Abstract
Context
To better guide strategies intended to reduce high rates of 30-day readmission after hospitalization for heart failure, acute myocardial infarction, or pneumonia, further information is needed about readmission diagnoses, readmission timing, and the relationship of both to patient age, sex, and race.
Objective
To examine readmission diagnoses and timing among Medicare beneficiaries readmitted within 30 days after hospitalization for heart failure, acute myocardial infarction, or pneumonia.
Design, Setting, and Patients
We analyzed 2007 to 2009 Medicare Fee-For-Service claims data to identify patterns of 30-day readmission by patient demographic characteristics and time after hospitalization for heart failure, acute myocardial infarction, or pneumonia. Readmission diagnoses were categorized using an aggregated version of the Centers for Medicare & Medicaid Services’ Condition Categories. Readmission timing was determined by day after discharge.
Main Outcomes Measures
We examined (1) the percentage of 30-day readmissions occurring on each day (0–30) after discharge; (2) the most common readmission diagnoses occurring during cumulative time periods (days 0–3, 0–7, 0–15, and 0–30) and consecutive time periods (days 0–3, 4–7, 8–15, and 16–30) after hospitalization; (3) median time to readmission for common readmission diagnoses; and (4) the relationship between patient demographic characteristics and readmission diagnoses and timing.
Results
From 2007 to 2009, we identified 329,308 30-day readmissions after 1,330,157 heart failure hospitalizations (24.8% readmitted), 108,992 30-day readmissions after 548,834 acute myocardial infarction hospitalizations (19.9% readmitted), and 214,239 30-day readmissions after 1,168,624 pneumonia hospitalizations (18.3% readmitted). The proportion of patients readmitted for the same condition was 35.2% after index heart failure hospitalization, 10.0% after index acute myocardial infarction hospitalization, and 22.4% after index pneumonia hospitalization. Of all readmissions within 30 days, 61.0%, 67.6%, and 62.6% occurred with 15 days of discharge after hospitalization for heart failure, acute myocardial infarction, or pneumonia, respectively. The diverse spectrum of readmission diagnoses was largely similar in both cumulative (days 0–3, 0–7, 0–15, and 0–30) and consecutive (days 0–3, 4–7, 8–15, and 16–30) time periods after discharge. Median time to 30-day readmission was 12 days, 10 days, and 12 days for patients initially hospitalized with heart failure, acute myocardial infarction, or pneumonia, respectively, and was comparable across common readmission diagnoses. Neither readmission diagnoses nor timing substantively varied by age, sex, or race.
Conclusions
Among Medicare Fee-for-Service beneficiaries hospitalized for heart failure, acute myocardial infarction, or pneumonia, 30-day readmissions are frequent throughout the month following hospitalization and result from a similar spectrum of readmission diagnoses regardless of age, sex, race, or time after discharge.
INTRODUCTION
Hospital readmissions are common1–4 and can be a marker of poor health care quality and efficiency.5–9 To lower readmission rates, the Centers for Medicare & Medicaid Services (CMS) began publicly reporting 30-day risk-standardized readmission rates for heart failure (HF), acute myocardial infarction (AMI), and pneumonia after these measures were endorsed by the National Quality Forum.10–13 These measures are part of a federal strategy to provide incentives to improve quality of care by reducing preventable readmissions.14
Critical to the development of effective programs to reduce readmission is an understanding of the diagnoses and timing associated with these events. Using 2003 to 2004 Medicare data, Jencks and colleagues identified the most frequent diagnoses accounting for readmission within 30 days after hospitalization for 10 common conditions.15 Yet unanswered questions remain that may be pertinent when planning targeted interventions and benchmarking performance. For example, within the 30-day period after hospitalization, do certain time periods have higher numbers of readmissions and therefore merit even greater attention to readmission risk? Second, do the diagnoses responsible for readmission change to a significant degree over the month following discharge, indicating a need to tailor interventions to the time after hospitalization? Finally, do the diagnoses and timing of 30-day readmissions substantively vary by patient age, sex, or race, thereby suggesting that interventions be guided by patient demographic characteristics? These insights into the diversity and variation of readmission diagnoses can illustrate the potential benefits of general versus disease-specific interventions in reducing the overall number of readmissions.
We therefore studied Medicare beneficiaries who were readmitted within 30 days after hospitalization for HF, AMI, or pneumonia from 2007 to 2009 to describe readmission causes and timing for each condition. These 3 conditions are primarily responsible for almost 15% of hospitalizations in older persons16 and are the focus of current public reporting efforts.14
METHODS
Study Sample
We used Medicare Standard Analytic and Denominator files to identify hospitalizations to acute care hospitals from 2007 to 2009 with a principal discharge diagnosis of HF, AMI, or pneumonia. Cohorts were defined with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes identical to those used in the CMS publicly reported readmission measures (online supplementary appendix eTable 1).11–13 We included hospitalizations among patients 65 years of age and older with a complete claims history for 1 year preceding admission. Reasons for exclusion included in-hospital death, less than 30 days post-discharge enrollment in Medicare Fee-For-Service, transfer to another acute care facility, and discharge against medical advice. We then used definitions consistent with the CMS measures to identify all readmissions due to any cause occurring within 30 days of hospitalization.11–13 As with the CMS measures, only the first rehospitalization within 30 days of discharge was considered a 30-day readmission. Additional rehospitalizations within this 30-day period were not counted as 30-day readmissions or index hospitalizations for the same condition. Subsequent hospitalizations occurring after 30 days from discharge were counted as index admissions if they met inclusion criteria. All study analyses were performed on the whole population of readmitted patients.
Sample Classification
We categorized readmission diagnoses using a modified version of the CMS Condition Categories (CCs).17 Each of the 189 CC groups is structured around a reasonably well-specified disease or medical condition.17 However, as nearly 90% of the 189 CC groups each accounted for less than 1% of all readmissions, we consolidated related diagnoses into a shorter list of 30 modified CCs (mCCs) to make data presentation more clinically meaningful. Based on our expert opinion, these 30 mCCs were designed to be clinically internally consistent and capture the most common readmission diagnoses after discharge from HF, AMI, and pneumonia hospitalizations. The specific diagnoses comprising each mCC are presented in eTable 2. Cardiopulmonary diagnoses were described with relatively greater granularity given their expected importance following index hospitalization for HF, AMI, or pneumonia.
Outcomes
We examined the following outcomes:
Readmission Diagnoses
We identified the percentage of observed 30-day readmissions due to the 30 most common reasons for readmission by mCC for the HF, AMI, and pneumonia cohorts. We noted the percentage of observed 30-day readmissions due to cardiovascular diagnoses after hospitalizations for HF and AMI, and pulmonary diagnoses following hospitalizations for pneumonia (mCC groups comprising cardiovascular and pulmonary diseases are listed in eTables 3 and 4).
Readmission Timing
We identified the percentage of 30-day readmissions occurring on each day (0–30) after discharge.
Readmission Diagnoses by Time after Discharge
We identified the 10 most common readmission diagnoses by mCC during cumulative time periods after discharge (days 0–3, 0–7, 0–15, and 0–30) that may occur before outpatient follow-up and therefore be of particular importance to discharging hospitals. We also examined the 10 most common readmission diagnoses by mCC in consecutive time periods after discharge (days 0–3, 4–7, 8–15, and 16–30) that could coincide with outpatient visits and therefore be of particular value to ambulatory care providers. We intentionally constructed shorter time intervals during days 0–15 compared with days 16–30 after discharge to provide greater granularity of information for hospitals and providers engaging in early outpatient follow-up. Lastly, we investigated whether the median time to readmission differed for the 5 most common readmission diagnoses.
Patient Demographic Characteristics and Readmission
We examined whether patient age, sex, and race were associated with readmission timing and the pattern of readmission diagnoses.
Statistical Analyses
Readmission Diagnoses and Timing
We calculated summary statistics for readmission diagnoses by mCC, readmission timing by day (0–30) after discharge, and readmission diagnoses by days 0–3, 0–7, 0–15, 0–30, 4–7, 8–15, and 16–30 after discharge. We then estimated Kaplan-Meier survival curves for the 10 most common readmission diagnoses as categorized by mCC for each condition. The outcome was readmission. Survival time was the number of days from discharge to readmission. Data were censored at the time of death or at 30 days, whichever occurred first. We also calculated the median time to readmission for all patients in the HF, AMI, and pneumonia cohorts as well as for those readmitted with the 5 most common readmission diagnoses for each condition.
Patient Demographic Characteristics and Readmission Diagnoses
To examine the association of patient demographic characteristics with readmission diagnoses and timing among 30-day readmissions, we first fit extended logistic regression models for the top 5 readmission diagnoses for each condition. We used a generalized estimating equation approach because of the clustering of hospitalizations within hospitals. Patient characteristics included age (65–74, 75–84, and ≥85 years), sex, and race (white, black, other). Further subdivision of race categories using CMS data is unreliable.18 We adjusted for the comorbidities used by CMS in its calculations of hospital risk-standardized readmission rates for HF, AMI, and pneumonia.11–13
We then illustrated the association of patient demographic characteristics with the marginal number of rehospitalizations due to common readmission diagnoses through use of a least squares means method.19 We first calculated the predicted population probability of readmission due to these common diagnoses by applying the estimates (beta coefficients) from the logistic models to hypothetical readmission cohorts with balanced patient characteristics. For ease of data presentation and understanding, we assumed cohort sizes of 100 readmissions. To isolate the association of each patient demographic characteristic with the marginal number of rehospitalizations due to common readmission diagnoses, we assumed that the marginal prevalence of the remaining demographic characteristics in each cohort was equal to the marginal prevalence of these patient characteristics in the overall HF, AMI, and pneumonia readmission populations. We then calculated the predicted number of patients readmitted for common diagnoses by multiplying the predicted population probability by 100, the total number of readmissions in each hypothetical cohort. For example, to identify the association of sex with the number of readmissions for recurrent HF, we compared the predicted number of readmissions due to recurrent HF among 100 readmitted women versus 100 readmitted men. These 2 groups had a marginal prevalence of age, race, and comorbidities that was identical to that of the overall HF readmission population.
Patient Demographic Characteristics and Readmission Timing
We fit extended Cox proportional hazards models to determine the association of patient characteristics with readmission timing by estimating comorbidity-adjusted hazard ratios for each patient characteristic. We used a generalized estimating equation approach. We confirmed the proportional hazards assumption by log-log plotting and based survival time on the number of days from discharge to readmission. Data were censored at the time of death or at 30 days, whichever occurred first.
All significance levels for logistic and Cox proportional hazards models were 2-sided with a p value <0.05. Analyses were primarily conducted by A.F.H. and Z.L. using SAS 9.2 (SAS Institute, Cary, North Carolina). We obtained Institutional Review Board approval, including waiver of the requirement for participant informed consent, through the Yale University Human Investigation Committee.
RESULTS
We identified 329,308 30-day readmissions after 1,330,157 hospitalizations for HF (24.8% readmitted), 108,992 30-day readmissions after 548,834 hospitalizations for AMI (19.9% readmitted), and 214,239 30-day readmissions after 1,168,624 hospitalizations for pneumonia (18.3% readmitted). The average patient age and associated standard deviation (SD) of each readmission cohort was 80.3 years (SD=7.9 years) for HF, 79.8 years (SD=8.0 years) for AMI, and 80.0 years (SD=8.0 years) for pneumonia. Common comorbidities among readmissions are listed in eTable 5.
Readmission Diagnoses
Ranked reasons for readmission for all 30 mCCs are presented in eTable 6. Following hospitalization for HF and AMI, readmission was most often due to HF (35.2% and 19.3% of readmissions, respectively). Following hospitalization for pneumonia, readmission was most likely for recurrent pneumonia (22.4%). The percentage of readmissions due to cardiovascular disease was 52.8% and 53.4% for the HF and AMI cohorts, respectively. The percentage of readmissions due to respiratory disease was 38.5% for the pneumonia cohort. The 5 most common readmission diagnoses among HF, AMI, and pneumonia cohorts comprised 55.9%, 44.3%, and 49.6% of all 30-day readmissions, respectively.
Readmission Timing
We found 61.0%, 67.6%, and 62.6% of all 30-day readmissions for the HF, AMI, and pneumonia cohorts, respectively, to have occurred during days 0–15 following discharge (Figure 1). More than 30% of 30-day readmissions occurred during days 16–30 for all 3 cohorts.
Figure 1.
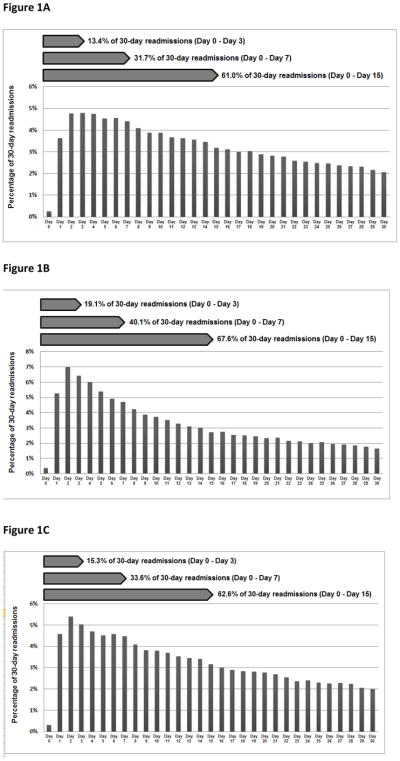
The percentage of 30-day readmissions by day (0–30) following hospitalization for heart failure, acute myocardial infarction, or pneumonia. The denominators used to calculate the percentage of 30-day readmissions on each day after hospitalization were 329,308 30-day readmissions following HF hospitalization, 108,992 30-day readmissions following AMI hospitalization, and 214,239 30-day readmissions following pneumonia hospitalization.
Readmission Diagnoses by Time after Discharge
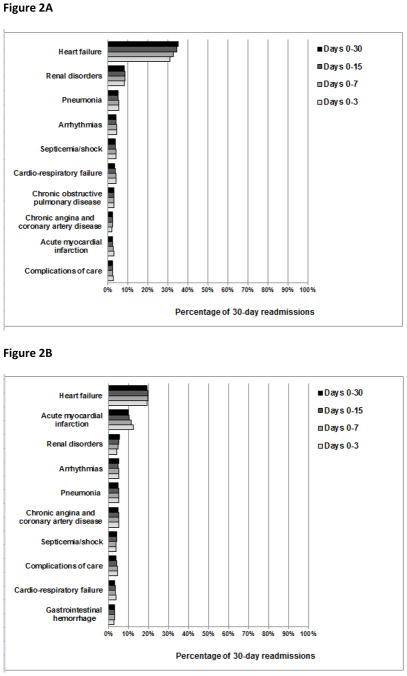
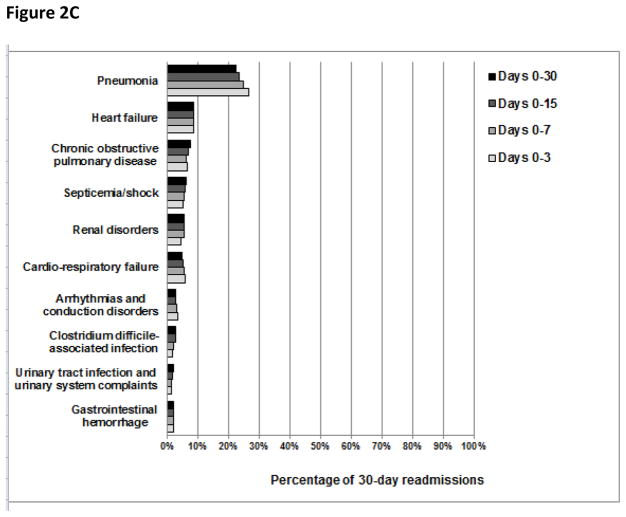
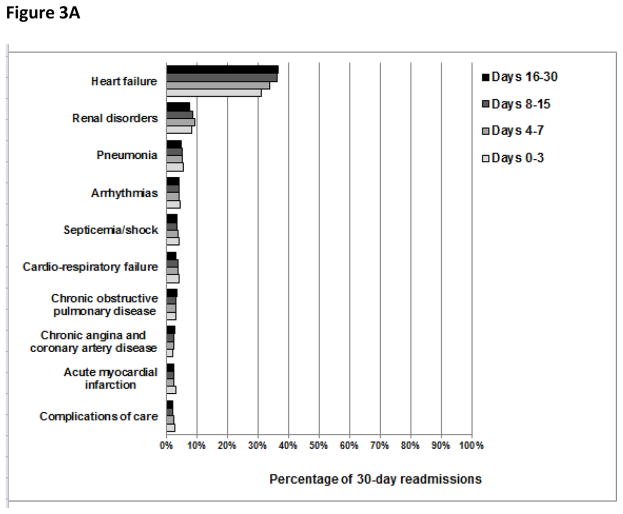
The overall pattern of readmission diagnoses was largely similar in both cumulative and consecutive time periods after discharge (Figures 2 and 3, respectively). However, we did note that the percentage of readmissions due to recurrent HF and recurrent pneumonia changed slightly with time. For example, the percentage of readmissions due to recurrent HF was 31%, 33%, 34%, and 35% during days 0–3, 0–7, 0–15, and 0–30 after discharge, respectively, and the percentage of readmissions due to recurrent pneumonia was 27%, 23%, 21%, and 21% during days 0–3, 4–7, 8–15, and 16–30 following hospitalization, respectively.
Figure 2.
The percentage of patients readmitted with common readmission diagnoses during cumulative time periods following hospitalization for heart failure, acute myocardial infarction, or pneumonia. The denominators used to calculate the percentage of 30-day readmissions due to common readmission diagnoses during each cumulative time period after hospitalization for HF were 44,257 readmissions for days 0–3, 104,362 readmissions for days 0–7, 201,005 readmissions for days 0–15, and 329,308 readmissions for days 0–30. Analogously, following AMI hospitalization, the denominators used were 20,801 readmissions for days 0–3, 43,687 readmissions for days 0–7, 73,641 readmissions for days 0–15, and 108,992 readmissions for days 0–30. Following pneumonia hospitalization, the denominators used were 32,829 readmissions for days 0–3, 71,995 readmissions for days 0–7, 134,033 readmissions for days 0–15, and 214,239 readmissions for days 0–30.
Figure 3.
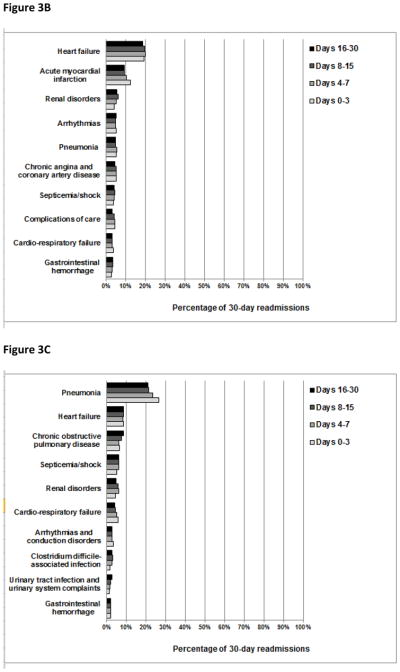
The percentage of patients readmitted with common readmission diagnoses during consecutive time periods following hospitalization for heart failure, acute myocardial infarction, or pneumonia. The denominators used to calculate the percentage of 30-day readmissions due to common readmission diagnoses during each consecutive time period after hospitalization for HF were 44,257 readmissions for days 0–3, 60,105 readmissions for days 4–7, 96,643 readmissions for days 8–15, and 128,303 readmissions for days 16–30. Analogously, following AMI hospitalization, the denominators used were 20,801 readmissions for days 0–3, 22,886 readmissions for days 4–7, 29,954 readmissions for days 8–15, and 35,531 readmissions for days 16–30. Following pneumonia hospitalization, the denominators used were 32,829 readmissions for days 0–3, 39,166 readmissions for days 4–7, 62,038 readmissions for days 8–15, and 80,206 readmissions for days 16–30.
Median times to readmission were 12 days, 10 days, and 12 days for patients initially hospitalized with HF, AMI, or pneumonia, respectively (eTable 7). Median times to readmission for the 5 most common readmission diagnoses ranged from 11 to 13 days, 9 to 11 days, and 11 to 14 days for the HF, AMI, and pneumonia cohorts, respectively.
Patient Demographic Characteristics and Readmission Diagnoses
Even when patient age, sex, or race was associated with the comorbidity-adjusted odds of readmission for a particular diagnosis, neither the predicted number of readmissions due to this diagnosis nor the overall spectrum of readmission diagnoses differed to a clinically significant degree. For example, among readmissions following index hospitalization for HF, increasing patient age was associated with higher adjusted odds of readmission for pneumonia (adjusted odds ratios 1.21 and 1.59 with increasing age group) (eTable 8). However, increasing age was associated with only 2.1 more predicted pneumonia readmissions among 100 rehospitalizations in patients aged 85 years and older compared with those aged 65–74 years (from 3.6 to 5.7 readmissions) (Table 1). The greatest change in predicted readmission number due to variation in any demographic characteristic was 5.6 additional predicted readmissions for HF among 100 rehospitalizations following the index AMI hospitalization for patients aged 65–74 years versus those aged 85 years and older (from 16.3 readmissions to 21.9 readmissions). The association of patient age, sex, and race with the overall spectrum of readmission diagnoses was always small, even when corresponding odds ratios appeared prominent, as the great majority of readmission diagnoses constituted only a small proportion of all 30-day readmissions.
Table 1.
Association of Patient Age, Sex, and Race with the Predicted Number of Common Readmission Diagnoses among 100 Readmissions Following Hospitalization for Heart Failure, Acute Myocardial Infarction, or Pneumonia.
| Readmission diagnosis by modified CC | Number of readmissions by patient age in years | Number of readmissions by patient sex | Number of readmissions by patient race | |||||
|---|---|---|---|---|---|---|---|---|
| 65–74 | 75–84 | ≥85 | Male | Female | White | Black | Other | |
| Heart failure cohort | ||||||||
| Heart failure | 34.0 | 33.8 | 35.6 | 34.8 | 34.0 | 33.8 | 38.4 | 35.3 |
| Renal disorders | 7.5 | 7.8 | 7.9 | 7.7 | 7.8 | 7.7 | 8.0 | 7.8 |
| Pneumonia | 3.6 | 4.4 | 5.7 | 5.0 | 4.1 | 4.7 | 3.6 | 4.3 |
| Arrhythmias and conduction disorders | 4.0 | 3.7 | 3.1 | 3.4 | 3.8 | 3.7 | 2.9 | 3.0 |
| Septicemia/shock | 2.9 | 3.3 | 3.6 | 3.4 | 3.1 | 3.2 | 3.2 | 3.9 |
| Acute myocardial infarction cohort | ||||||||
| Heart failure | 16.3 | 18.7 | 21.9 | 18.3 | 19.4 | 18.9 | 18.8 | 19.6 |
| Acute myocardial infarction | 8.3 | 8.6 | 12.1 | 10.0 | 9.1 | 9.5 | 9.7 | 9.8 |
| Renal disorders | 4.7 | 5.3 | 5.4 | 5.3 | 5.0 | 5.0 | 6.8 | 5.5 |
| Arrhythmias and conduction disorders | 5.0 | 4.8 | 4.2 | 5.0 | 4.5 | 4.9 | 2.9 | 4.2 |
| Pneumonia | 3.6 | 4.3 | 5.5 | 5.3 | 3.7 | 4.4 | 4.0 | 4.3 |
| Pneumonia cohort | ||||||||
| Pneumonia | 20.6 | 21.4 | 23.1 | 24.1 | 19.7 | 22.0 | 18.5 | 21.8 |
| Heart failure | 5.7 | 6.5 | 8.0 | 6.1 | 7.3 | 6.7 | 7.4 | 6.2 |
| Chronic obstructive pulmonary disease/asthma | 5.6 | 4.7 | 3.4 | 4.4 | 4.5 | 4.5 | 4.4 | 4.3 |
| Septicemia/shock | 5.0 | 5.3 | 5.5 | 5.7 | 4.9 | 5.1 | 6.3 | 6.3 |
| Renal disorders | 4.4 | 4.9 | 5.4 | 4.8 | 5.0 | 4.8 | 5.9 | 5.0 |
When illustrating the influence of age on the number of patients readmitted with common diagnoses, patient populations were made identical to the overall readmission cohorts in sex, race, and comorbidity. To isolate the influence of age, our cohort consisted of 100 patients aged 65–74 years, 100 patients aged 75–84 years, and 100 patients aged ≥85 years. Analogously, populations were identical in age, race, and comorbidity when illustrating the influence of sex. To isolate the influence of sex, our cohort consisted of 100 men and 100 women. Lastly, populations were identical in age, sex, and comorbidity when illustrating the influence of race. To isolate the influence of race, our cohort consisted of 100 white patients, 100 black patients, and 100 patients of other race.
CC: condition category
Patient Demographic Characteristics and Readmission Timing
Comorbidity-adjusted hazard ratios did not appear to differ by patient age, sex, or race to a clinically significant degree (Table 2).
Table 2.
Association of Patient Demographic Characteristics with Comorbidity-adjusted Hazard Ratios for Patients Readmitted within 30 Days after Hospitalization for Heart Failure, Acute Myocardial Infarction, or Pneumonia.
| Patient demographic characteristics | Heart failure cohort (329,308 readmissions) | AMI cohort (108,992 readmissions) | Pneumonia cohort (214,239 readmissions) | |||
|---|---|---|---|---|---|---|
| Number of readmissions | Hazard ratio (95% CI) | Number of readmissions | Hazard ratio (95% CI) | Number of readmissions | Hazard ratio (95% CI) | |
| Age in years | ||||||
| 65–74 | 85,662 | 1 | 31,143 | 1 | 59,144 | 1 |
| 75–84 | 136,785 | 1.02 (1.01, 1.03) | 44,609 | 1.01 (0.99, 1.02) | 88,642 | 1.02 (1.01, 1.03) |
| ≥85 | 106,861 | 1.02 (1.01, 1.03) | 33,240 | 1.00 (0.98, 1.01) | 66,453 | 1.04 (1.03, 1.05) |
| Sex | ||||||
| Male | 145,270 | 1 | 50,570 | 1 | 100,077 | 1 |
| Female | 184,038 | 0.98 (0.97, 0.99) | 58,422 | 0.99 (0.98, 1.00) | 114,162 | 1.00 (1.00, 1.01) |
| Race | ||||||
| White | 272,045 | 1 | 94,275 | 1 | 187,288 | 1 |
| Black | 42,465 | 0.97 (0.96, 0.99) | 9,764 | 0.98 (0.97, 1.00) | 17,412 | 0.99 (0.98, 1.01) |
| Other | 14,798 | 0.99 (0.97, 1.00) | 4,953 | 0.98 (0.96, 1.01) | 9,539 | 0.99 (0.97, 1.01) |
AMI: acute myocardial infarction; CI: confidence interval
COMMENT
Medicare Fee-For-Service beneficiaries are readmitted within 30 days after hospitalization for HF, AMI, or pneumonia with a diverse spectrum of diagnoses that usually differ from the cause of the index hospitalization. We have extended prior literature by revealing that the overall pattern of diagnoses responsible for readmission does not substantively differ by patient demographic characteristics or time after discharge. We have also shown that although a disproportionately high number of readmissions occur soon after hospitalization, readmissions remain frequent throughout the month. These findings imply that the entire 30-day period post-discharge is one of heightened vulnerability to readmission from a wide variety of illnesses. Programs to reduce 30-day readmissions should therefore be correspondingly broad in scope in the diagnoses they target and effective for at least the full month following hospitalization. Interventions targeted at specific diseases or time periods responsible for only a fraction of all 30-day readmissions may be less efficacious unless they provide broader collateral benefits.
Similar to previous work, we demonstrated in a contemporary cohort that readmission diagnoses usually differ from the specific diagnosis responsible for the index hospitalization and often involve different physiologic systems.15,20–22 For example, only 22% of readmissions after hospitalization for pneumonia were due to recurrent pneumonia and less than 40% were due to pulmonary disease. Moreover, only a minority of readmissions were attributable to the 5 most common readmission diagnoses among patients initially hospitalized for AMI or pneumonia. No diagnosis was responsible for more than 5% of the remaining readmissions.
We additionally found that the overall pattern of diagnoses responsible for readmission did not substantively differ by patient demographic characteristics or time after discharge. This observation suggests that hospitals should account for a fairly standard spectrum of readmission diagnoses when designing and implementing interventions to prevent rehospitalization regardless of patient age, sex, race, or anticipated follow-up date in the month after hospitalization. Similarly, ambulatory providers seeing patients at different time periods after discharge should be aware that the diverse spectrum of readmission diagnoses is largely stable over time, and perform their surveillance and preventive measures accordingly. While we did find that readmissions for recurrent HF were more likely to occur later in the month and that readmissions for recurrent pneumonia were more likely to occur soon after discharge, these differences involved less than 6% of all readmissions. It may be that recurrent volume overload is a progressive process that takes some time to manifest,23 while recurrent pneumonia is greatest in recently hospitalized patients who are often colonized with drug-resistant pathogens.24
The broad range of acute conditions responsible for rehospitalization may reflect a generalized vulnerability to illness among recently discharged patients, many of whom have developed new impairments both during and after hospitalization. Inpatients frequently experience loss of strength and mobility25 and develop new disabilities and difficulties in performing activities of daily living.26–28 Hospitalized patients may suffer from nutritional deficits due to reduced appetite and imposed caloric restriction.29 Sleep deprivation may occur.30 Delirium often continues even after hospitalization.31 Adverse effects of commonly used pharmacotherapies started in the hospital and continued at discharge may exacerbate all of these conditions.32–34
This heightened vulnerability to a diversity of illnesses may explain why interventions that are broadly applicable to many conditions with multiple components or are delivered by a multidisciplinary team are more likely to reduce readmissions.35,36 Rich et al. demonstrated that the combination of general HF education by a registered nurse, dietary education by a registered dietician, consultation with a social worker, medication management by a geriatric cardiologist, and home visits reduced the number of all-cause readmissions.37 Similarly, Jack et al. demonstrated that patient education, care coordination, and confirmation of a specific medication plan by trained registered nurses plus medication education, reconciliation, and compliance assessment by a clinical pharmacist led to reductions in emergency department visits and readmissions.38 In contrast, single randomized interventions or strategies delivered by one expert have more often failed.35,36,39–42
The timing of 30-day readmissions highlights the importance of both transitional care and longitudinal strategies that are effective for at least the full month following hospitalization. We found that a high proportion of 30-day readmissions occurred relatively soon after discharge, which may explain why hospitals least likely to provide outpatient follow-up within 7 days after hospitalization for HF had the highest rates of 30-day readmission.43 The preponderance of early readmissions may also explain why exclusively outpatient interventions have often been ineffective in reducing 30-day readmissions that may have occurred before initial follow-up.41,42 In contrast, strategies involving the combination of inpatient and early outpatient interventions with the use of tools that facilitate cross-site communication have lowered readmissions that occur soon after discharge.44,45 However, as about one-third of 30-day readmissions occur during days 16–30 after hospitalization, many patients require substantial attention well beyond the initial follow-up visit.
There are some additional factors to consider when interpreting this study. Data were limited to Medicare Fee-For-Service beneficiaries and conclusions drawn from this population may not apply to others. Nevertheless, this is the population that is the focus of recent federal policies. Also, we relied on claims data to assign diagnoses for both index hospitalizations and readmissions. However, administrative codes have been shown to be accurate for cardiovascular and pulmonary diagnoses.46–48 Finally, CMS claims data contain relatively limited information on patient social factors potentially associated with readmission patterns,49 and we did not develop proxy measures to test the relationship between these variables and readmission diagnoses and timing.
The diagnoses associated with 30-day readmission are diverse and are not associated with patient demographic characteristics or time after discharge for older patients initially hospitalized with HF, AMI, or pneumonia. Although a high percentage of 30-day readmissions occur relatively soon after hospitalization, readmissions remain frequent during days 16–30 after discharge regardless of patient age, sex, or race. This heightened vulnerability of recently hospitalized patients to a broad spectrum of conditions throughout the post-discharge period favors a generalized approach to preventing readmissions that is broadly applicable across potential readmission diagnoses and effective for at least the full month after hospitalization. Strategies that are specific to particular diseases or time periods may only address a fraction of patients at risk for rehospitalization.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by grant 1U01HL105270-03 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr. Dharmarajan is supported by grant T32 HL007854-16A1 from the Division of Cardiology at Columbia University; he is also supported as a Centers of Excellence Scholar in Geriatric Medicine at Yale by the John A. Hartford Foundation and the American Federation for Aging Research. Drs. Ross and Horwitz are supported by grants K08 AG032886 and K08 AG038336 from the National Institute on Aging and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program, respectively. Dr. Barreto-Filho is supported by grant 3436-10-1 from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Ministry of Education, Brazil) and the Federal University of Sergipe, Brazil. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest Disclosures: Drs. Bernheim, Drye, Horwitz, Kim, Krumholz, Lin, Ross, and Suter work under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr. Bueno has received speaking or advisory fees from Astra-Zeneca, Bayer, Daiichi-Sankyo, Eli Lilly, Novartis, and Roche. Dr. Krumholz is chair of a cardiac scientific advisory board for UnitedHealth, and Dr. Ross is a member of a scientific advisory board for FAIR Health. Drs. Krumholz and Ross are the recipients of a research grant from Medtronic through Yale University.
Previous Presentation: These data were presented in part at the Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke Conference of the American Heart Association; May 9–12, 2012; Atlanta, Georgia.
Author Contributions: Drs. Dharmarajan and Krumholz had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dharmarajan, Lin, Bueno, Krumholz
Acquisition of data: Krumholz
Analysis and interpretation of data: Dharmarajan, Hsieh, Lin, Bueno, Ross, Horwitz, Barreto-Filho, Kim, Bernheim, Suter, Drye, Krumholz
Drafting of the manuscript: Dharmarajan, Krumholz
Critical revision of the manuscript for important intellectual content: Dharmarajan, Hsieh, Lin, Bueno, Ross, Horwitz, Barreto-Filho, Kim, Bernheim, Suter, Drye, Krumholz
Statistical analysis: Hsieh, Lin
Obtained funding: Krumholz
Administrative, technical, or material support: Krumholz
Study supervision: Krumholz
References
- 1.Lindenauer PK, Bernheim SM, Grady JN, et al. The performance of US hospitals as reflected in risk-standardized 30-day mortality and readmission rates for Medicare beneficiaries with pneumonia. J Hosp Med. 2010;5(6):E12–18. doi: 10.1002/jhm.822. [DOI] [PubMed] [Google Scholar]
- 2.Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3(5):459–467. doi: 10.1161/CIRCOUTCOMES.110.957613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3(1):97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services. Medicare Hospital Quality Chartbook 2011: Performance Report on Readmission Measures for Acute Myocardial Infarction, Heart Failure, and Pneumonia. Washington, DC: Centers for Medicare & Medicaid Services; 2011. [Google Scholar]
- 5.Ashton CM, Wray NP. A conceptual framework for the study of early readmission as an indicator of quality of care. Soc Sci Med. 1996;43(11):1533–1541. doi: 10.1016/s0277-9536(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 6.Ashton CM, Kuykendall DH, Johnson ML, Wray NP, Wu L. The association between the quality of inpatient care and early readmission. Ann Intern Med. 1995;122(6):415–421. doi: 10.7326/0003-4819-122-6-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ashton CM, Del Junco DJ, Souchek J, Wray NP, Mansyur CL. The association between the quality of inpatient care and early readmission: a meta-analysis of the evidence. Med Care. 1997;35(10):1044–1059. doi: 10.1097/00005650-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Medicare Payment Advisory Commission. Promoting Greater Efficiency in Medicare, Report to the Congress. Washington, DC: MedPAC; 2007. [Accessed September 12, 2012]. http://www.medpac.gov/documents/Jun07_EntireReport.pdf. [Google Scholar]
- 9.Centers for Medicare & Medicaid Services Hospital Pay-for-Performance Workgroup. US Department of Health and Human Services Medicare Hospital Value-Based Purchasing Plan Development, Issues Paper, 1st Public Listening Session. Washington, DC: Centers for Medicare & Medicaid Services; 2007. [Accessed September 12, 2012]. http://www.cms.hhs.gov/AcuteInpatientPPS/Downloads/Hospital_VBP_Plan_Issues_Paper.pdf. [Google Scholar]
- 10.National Quality Forum. National Voluntary Consensus Standards for Hospital Care 2007: Performance Measures - A Consensus Report. Washington, DC: National Quality Forum; Aug, 2008. [Google Scholar]
- 11.Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1(1):29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4(2):243–252. doi: 10.1161/CIRCOUTCOMES.110.957498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindenauer PK, Normand SL, Drye EE, et al. Development, validation, and results of a measure of 30-day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6(3):142–150. doi: 10.1002/jhm.890. [DOI] [PubMed] [Google Scholar]
- 14.Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306(16):1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 15.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality. Statistical Brief #66. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; Jan, 2009. [Accessed November 28, 2012]. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb66.jsp. [Google Scholar]
- 17.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 18.McBean AM. Improving Medicare’s data on race and ethnicity. Medicare Brief. 2006;(15):1–7. [PubMed] [Google Scholar]
- 19.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34(4):216–221. [Google Scholar]
- 20.O’Connor CM, Miller AB, Blair JE, et al. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159(5):841–849. e841. doi: 10.1016/j.ahj.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Jasti H, Mortensen EM, Obrosky DS, Kapoor WN, Fine MJ. Causes and risk factors for rehospitalization of patients hospitalized with community-acquired pneumonia. Clin Infect Dis. 2008;46(4):550–556. doi: 10.1086/526526. [DOI] [PubMed] [Google Scholar]
- 22.Dunlay SM, Weston SA, Killian JM, Bell MR, Jaffe AS, Roger VL. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11–18. doi: 10.7326/0003-4819-157-1-201207030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman MM. Older adults’ symptoms and their duration before hospitalization for heart failure. Heart Lung. 1997;26(3):169–176. doi: 10.1016/s0147-9563(97)90053-4. [DOI] [PubMed] [Google Scholar]
- 24.Poch DS, Ost DE. What are the important risk factors for healthcare-associated pneumonia? Semin Respir Crit Care Med. 2009;30(1):26–35. doi: 10.1055/s-0028-1119806. [DOI] [PubMed] [Google Scholar]
- 25.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 26.Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in disability after hospitalization or restricted activity in older persons. JAMA. 2010;304(17):1919–1928. doi: 10.1001/jama.2010.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292(17):2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 28.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA. 1999;281(21):2013–2019. doi: 10.1001/jama.281.21.2013. [DOI] [PubMed] [Google Scholar]
- 30.Yoder JC, Staisiunas PG, Meltzer DO, Knutson KL, Arora VM. Noise and sleep among adult medical inpatients: far from a quiet night. Arch Intern Med. 2012;172(1):68–70. doi: 10.1001/archinternmed.2011.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiely DK, Bergmann MA, Murphy KM, Jones RN, Orav EJ, Marcantonio ER. Delirium among newly admitted postacute facility patients: prevalence, symptoms, and severity. J Gerontol A Biol Sci Med Sci. 2003;58(5):M441–445. doi: 10.1093/gerona/58.5.m441. [DOI] [PubMed] [Google Scholar]
- 32.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart. 2006;92(4):481–486. doi: 10.1136/hrt.2004.058073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 35.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 36.Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health Aff (Millwood) 2009;28(1):179–189. doi: 10.1377/hlthaff.28.1.179. [DOI] [PubMed] [Google Scholar]
- 37.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 38.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the Telemedical Interventional Monitoring in Heart Failure Study. Circulation. 2011;123(17):1873–1880. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 41.Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Card Fail. 2006;12(3):211–219. doi: 10.1016/j.cardfail.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008;62(5):585–595. doi: 10.1111/j.1365-2648.2008.04631.x. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 44.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 45.Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994;120(12):999–1006. doi: 10.7326/0003-4819-120-12-199406150-00005. [DOI] [PubMed] [Google Scholar]
- 46.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 47.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Skull SA, Andrews RM, Byrnes GB, et al. ICD-10 codes are a valid tool for identification of pneumonia in hospitalized patients aged > or = 65 years. Epidemiol Infect. 2008;136(2):232–240. doi: 10.1017/S0950268807008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvillo-King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2012 Oct 6; doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.