusting for competing cardiovascular risks. (Appendix; Calibrating Rates of Non-Cardiovascular Death)
Table 1.
Model Inputs
| Intervention effects: | (Value) | (Range) | |
|---|---|---|---|
| CV relative risk reduction from statins (stage 3 CKD)1 | 0.80 | 0.66 | 0.85 |
| CV relative risk reduction from statins (stage 4 CKD) | 0.83 | 0.74 | 1.00 |
| Rate of rhabdomyolysis (per 10,000 person-years) | 4.64 | 0.46 | 46.40 |
| Probability of death from rhabdomyolysis | 0.08 | 0.07 | 0.08 |
| Probability of myopathy on statins | 0.09 | 0.02 | 0.20 |
| Non-CKD Natural History Parameters: | |||
| Age-based probability of death in healthy individuals | (U.S. Life Tables) | (+−10%) | |
| Probability of myocardial infarction (MI) and stroke | (Framingham model)† | (+−20%) | |
| Probability of death from acute MI | 0.07 | 0.06 | 0.11 |
| Increased hazard of death after MI - hazard ratio2 | 1.40 | 1.07 | 2.04 |
| Probability of death from acute stroke | 0.12 | 0.10 | 0.19 |
| Increased hazard of death after Stroke - hazard ratio2 | 2.30 | 2.00 | 2.70 |
| CKD-Specific Natural History Parameters: | |||
| Increased risk of all-cause mortality (CKD stage 3a) - hazard ratio | 1.20 | 1.10 | 1.30 |
| Increased risk of all-cause mortality (CKD stage 3b) - hazard ratio | 1.80 | 1.70 | 1.90 |
| Increased risk of all-cause mortality (CKD stage 4) - hazard ratio | 3.20 | 3.10 | 3.40 |
| Probability of death from ESRD | (see appendix) | (+−20%) | |
| Increased risk of MI and Stroke (CKD stage 3 a) - hazard ratio | 1.40 | 1.30 | 1.50 |
| Increased risk of MI and Stroke (CKD stage 3b) - hazard ratio | 2.00 | 1.90 | 2.10 |
| Increased risk of MI and Stroke (CKD stage 4) - hazard ratio | 2.80 | 2.60 | 2.90 |
| Increased hazard of death from acute MI due to CKD (all stages)3 | 1.40 | 1.20 | 1.60 |
| Increased hazard of death from acute stroke due to CKD (all stages)(38)3 | 1.86 | 1.00 | 2.00 |
| Rate of CKD progression (ml/min/1.73m2/yr)† | 1.36 | 1.00 | 2.00 |
| Quality of life assumptions | (QALY) | (Range) | |
|---|---|---|---|
| CKD stage IIIa† | 0.96 | 0.86 | 1.00 |
| CKD stage IIIb† | 0.92 | 0.82 | 1.00 |
| CKD Stage IV | 0.88 | 0.71 | 1.00 |
| ESRD with no prior CV event | 0.70 | 0.50 | 0.84 |
| Following MI | 0.80 | 0.50 | 0.90 |
| Following Stroke | 0.80 | 0.40 | 0.92 |
| While experiencing myalgia | 0.80 | 0.70 | 0.90 |
| ESRD with prior CV event | 1.00 | 0.50 | 1.00 |
| Cost assumptions ($2010) | ($) | (Range) | |
|---|---|---|---|
| Annual Cost Following MI | 4,343 | (50%–200%) | |
| Annual Cost Following Stroke | 6,060 | (50%–200%) | |
| Annual Cost of Stage IIIa CKD† | 1,833 | (50%–200%) | |
| Annual Cost of Stage IIIb CKD† | 4507 | (50%–200%) | |
| Annual Cost of Stage IV CKD | 5,844 | (50%–200%) | |
| Acute cost of MI | 11,071 | (50%–200%) | |
| First year after MI | 7,747 | (50%–200%) | |
| Acute cost of Stroke | 18,516 | (50%–200%) | |
| First year after stroke | 7770 | (50%–200%) | |
| Cost of Rhabdomyolysis | 55,794 | (50%–200%) | |
| Monthly cost of Statin | 4 | 4 | 166 |
| Annual laboratory + physician monitoring fees† | 43 | ||
See Appendix for more detail about data sources and model assumptions
CV – cardiovascular; CKD – chronic kidney disease. Asymmetric ranges appear in some deterministic sensitivity analyses when ranges are obtained from literature. See appendix for additional references
Applies to CKD stages 3a and 3b, and patients without CKD.
Estimates of increased probability of death following MI in CKD vs. non-CKD populations are comparable to the elevated risk of all-cause mortality in CKD. Consequently, baseline all-cause mortality hazard from CKD was substituted to approximate CKD-specific increased hazard of death following MI and stroke and was multiplied by post-MI and post-stroke hazards of death to obtain overall hazard of death in CKD patients following cardiovascular event.
We did not vary hazards across stages due to a lack of data addressing outcomes from stroke by CKD stage and data demonstrating only mild variation across CKD stage following MI(39).
Note: Quality-of-life assumed to decrease as patients age and differs by sex as observed in the Beaver Dam Study.(40) Decreases in health-related quality-of-life were applied multiplicatively for patients with more than one illness and depended on age and sex. Costs included background medical costs (see appendix) End-stage renal disease (ESRD) costs and mortality stratified by age and ESRD duration are derived from national averages published by USRDS(41) Statin costs were not added to these for patients with stage 5 CKD. Patients experience 0 QALYs for the 2 weeks of hospitalization with rhabdomyolysis. See Appendix for additional references.
See Appendix for more detail.
Statin Benefits
Relative risk reductions for MI and stroke in stages 3a and 3b CKD were obtained from a meta-analysis (11) and were similar to the treatment effect observed in patients with CKD from the Pravastatin Pooling Project and in two other meta-analyses(9,12,13). (Appendix Table S1) While lacking definitive evidence, evaluation of clinical trials suggests a trend towards decreased CVD risk reduction from statins for patients with stage 4 CKD(11,15). Consequently, we assumed the CV relative risk reduction equaled that reported in the SHARP trial for patients with stage 4 CKD(15). Additionally, we assumed that statins do not reduce CV risks for stage 5 CKD – i.e., these patients did not incur added costs or receive added health benefits from statins(5,6). We assumed that all patients, unless contraindicated, would be prescribed statins after experiencing a CV event and that published hazards of death and costs of medical care following CV events reflect the effects of statins. In our base case, we assumed statins do not influence the likelihood or rates of CKD progression(11).
Adverse Effects of Statins
Rates of muscle-related toxicity from statins are elevated in patients with CKD(16). We assumed patients with CKD on statins experience a one-time risk of myalgias upon initiating statin therapy and an ongoing risk of rhabdomyolysis. Since previous analyses show that statins for primary prevention are not cost-effective if patients experience even minor quality-of-life decrements from myalgias (23), we assumed patients who experience myalgias discontinue statins. Patients who developed myalgias incurred a cost for checking muscle enzyme levels and a three-month quality-of-life decrement due to the muscle pain. In the event of rhabdomyolysis, patients experience a risk of death from the acute illness, and for those surviving, statins were permanently discontinued.
Costs and Quality-of-Life
We included costs and quality-of-life decrements for each CKD stage, MI, stroke, rhabdomyolysis, and for patients surviving an MI or stroke. (Table 1; Appendix: Selected Model Assumptions) In the base case, statin costs included 40mg of generic pravastatin daily available at discount retailers and integrated health systems in addition to laboratory monitoring and clinical follow-up(24–27).
Alternative Assumptions about CKD and Model Validation
To understand how CKD progression affects the health benefit and cost-effectiveness of statins, we assessed outcomes in otherwise similar patient groups with non-progressive CKD and without CKD. To verify that our assumptions accurately describe mortality in patients with CKD, we compared life expectancies produced from each patient group to U.S. life tables, demonstrating stepwise decreases following additions of hypertension, non-progressive CKD and progressive CKD. (Appendix Tables S2, S3)
Sensitivity Analyses
All model inputs were varied in sensitivity analyses. The effect of statin costs was tested for a range of baseline cardiovascular risks. Probabilistic sensitivity analysis evaluated how the simultaneous uncertainties about model parameters might influence outcomes. We examined scenarios in which statins were assumed to slow the rate of CKD progression by up to 19% and scenarios in which statins increased the likelihood of developing either diabetes or memory loss (28–30).
Results
Initiating statin therapy for patients with mild-to-moderate (stage 3a) CKD, moderate hypertension and no other cardiovascular risk factors led to a modest improvement in health outcomes for 50–85 year-old men and women. (Table 2) For 65 year-old men, statin therapy increased non-discounted life expectancy by 50 days (from 11.65 to 11.78 years) and increased discounted QALYs by 36 days (from 7.21 to 7.31 QALYs). Statins reduced the risk of having at least one MI or stroke over a lifetime (or before progression to ESRD) from 39.8% to 34.7%. Relative benefits were smaller in patients with lower CV risk. For instance, for 65 year-old women, statin therapy increased non-discounted life expectancy by 34 days (from 13.74 to 13.84 years) and increased QALYs by 20 discounted days (from 8.20 to 8.26 QALYs). The corresponding risk of at least one MI or stroke prior to development of ESRD was reduced from 24.1% to 20.6%.
Table 2.
Health Benefits, Costs, and Incremental Cost-Effectiveness Ratio from Statin Therapy for Patients with Different Age, Sex, and Cardiovascular Risk Profiles
| Starting Age | 10-Year Probability of MI1 % | Increased Cost ($) | Gain in QALYs (discounted) | Increased life expectancy (undiscounted) (months) | Reduced risk of MI or stroke (percent)2 | Incremental cost-effectiveness ratio ($/QALY) |
|---|---|---|---|---|---|---|
| Men | ||||||
| 50 | 6 | 1,700 | 0.09 | 1.6 | 4.4 | 20,500 |
| 55 | 10 | 1,800 | 0.09 | 1.7 | 4.8 | 19,600 |
| 60 | 12 | 1,800 | 0.10 | 1.7 | 5.0 | 18,900 |
| 65 | 16 | 1,800 | 0.10 | 1.6 | 5.1 | 18,000 |
| 70 | 17 | 1,500 | 0.09 | 1.5 | 5.0 | 16,900 |
| 75† | 20 | 1,300 | 0.08 | 1.2 | 4.7 | 16,300 |
| 80† | 20 | 900 | 0.06 | 0.9 | 4.1 | 16,100 |
| 85† | 20 | 600 | 0.04 | 0.6 | 3.3 | 15,400 |
| Women | ||||||
| 50 | 1 | 1,700 | 0.03 | 0.7 | 2.1 | 56,800 |
| 55 | 2 | 1,800 | 0.04 | 0.9 | 2.6 | 46,200 |
| 60 | 3 | 1,900 | 0.05 | 1.0 | 3.1 | 39,200 |
| 65 | 5 | 1,900 | 0.06 | 1.1 | 3.5 | 33,400 |
| 70 | 8 | 1,700 | 0.06 | 1.1 | 3.7 | 29,300 |
| 75 | 14 | 1,400 | 0.06 | 1.1 | 3.8 | 25,000 |
| 80 | 14 | 1,100 | 0.05 | 0.9 | 3.6 | 21,300 |
| 85 | 14 | 700 | 0.04 | 0.6 | 3.0 | 19,800 |
For more detailed results see Supplement Table S5. Because costs are rounded to the nearest $100, incremental cost-effectiveness ratios may be slightly different than the incremental costs and QALYs in the table suggest.
Based from a Framingham risk score(19)
Combined reduction in myocardial infarction (MI) and stroke before development of end-stage renal disease
ATPIII currently recommends that individuals with risk level of 20% or more should be treated with statins. All other groups of CKD patients in the table would not have statin therapy currently recommended if they had no other CV risk equivalents.
Treatment with statins increased total lifetime costs for all patient groups. (Table 2) Combining health benefits and costs, statin therapy for 65 year-old men with mild-to-moderate CKD and moderate hypertension cost $18,000 per QALY gained and for 65 year-old women cost $33,400 per QALY gained, assuming the cost of statins at $4 per month.(24,25)
Influence of CKD progression
Patients with advanced CKD and ESRD experience lower health-related quality-of-life, higher rates of costly morbidities, and premature mortality compared to patients without CKD. Although the increased CV risk in patients with CKD means that statins provide a larger absolute risk reduction in CV events compared to patients without CKD, higher “downstream” costs and worse health outcomes associated with progressive CKD blunted the cost-effectiveness of interventions to avert CV events in patients with less severe CKD. In 65 year-old men and women without CKD and moderate hypertension, statins cost $10,200 per QALY gained. In patients of similar age and hypertension severity with mild-to-moderate, non-progressive CKD, statins cost $16,400 per QALY gained, slightly more than in patients without CKD. With CKD progression, the cost-effectiveness attenuated further, reflecting the greater costs and health decrement experienced due to progressive CKD for patients whose CV events were averted with statins. For a cohort of 65 year-olds with moderate hypertension, CKD, and the possibility of CKD progression, statins cost $25,800 per QALY gained. (Appendix Figure S2). In other words, statins will allow more patients with mild-to-moderate CKD to survive free from CV events who later experience more morbid and costly stages of advanced CKD and ultimately ESRD.
Age and baseline cardiovascular risk
Older individuals with CKD have substantially shorter life expectancies, higher ongoing medical costs, decreased likelihood of surviving long enough to progress to ESRD, and higher CV risks compared to younger individuals. In net, there was a trend toward statin use being more cost-effective for older patients with CKD. (Table 2)
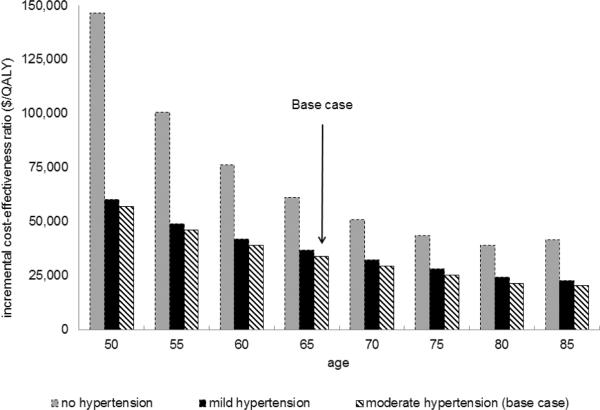
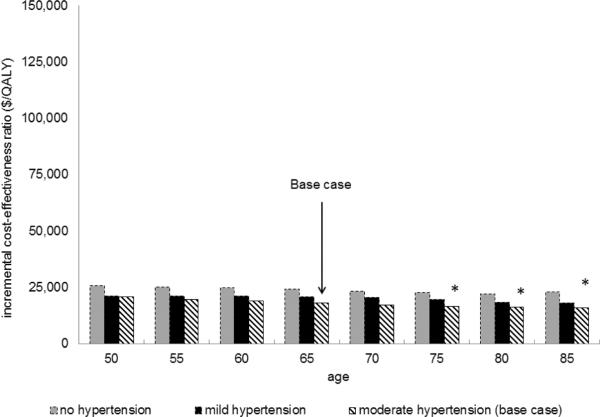
Statins were less cost-effective in groups with lower baseline CV risk. When varying the range of baseline CV risks (designated by the presence and severity of hypertension in conjunction with sex and age at statin initiation), statin therapy cost between $16,100 and $146,700 per QALY gained. (Figure 1)
Figure 1. Statin Cost-effectiveness – Differing Cardiovascular Risk Groups.
Figure 1a Lower Cardiovascular Risk (women)
Figure 1b Higher Cardiovascular Risk (men)
Moderate HTN: SBP 130-140 on treatment; Mild HTN: SBP 120–130 on treatment; No HTN: SBP 120–130 without treatment.
*Indicates patients for whom statins are currently recommended according to ATP III guidelines due to 10-year probability of myocardial infarction (based on traditional cardiovascular risk factors) ≥ 20%.
Statin Prices
When purchased at average retail prices, the cost-effectiveness of statin therapy compared favorably to other commonly accepted treatments in patients at higher CV risks. (Figure 2) If 40mg of pravastatin is purchased at $47 per month, statin therapy cost between $51,700–$87,700 per QALY gained in 50–85 year-old men with mild (SBP 120–130 mmHg on treatment) or moderate hypertension and $81,600–$112,700 per QALY without hypertension; in women 70 and older with mild or moderate hypertension statin therapy cost between $74,300–$110,900 per QALY gained. Due to their lower baseline CV risk, statins were less favorable in younger women with and without hypertension, where the cost was $117,700–$746,700 per QALY gained. (Appendix Table S4; Figures S3–S4) Despite its greater potential efficacy (owing to more potent lowering of LDL cholesterol), brand name rosuvastatin cost more per QALY gained, ranging from $80,500 per QALY gained in 65 year-old men to $390,500 per QALY in 50 year-old women.
Figure 2. Price Sensitivity Analysis – Cost-effectiveness at Different Statin Prices.
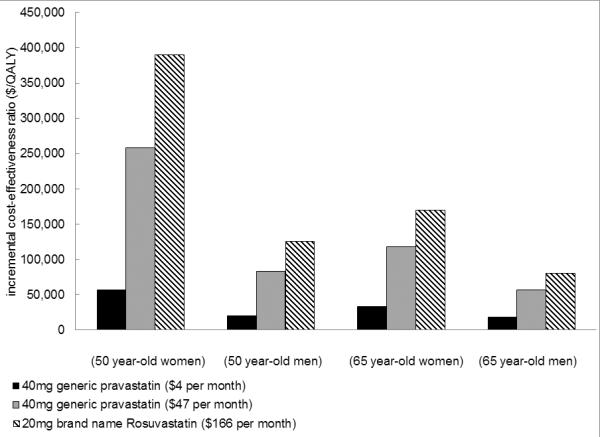
Treatment effect for Pravastatin (RR 0.8) obtained from a meta-analysis of statin trials in patients with CKD, weighted heavily by the Pravastatin Pooling Project.(11) Treatment effect for Rosuvastatin obtained from an analysis of the CKD population from the Jupiter Trial in patients with high CRP(7). $4 per month prices obtained from Wal-Mart Retail Prescription Drug Program List (24) are comparable to those paid by Veterans Affairs facilities (25) while remaining prices are average retail prices obtained from consumer reports(37).
Sensitivity analyses
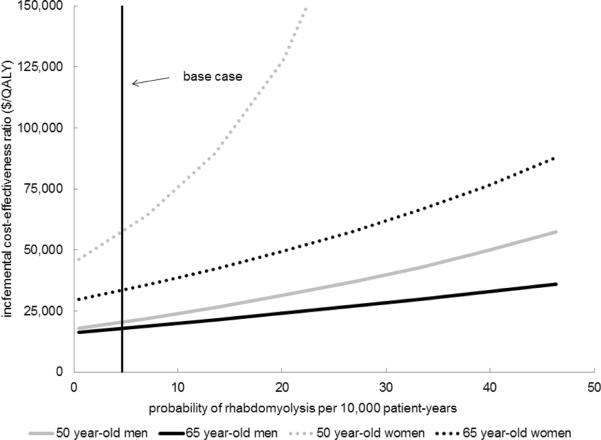
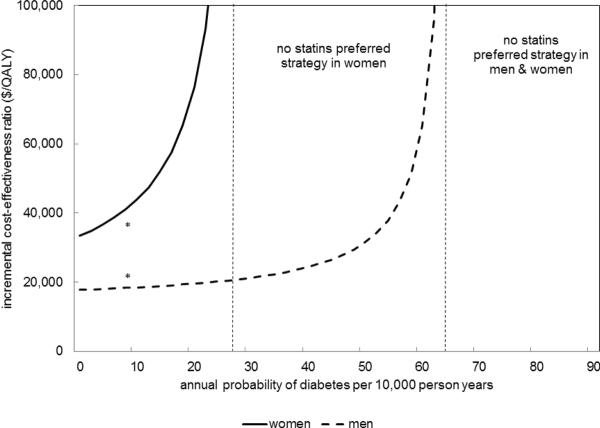
Results were sensitive to the range of rhabdomyolysis risk in CKD, particularly in younger, lower risk groups facing prolonged exposure to statins. (Figure 3) Due to higher healthcare costs, lower quality-of-life, and elevated risk of non-CV mortality in more advanced CKD, statins were less cost-effective when started in patients with lower GFR, despite higher CV risks. (Appendix Figure S5) Although not definitive, evidence suggests that statins may reduce the rate of CKD progression(28). If statins were to reduce CKD progression rates by 19%, they are cost-saving in 65-year old men and women. (Appendix Figure S6) When we considered the possibility of statins causing either diabetes or permanent memory loss, the cost-effectiveness of statins did not substantially change for CKD patients with higher CV risk.(Appendix Figures S7–8) However, for CKD patients with lower CV risks, statin therapy was substantially less cost-effective (or dominated by a strategy of no statins). In 65 year-old men and women (who are at higher CV risk), if the long-term risk of diabetes from statins is 2-fold higher in women and 6-fold higher in men than described, no statins becomes the preferred strategy as it yields longer quality-adjusted life expectancy at a lower cost. (Figure 4)
Figure 3. Sensitivity Analysis.
Cost-effectiveness of statins was most sensitive to the risk of rhabdomyolysis in patients at lower baseline cardiovascular risk; an increase in the rate of rhabdomyolysis in 50 year-old women with moderate hypertension makes statins considerably less cost-effective.
Figure 4. Exploratory Analysis – Cost-effectiveness if Statins Cause Diabetes in 65 year-old men and women.
*estimate of statin-induced diabetes incidence from a meta-analysis Cost-effectiveness is not sensitive to the long-term risk of diabetes at rates that have been described. If the long-term risk of diabetes from statins is more than 2-fold higher in women and 6-fold higher in men than described, no statins becomes the preferred strategy as it yields longer quality-adjusted life expectancy at a lower cost
Probabilistic sensitivity analysis
Considering the simultaneous uncertainty in all model parameters, statins cost less than $50,000 per QALY gained in over 99% of simulations for 50 –and 65 –year-old men, and in 95% of simulations for 65 year-old women. In 50 year-old women statins cost less than $50,000 per QALY gained in 52% of simulations, and cost less than $100,000 per QALY gained in 91% of simulations. (Appendix Figures S9–S12)
Discussion
Statins for primary prevention of CVD in patients with moderate hypertension and mild-to-moderate CKD can increase life expectancy by 0.5 to 1.9 months and prevent MI or stroke for 2 to 5% of patients prior to developing ESRD. If generic statins were obtained at $4 per month (available from discount retail programs and integrated health systems)(24,25), they were cost-effective for patients with a wide range of CV risks, costing less than $25,803 per QALY in men with and without hypertension and ranging from $20,152–$60,043 per QALY in women with mild or moderate hypertension, comparing favorably with many commonly-accepted treatments.
Statins are less cost-effective when their prices are higher and in patients with lower baseline CV risks. At average retail prices, statin therapy costs between $51,835–$97,314 per QALY in men with mild or moderate hypertension and in women above age 70 with moderate hypertension. For patients with lower baseline CV risk (i.e., women below age 70 with mild or moderate hypertension, women of all ages without hypertension, and men below age 60 without hypertension), statin therapy cost between $100,818–$746,741 per QALY.
In addition to baseline CV risk and statin price, the cost-effectiveness of statins was particularly sensitive to drug-related toxicity. This highlights the importance of exercising caution when prescribing statins for primary cardiovascular prevention to patients with CKD. An increase from the baseline incidence of rhabdomyolysis makes statins substantially less cost-effective when given to younger patients with lower CV risk. As lower costs make statins affordable to larger segments of the population, patient education about signs and symptoms of muscle toxicity as well as prompt discontinuation of therapy at the first sign of muscle injury will be increasingly important. More knowledge about the relative risks of rhabdomyolysis from different statins in patients with CKD would be informative. Additionally, it will be important to verify that long-term statin risks such as diabetes and memory loss are low.
Our findings suggest that CKD may be different from traditional CV risk factors when considering statin cost-effectiveness. Current ATP III guidelines recommend initiating statin therapy in the general population according to a person's risk of future cardiovascular events as determined by a Framingham CV risk score(31). This recommendation is based in part on the observation that the relative reduction in risk of CVD appears to be independent of the baseline risk(8,32). This observation has led to past findings that statins are more cost-effective in higher-risk populations where the absolute risk reduction is the largest (23,33,34).
Our analysis reaches a different conclusion for adults with CKD, hypertension, and no other traditional CV risk factors – despite an increased risk of MI and stroke in patients with CKD, statins are not more cost-effective in patients with CKD than they are in the general population after considering traditional CV risk factors. This is because the presence of CKD introduces the following combination of offsetting health and economic influences: 1) Patients with CKD are at an increased risk of CV disease and related mortality, increasing statins' net benefit; 2) Patients with CKD are at an increased risk of muscle toxicity, reducing statins' net benefit; 3) CKD progression leads to increased medical costs, decreased quality-of-life, increased non-cardiovascular mortality, and apparent ineffectiveness of statins once patients develop ESRD, reducing statins' net benefit. These partly offsetting health and economic influences are most pronounced when considering statin therapy in patients with lower baseline CV risks and when statins are obtained at higher prices.
This study has several limitations. First, it is unknown precisely how the benefits from statin therapy decline as CKD advances. While clinical trials demonstrate that statins are effective for CV prevention in stage 3 CKD (7–10), statin trials in patients with ESRD have not shown a benefit (5,6). Because of these disparate observations – and evidence that CVD may have a different pathophysiologic basis in more advanced CKD (35) – we assume the treatment effect from statins diminishes in advanced CKD stages. While this assumption reflects current observations, the assumed step-wise decrease in treatment effect has not been demonstrated definitively. Second, when patients with CKD progress to ESRD, medical care becomes substantially more expensive. By prolonging life in patients with CKD, a part of statins' incremental cost is due to the high downstream costs of dialysis. Manns et al. discuss how including downstream health care costs in economic evaluations of patients on dialysis are “methodologically correct [but] may mitigate against the acceptance of interventions that are relatively inexpensive themselves but which improve patient survival(36)”. This may pose challenges for policymakers because, as a society, we currently pay for the high cost of ESRD care. It is counter-intuitive that a strategy aimed at prolonging life for patients with non-dialysis-requiring CKD would be subjected to a more stringent criterion; a higher cost-effectiveness threshold for statins in this population may therefore be justified. Understanding whether there is a direct benefit of statins on CKD progression would be particularly informative in this regard. Finally, the incidence of diabetes due to statins has been described for the general population, but is not well described in the CKD population. However, in our exploratory analysis we found statins to be cost-effective in the base case despite using cost, quality of life, and hazard estimates that were weighted towards making diabetes more severe in its health and economic ramifications.
While statins reduce absolute CVD risk in patients with CKD, increased risk of rhabdomyolysis, and competing risks associated with progressive CKD, partly offset these gains. Nevertheless, low-cost generic statins are cost-effective for primary prevention of CVD in patients with mild-to-moderate CKD and hypertension. At average retail prices it could be argued that statin therapy for patients with CKD should be reserved for subgroups defined by higher cardiovascular risk and greater potential to benefit. Statins are only cost-effective in populations at lower CV risk if they have low risk of rare but clinically important side effects.
Supplementary Material
Acknowledgements
The authors thank Arjun Adhikari, MS, and Joshua Glucoft, MS for their contribution to early model design and data collection.
Funding Sources: F32 HS019178 from AHRQ (Dr. Erickson); DK085446 (Dr. Chertow); AG037593 (Dr. Goldhaber-Fiebert) Dr. Owens was supported by the Department of Veterans Affairs.
Abbreviations List
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- ESRD
End-stage renal disease
- SHARP
Study of Heart and Renal Protection
- CHD
Coronary heart disease
- eGFR
Estimated glomerular filtration rate
- QALY
Quality adjusted life year
- ICER
Incremental cost-effectiveness ratio
- ATP
Adult Treatment Panel
- CV
Cardiovascular
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Hsu C-y, Vittinghoff E, Lin F, Shlipak MG. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Annals of Internal Medicine. 2004;141:95–101. doi: 10.7326/0003-4819-141-2-200407200-00007. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. Journal of the American Society of Nephrology. 2002;13:745–53. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. Erratum appears in N Engl J Med. 2008;18(4):4. [DOI] [PubMed] [Google Scholar]
- 5.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. New England Journal of Medicine. 2009;360:1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 6.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. New England Journal of Medicine. 2005;353:238–48. doi: 10.1056/NEJMoa043545. Erratum appears in N Engl J Med. 2005 Oct 13;353(15):1640. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. Journal of the American College of Cardiology. 2010;55:1266–73. doi: 10.1016/j.jacc.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Heart Protection Study Collaborative G MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. Summary for patients in Curr Cardiol Rep. 2002 Nov;4(6):486-7; PMID: 12379169. [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557–63. doi: 10.1161/01.CIR.0000143892.84582.60. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Kastelein JJP, Bittner V, et al. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: the TNT (Treating to New Targets) study. Journal of the American College of Cardiology. 2008;51:1448–54. doi: 10.1016/j.jacc.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 11.Navaneethan SD, Pansini F, Perkovic V, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews. 2009:CD007784. doi: 10.1002/14651858.CD007784. [DOI] [PubMed] [Google Scholar]
- 12.Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GFM. Benefits and Harms of Statin Therapy for Persons With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Annals of Internal Medicine. 2012;157:263–75. doi: 10.7326/0003-4819-157-4-201208210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhyay A, Earley A, Lamont JL, Haynes S, Wanner C, Balk EM. Lipid-Lowering Therapy in Persons With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Annals of Internal Medicine. 2012;157:251–62. doi: 10.7326/0003-4819-157-4-201208210-00005. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli M, Moye L, Sacks FM, et al. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Annals of Internal Medicine. 2003;138:98–104. doi: 10.7326/0003-4819-138-2-200301210-00010. Summary for patients in Ann Intern Med. 2003 Jan 21;138(2):I28; PMID: 12529112. [DOI] [PubMed] [Google Scholar]
- 15.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClure DL, Valuck RJ, Glanz M, Murphy JR, Hokanson JE. Statin and statin-fibrate use was significantly associated with increased myositis risk in a managed care population. Journal of Clinical Epidemiology. 2007;60:812–8. doi: 10.1016/j.jclinepi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. Erratum appears in Circulation. 2005 Apr 19;111(15):2013. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney F K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39:S1–266. [PubMed] [Google Scholar]
- 19.Expert Panel on Detection E Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 20.Hyre AD, Fox CS, Astor BC, Cohen AJ, Muntner P. The impact of reclassifying moderate CKD as a coronary heart disease risk equivalent on the number of US adults recommended lipid-lowering treatment. American Journal of Kidney Diseases. 2007;49:37–45. doi: 10.1053/j.ajkd.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 22.Elizabeth Arias PD. United States Life Tables, 2006. National Vital Statistics Reports. 2010;58 [PubMed] [Google Scholar]
- 23.Lee KK, Cipriano LE, Owens DK, Go AS, Hlatky MA. Cost-effectiveness of using high-sensitivity C-reactive protein to identify intermediate- and low-cardiovascular-risk individuals for statin therapy. Circulation. 2010;122:1478–87. doi: 10.1161/CIRCULATIONAHA.110.947960. [DOI] [PubMed] [Google Scholar]
- 24.Walmart Pharmacy Retail Prescription Drug Program List. 2012 Retreived from http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf.
- 25.ZenRx Research Prices for Pravastatin. 2012 Mar 7; Retreived from http://zenrx.org/register.asp?reg=0&a=y&c=289&b=y&x=165&y=85&w=Pravastatin-40.
- 26.Department of Health and Human Resources: Centers for Medicare and Medicaid Services Clinical Laboratory Fee Schedule. 2010 Reterived from http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html.
- 27.Department of Health and Human Resources: Centers for Medicare and Medicaid Services Physician Fee Schedule. 2011 Retreived from http://www.cms.gov/apps/physician-fee-schedule/overview.aspx.
- 28.Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112:171–8. doi: 10.1161/CIRCULATIONAHA.104.517565. [DOI] [PubMed] [Google Scholar]
- 29.Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–64. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 30.Glasser SP, Wadley V, Judd S, et al. The association of statin use and statin type and cognitive performance: analysis of the reasons for geographic and racial differences in stroke (REGARDS) study. Clinical Cardiology. 2010;33:280–8. doi: 10.1002/clc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 32.Baseline risk factors and their association with outcome in the West of Scotland Coronary Prevention Study. The West of Scotland Coronary Prevention Study Group. American Journal of Cardiology. 1997;79:756–62. doi: 10.1016/s0002-9149(96)00863-6. [DOI] [PubMed] [Google Scholar]
- 33.Prosser LA, Stinnett AA, Goldman PA, et al. Cost-effectiveness of cholesterol-lowering therapies according to selected patient characteristics. Annals of Internal Medicine. 2000;132:769–79. doi: 10.7326/0003-4819-132-10-200005160-00002. [DOI] [PubMed] [Google Scholar]
- 34.Pharoah PD, Hollingworth W. Cost effectiveness of lowering cholesterol concentration with statins in patients with and without pre-existing coronary heart disease: life table method applied to health authority population. BMJ. 1996;312:1443–8. doi: 10.1136/bmj.312.7044.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz U, Buzello M, Ritz E, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrology Dialysis Transplantation. 2000;15:218–23. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 36.Manns B, Meltzer D, Taub K, Donaldson C. Illustrating the impact of including future costs in economic evaluations: an application to end-stage renal disease care. Health Economics. 2003;12:949–58. doi: 10.1002/hec.790. [DOI] [PubMed] [Google Scholar]
- 37.Consumer Reports Health Best Buy Drugs. Statin Drugs to Treat High Cholesterol and Heart Disease. 2010 Retreived from http://www.consumerreports.org/health/resources/pdf/best-buy-drugs/StatinsUpdate-FINAL.pdf.
- 38.USRDS . United States Renal Data System. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 39.Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Annals of Internal Medicine. 2002;137:563–70. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 40.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 41.Leibson CL, Hu T, Brown RD, Hass SL, OFallon WM, Whisnant JP. Utilization of acute care services in the year before and after first stroke: A population-based study. Neurology. 1996;46:861–869. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.