Highlights
-
•
Pain-related evoked potentials were similarly influenced by emotional priming.
-
•
The amplitude of both N2 and P2 decreased monotonically from viewing neutral to pleasant to unpleasant pictures.
-
•
Cortical processing of trigeminal nociception is modulated by emotion.
-
•
Reductions in pain-related evoked potentials may be explained by the ability of affective pictures to grab attention.
Keywords: Attention, Emotion, Nociception
Abstract
To investigate whether cortical processing of trigeminal nociception is modulated by emotion, the N2 and P2 components of the pain-related evoked potential (PREP) were recorded in response to noxious stimulation of the supraorbital nerve while participants viewed neutral, pleasant and unpleasant pictures. The nerve was stimulated at 125% of pain threshold via a nociceptive-specific concentric electrode to selectively activate A-delta pain fibres. The N2 and P2 pain-related evoked potentials were similarly influenced by emotional priming: the amplitude of both potentials decreased monotonically from viewing neutral to pleasant to unpleasant pictures. These findings show that cortical processing of trigeminal nociception is modulated by emotion. We explain our findings in terms of the effects of picture viewing on attention.
1. Introduction
The affective picture viewing paradigm has been used to study the emotion-pain relationship. Electrical pain is highest while viewing unpleasant pictures and lowest while viewing pleasant pictures (Kenntner-Mabiala, Andreatta, Wieser, Muhlberger, & Pauli, 2008; Kenntner-Mabiala & Pauli, 2005; Mini, Rau, Montoya, Palomba, & Birbaumer, 1995). However, few studies have examined pain-related evoked potentials (PREPs) in this context and none has investigated trigeminal stimulation. The present study was designed to fill this gap.
Cortical evoked potentials to noxious stimuli characterize the central processes associated with nociception. The most commonly studied PREPs are the N2 and P2, which refer to the second negative and positive peaks, respectively, of the cortical response to a noxious stimulus and represent the cortical activity that results from processing a painful stimulus (Edwards, Inui, Ring, Wang, & Kakigi, 2008). The N2 and P2 are generated mainly in anterior cingulate cortex whereas N2 is also shaped by secondary somatosensory and insula cortexes (Bromm & Chen, 1995; Tarkka & Treede, 1993; Valeriani, Rambaud, & Mauguiere, 1996). A review of cortical areas activated by painful stimuli points to the possible functional significance of N2 and P2: anterior cingulate is influenced by affect and attention; secondary somatosensory cortex is implicated in spatially directed attention and inter-modal sensory integration; and insula cortex reflects limbic integration, visceral sensorimotor processes, and inter-modality sensory integration (Treede, Kenshalo, Gracely, & Jones, 1999).
Two studies have documented effects of affective picture viewing on PREPs elicited by forearm electrical stimulation. First, N2 (N150) amplitude was greater (more negative) when viewing unpleasant than pleasant pictures, whereas P2 (P260) amplitude was smaller (less positive) when viewing pleasant than neutral pictures (Kenntner-Mabiala & Pauli, 2005). Second, N2 amplitude was again greater when viewing unpleasant than pleasant pictures, but P2 amplitude was smaller when viewing unpleasant and pleasant than neutral pictures (Kenntner-Mabiala et al., 2008). These findings together suggest that N2 was influenced by emotional valence whereas P2 was influenced by emotional arousal. Both studies electrically stimulated the skin using a bar electrode, which at high currents activates multiple sensory fibers, including A-beta non-pain fibres. To avoid this issue, de Tommaso et al. (2009) used a laser to selectively stimulate only A-delta pain fibers. However, they found no effects of viewing affective pictures on PREPs. Given these discrepant findings, further investigation seems warranted.
The present study was designed with this in mind. Using a concentric electrode to selectively stimulate A-delta pain fibres (Katsarava et al., 2006; Kaube, Katsarava, Kaufer, Diener, & Ellrich, 2000) we investigated whether cortical processing of trigeminal nociception is modulated by emotion1. Specifically, we assessed N2 and P2 during affective picture viewing. Given discrepancies among previous studies, we made no explicit predictions.
2. Methods
2.1. Participants
Ninety-six (48 males, 47 females) healthy adults (M = 21 years) who played competitive team sport participated.
2.2. Noxious stimulation
The noxious electrical stimulus comprised two 500 μs rectangular wave pulses separated by 100 μs delivered via a Digitimer constant current stimulator and nociceptive-specific concentric electrode (Katsarava et al., 2006; Kaube et al., 2000) secured over the supraorbital nerve above the left eye. It was perceived as a single pinprick-like pain.
2.3. Pain threshold
The pain threshold was determined using an ascending method of limits followed by an up–down staircase (Kavussanu, Willoughby, & Ring, 2012). The mean (SD) pain threshold was 1.34 (0.86) mA.
2.4. Pain-related evoked potential
The electroencephalogram (EEG) and electrooculogram were recorded using a BioSemi ActiveTwo system (for details see Kavussanu et al., 2012). The EEG was recorded at 512 Hz and re-referenced to average earlobe electrodes offline when the data were scored using EEGLAB (Delorme & Makeig, 2004). To score the PREPs, the EEG was high-pass filtered using a finite impulse response windowed-sinc filter with a half-amplitude cut-off at 1 Hz and a 0.4 Hz transition band. Artifact rejection comprised removal of epochs containing excessive noise or paroxysmal artifact followed by independent components analysis. N2 and P2 amplitudes at Cz were calculated as the average of seven data-points around the peak 100–200 ms and 200–300 ms post-stimulation, respectively, relative to a 100 ms pre-stimulus baseline (Inui & Kakigi, 2011). Peak latencies were also determined.
2.5. Picture viewing task
The task comprised 3 habituation pictures, randomly followed by 20 neutral (e.g., players standing or moving), 20 pleasant (e.g., players celebrating, semi-naked players), and 20 unpleasant (e.g., players being hurt, badly injured players) pictures (for previous valence and arousal ratings see Stanger, Kavussanu, Willoughby, & Ring, 2012). Each picture was presented on a monitor for 6 s with a 16–20 s inter-picture interval. A noxious electrical stimulus (125% of pain threshold) was delivered 3–5 s after picture onset on 90% and 8–10 s after picture offset on 10% of trials.
2.6. Manipulation checks
Participants used a Self Assessment Manikin (Bradley & Lang, 1994) to rate each picture for valence (1, very unpleasant; 9, very pleasant) and arousal (1, very calming; 9, very exciting). The Late Positive Potential (LPP), at Pz with 0.1 Hz high-pass filtering, assessed sustained positivity in the cortical response to picture viewing (Hajcak, MacNamara, & Olvet, 2010; Palomba, Angrilli, & Mini, 1997).
2.7. Procedure
Following instrumentation, pain threshold determination, rest, and instruction, participants completed the picture viewing task. Finally, they reviewed and rated each picture for valence and arousal.
2.8. Data analysis
Analyses of Variance (ANOVAs), with picture category (neutral, pleasant, unpleasant) as within-subjects factor, were conducted using the multivariate method (Vasey & Thayer, 1987). Significant effects were followed by Newman-Keuls post hoc comparisons.
3. Results
3.1. Evoked potentials and ratings
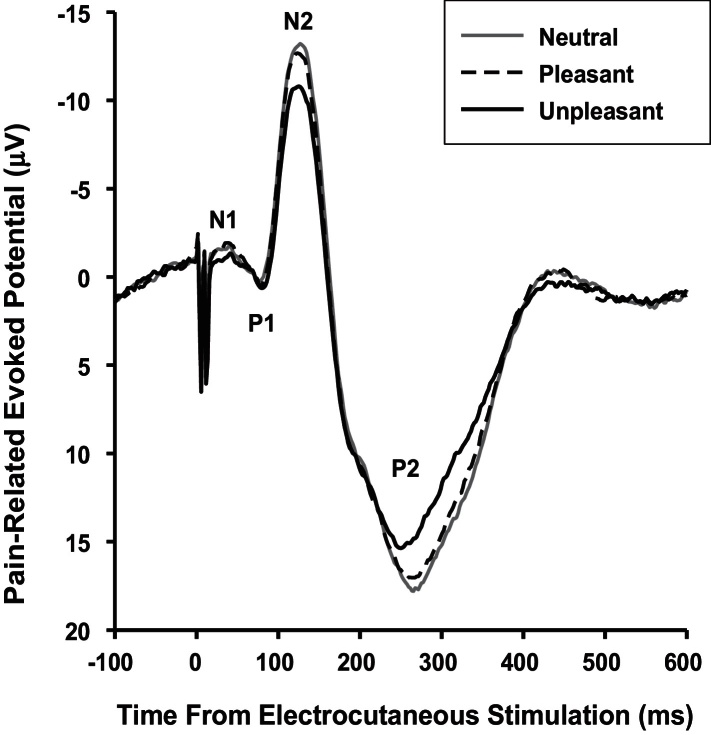
A series of 3 Picture Category ANOVAs revealed picture category effects for N2 amplitude, P2 amplitude, and P2 latency (Table 1 and Fig. 1). Both N2 and P2 amplitudes were smaller for unpleasant than pleasant and neutral pictures and smaller for pleasant than neutral pictures. P2 latency was shorter for unpleasant than neutral and pleasant pictures. As expected, there were picture category effects for valence and arousal (Table 1), confirming that the pictures elicited the expected emotion ratings (cf. Stanger et al., 2012). Finally, an ANOVA yielded category effects for the LPP (Table 1): Pz activity 400–1000 ms after picture onset was more positive for unpleasant than pleasant and neutral pictures and more positive for pleasant than neutral pictures.
Table 1.
Evoked potentials and emotion ratings as a function of picture category.
| Neutral pictures |
Pleasant pictures |
Unpleasant pictures |
ANOVA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F (2, 93) | p | η2 | |
| Amplitude | |||||||||
| N2 (μV) | −16.21 | 9.29 | −15.39 n | 9.26 | −13.62 np | 8.86 | 14.63 | .001 | .24 |
| P2 (μV) | 21.69 | 10.72 | 20.75 n | 10.66 | 19.14 np | 10.26 | 11.44 | .001 | .20 |
| LPP (μV) | 10.69 | 6.23 | 12.01n | 7.26 | 15.51 np | 6.54 | 36.21 | .001 | .44 |
| Latency | |||||||||
| N2 (ms) | 128.62 | 17.76 | 129.38 | 16.95 | 127.08 | 17.58 | 3.20 | .05 | .06 |
| P2 (ms) | 255.53 | 30.21 | 256.09 | 31.00 | 249.49 np | 30.20 | 1.02 | .36 | .02 |
| Ratings | |||||||||
| Valence | 5.26 | 0.68 | 7.15 n | 0.66 | 2.46 np | 0.68 | 1022.37 | .001 | .96 |
| Arousal | 4.33 | 0.88 | 6.42 n | 0.85 | 6.16 n | 1.20 | 216.31 | .001 | .82 |
Note: Letters n and p denote significant (p < 0.05) differences from the neutral and pleasant categories, respectively.
Fig. 1.
Grand average evoked potential waveforms recorded at Cz and elicited by noxious electrocutaneous stimulation of the supraorbital nerve while viewing pleasant, neutral and unpleasant pictures.
3.2. Control analyses
We analyzed the EEG uncorrected for ocular activity to determine whether the aforementioned effects were an artefact of the eye-movement and blink correction procedure. All category effects remained significant, confirming that effects of picture viewing on N2 and P2 were not an artifact of ocular activity (cf. Cuthbert, Schupp, Bradley, McMamis, & Lang, 1998).
4. Discussion
Our primary purpose was to investigate whether cortical processing of trigeminal nociception is modulated by emotion. That both N2 and P2 amplitudes were smaller while viewing unpleasant compared to pleasant compared to neutral pictures indicates a global inhibitory effect of affective picture processing on pain-related cortical processing of trigeminal nociceptive stimulation.
Our N2 findings agree in part with a study that used intracutaneous electrical stimulation: Mini et al. (1995) found that baroreceptor activation produced smaller N2 amplitudes while participants viewed unpleasant compared to pleasant and neutral pictures. Our findings are also in line with studies showing that PREPs are similarly affected when attention is diverted from a painful stimulus (Lorenz & Garcia-Larrea, 2003; Miltner, Johnson, Braun, & Larbig, 1989). However, these findings contrast with reports that N2 was greater for unpleasant than pleasant pictures (Kenntner-Mabiala et al., 2008; Kenntner-Mabiala & Pauli, 2005). Our P2 findings are broadly consistent with previous studies showing that P2 was smaller for unpleasant than neutral (Kenntner-Mabiala et al., 2008) and smaller for pleasant than neutral pictures (Kenntner-Mabiala et al., 2008; Kenntner-Mabiala & Pauli, 2005).
These small discrepancies could be explained by methodological factors. First, we stimulated the supraorbital nerve at low currents using a nociceptive-specific electrode to selectively examine cortical processing of trigeminal nociception. Second, we did not collect subjective pain ratings, which may have affected relative depth of processing of the electrical and visual stimuli or amount of attention paid to these two modalities. Finally, our pleasant pictures were rated somewhat more pleasant and arousing than in previous studies and our unpleasant pictures more (or equally) unpleasant and arousing compared to theirs, so our pictures might have grabbed more attention and thereby reduced PREPs more than previous studies to investigate emotional modulation of pain-related evoked potentials (cf. Kenntner-Mabiala et al., 2008; Kenntner-Mabiala & Pauli, 2005).
Our findings demonstrated that N2 and P2 were similarly influenced by emotional priming: Their amplitudes decreased monotonically from viewing neutral to pleasant to unpleasant pictures. N2 and P2 reflect pain-related activity in three key brain areas (see Introduction), which have been linked with various roles, including affect and attention (anterior cingulate), attention and inter-modal sensory integration (secondary somatosensory cortex), and limbic integration and inter-modal sensory integration (insula cortex). Accordingly, our findings are compatible with the hypothesis that the target site of the interaction between the processing of affective stimuli and nociceptive stimuli can be localized in one or more of these areas. Since the LPP tracks the sustained increase in attention toward and processing of intrinsically motivating stimuli (Hajcak et al., 2010), our LPP findings suggest that the unpleasant pictures grabbed more attention and were processed deeper than the pleasant pictures which, in turn, were more attention grabbing than the neutral pictures. Accordingly, the present modulation of PREPs may be explained best in terms of changes in emotion-dependent attentional focus.
Acknowledgments
This research was supported by a project grant (ES/G007888/1) awarded to the second author by the Economic and Social Research Council.
The authors would like to thank Professor Holger Kaube for supplying and advising us on the use of his concentric nociceptive-specific electrode.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
This report is based on data collected as part of a large project that examined psychological correlates of emotional reactivity. The study protocol employed a priming manipulation prior to the picture viewing task; however, the effects of picture category on pain-related evoked potentials (i.e., the findings reported here) were not moderated by this manipulation.
References
- Bradley M.M., Lang P.J. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy & Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bromm B., Chen A.C.N. Brain electrical source analysis of laser evoked-potentials in response to painful trigeminal nerve-stimulation. Electroencephalography and Clinical Neurophysiology. 1995;95:14–26. doi: 10.1016/0013-4694(95)00032-t. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M., McMamis M., Lang P.J. Probing affective pictures: Attending startle and tone probes. Psychophysiology. 1998;35:344–347. doi: 10.1017/s0048577298970536. [DOI] [PubMed] [Google Scholar]
- de Tommaso M., Calabrese R., Vecchio E., Francesco V.D.V., Lancioni G., Livrea P. Effects of affective pictures on pain sensitivity and cortical responses induced by laser stimuli in healthy subjects and migraine patients. International Journal of Psychophysiology. 2009;74:139–148. doi: 10.1016/j.ijpsycho.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Edwards L., Inui K., Ring C., Wang X., Kakigi R. Pain-related evoked potentials are modulated across the cardiac cycle. Pain. 2008;137:488–494. doi: 10.1016/j.pain.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology. 2010;35:129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Inui K., Kakigi R. Pain perception in humans. Journal of Neurology Neurosurgery and Psychiatry. 2011 doi: 10.1136/jnnp-2011-301484. [DOI] [PubMed] [Google Scholar]
- Katsarava Z., Ayzenberg I., Sack F., Limmroth V., Diener H.C., Kaube H. A novel method of eliciting pain-related potentials by transcutaneous electrical stimulation. Headache. 2006;46:1511–1517. doi: 10.1111/j.1526-4610.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- Kaube H., Katsarava Z., Kaufer T., Diener H.C., Ellrich J. A new method to increase nociception specificity of the human blink reflex. Clinical Neurophysiology. 2000;111:413–416. doi: 10.1016/s1388-2457(99)00295-3. [DOI] [PubMed] [Google Scholar]
- Kavussanu M., Willoughby A.R., Ring C. Moral identity and emotion in athletes. Journal of Sport & Exercise Psychology. 2012;34:695–714. doi: 10.1123/jsep.34.6.695. [DOI] [PubMed] [Google Scholar]
- Kenntner-Mabiala R., Andreatta M., Wieser M.J., Muhlberger A., Pauli P. Distinct effects of attention and affect on pain perception and somatosensory evoked potentials. Biological Psychology. 2008;78:114–122. doi: 10.1016/j.biopsycho.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Kenntner-Mabiala R., Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology. 2005;42:559–567. doi: 10.1111/j.1469-8986.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- Lorenz J., Garcia-Larrea L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Clinical Neurophysiology. 2003;33:293–301. doi: 10.1016/j.neucli.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Miltner W., Johnson R., Braun C., Larbig W. Somatosensory event-related potentials to painful and non-painful stimuli: Effects of attention. Pain. 1989;38:303–312. doi: 10.1016/0304-3959(89)90217-0. [DOI] [PubMed] [Google Scholar]
- Mini A., Rau H., Montoya P., Palomba D., Birbaumer N. Baroreceptor cortical effects, emotion and pain. International Journal of Psychophysiology. 1995;19:67–77. doi: 10.1016/0167-8760(94)00084-r. [DOI] [PubMed] [Google Scholar]
- Palomba D., Angrilli A., Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27:55–67. doi: 10.1016/s0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Stanger N., Kavussanu M., Willoughby A., Ring C. Psychophysiological responses to sport-specific affective images: A study of morality and emotion in athletes. Psychology of Sport and Exercise. 2012;13:840–848. [Google Scholar]
- Tarkka I.M., Treede R.D. Equivalent electrical source analysis of pain-related somatosensory-evoked potentials elicited by a CO2-laser. Journal of Clinical Neurophysiology. 1993;10:513–519. doi: 10.1097/00004691-199310000-00009. [DOI] [PubMed] [Google Scholar]
- Treede R-D., Kenshalo D.R., Gracely R.H., Jones A.K.P. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Valeriani M., Rambaud L., Mauguiere F. Scalp topography and dipolar source modelling of potentials evoked by CO2 laser stimulation of the hand. Electroencephalography & Clinical Neurophysiology/Evoked Potentials Section. 1996;100:343–353. doi: 10.1016/0168-5597(96)95625-7. [DOI] [PubMed] [Google Scholar]
- Vasey M.W., Thayer J.F. The continuing problem of false positives in repeated measures ANOVA in psychophysiology: A multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]