Summary
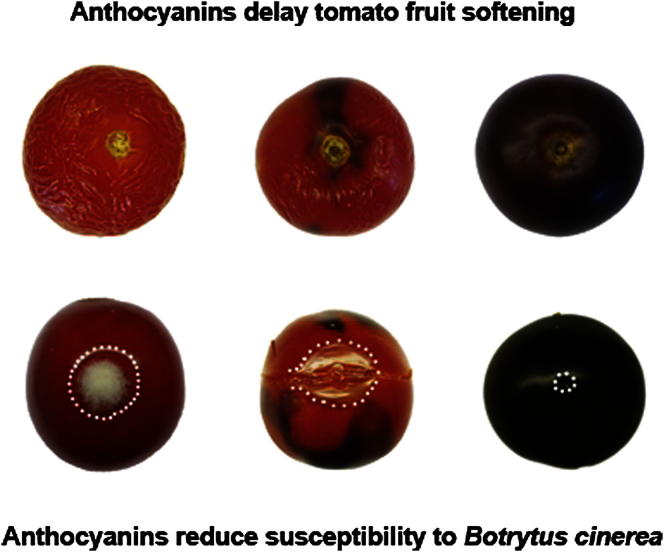
Shelf life is an important quality trait for many fruit, including tomatoes. We report that enrichment of anthocyanin, a natural pigment, in tomatoes can significantly extend shelf life. Processes late in ripening are suppressed by anthocyanin accumulation, and susceptibility to Botrytis cinerea, one of the most important postharvest pathogens, is reduced in purple tomato fruit. We show that reduced susceptibility to B. cinerea is dependent specifically on the accumulation of anthocyanins, which alter the spreading of the ROS burst during infection. The increased antioxidant capacity of purple fruit likely slows the processes of overripening. Enhancing the levels of natural antioxidants in tomato provides a novel strategy for extending shelf life by genetic engineering or conventional breeding.
Graphical Abstract
Highlights
-
•
Anthocyanin accumulation doubles the postharvest storage time of tomato fruit
-
•
Anthocyanin enrichment reduces susceptibility of tomato fruit to Botrytis cinerea
-
•
Longer shelf life is associated with high hydrophilic antioxidant capacity of fruit
-
•
Anthocyanins perturb the dynamics of the ROS burst during infection by B. cinerea
Results and Discussion
Important challenges for the cultivation of tomatoes include postharvest losses and reduced quality due to fruit senescence and pathogen infection. Many tomatoes grown for fresh consumption are picked when still firm and green, stored at low temperature, and exposed to exogenous ethylene to induce color and ripeness before reaching the supermarket shelf. Although effective in limiting postharvest losses, these procedures negatively affect tomato flavor, aroma, and texture [1]. The common use of mutants affected in ripening has similar negative impacts on flavor. Over the last two decades, genetic engineering has been used to extend tomato shelf life by reducing the activity of cell-wall-degrading enzymes [2–5] and enhancing the levels of specific metabolites [6, 7].
Anthocyanins are water-soluble pigments responsible for the red, purple, and blue colors of many flowers and fruit [8]. They are produced by plants to attract pollinators and seed dispersers [9]. Anthocyanin production is also commonly induced under stress conditions [10] and infection by pathogens [11]. Besides physiological roles in plants, dietary anthocyanins are associated with protection against certain cancers [12], cardiovascular diseases [13], and other chronic human disorders [13].
We have shown that ectopic expression of two genes encoding transcription factors, Delila (Del) and Rosea1 (Ros1), from snapdragons, under the control of the fruit-specific E8 promoter, results in increased expression of all the genes committed to anthocyanin biosynthesis to create intensely purple tomato fruit [14]. While growing the purple tomatoes, we observed that they had improved shelf life compared to wild-type, red fruit. The shelf life of food is defined as the period during which a stored product remains suitable for consumption and is normally determined by the degree of softening, shriveling, and rotting of fruit. Consequently, both fruit softening late during ripening and pathogen infection influence the shelf life of tomatoes. Purple fruit from Del/Ros1 tomato plants have normal size, shape, and number of seeds. However, purple fruit exhibit delayed ripening after breaker compared to red fruit. This is evident from the appearance of the purple fruit both on the vine and during postharvest storage and from a reduced level of fungal infection under either condition (Figures 1A and 1B).
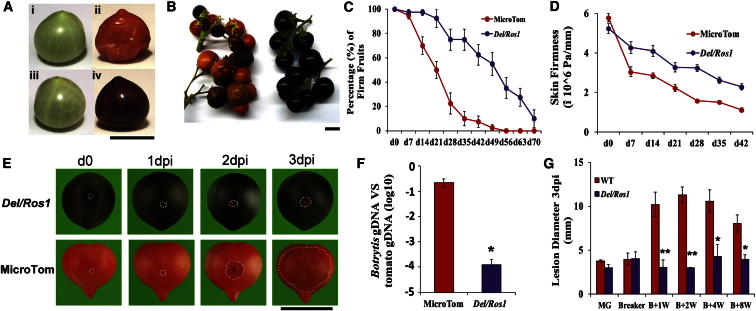
Figure 1.
Accumulation of Anthocyanins in Tomato Fruit Delays Late Ripening and Decreases Pathogen Susceptibility
(A) Wild-type, red (i and ii) and transgenic, purple (iii and iv) tomato fruits were tagged during the initial stages of development and harvested and photographed at the end of the green stage (i and iii). The same fruit, stored at room temperature, was rephotographed after 2 months (ii and iv). The scale bar represents 2 cm.
(B) Severe symptoms of opportunistic infection normally associated with overripe red, wild-type tomato fruit on the vine (left) were not observed in purple, Del/Ros1 tomato fruit of the same age grown under identical greenhouse conditions (right). The scale bar represents 2 cm.
(C) Purple fruit showed slower softening as determined by visual inspection compared to wild-type, red fruit. Percentages of fruit showing overripening symptoms were assessed every week during storage tests. Error bars show the SEM (n = 4). Fruits were harvested at 14 days postbreaker (d0 = 14 dpb).
(D) Texture strength changes in MicroTom and Del/Ros1 fruits during storage tests. Average values were calculated for at least eight individual fruits, and error bars indicate the SEM.
(E) Symptoms of wounded red and purple fruits after inoculation with B. cinerea B05.10. White dots represent the lesion margins.
(F) Quantitative PCR revealed more Botrytis growing on the WT tomatoes than on purple fruit, 3 dpi. Botrytis growth was calculated by comparison of the ratio of Botrytis DNA to tomato DNA. Error bars show the SEM (n = 3). ∗p < 0.05 compared to control red tomato.
(G) The ripening-related increase in susceptibility to Botrytis did not occur in purple fruit. Lesion diameter was measured 3 dpi. Error bars show the SEM (n ≥ 3). The scale bar represents 2 cm. ∗p < 0.05 and ∗∗p < 0.01 for values for purple tomatoes compared to red tomatoes at the same stage.
See also Figures S1 and S2.
Both wild-type (WT) and purple tomatoes were harvested when ripe and stored under sterile conditions. For purple fruit, 49 days of storage at 18°C were required to observe 50% of the fruit softened, equivalent to the level of softening observed in red fruit at 21 days. Complete collapse was observed in purple fruit after 10 weeks storage, compared to 5 weeks for red fruit. With a texture analyzer, the firmness of red fruit was measured as 50% lower than that at breaker, after 2 weeks at 18°C, whereas the same reduction in firmness was reached after 5 weeks storage of purple fruit. These results indicated that expression of Del and Ros1 can more than double the shelf life of tomato fruit (Figure 1C). These differences were accompanied by greater ability to resist tensile forces in purple tomatoes compared to red fruit of equivalent age (Figure 1D).
Production of ethylene, required for full ripening in climacteric fruit such as tomato, increased just after breaker and was 2-fold greater in purple fruit than in red fruit (Figure S1A available online). Measurements of cuticle thickness revealed no differences between WT and purple tomato (Figures S1B–S1D). In addition, Fourier transform infrared (FT-IR) spectroscopy indicated that there were no significant cell wall compositional differences between purple tomato peel and red tomato peel 1 week after breaker (Figure S2E). These observations implied that the extended shelf life of purple fruit was due to neither impaired ethylene production nor altered cuticle/peel composition.
The susceptibility of purple fruit to pathogens was investigated by infection of intact or wounded tomatoes with B. cinerea, the causal agent of gray mold disease, one of the most important postharvest pathogens of tomatoes [15]. When intact fruit were sprayed with a B. cinerea spore suspension without wounding, the proportion of purple fruit showing severe symptoms of infection was substantially lower than for red fruit (Figures S2A and S2B). When wounded fruit were inoculated with the B. cinerea spore suspension, the size of the lesions did not increase 1 day postinoculation (dpi) in either fruit type, indicating that the fungus needs about 24 hr to establish after inoculation. From 2 dpi, however, there was greater spread of infection in red fruit than in purple fruit. At 3 dpi, the average size of the lesions in purple tomatoes was significantly smaller than in red fruit, indicating reduced susceptibility to B. cinerea infection (Figure 1E). Quantitative PCR with DNA extracted from infected tomatoes confirmed that there was significantly more Botrytis growing on red fruit than on purple fruit at 3 dpi (Figure 1F). Reduced pathogen susceptibility was also observed in purple fruit introgressed into the MoneyMaker genetic background (Figure S2C), indicating that the lower susceptibility of purple tomatoes to B. cinerea is not dependent on a specific genetic background.
The susceptibility of tomato fruit to necrotrophic pathogens increases during ripening [16, 17]. A correlation between fruit age late in ripening and increased susceptibility was observed in red fruit. However, in purple fruit, susceptibility to B. cinerea did not increase from the breaker stage when anthocyanin production was induced (Figure 1G). This observation suggested a specific role for anthocyanins in limiting the spread of fungal infection, as supported by the intermediate susceptibility displayed by two different Del/Ros1 lines (C and Y) that produce lower levels of anthocyanins than line N (used for the initial tests) [14] (Figures S2D–S2F).
To ensure that the effects on delayed ripening and pathogen susceptibility were compared at exactly the same developmental stage, we used virus-induced gene silencing (VIGS) to silence the expression of Del and Ros1 in purple fruit in the MoneyMaker background (in which large fruit size allows dissection of tissue sectors relatively easily). Agro-infiltrated Del/Ros1 fruit showed a phenotype of purple and red sectors, the latter defining those parts of the fruit where Del and Ros1 had been silenced [18] and hydrophilic antioxidant capacity was reduced (Figures 2A and 2B). In older fruit, the red sectors were clearly softer and the tissues were more collapsed than in purple sectors, demonstrating the shorter storage life of red sectors compared to purple sectors (Figure 2A). Red sectors also showed greater susceptibility to B. cinerea than purple sectors (Figure 2A).
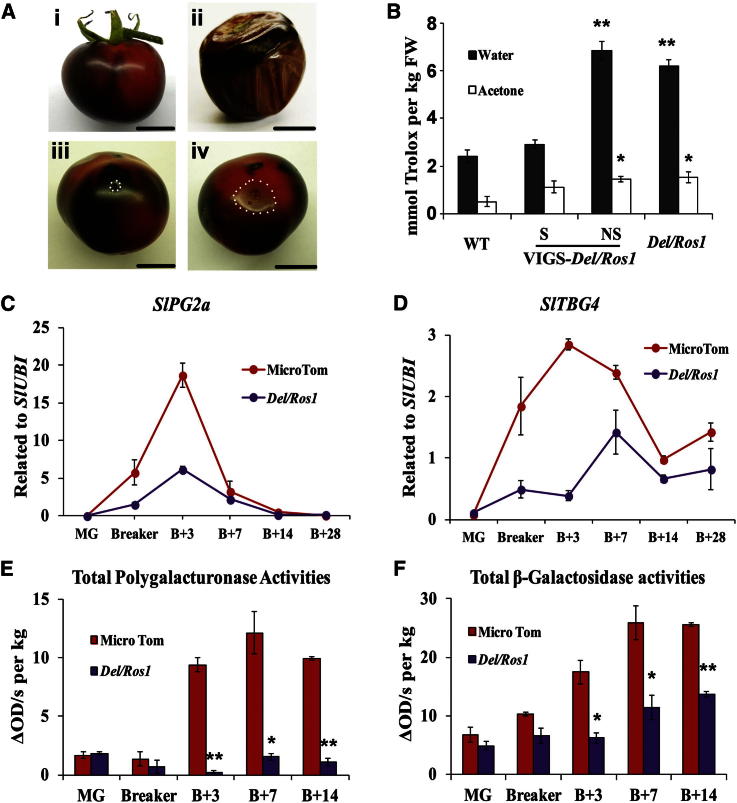
Figure 2.
Delayed Ripening and Reduced Pathogen Susceptibility Are Associated with the Accumulation of Anthocyanins, and Expression of Ripening-Related Genes Is Suppressed in Purple Tomatoes
(A) VIGS-Del/Ros1tomato fruits showed reduced accumulation of anthocyanins in silenced areas (i, pictures taken 14 days after breaker). The red sectors showed quicker softening than purple sectors (ii, pictures taken 42 days after breaker). Purple sectors showed reduced susceptibility to B. cinerea 3 dpi (iii). Red sectors of VIGS-silenced tomatoes were more susceptible to B. cinerea 3 dpi (iv). All scale bars represent 2 cm.
(B) VIGS-Del/Ros1 silenced sectors had reduced hydrophilic antioxidant capacity compared to purple sectors. Error bars show the SEM (n = 3). S, silenced sectors; NS, nonsilenced sectors. ∗p < 0.05 and ∗∗p < 0.01 compared to the WT.
(C and D) Quantitative RT-PCR analysis of genes encoding cell-wall-modifying enzymes in WT and Del/Ros1 fruits during ripening. Polygalacturonase 2a (SlPG2a) (C) and β-galactosidase 4 (SlTBG4) (D) are shown. Error bars show the SEM (n = 3).
(E and F) Total polygalacturonase (E) and β-galactosidase (F) activities in red and purple fruit at different stages during ripening. Error bars show the SEM (n = 3). ∗p < 0.05 and ∗∗p < 0.01 compared to the WT at the same stage.
See also Figure S3, Table S1, and Data Set S1.
Gene expression profiles of red and purple sectors of VIGS-Del/Ros1 fruit were compared. Samples were harvested at 8, 30, and 45 days after breaker. A 3-fold difference in expression levels (purple versus red) was set as the threshold for significant changes detected using the TOM2 microarray. Two hundred and forty one genes showed significant differences in expression between purple and red sectors over at least two stages (Figure S3A). Functional annotation revealed that many of these genes are involved in primary and secondary metabolism, cell wall modification, oxidative stress, and pathogen resistance (Figures S3B and S3C and Data Set S1). Reduced expression of many genes known to be involved in overripening was observed in purple sectors (Figures S3B and S3C), indicating that the suppression of expression of these genes in purple tomatoes contributes to the extended shelf life of the fruit.
The suppression of genes involved in overripening in purple fruit was confirmed by quantitative RT-PCR. Genes encoding polygalacturonase (SlPG2a) [4] and β-galactosidase (SlTBG4) [5], involved in cell wall softening, showed substantially lower expression in purple fruit during ripening (Figures 2C and 2D). The lower levels of gene expression resulted in lower total activities of polygalacturonase and β-galactosidase in purple tomatoes compared to red tomatoes (Figures 2E and 2F). Although the silencing of the individual genes might only have minor effects on softening [3, 5], the combined suppression of a number of different cell wall modification enzymes likely reduces significantly the rate of fruit softening.
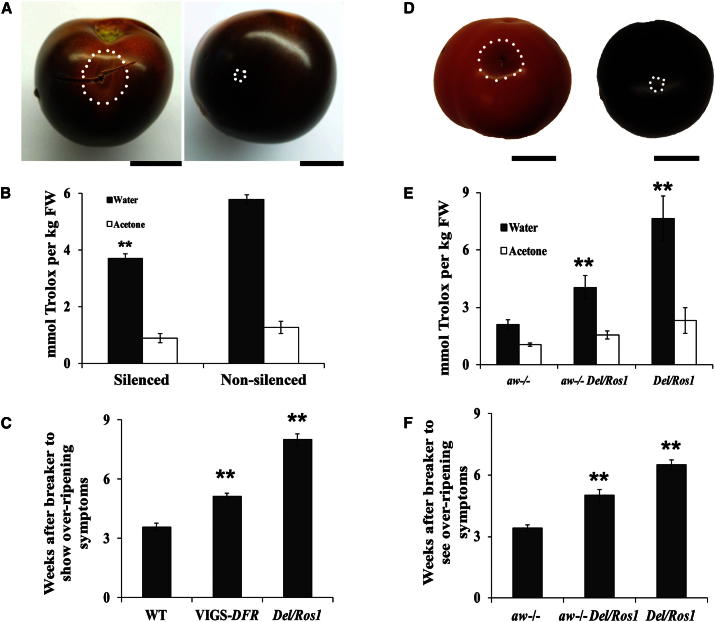
To identify specific effects of anthocyanins on extension of shelf life, we silenced dihydroflavonol 4-reductase (SlDFR), a key gene in anthocyanin biosynthesis, using VIGS in purple tomatoes. On the same fruit, VIGS-SlDFR-silenced, orange sectors showed similar expression levels of Del and Ros1 to nonsilenced, purple sectors, whereas SlDFR expression was substantially reduced (Figure S4A). Anthocyanin levels were reduced by 80%, although other flavonoids accumulated in the silenced sectors, giving them an orange color (Figures S4B and S4C). SlDFR-silenced sectors were sensitive to B. cinerea, whereas purple sectors on the same fruit remained resistant (Figure 3A). Compared to nonsilenced sectors, SlDFR-silenced sectors had reduced hydrophilic antioxidant capacity (Figure 3B), although this was higher than the hydrophilic antioxidant capacity of WT red fruit, due to the accumulation of flavonols. Storage tests indicated that VIGS-SlDFR-silenced fruit could be kept longer than WT fruit but not as long as purple tomatoes (Figure 3C). We confirmed these observations by crossing Del/Ros1 plants to the aw mutant of tomato in the Ailsa Craig genetic background, which lacks DFR activity and cannot make anthocyanins [19]. In the F2, the plants that contained Del/Ros1 but lacked DFR activity (aw−/−) produced orange fruit due to high levels of flavonols. Like the VIGS-SlDFR-silenced sectors, the aw−/−, Del/Ros1 fruit were no less susceptible to B. cinerea than were red tomatoes (Figure 3D). The orange fruit had 2-fold higher hydrophilic antioxidant capacity than the parental aw−/− line (Figure 3E) and they could be kept longer postharvest, although not as long as purple tomatoes (Figure 3F). Consequently, the delay in overripening and the enhanced pathogen resistance of purple tomatoes are not due to off-targets of the Del and Ros1 transcription factors. Resistance to B. cinerea is specifically the result of the accumulation of anthocyanins, whereas the delay in overripening is most likely associated with the increased hydrophilic antioxidant capacity of the fruit.
Figure 3.
Inhibition of Anthocyanin Biosynthesis in Purple Tomatoes Alters Susceptibility to Botrytis and Postharvest Storage
(A) VIGS-SlDFR silenced sectors had increased susceptibility to B. cinerea compared to nonsilenced sectors on the same fruit. Pictures were taken at 3 dpi. White dots indicate lesion sizes. Scale bars represent 2 cm.
(B) The hydrophilic antioxidant capacity of VIGS-SlDFR-silenced sectors was lower than that of nonsilenced sectors, although still higher than that of WT fruit due to the accumulation of flavonols. Error bars show the SEM, n = 3. ∗∗p < 0.01 for differences in TEAC values of hydrophilic extracts of silenced and nonsilenced tissues.
(C) Storage tests indicate VIGS-SlDFR-silenced fruit can be kept for longer than WT fruit but for less time than nonsilenced purple fruit. Fruits were harvested 2 weeks after breaker, and the times to show overripening symptoms (visual rotting and collapse of fruit) were recorded. Error bars show the SEM, n = 7. ∗∗p < 0.01 compared with WT, red fruit.
(D) High levels of flavonols accumulate in aw−/−Del/Ros1 F2 tomato fruit obtained by crossing Del/Ros1MicroTom with aw−/− (DFR–) mutants. The orange, flavonols-enriched tomato (left) was more susceptible to B. cinerea. Pictures were taken at 3 dpi. White dots show lesion boundries. Scale bars represent 2 cm.
(E) Comparisons of antioxidant capacities of aw−/−,aw−/−Del/Ros1and Del/Ros1fruit. Error bars show the SEM, n = 3. Solid bars show hydrophilic antioxidant capacity, and open bars show lipophilic antioxidant capacity. ∗∗p < 0.01 compared with parental aw−/− fruit.
(F) aw−/−Del/Ros1 fruit had a longer shelf life than parental aw−/− fruit but a shorter shelf life than Del/Ros1 fruit. Times (after breaker) for fruit to show overripening symptoms (visual rotting and collapse of fruit) were recorded. Error bars show the SEM, n = 10. ∗∗p < 0.01 compared with parental aw−/− fruit.
See also Figure S4.
Levels of oxidative stress increase markedly in the later stages of ripening and may facilitate many of the metabolic changes associated with maturation of tomato fruit [20]. Comparison of a cultivar with shorter shelf life to one with longer shelf life showed reduced scavenging ability and increased levels of reactive oxygen species (ROS) [21]. Accordingly, increase of antioxidant capacity or reduction of levels of ROS with different antioxidants can extend shelf life [6, 22, 23]. Taken together, our data suggest that elevation of the levels of antioxidants in fruit reduces the tissue-damaging activity of oxidative stress and thus is the most likely cause of the delay in overripening observed in purple (Del/Ros1) and orange (VIGS-SlDFR and Del/Ros1,aw−/−) tomatoes.
Malondialdehyde (MDA) is a byproduct of lipid peroxidation and can be used to measure damage resulting from oxidative stress during tissue senescence [21, 24]. MDA levels in red MicroTom fruit increased late in ripening. In purple tomatoes, however, MDA levels did not increase significantly up to 4 weeks after breaker (Figure 4A). Lower oxidative damage in purple tomato was associated closely with increased total antioxidant capacity during overripening, which resulted principally from the accumulation of anthocyanins (Figure 4B). Higher hydrophilic antioxidant capacity/lower ROS levels were associated with suppression of ripening-related enzyme activities such as polygalacturonase and β-galactosidase, an effect likely to be of importance in extending shelf life, since downregulation of some of the corresponding genes by antisense has been shown to result in fruit that are firmer for longer than controls [3, 5] and their combined suppression may extend shelf life yet further. One explanation for the induced expression of these genes, late in ripening, is that it is the result of increased ROS signaling. Our data suggest that ROS signaling is an important determinant of the rate of ripening, late in fruit development. High hydrophilic antioxidant capacity can suppress both ROS activity and signaling and consequently may delay the processes of overripening, both directly and indirectly.
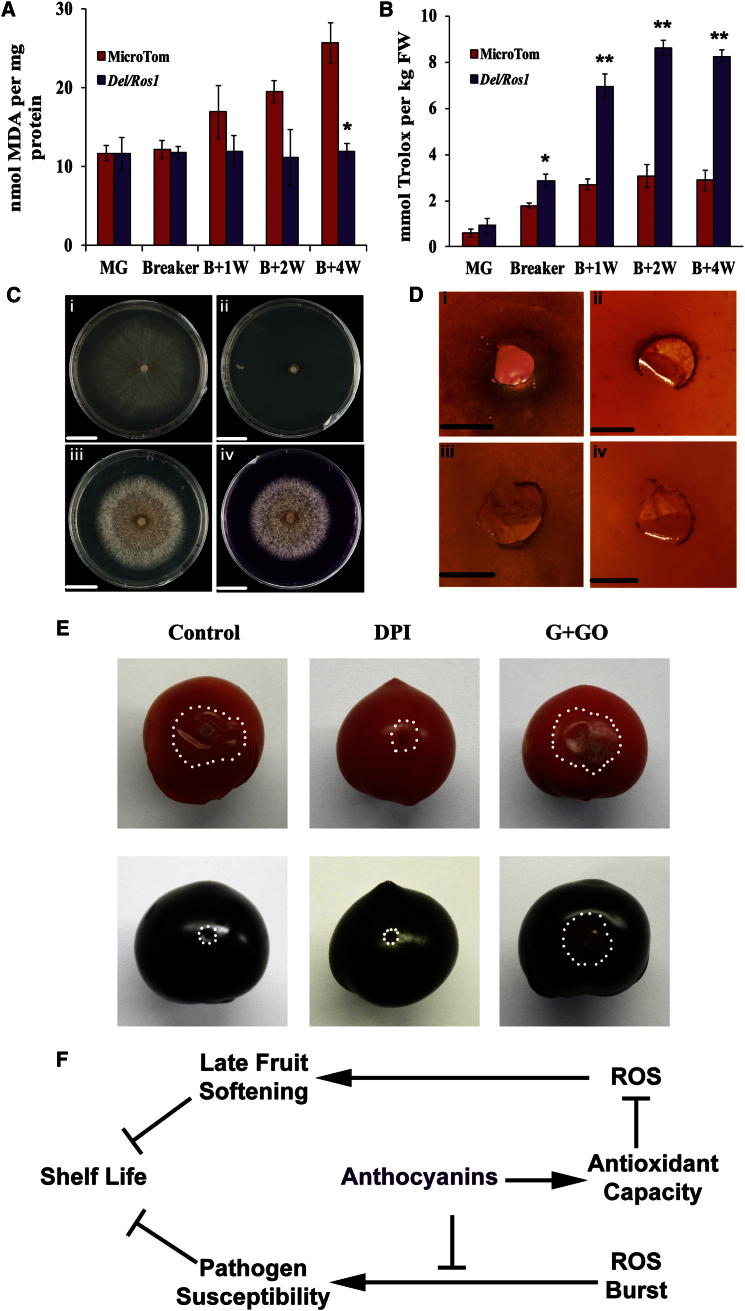
Figure 4.
Extended Shelf Life in Purple Tomatoes Is Associated with Their High Antioxidant Capacity
(A) Malondialdehyde levels in pericarp of red and purple Microtom fruit during ripening. Error bars show the SEM (n = 3). ∗p < 0.05 compared with WT, red fruit at same stage
(B) Trolox equivalent total antioxidant capacity (TEAC) of water extracts from red and purple tomatoes during ripening. Error bars show the SEM (n = 3). ∗p < 0.05 and ∗∗p < 0.01 in comparison to WT, red fruit at the same stages.
(C) Addition of juice from either red or purple tomatoes to the growth medium had no effect on growth of B. cinerea. PDA medium (i), PDA with 15 mg/liter Triademinol (an inhibitor of fungal growth) (ii), PDA supplemented with 50% red juice (iii), and PDA supplemented with 50% purple juice (iv) are shown. Pictures were taken 3 days after plate inoculation. Scale bars represent 2 cm.
(D) 3,3′-diaminobenzidine (DAB) staining of hydrogen peroxide produced 24 hr after inoculation of B. cinerea: red (i) and purple (ii) fruits stained with DAB, 24 hr after inoculation, wound only red (iii) and purple (iv) fruit stained 24 hr after wounding. Scale bars represent 1 mm.
(E) The levels of ROS in red and purple tomatoes were altered by infiltration of a water control, 10 mM diphenyleneiodonium chloride (DPI, ROS inhibitor), or 50 units/ml glucose oxidase plus 1% glucose (G+GO, ROS inducer). Fruits were wounded and infiltrated 1 hr prior to B. cinerea inoculation. Pictures were taken 3 dpi. White dotted lines represent lesion margin. All scale bars represent 2 cm.
(F) Model for the mechanism of shelf life extension in purple, high-anthocyanin tomatoes.
Reduced susceptibility to B. cinerea is associated specifically with anthocyanin accumulation. Anthocyanin levels have been associated with reduced susceptibility to Botrytis in grapes [25] and may reduce postharvest spoilage of fruits in general by Botrytis. When we grew B. cinerea on agar plates supplemented with red and purple fruit juice, neither extract inhibited the growth of the fungus (Figure 4C). This indicates that anthocyanins do not suppress the growth of B. cinerea directly and that the resistance requires living host cells. Between 24 and 48 hr after infection with B. cinerea, lesions on red fruit spread quickly, while on purple fruit their size remained small (Figure 1E). 3,3′-diaminobenzidine (DAB) staining of H2O2 in infected red and purple fruits during this period showed that a ROS burst was generated at the infection site. However, the ROS burst on red fruit spread widely, whereas on purple fruit strong ROS induction was restricted to the inoculation site (Figure 4D). The oxidative burst is thought to potentiate infection by necrotrophic pathogens that feed on dead tissue, facilitating the expansion of disease lesions [26–28]. Vacuum infiltration of diphenyleneiodonium chloride (DPI), an NADPH oxidase inhibitor, into red fruit prior to B. cinerea inoculation restricted the spread of lesions, whereas infiltration of purple tomatoes with glucose and glucose oxidase (which induce ROS, through the generation of H2O2) increased lesion growth in purple fruit (Figure 4E). These data suggest that in purple tomatoes, anthocyanins alter the dynamics of the ROS burst generated by B. cinerea infection and limit the induction of cell death necessary for growth of the necrotroph.
In addition to their high nutritional value [14], anthocyanin-rich purple tomatoes have 2-fold longer shelf life, the combined result of increased resistance to opportunistic pathogens and slower ripening at late stages. These traits are associated with the accumulation of anthocyanins in tomatoes. Anthocyanins specifically alter the spread of the ROS burst generated as part of necrotrophic infection and so reduce susceptibility to B. cinerea. Accumulation of anthocyanins results in high hydrophilic antioxidant capacity, which reduces the increase in ROS levels, that occurs late in fruit development, and the reduction in ROS may suppress the later stages of ripening (Figure 4F). The association of slower ripening with elevated hydrophilic antioxidant capacity of fruit offers new, yet broad, targets for breeders to extend the postharvest shelf life of fruit. Additionally, anthocyanins could be used to reduce the susceptibility of ripe fruit specifically to Botrytis cinerea, the most important fungal pathogen of soft fruit.
Acknowledgments
E.B. and C.M. were supported by the European Union FP6 FLORA project (FOOD-CT-01730), and Y.Z., E.B., and C.M. are supported by the European Union FP7 ATHENA collaborative project (grant agreement 245121). Y.Z. is also supported by a Rotation Studentship from the John Innes Foundation. C.M. and E.B. were supported by the core strategic grant of the Biological and Biotechnological Science Research Council (BBSRC) to the John Innes Centre and are currently supported by the Institute Strategic Program Understanding and Exploiting Plant and Microbial Secondary Metabolism (BB/J004596/1) from the BBSRC. E.B. was supported by a short-term EMBO fellowship for undertaking part of the research reported in this paper. H.-J.S. was supported by grant BB/G042960/1 from the BBSRC and the John Innes Foundation, and A.G. and D.O. are supported by the Fundación Genoma (Calitom project) and the Spanish Ministry of Science and Education (project BIO2010-15384). This work benefited from the networking activities within the European-funded COST ACTION FA1106 QualityFruit. We thank Andrew Davis for photography. C.M. and J.D.G.J. are Directors of Norfolk Plant Sciences, a company bringing biotechnological improvements in crops to market.
Published: May 23, 2013
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, one table, and one data set and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.04.072.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Accession Numbers
The GEO accession number for the TOM2 microarray data reported in this paper is GSE46341.
Supplemental Information
References
- 1.Baldwin E., Plotto A., Narciso J., Bai J. Effect of 1-methylcyclopropene on tomato flavour components, shelf life and decay as influenced by harvest maturity and storage temperature. J. Sci. Food Agric. 2011;91:969–980. doi: 10.1002/jsfa.4281. [DOI] [PubMed] [Google Scholar]
- 2.Meli V.S., Ghosh S., Prabha T.N., Chakraborty N., Chakraborty S., Datta A. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proc. Natl. Acad. Sci. USA. 2010;107:2413–2418. doi: 10.1073/pnas.0909329107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powell A.L., Kalamaki M.S., Kurien P.A., Gurrieri S., Bennett A.B. Simultaneous transgenic suppression of LePG and LeExp1 influences fruit texture and juice viscosity in a fresh market tomato variety. J. Agric. Food Chem. 2003;51:7450–7455. doi: 10.1021/jf034165d. [DOI] [PubMed] [Google Scholar]
- 4.Smith C.J.S., Watson C.F., Ray J., Bird C.R., Morris P.C., Schuch W., Grierson D. Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature. 1988;334:724–726. [Google Scholar]
- 5.Smith D.L., Abbott J.A., Gross K.C. Down-regulation of tomato beta-galactosidase 4 results in decreased fruit softening. Plant Physiol. 2002;129:1755–1762. doi: 10.1104/pp.011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nambeesan S., Datsenka T., Ferruzzi M.G., Malladi A., Mattoo A.K., Handa A.K. Overexpression of yeast spermidine synthase impacts ripening, senescence and decay symptoms in tomato. Plant J. 2010;63:836–847. doi: 10.1111/j.1365-313X.2010.04286.x. [DOI] [PubMed] [Google Scholar]
- 7.Centeno D.C., Osorio S., Nunes-Nesi A., Bertolo A.L., Carneiro R.T., Araújo W.L., Steinhauser M.C., Michalska J., Rohrmann J., Geigenberger P. Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell. 2011;23:162–184. doi: 10.1105/tpc.109.072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grotewold E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- 9.Shang Y., Venail J., Mackay S., Bailey P.C., Schwinn K.E., Jameson P.E., Martin C.R., Davies K.M. The molecular basis for venation patterning of pigmentation and its effect on pollinator attraction in flowers of Antirrhinum. New Phytol. 2011;189:602–615. doi: 10.1111/j.1469-8137.2010.03498.x. [DOI] [PubMed] [Google Scholar]
- 10.Gould K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004;2004:314–320. doi: 10.1155/S1110724304406147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenc-Kukuła K., Jafra S., Oszmiański J., Szopa J. Ectopic expression of anthocyanin 5-o-glucosyltransferase in potato tuber causes increased resistance to bacteria. J. Agric. Food Chem. 2005;53:272–281. doi: 10.1021/jf048449p. [DOI] [PubMed] [Google Scholar]
- 12.Wang L.S., Stoner G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuda T., Horio F., Uchida K., Aoki H., Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003;133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]
- 14.Butelli E., Titta L., Giorgio M., Mock H.P., Matros A., Peterek S., Schijlen E.G., Hall R.D., Bovy A.G., Luo J., Martin C. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008;26:1301–1308. doi: 10.1038/nbt.1506. [DOI] [PubMed] [Google Scholar]
- 15.Williamson B., Tudzynski B., Tudzynski P., van Kan J.A.L. Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 2007;8:561–580. doi: 10.1111/j.1364-3703.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 16.Cantu D., Blanco-Ulate B., Yang L., Labavitch J.M., Bennett A.B., Powell A.L. Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or ethylene. Plant Physiol. 2009;150:1434–1449. doi: 10.1104/pp.109.138701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantu D., Vicente A.R., Greve L.C., Dewey F.M., Bennett A.B., Labavitch J.M., Powell A.L. The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc. Natl. Acad. Sci. USA. 2008;105:859–864. doi: 10.1073/pnas.0709813105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orzaez D., Medina A., Torre S., Fernández-Moreno J.P., Rambla J.L., Fernández-Del-Carmen A., Butelli E., Martin C., Granell A. A visual reporter system for virus-induced gene silencing in tomato fruit based on anthocyanin accumulation. Plant Physiol. 2009;150:1122–1134. doi: 10.1104/pp.109.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldsbrough A., Belzile F., Yoder J.I. Complementation of the Tomato anthocyanin without (aw) Mutant Using the Dihydroflavonol 4-Reductase Gene. Plant Physiol. 1994;105:491–496. doi: 10.1104/pp.105.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez A., Creissen G., Kular B., Firmin J., Robinson S., Verhoeyen M., Mullineaux P. Changes in oxidative processes and components of the antioxidant system during tomato fruit ripening. Planta. 2002;214:751–758. doi: 10.1007/s004250100667. [DOI] [PubMed] [Google Scholar]
- 21.Mondal K., Sharma N.S., Malhotra S.P., Dhawan K., Singh R. Antioxidant Systems in Ripening Tomato Fruits. Biologia Plantarum. 2004;48:49–53. [Google Scholar]
- 22.Zidenga T., Leyva-Guerrero E., Moon H., Siritunga D., Sayre R. Extending cassava root shelf life via reduction of reactive oxygen species production. Plant Physiol. 2012;159:1396–1407. doi: 10.1104/pp.112.200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhagwan A., Reddy Y.N., Rao P.V., Mohankumar K.C. Shelf life extension of tomato fruits by postharvest antioxidant application. Journal of Applied Horticulture. 2000;2:88–91. [Google Scholar]
- 24.Dhindsa R.S., Plumb-Dhindsa P., Thorpe T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981;32:93–101. [Google Scholar]
- 25.Iriti M., Rossoni M., Borgo M., Faoro F. Benzothiadiazole enhances resveratrol and anthocyanin biosynthesis in grapevine, meanwhile improving resistance to Botrytis cinerea. J. Agric. Food Chem. 2004;52:4406–4413. doi: 10.1021/jf049487b. [DOI] [PubMed] [Google Scholar]
- 26.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 27.Govrin E.M., Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 28.Segmüller N., Kokkelink L., Giesbert S., Odinius D., van Kan J., Tudzynski P. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant Microbe Interact. 2008;21:808–819. doi: 10.1094/MPMI-21-6-0808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.