Mutations in the isocitrate dehydrogenase (IDH) 1 and 2 genes are recurrent, frequent and prognostic in patients with acute myeloid leukemia (AML). IDH1 and IDH2 encode nicotinamide adenine dinucleotide phosphate (NADP +) dependent enzymes responsible for the oxidative decarboxylation of isocitrate to α-ketoglutarate (AKG) [1]. Heterozygous point mutations in IDH1 and IDH2 result in the loss of this activity, concomitant with a neomorphic ability to convert AKG to 2-hydroxyglutarate (2HG), resulting in significant elevations in this oncometabolite, which is not detectable in patients with AML with wild type IDH [2,3]. The ten-eleven translocation (TET) family proteins convert 5-methylcytosine (5MC) to 5-hydroxymethylcytosine (5HMC), leading to DNA demethylation [4]. TET2 encodes a dioxygenase that is dependent on AKG, and therefore, increased levels of 2HG may disrupt TET2 function [5]. We explored the potential of 2HG to serve as a clinical biomarker of response to therapy in patients with AML with IDH mutations, and correlated fluctuations of 2HG with changes in 5MC and 5HMC levels in a clinical setting.
A clinical trial of elderly, previously untreated patients with AML who received sequential azacitidine and lenalidomide was approved by the Stanford University Institutional Review Board. Bone marrow aspirate was collected at specified pre- and post-treatment time points with the informed consent of the patients, in accordance with the Declaration of Helsinki. Plasma and mononuclear cells were isolated and preserved as previously described [6]. DNA and RNA were extracted with the use of Qiagen kits (Qiagen, Valencia, CA).
Genotyping for IDH1/2, TET2 , FMS-like tyrosine kinase 3 internal tandem duplication (FLT-3–ITD) and D835 tyrosine kinase domain mutation (FLT-3–TKD), nucleophosmin (NPM) and CCAAT enhancer binding protein alpha (CEBPA) were performed as previously described [6]. 2HG levels were measured as previously described by liquid chromatography-mass spectrometry from plasma obtained from bone marrow aspirations [2], and normalized according to the total blast count. 5HMC and 5MC levels were measured as previously described by high performance liquid chromatography (HPLC) coupled to tandem mass spectrometry from DNA extracted from bone marrow aspirate mononuclear cells [7].
Six patients with IDH mutations had pre- and post-treatment samples available for comparison. Clinical responses were determined according to European Leukemia Net criteria [8]. The median age was 77, and 3/6 were female. Three had a normal karyotype, and three had other coexisting mutations. Two had IDH1 mutations (R132), and four had IDH2 mutations (R140 n = 3, R172 n = 1) (Table I).
Table I.
Characteristics of patients with IDHMUT with pre- and post-treatment samples available for comparison.
| Patient | Age | Sex | IDH mutation | Coexisting mutations* | Cytogenetics | Best response |
|---|---|---|---|---|---|---|
| A | 82 | F | IDH2 R140 | FLT-3–ITDMUT | NK | RD |
| NPM1MUT | ||||||
| B | 77 | M | IDH1 R132 | FLT-3–ITDMUT | NK | CR |
| C | 80 | F | IDH2 R140 | TET2MUT (S214X) | del(7) | CR |
| D | 70 | M | IDH1 R132 | None | NK | RD |
| E | 70 | M | IDH2 R172 | None | del(13q) | RD |
| F | 76 | F | IDH2 R140 | None | +8 | RD |
F, female; M, male; NK, normal karyotype; RD, resistant disease; CR, complete response.
All patients were screened for FMS-like tyrosine kinase 3 internal tandem duplication (FLT-3–ITD) and D835 tyrosine kinase domain mutation (FLT-3–TKD), nucleophosmin (NPM1), CCAAT enhancer binding protein alpha (CEBPA) and ten-eleven translocation 2 (TET2).
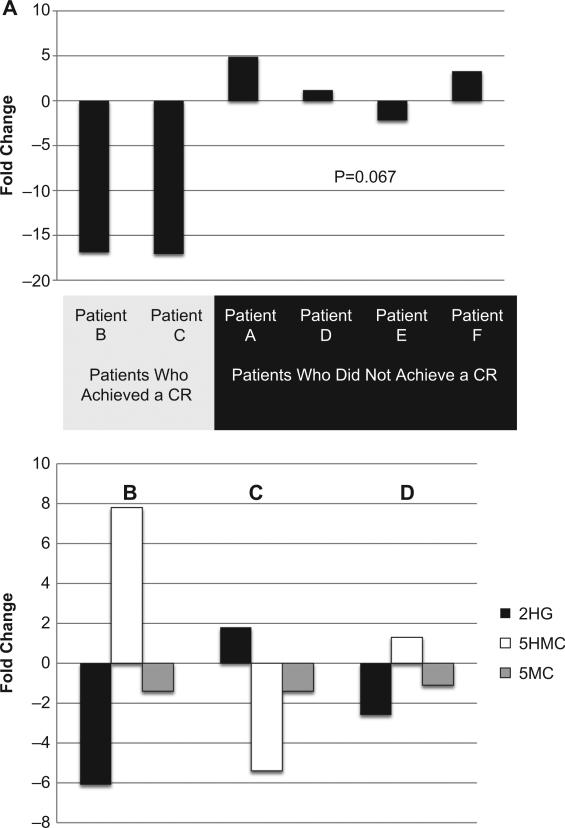
Two of the six patients achieved a complete remission (CR), one with an IDH1 R132 (patient B) and the other with an IDH2 R140 (patient C). A comparison of pre- and post-treatment samples from these patients revealed decreases in 2HG levels of –16.9- and –17.9-fold after three cycles of therapy. Four patients did not achieve a CR, although some did experience decreases in the percentage of blasts. These patients had substantially smaller decreases, or increases, in 2HG levels when measured after one cycle (non-responding patients did not receive more than one cycle of therapy) [Figure 1(A)]. A non-parametric comparison (Mann–Whitney U-test) in this small sample set showed a trend toward a significant change in pre-versus post-treatment 2HG levels between patients who experienced a CR and those who did not (p = 0.067).
Figure 1.
(A) Fold changes in 2-hydroxyglutarate in patients with AML with IDH mutations. (B–D) Fold changes in 2-hydroxyglutarate, 5-hydroxymethylcytosine and 5-methylcytosine in patient B (IDHMUT TET2WT) after achieving a post-cycle 1 complete remission (B) and at relapse (C), and in patient C (IDHMUT TETMUT) after achieving a post-cycle 1 complete remission (D).
Both patients who achieved a CR continued to have detectable 2HG levels. Furthermore, patient B ultimately relapsed, and at the time of this relapse, which was unexpected based on clinical indicators or routine peripheral blood laboratory findings, there was a 1.8-fold increase in 2HG levels.
We hypothesized that decreases in 2HG would allow functional TET2 activity, resulting in increased 5HMC and decreased 5MC. Therefore, in the patients who achieved a CR, we correlated changes in 2HG levels with changes in global 5MC and 5HMC levels. After one cycle of therapy, which resulted in a CR, patient B (TET2WT) experienced a 6.1-fold decrease in 2HG; 5HMC increased 7.8-fold and 5MC decreased 1.4-fold. At relapse, when 2HG rose 1.8-fold, 5HMC decreased 5.4-fold [Figure 1(B) and 1(C)]. Patient C (TET2MUT) had a 2.6-fold decrease in 2HG after one cycle of therapy, which resulted in a CR, with a comparatively smaller 1.3-fold increase in 5HMC [Figure 1(D)].
IDH mutations in AML cause dramatic elevations in 2HG [2]; the full implications of this metabolic derangement on the initiation or propagation of malignant cells remains a question of active interest. Our results suggest that 2HG levels may correlate with leukemic blast clearance, raising the possibility that this may serve as a surrogate for response in this subset of patients with AML. It remains unclear whether the degree of baseline 2HG elevation is predictive of outcome. Furthermore, measuring 2HG may present opportunities to utilize a non-invasive marker of minimal residual disease, based on our observation that patients who achieve a morphological remission continue to have detectable levels. However, all patients reported here were treated with non-intensive regimens, and whether 2HG would remain detectable in patients in CR after intensive induction regimens is not known.
By assaying fold changes in 2HG, 5HMC and 5MC from serial patient samples, we were able to test the hypothesis that 2HG inhibits TET2 function in a clinical setting. Patient B, who was wild-type for TET2 , had a decrease in 2HG at the time of remission. Concomitantly, this patient had an increase in 5HMC and a decrease in 5MC, ostensibly due to the recovery of TET2 function. However, at relapse, 2HG levels rose, once again inhibiting TET2 and causing a relative decrease in 5HMC; the expected increase in 5MC was not observed [Figure 1(B) and 1(C)]. Although concomitant mutations in IDH and TET2 are rare [9], patient C did have this genotype, which allowed for an instructive negative control. After one cycle of therapy, 2HG decreased 2.6-fold. However, in contrast to patient B, in whom it appeared at CR that TET2 regained function (suggested by the increase in 5HMC), patient C's mutant TET2 resulted in marginal changes in 5HMC and 5MC levels (1.3-fold increase and 1.1-fold decrease, respectively) [Figure 1(D)].
Therefore, the unique elevations in 2HG that typify AML with IDH mutations may be useful markers of response to therapy or minimal residual disease. These results suggest that the interactions between 2HG and TET2 function are dynamic and clinically relevant, with structural epigenetic consequences that may impact clinical outcomes and be exploited therapeutically.
Supplementary Material
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito S, D'Alessio AC, Taranova OV, et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollyea DA, Kohrt HE, Gallegos L, et al. Safety, efficacy and biological predictors of response to sequential azacitidine and lenalidomide for elderly patients with acute myeloid leukemia. Leukemia. 2012;26:893–901. doi: 10.1038/leu.2011.294. [DOI] [PubMed] [Google Scholar]
- 7.Pronier E, Almire C, Mokrani H, et al. Inhibition of TET2-mediated conversion of 5-methylcytosine to 5-hydroxymethylcytosine disturbs erythroid and granulomonocytic differentiation of human hematopoietic progenitors. Blood. 2011;118:2551–2555. doi: 10.1182/blood-2010-12-324707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.