Abstract
Background
Aggressive glycemic control has been hypothesized to prevent renal disease in type 2 diabetics. A systematic review was conducted to summarize the benefits of intensive versus conventional glucose control on kidney-related outcomes for adults with type 2 diabetes.
Methods
Three databases were systematically searched (January 1950 to December 2010) with no language restrictions to identify randomized trials that compared surrogate renal endpoints (micro and macroalbuminuria) and clinical renal endpoints (doubling of serum creatinine, End Stage Renal Disease [ESRD] and death from renal disease) in patients with type 2 diabetes receiving intensive glucose control versus receiving conventional glucose control.
Results
Seven trials involving 28,065 adults who were followed-up for 2 to 15 years. Compared with conventional control, intensive glucose control reduced the risk for microalbuminuria (risk ratio [RR], 0.86 [95% CI, 0.76 to 0.96]) and macroalbuminuria (RR 0.74 [95% CI, 0.65–0.85]), but not doubling of serum creatinine (RR 1.06 [95% CI, 0.92 to 1.22]), ESRD (RR 0.69 [95% CI, 0.46–1.05]), or death from renal disease (RR 0.99 [95% CI 0.55–1.79]). Meta-regression revealed that larger differences in HbA1C between intensive and conventional therapy at the study level were associated with greater benefit for both micro- and macroalbuminuria. The pooled cumulative incidence of doubling of creatinine, ESRD, and death from renal disease was low (< 4%, <1.5%, and <0.5%, respectively) compared with the surrogate renal endpoints of micro- (23%) and macroalbuminuria (5%).
Conclusion
Intensive glucose control reduces the risk for microalbuminuria and macroalbuminuria but evidence is lacking that intensive glycemic control reduces the risk for significant clinical renal outcomes such as doubling of creatinine, ESRD or death from renal disease during the years of follow-up of the trials.
Keywords: Proteinuria, creatinine, chronic kidney disease, end-stage renal disease, prognosis
INTRODUCTION
Epidemiologic studies have demonstrated an association between poor glycemic control and microvascular complications in patients with type 2 diabetes.1, 2 Randomized controlled trials have demonstrated that intensive glycemic control reduces albuminuria.3–5 Less clear, however, is whether intensive glycemic control actually prevents clinical renal endpoints (e.g., progressive loss in glomerular filtration rate) beyond albuminuria in type 2 diabetics. Despite the lack of strong evidence, expert panels and guidelines continue to recommend a target HbA1C < 7.0% for prevention of renal disease and other microvascular complications. The 2007 National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease (CKD)6 endorse intensive glycemic control, and these recommendations are reinforced by the 2011 American Diabetes Association (ADA) guidelines. As stated in the guidelines, recommendations for intensive glycemic control for prevention of renal disease are based on studies that have demonstrated an improvement in albuminuria, a surrogate endpoint.
Furthermore, in light of the fact that intensive glycemic control increased the risk for death by 22% in the ACCORD trial,7 and, pooling the data from all studies did not reduce cardiovascular-related death or all-cause mortality,8 it is increasingly problematic for clinicians to continue aggressive glycemic control for the treatment of renal outcomes. The reasons for the lack of clinical benefits are unclear. A recent study demonstrated that despite substantial increases in the use of glucose-lowering medications (and inhibitors of the renin-angiotensin-aldosterone system) from 1988 to 2008, the prevalence of CKD in diabetics actually increased.9
The recent publication of several large randomized controlled multicenter trials of intensive glycemic control in type 2 diabetes7, 10, 11 may allow an assessment of the effects intensive glycemic control on clinical renal endpoints. Thus, in the context of strategies employed in these studies for intensive control of glycemia, we sought to examine whether this form of therapy was associated with benefits on clinically relevant renal outcomes among patients with type 2 diabetes via a systematic review and meta-analysis.
METHODS
Data Sources and Searches
In collaboration with an expert librarian, we searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials to identify randomized controlled trials that compared the effects of intensive glucose control and conventional glucose control on renal events in patients with type 2 diabetes. Inclusion criteria and methods of analysis were specified in advance and documented in a protocol available on request. Investigators searched the PUBMED central for publications (January 1950 through December 2010) using the Medical Subject Headings(MeSH) chronic kidney disease; diabetes mellitus type 2; hypoglycemic agents; creatinine as well as keywords chronic kidney disease, albuminuria, proteinuria, protein to creatinine ratio, albumin to creatinine ratio, glucose control, and glycemic control. The search was restricted to randomized, controlled trials conducted among human adults (age≥ 19 years), with no journal group, language or gender restrictions. We also checked the reference lists of identified articles, previous meta-analyses, and original studies identified by the electronic search to find other potentially eligible studies. We searched review articles and the Web of Science database to find all relevant follow-up articles.
Study Selection
Two investigators independently reviewed the contents of 751 abstracts or full text manuscripts identified through the literature search to determine whether they met the eligibility criteria. He predefined inclusion criteria required the clinical trials to: (1) randomly assign individuals with type 2 diabetes mellitus either to an intensive lowering of glucose versus a standard regimen (placebo, standard care, or glycemic control of reduced intensity), (2) address the progression or development of kidney disease either as a primary or a surrogate outcome, and report complete information about effect measures or provide information to allow calculation of effect estimates for progression or new diagnosis of kidney disease, and (3) involve stable patients in the outpatient setting only, excluding studies in an acute hospital setting. The risk of bias was assessed by using the components recommend by the Cochrane Collaboration: sequence generation by allocation; allocation concealment; blinding of participants, staff and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias.
Data Extraction and Risk of Bias in Included Studies
We entered data from the trials into an electronic database with validity checks. The data abstraction and data entry were confirmed by a second reviewer who cross-checked 100% of selected articles.
Variables including details of the trials, details of the intervention, renal end-points were abstracted. The corresponding primary author of the article was contacted to clarify details or confirm outcomes for two trials.7, 10
The surrogate endpoints were development of microalbuminuria and macroalbuminuria. The clinical endpoints included doubling of serum creatinine, end-stage renal disease (ESRD), and death from renal disease.
Statistical Analysis
We examined the relationship between intensive glucose control and risk for all the study outcomes using relative risk and risk difference measures. Forest plots were created to determine pooled measures. Heterogeneity was assessed with I2 statistics, ranging from 0% to 100%. I2 demonstrates the percentage of total variation across studies due to heterogeneity and was used to judge the consistency of evidence. I2 values of 50% and more indicate a substantial level of heterogeneity.12 Random effects models were used to combine data on outcomes in Review Manager 5.0 (The Cochrane Collaboration). The meta-analysis was performed in line with recommendations from the Cochrane Library. A P-value <0.05 was considered statistically significant. Analyses were stratified by risk of bias in subsequent analyses. We also performed meta-regression using SAS 9.1 (Cary, NC) on the 5 study-level variables (median date of enrollment, years since diabetes diagnosis, duration of therapy, difference in achieved HbA1C, and median achieved HbA1C) to determine the relationship between these variables and the relative risk for each endpoint. Regression lines were plotted and bubbles were weighted for the inverse of the variance of the individual relative risks of each endpoint in each trial via Microsoft Excel 2007 (Redmond, Washington).
RESULTS
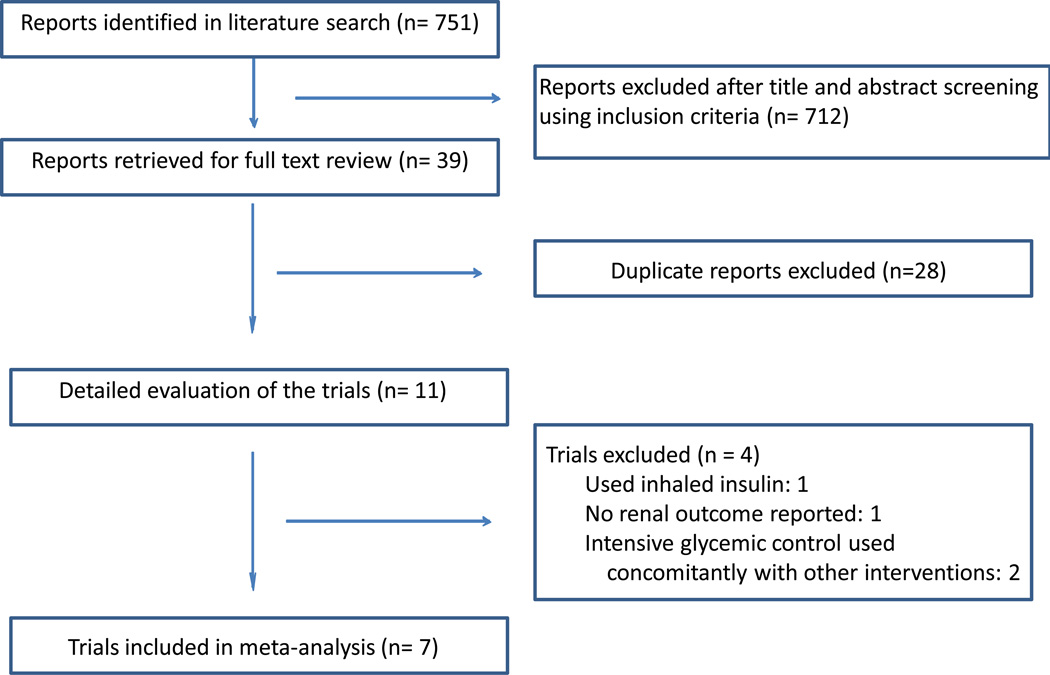
Figure 1 depicts the study selection process. The current meta-analysis included a total of seven trials conducted among 28,065 participants.
Figure 1. Literature search and selection.
Description of Studies
Table 1 presents the characteristics of the 7 randomized, controlled trials and trial participants.4, 5, 7, 10, 11, 13–16 The number of trial participants ranged from 110 to 11,140. Mean baseline serum creatinine ranged from 81 to 88.4 µmol/L in the trials. Mean duration of diabetes before enrollment ranged from 8 to 18 years with the exception of UKPDS 33 and UKPDS 34, which enrolled patients with newly-diagnosed T2DM. The interventions to achieve glycemic control varied across studies (Table 1). HbA1C (or FPG) targets varied as well in all of the studies. The highest HbA1C target in the intensive arms of the trials was < 7%,4 and the lowest HbA1C targets were < 6% in the ACCORD study13 and VADT10and ≤ 5.1% in the Veterans Affairs (VA) Diabetes Feasibility Trial.5
Table 1.
Characteristics of Randomized, Controlled Trials of Intensive Glucose Control
| CHARACTERISTIC | Kumamoto4 | UKPDS 3314 | UKPDS 3415 | VA Diabetes Feasibility Trial5 |
ACCORD7 | ADVANCE11 | VADT10 |
|---|---|---|---|---|---|---|---|
| Year of Publication | 1995 | 1998 | 1998 | 2000 | 2008 | 2008 | 2009 |
| Participants, n | 110 | 3867 | 753 | 153 | 10,251 | 11,140 | 1791 |
| Country | Japan | UK | UK | USA | USA & Canada | Multi-national | USA |
| Median Year of Enrollment | 1991 | 1984 | 1984 | 1991 | 2003 | 2002 | 2002 |
| Age (mean, yrs) | 49 | 53.3 | 53 | 60.1 | 62.2 | 66 | 60.4 |
| Race-ethnicity (%) | |||||||
| Non-Hispanic white | 0 | 81 | 85 | … | 64 | … | 62 |
| Hispanic white | 0 | … | … | … | 7 | … | 16 |
| Black | 0 | 8 | 10 | … | 19 | … | 17 |
| Asian | 100 | 10 | 4 | … | … | … | … |
| Mean duration of diabetes (years) | 6.5/10.2 | 0 | 0 | 8 | 10 | 8 | 12 |
| Men (%) | 45 | 61 | 46 | 100 | 61 | 58 | 97 |
| Current Smokers (%) | … | 31 | 39 | 26 | 14 | 14 | 17 |
| Hypertension (%) | … | 12 | 16 | … | 85 | … | 72 |
| Hx of CVD (%) | … | … | … | 13 | 35 | 32 | 40 |
| SBP (mm Hg) | 120/122 | 135 | 140 | 135 | 136 | 145 | 132 |
| DBP (mm Hg) | 70/70 | 82 | 85 | 81 | 75 | 80 | 76 |
| Mean LDL (mmol/L) | 2.7 | 3.5 | 3.7 | … | 2.7 | 3.1 | 2.8 |
| Body weight (kg) | … | 77.5 | 87 | … | 94 | 78 | 97 |
| BMI (kg/sq meter) | 21.4/19.3 | 27.5 | 31.6 | 31 | 32.2 | 28 | 31.3 |
| HbA1C (%) | 9.1/9.2 | 7 | 7.3 | 9.7 | 8.3 | 7.2 | 9.4 |
| Creatinine (µmol/L) | … | 81 | 77 | 83 | 80 | 87 | 88.4 |
| Macroalbuminuria (%) | 0 | 1.6% | 2% | … | 6 | 3.6 | … |
| Microalbuminuria (%) | 0 | 10.5% | < 7.6% | 38 | 27 | 25.6 | … |
| Urine Alb:creat | 13/43 | … | … | 0.042 | 1.54 | … | … |
| Fasting plasma glucose (mmol/L) | … | 8 | 8.1 | 11 | 9.3 | 7.9 | … |
| GFR (ml/min) | … | … | … | … | 90 | … | … |
| ARB/ACEI (%) | … | … | … | 37 | 53 | … | … |
| Statins (%) | … | … | … | … | 62 | 28 | … |
| Target HbA1C% in intensive glucose group | <7% | FPG < 6 mmol/L | FPG < 6 mmol/L | 5% | <6% | ≤6.5% | < 6% and 1.5% less than conventional |
| Target HbA1C% in conventional glucose group | …* | FPG 6.1–15 mmol/L | FPG 6.1–15 mmol/L | Avoidance of excessive hyperglycemia | 7–7.9% | Local standards | < 9% and 1.5% higher than intensive |
| Primary agent in intensive arm | Insulin | Sulfonylurea or insulin | Metformin plus sulfonylurea | Insulin | Multiple Drugs | Gliclazide | BMI ≥ 27: metformin & rosiglitazone BMI < 27: glimerpiride & rosiglitazone |
| Median duration of treatment (years) | 6 | 11.1 | 10.7 | 2 | 3.7 | 5 | 5.6 |
| Median follow-up (years) | 8 | 11.1 | 10.7 | 2 | 5 | 5.0 | 5.6 |
2 separate numbers reported for primary prevention arm (n=55 in each); The clinical and glycemic goals for the intensive therapy group in the Kumomato study was to maintain the blood glucose control as close to the fasting blood glucose concentration of < 140 mg/dL, 2 hour post-prandial blood glucose concentration < 200 mg/dL, HbA1C < 7.0% and mean amplitude of glycemic excursions < 100 mg/dL. The goals in the conventional arm was to show no symptoms of hyperglycemia or hypoglycemia, and glycemic control as close to the fasting glucose concentration of < 140 mg/dL.
…= Not Reported
Abbreviations: CAD- coronary artery disease, SBP- systolic blood pressure, DBP- diastolic blood pressure, LDL- low density lipoprotein, BMI- body mass index, GFR- glomerular filtration rate, ARB- angiotensin receptor blocker, ACEI- angiotensin converting enzyme inhibitor; FPG- fasting plasma glucose.
The median HbA1C values during the trials were lower in the intensive group in all studies, and 4 studies achieved HbA1C difference of > 1% compared to the control group (Table 2).4, 5, 10, 13 Three studies achieved median HbA1C < 7% in the intensive glycemic control group. Follow-up time was shortest in the VA Diabetes Feasibility Trial (2 years),5 and was 5 years or more in all other studies. UKPDS 33 and 34 had the longest follow-up times (up to 15 years).14, 15 The cumulative incidence of renal endpoints were as follows: microalbuminuria, range 5.8–53%; macroalbuminuria, range from 2.6–8.5%; doubling of serum creatinine, 1.1–8.8%; and ESRD 0.5–2.8% (Table 3). The cumulative incidence of morality was lowest in ACCORD (5.0% and 3.9% in intensive and standard therapy groups, respectively), and highest in UKPDS 34 (14.6% and 21.7%, respectively).
Table 2.
Risk factors for renal disease in trial participants after the intervention
| Kumamoto | UKPDS 33 | UKPDS 34 | VA Diabetes Feasibility Trial |
ACCORD | ADVANCE | VADT | |
|---|---|---|---|---|---|---|---|
| Median HbA1C (%) | |||||||
| Intensive | 7.1 | 7.0 | 7.4 | 7.1 | 6.4 | 6.5 | 6.9 |
| Standard | 9.4 | 7.9 | 8.0 | 9.1 | 7.5 | 7.3 | 8.4 |
| Mean LDL (mmol/L) | |||||||
| Intensive | … | 3.26 | 3.37 | … | 2.59 | 2.64 | 2.07 |
| Standard | … | 3.26 | 3.34 | … | 2.61 | 2.65 | 2.07 |
| Mean SBP (mm Hg) | |||||||
| Intensive | … | 139 | 141 | 135 | 135 | 135.5 | 127 |
| Standard | … | 138 | 139 | 142 | 135 | 138 | 125 |
| Mean DBP (mm Hg) | |||||||
| Intensive | … | 77 | 78 | 82 | 75 | 73.5 | 68 |
| Standard | … | 77 | 77 | 82 | 75 | 74.3 | 69 |
…= Not Reported
Abbreviations: SBP- systolic blood pressure, DBP- diastolic blood pressure, LDL- low density lipoprotein
Table 3.
Cumulative Incidence of Renal Outcomes in the Trials (Combined incidence in treatment and control arms)
| Kumamoto | UKPDS 33 |
UKPDS 34 |
VA Diabetes Feasibility Trial |
ACCORD | ADVANCE | VADT | |
|---|---|---|---|---|---|---|---|
| Median length of follow-up (yrs) | 6 | 10 | 10.7 | 2 | 5 | 5 | 6 |
| Microalbuminuria | 15.6% | 21.0%* | 23% | 44% | 23.5% | 24.7% | 11.5% |
| Macroalbuminuria | 3.9% | 5.0%* | … | 8.5% | 4.6% | 3.5% | 4.0% |
| Doubling of Creatinine | … | 1.0%* | … | … | 7.4% | 1.1% | 8.8% |
| ESRD | … | 0.6%† | 0.5% | … | 2.8% | 0.5% | 1.0% |
| Death from Renal Failure | … | 0.3%† | 0.4% | … | … | 0.5% | … |
At 9 years follow-up
At study end (median 11.1 years of follow-up)
…=Not Reported
Outcomes
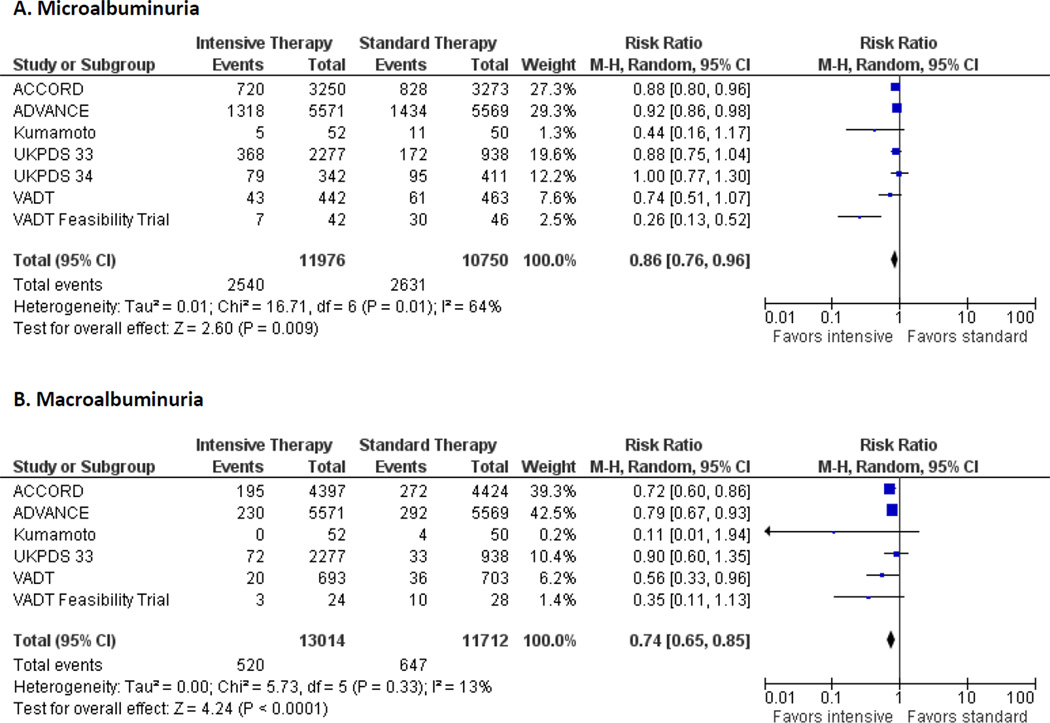
Figure 2 presents the individual and pooled relative risks and risk differences of microalbuminuria (Panel A) and macroalbuminuria (Panel B). Figure 3 presents the same for the clinical renal endpoints of doubling of serum creatinine (Panel A), renal failure/ESRD (Panel B), and death from renal disease (Panel C). Overall analyses indicated that patients randomly assigned to intensive glucose control had reduced risk for microalbuminuria (7 studies: risk ratio [RR], 0.86 [95% CI, 0.76 to 0.96; risk difference [RD] −0.04 [CI −0.08 to −0.01]) and macroalbuminuria (6 studies: 0.74 [CI, 0.65–0.85]; RD −0.01 [CI −0.02 to −0.01]); but not doubling of serum creatinine (4 studies: RR, 1.06 [CI, 0.92 to 1.22]; RD 0.0 [CI 0.0 to 0.1]; ESRD (5 studies: RR, 0.69 [CI, 0.46–1.05]; RD 0.0 [CI −0.01 to 0.0]), or death from renal disease (3 studies: RR, 0.99 [95% CI 0.55–1.79]; RD 0.0 [CI 0.0 to 0.0]) compared with participants in the conventional treatment groups. We identified possible heterogeneity for the endpoint of microalbuminuria (I2 = 69%), whereas statistical heterogeneity was low for all other analyses.
Figure 2. Pooled Risk Ratios, with 95% CI, by trial for end-points of micro and macroalbuminuria.
Footnote: Data on the incidence of micro- and macroalbuminuria from UKPDS 33 was reported in 3 year intervals. Due to the marked drop-off of patients with outcomes reported at 9 years and beyond, the data from the 6 year time-point was chosen for the endpoints of micro- and macroalbuminuria. The incidence of microalbuminuria at 9, 12, and 15 years were 19.2%, 23.0%, and 27.1% in the intensive group and 25.4%, 34.2%, and 39% in the conventional group. The incidence of macroalbuminuria at 9, 12, and 15 years was 4.4%, 6.5%, and 7.9% in the intensive group and 6.5%, 10.3%, and 12.6% in the conventional group. Intensive therapy was stopped earlier than planned in ACCORD. Data on renal outcomes were reported at transition to standard therapy (median follow-up 3.5 years) and at study end (median follow-up 5 years). The incidence of outcomes was taken from study end for the main analyses. Utilization of data from transition did not change the results for macroalbuminuria (pooled RR 0.83, 95% CI 0.72–0.95, I2 = 68%) or macroalbuminuria (pooled RR 0.74, 95% CI 0.65–0.84, I2 = 17%).
Figure 3. Pooled Risk Ratios, with 95% CI, by trial for clinical renal endpoints (doubling of serum creatinine and ESRD).
Footnote: Data on the incidence of doubling of serum creatinine from UKPDS 33 was reported in 3 year intervals. Due to the marked drop-off of patients with outcomes reported at 9 years and beyond, the data from the 6 year time-point (n= 3,045) was chosen for inclusion in the summary data above. There was no difference in the magnitude or direction of effect at 9 and 12 years. At 9 years (n= 2,172), 0.71% vs. 1.76% (RR 0.40, 95% CI 0.14–1.20), and at 12 years (n=1,054), 0.91% and 3.5% (RR 0.25, 95% CI 0.07–0.91)had doubling of serum creatinine in the intensive vs. conventional groups. At 15 years (n=170), 3.52% in the intensive group and 2.8% in the convention group had doubling of serum creatinine (RR 1.25, 95% 0.16–9.55). Data on the incidence of ESRD and Death from Renal Disease are reported from the end of the study period. Intensive therapy was stopped earlier than planned in ACCORD. Data on renal outcomes were reported at transition to standard therapy (median follow-up 3.5 years) and at study end (median follow-up 5 years). The incidence of outcomes was taken from study end for the main analyses. Utilization of data from transition did not change the results for doubling of serum creatinine (pooled RR 1.08, 95% CI 0.95–1.23, I2 = 19%) or ESRD (pooled RR 0.70, 95% CI 0.45–1.08, I2 = 45%).
Sensitivity Analyses & Meta-Regression
In order to determine the reasons for heterogeneity for our analyses of the effect on intense glucose therapy on the outcome of microalbuminuria, we excluded each study one by one. Elimination of the VA Diabetes Feasibility Trial5 from the microalbuminuria analysis reduced the I2 to 0%. This study was one of the smallest studies and had a short duration of follow-up (2 years). However, even after exclusion of VA Diabetes Feasibility Trial, the pooled RR was not measurably different (RR 0.91, CI 0.85–0.96).
We formally examined the relationship between the 5 study level variables as continuous variables and the risk for each of the renal endpoints (eFigure 1). The median year of enrollment, the years since diabetes diagnosis, and the duration of therapy (eFigure 1A–C) were only associated with one endpoint: risk for doubling of serum creatinine. Furthermore, these three meta-regressions were largely driven by UKPDS 33, as this study had the earliest median year (1984), the shortest duration of years since diagnosis (zero), and the longest duration of therapy (11 years). The difference in achieved HbA1C was associated with greater benefit from intensive glycemic control for both microalbuminuria (β = −0.401 for every percent difference in HbA1C, p = 0.01) and macroalbuminuria (β = −0.474, p=0.008; eFigure 1D). The median HbA1C achieved in the intensive glycemic group was not associated with magnitude of the relative risk for any of the endpoints (eFigure 1E).
Risk of Bias Assessment
The studies were generally of good methodological quality (eFigure 2 and 3).
Allocation
Two4, 5 of the seven trials did not clearly state their methods for allocation concealment. The results were not quantitatively or qualitatively changed when those two trials were excluded from the analyses.
Blinding
Only UKPDS 33 and 34 ensured blinding of participants and personnel. Participants in the intensive glycemic control arms in the other five trials4, 5, 7, 10, 11 had more frequent study visits with personnel than participants in the control arms. When only UKPDS 33 and 34 were considered, the results were not qualitatively different for ESRD (RR 0.80, 95% CI 0.38–1.69), however the benefit of intensive glycemic control for the endpoint of macroalbuminuria was lost (RR 0.91, 95% CI 0.79–1.05). Blinding of outcome assessment was reported in all included studies except one.4
Incomplete outcome data
There was a significant amount of incomplete outcome data from several of the studies. For example, between 20–40% of participants were not assessed for the endpoints of micro and macroalbuminuria in ACCORD, UKPDS 33, UKPDS 34, and VADT. However, the proportions with assessment of these endpoints were equal in both arms of each of these studies, indicating low risk of bias. Sensitivity analyses with exclusion of the 4 aforementioned studies resulted in similar results for micro and macroalbuminuria.
The proportion missing serum creatinine values in follow-up was < 5% in ACCORD and VADT, but was 45% at 9 years in UKPDS 33. Again, however, the proportion that were missing values were equal in both arms, thus the risk of bias was low. Due to the fact that patients are unaware of either subnephrotic proteinuria levels or of serum creatinine values, and the equal proportions of missingness, we felt that the missing data occurred at random and was not due to differences in the outcomes themselves in those without the assessments. Nevertheless, a sensitivity analysis excluding UKPDS 33 from the analysis did not change the results qualitatively or quantitatively. The ascertainment for the outcome of ESRD was complete in all of the studies that reported the endpoint.
Selective reporting
There was no evidence of selective reporting in the included studies.
Other potential sources of bias
There was evidence of publication bias by funnel plot analysis for the outcomes of microalbuminuria, macroalbuminuria, and doubling of serum creatinine, as small studies with risk ratio greater than the summary estimates were missing for these outcomes.
COMMENT
In this systematic review and meta-analysis of 7 RCTs of intensive glycemic control in type 2 diabetes, a statistically significant reduction in micro and macroalbuminuria occurred with intensive therapy. However, the data were inconclusive in regards to the effect of intensive glycemic control on clinical renal outcomes defined as doubling of serum creatinine, ESRD, or death from renal disease. Our analysis demonstrates that after 163,828 patient-years of follow-up in the 7 studies examined, intensive glycemic control improves albuminuria but data is lacking for evidence of a benefit for clinically important renal endpoints. There was a non-significant trend towards reduction of the endpoint of ESRD, a surprising observation given the very tight precision and null findings for the endpoint of doubling of creatinine which must temporally precede ESRD. However, it is worth noting that that the absolute rate of clinical renal outcomes in the published studies is relatively low: the pooled cumulative incidence of doubling of creatinine in the standard treatment group of all of the trials that measured these outcomes was only 4.1%10, 11, 13, 14 and for ESRD was only 1.6%.10, 11, 13–15 The low incidence of these endpoints may render the number needed to treat too large to justify intensive insulin therapy (even assuming a treatment effect) given the risks of severe hypoglycemia and minimal benefit for cardiovascular outcomes and potential for increased risk of death.7
As further detailed below, multiple reasons may underlie the lack of evidence for a beneficial effect of tight glycemic control on clinically significant renal endpoints (i.e., doubling of serum creatinine or ESRD) in this setting. These include A) intensive glycemic control may have started too late in the course of the disease; B) the duration of glycemic treatment may have been insufficient; C) HbA1C levels were not reduced to normal; D) there may be a “ceiling effect” that once HbA1C is reduced to a moderate degree (e.g., < 7%), further reduction does not benefit the patient, especially in the setting of other interventions including use of statins and antihypertensive medications; E) competing risk of death; F) inadequate statistical power to detect a difference.
Is it possible that the glycemic interventions started too late in the disease process to prevent the development of clinical renal outcomes? More years since diagnosis of T2DM at time of enrollment trended towards less benefit for doubling of serum creatinine. In fact, the only RCT that did not have a relative risk ≥ 1 for doubling of creatinine enrolled NEWLY-diagnosed type 2 diabetics exclusively (UKPDS 33).14 The other studies had a mean duration of diabetes of 8–12 years at the time of enrollment.10, 11, 13 Thus, it is possible that despite normal GFR at the time of enrollment, there was already a significant amount of subclinical kidney damage that occurred over the 8+ years of “non-intensive” glycemic control, making it too late to change the natural history of the kidney disease despite aggressive glycemic management.
Alternatively, is it possible that the duration of intensive glycemic therapy (or the duration of follow-up) was too short to witness improvement in progressive CKD? Because the duration of therapy was not exceedingly long in any of the RCTs that enrolled patients with prevalent type 2 diabetes (generally ~5 years), it is impossible to answer this question with any degree of certainty. It is conceivable that longer duration of intensive therapy is required to demonstrate an effect on CKD or ESRD. Longer duration of therapy was associated with a reduction in doubling of serum creatinine, however, this was again driven by UKDPS 33, which also enrolled newly-diagnosed type 2 diabetics. Furthermore, there was no reduction in ESRD in UKPDS 33 or 34, despite the long duration of treatment. Regardless, given that a small and nearly equal percentage of participants in both glycemic treatment arms of all the studies examined developed CKD or ESRD, it can be surmised that any potential differential benefit from intensive treatment must be quite small. In contrast, data from patients with type 1 diabetes from the DCCT/EDIC study demonstrate that intensive glycemic control for 6.5 years reduced the incidence of impaired GFR by 50% over a median follow-up period of 22 years.17 An analysis at 14 years after the start of DCCT was not able to demonstrate a difference in the number of patients with doubling of serum creatinine.18 Thus, it may take 20+ years to witness the impact of intensive glycemic control on clinical renal outcomes.
Was the reduction in HbA1C achieved in the trials of sufficient magnitude? Four RCTs achieved a difference in HbA1C of > 1% with intensive therapy vs. standard therapy.4, 5, 10, 13 While there was a strong association between the difference in HbA1C in the intensive vs. standard groups and the risk for both micro- and macroalbuminuria, there was no association for the endpoints of doubling of serum creatinine or ESRD. Furthermore, although median HbA1C achieved in the intensive group was not statistically associated with any of the renal endpoints, qualitatively there was no greater benefit for development of micro- and macroalbuminuria and a trend towards harm for the endpoint of doubling of creatinine in studies with lower achieved median HbA1C values. This suggests that avoidance of excessive hyperglycemia is necessary, but aggressive glycemic control offers little advantage and may be deleterious when one accounts for risk for severe hypoglycemic events. Furthermore, given the multifactorial nature and complexity of mechanisms underlying the pathogenesis of T2DM, it is of importance to investigate whether control of other pathogeneic mechanisms - in addition to intensive treatment of hyperglycemia, hypertension, and dyslipidemia - might help prevent progressive CKD in patients with T2DM.
Could the lack of apparent convincing benefit for hard renal outcomes be due to competing risk of death? For this to be it operative, it would presume that those at risk of developing the renal endpoint are the same patients who are dying prematurely, and thus when outcomes are examined at the study level, the higher rate of death in one group vs. the other does not allow more participants in that group sufficient time to manifest the renal endpoint of interest. However, the pooled risk of death not different between the two groups (RR 0.98, 95% CI 0.84–1.15).8 If mortality was higher in the standard treatment group, there may have been a chance for competing risk of death to mask the renal benefit.
Finally, despite nearly 30,000 patients included in this meta-analysis, we may have lacked adequate statistical power to detect a difference in clinical renal endpoints between the two groups. The incidence of doubling of creatinine was 503 events in 12383 participants in the standard therapy group (4.1%). Given the number of patients and a 2-sided alpha of 0.05, we would have been able to detect at least a 16% difference in the relative risk of the outcome between the two groups with 80% power, if there would have been a difference. The incidence of ESRD was 204 in 13117 (1.55%) in the standard therapy group and 147 in 14643 participants (1.0%) in the intensive therapy group, yielding 98% power at a 2-sided alpha of 0.05 to detect whether this 31% relative risk reduction was statistically significant. Regardless, with a baseline rate of ESRD so low in the standard therapy group, and the overall lack of benefit for cardiovascular or all-cause mortality,8 it does not seem prudent to expose patients to this therapy to achieve an absolute risk reduction for ESRD that will be < 1% in a best-case scenario.
In conclusion, our systematic review and meta-analysis suggests that intensive glycemic control reduces albuminuria but evidence is lacking that it prevents clinically meaningful renal outcomes such as CKD, ESRD and renal-related death in patients with T2DM measured during the 3.5 to 10.7 years of the published trials. Acknowledging the low incidence of clinical renal outcomes coupled with the apparent lack of convincing benefit of intensive glycemic control to prevent CKD and ESRD in patients with newly-diagnosed or prevalent type 2 diabetes, there is little compelling reason to initiate intensive glycemic control in mid-stage of the disease with the aim of preventing renal failure.
Supplementary Material
Acknowledgments
We would like to thank Mark Gentry, Yale University School of Medicine Library, for his assistance with our search of the medical literature.
Funding: None
Funding/Support: Dr. Krumholz is supported by grant U01 HL105270-02 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute, and is the recipient of a research grant from Medtronic, Inc. through Yale University.
Footnotes
Author Contributions
Dr Coca had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Coca and Parikh.
Acquisition of data: Coca and Haq.
Analysis and interpretation of the data: Coca, Ismail-Beigi, Haq, Krumholz, and Parikh.
Drafting of the manuscript: Coca and Haq.
Critical revision of the article for important intellectual content: Coca, Ismail-Beigi, Krumholz, and Parikh.
Statistical analysis: Coca and Haq.
Obtained funding: None
Administrative, technical, or material support: Coca, Ismail-Beigi, and Parikh.
Study Supervision: Coca and Parikh
Financial Disclosure: Dr. Krumholz chairs a scientific advisory board for United Healthcare.
REFERENCES
- 1.Kawazu S, Tomono S, Shimizu M, et al. The relationship between early diabetic nephropathy and control of plasma glucose in non-insulin-dependent diabetes mellitus. The effect of glycemic control on the development and progression of diabetic nephropathy in an 8-year follow-up study. J Diabetes Complications. 1994;8(1):13–17. doi: 10.1016/1056-8727(94)90005-1. [8167381] [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [10938048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [8366922] [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–117. doi: 10.1016/0168-8227(95)01064-k. [7587918] [DOI] [PubMed] [Google Scholar]
- 5.Levin SR, Coburn JW, Abraira C, et al. Effect of intensive glycemic control on microalbuminuria in type 2 diabetes. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type 2 Diabetes Feasibility Trial Investigators. Diabetes Care. 2000;23(10):1478–1485. doi: 10.2337/diacare.23.10.1478. [11023140] [DOI] [PubMed] [Google Scholar]
- 6.KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2) Suppl 2:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [17276798] [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [18539917] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [19620144] [DOI] [PubMed] [Google Scholar]
- 9.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [21693741] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [19092145] [DOI] [PubMed] [Google Scholar]
- 11.Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [18539916] [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [20594588] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [9742976] [PubMed] [Google Scholar]
- 15.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. [9742977] [PubMed] [Google Scholar]
- 16.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–B29. [10860187] [PubMed] [Google Scholar]
- 17.Intensive Diabetes Therapy and Glomerular Filtration Rate in Type 1 Diabetes. N Engl J Med. 2011 doi: 10.1056/NEJMoa1111732. [22077236] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290(16):2159–2167. doi: 10.1001/jama.290.16.2159. [14570951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.