Highlights
-
•
Dual-process models of psychopathy postulate two underlying dimensions.
-
•
Fearless Dominance was associated with altered feedback processing.
-
•
Feedback-related negativity amplitudes were reduced.
-
•
Self-Centered Impulsivity was unrelated to feedback processing.
-
•
These findings provide further evidence for dual-process models of psychopathy.
Keywords: Psychopathy, Fearless Dominance, Dual-process models, Feedback processing, Event-related potentials, ERPs, sLORETA, FRN, ACC, RCZa
Abstract
Dual-process models of psychopathy postulate two etiologically relevant processes. Their involvement in feedback processing and its neural correlates has not been investigated so far. Multi-channel EEG was collected while healthy female volunteers performed a time-estimation task and received negative or positive feedback in form of signs or emotional faces. The affective-interpersonal factor Fearless Dominance, but not Self-Centered Impulsivity, was associated with reduced feedback-related negativity (FRN) amplitudes. This neural dissociation extends previous findings on the impact of psychopathy on feedback processing and further highlights the importance of distinguishing psychopathic traits and extending previous (neuroscientific) models of psychopathy.
1. Introduction
Psychopathy is a construct characterized by a number of deficits in adaptation and affective processing – lack of empathy, fearlessness, deficits in aversive and passive avoidance learning, and antisocial behavior among others (Cleckley, 1941; Hare, 2003; Hare & Neumann, 2008). Although primarily studied in offenders, there is a growing number of investigations in the general population, as psychopathy is not restricted to incarcerated offenders (Hall & Benning, 2006) but rather considered as a construct with a dimensional latent structure and not representing a qualitatively discrete group (Edens, Marcus, Lilienfeld, & Poythress, 2006; Marcus, John, & Edens, 2004). Moreover, this also indicates more than one causal factor in the etiology of psychopathy.
1.1. Dual-process models of psychopathy
Dual-process models (e.g., Fowles & Dindo, 2009; Patrick & Bernat, 2009) relate two potential etiological dimensions to the higher order factors of frequently applied psychometric instruments in the assessment of psychopathy in offenders, e.g. the PCL-R (Psychopathic Checklist-Revised; Hare, 2003) or in the general population, e.g. the PPI-R (Psychopathy Personality Inventory-Revised; Alpers & Eisenbarth, 2008; Lilienfeld & Andrews, 1996). The first model dimension (“Trait Fearlessness” in the model of Patrick & Bernat, 2009) focuses on emotional-interpersonal aspects and is related to an arrogant interpersonal style, lack of empathy and reduced fear reactivity. The second model dimension (“Externalizing Vulnerability”, Patrick & Bernat, 2009) is associated with an impulsive, socially deviant lifestyle. In the PPI-R, they are psychometrically operationalized in form of the higher-order factors Fearless Dominance and Self-Centered Impulsivity, respectively. Both dimensions of psychopathic personality are thought to reflect etiologic pathways that can be already found in childhood psychopathology (Fowles & Dindo, 2009). The label “Externalizing Vulnerability” emphasizes the link to externalizing psychopathology (Patrick, Hicks, Krueger, & Lang, 2005) – one of two broad factors underlying the most common mental disorders, in particular the one associated with conduct disorder, antisocial behavior, alcohol and drug abuse among others (Krueger, 1999). However, psychopathy cannot be sufficiently described by externalizing psychopathology because the latter was unrelated to the unique variance of the emotional-interpersonal dimension of psychopathy (Patrick et al., 2005).
A dual-process perspective might allow new insights in the (neurocognitive) mechanisms underlying these pathways to psychopathic personality and the core deficits of psychopathy such as deficits in behavioral adaptation or passive avoidance learning (Newman & Kosson, 1986; Newman, Patterson, Howland, & Nichols, 1990). Dinn and Harris (2000) suggested that behavioral adaptation deficits found in ASPD (antisocial personality disorder) individuals with psychopathic traits might be related to inadequate processing of feedback information. Previous studies already reported neurocognitive dissociations between the two dimensions of psychopathy, for instance in affect recognition (Gordon, Baird, & End, 2004) or executive functions such as attention and inhibition (Carlson & Thái, 2010; Carlson, Thái, & McLarnon, 2009). The aim of our study was to investigate now feedback processing – another potentially relevant neurocognitive mechanism – from a dual-process perspective of psychopathy.
1.2. Feedback processing and psychopathy
A brain structure that has been associated with feedback processing is the dorsal anterior cingulate cortex (dACC; Holroyd & Coles, 2002; Holroyd, Pakzad-Vaezi, & Krigolson, 2008; Miltner, Braun, & Coles, 1997; Ullsperger & Von Cramon, 2003). It is an area supposed to be fundamental to response-reinforcement associations (Rushworth, Behrens, Rudebeck, & Walton, 2007), behavioral monitoring and adaptation (e.g. Holroyd & Coles, 2002), and therefore a plausible candidate for explaining behavioral adaptation deficits in psychopathy.
Electrophysiologically, external feedback after the occurrence of an error elicits a negative event-related potential (ERP) called feedback-related negativity (FRN) with a typical peak amplitude within 200–300 ms. Behaviorally, the FRN was shown to be associated with the degree of learning from negative feedback in an emotion recognition task and a probabilistic learning task (Frank, D’Lauro, & Curran, 2007; Frank, Woroch, & Curran, 2005).
Reinforcement Learning Theory (RLT; Baker & Holroyd, 2009, 2011; Holroyd & Coles, 2002) suggests that reward-prediction error signals are transmitted via the mesencephalic dopamine system to the dACC eliciting the FRN. As the FRN is sensitive to the unpredictability of the outcome, its amplitude becomes smaller in the course of learning the specific action-outcome association, enabling a switch from external (i.e. via external feedback information) to internal error monitoring (i.e. comparing actual and intended behavior) indexed by a functional related component called error-related negativity (ERN), peaking earlier than the FRN, about 100 ms after erroneous response (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Gross, Coles, Meyer, & Donchin, 1993; Holroyd & Coles, 2002). This is called backward propagation after learning (Holroyd & Coles, 2002). In particular, the rostral cingulate zone anterior (RCZa), which is part of the dACC, is sensitive to both forms of error monitoring and also reflects these learning-dependent dynamics (Mars et al., 2005). However, van der Veen, Röde, Mies, van der Lugt, & Smits, (2011) proposed rather an involvement of the RCZ in remedial action than a signaling function as stated in the RLT.
Another ERP repeatedly investigated during feedback processing is the P3(b) component, peaking between 200 and 600 ms at posterior electrode sites (Yeung & Sanfey, 2004). This classical P3 component seems to index the task relevance of a stimulus (Coles, Smid, Scheffers, & Otten, 1995) and resource allocation (Israel, Chesney, Wickens, & Donchin, 1980; Kahneman, 1973). One influential theory links the classical P3 with context-updating of working memory, i.e. revisions of mental representations by stimuli classified as new after comparison with previous stimuli (Donchin & Coles, 1988, 1998; Polich, 2007).
These ERPs in error monitoring have been associated with several personality traits in previous studies, for instance with trait anxiety or anxiety disorders (Gu, Ge, Jiang, & Luo, 2010; Hajcak, McDonald, & Simons, 2003; Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006) and the Behavioral Inhibition System (BIS; Boksem, Tops, Wester, Meijman, & Lorist, 2006; De Pascalis, Varriale, & D’Antuono, 2010). The question arises if these electrophysiological components are also linked to psychopathy, in particular the FRN, consistent with the suggestion of Dinn and Harris (2000) of impaired feedback processing underlying the behavioral adaptation deficits found.
The majority of studies related to psychopathy investigated internal error monitoring (i.e. ERN; Brazil et al., 2009, 2011; Munro et al., 2007; von Borries et al., 2010) with inconsistent results. As far as feedback processing is concerned, two studies reported no FRN amplitude modulation related to psychopathy in a probabilistic gambling task (von Borries et al., 2010) and in a visual Go/No Go task (Varlamov, Khalifa, Liddle, Duggan, & Howard, 2010). With regard to the P3 component, but unrelated to feedback processing, PPI-R Self-Centered Impulsivity was associated with reduced frontal P3 amplitudes in an oddball task (Carlson et al., 2009), whereas PPI-R Fearless Dominance was associated with increased P3 amplitudes in a continuous performance task (Carlson & Thái, 2010). A meta-analysis of Gao and Raine (2009) showed inconsistent, task-dependent effects on the P3 for psychopathy.
1.3. The present study
Importantly, none of the studies investigating error monitoring focused on specific psychopathic traits in a multi-dimensional fashion, as also discussed in Pfabigan, Alexopoulus, Bauer, Lamm, and Sailer (2011). This creates two potential problems for investigating associations between psychopathy and feedback processing. First, psychopathic traits might be differentially related to error monitoring. Working with a unitary construct (i.e. total scores instead of specific psychopathic traits/higher-order factors) could obscure potential associations with both, the FRN and ERN. Second, categorical grouping of dimensional data (i.e. splitting subjects into low- and high-scoring groups) leads to a loss of information about individual differences (MacCallum et al., 2002). From a dual-process perspective, each individual is located on two functionally interrelated dimensions rather than belonging to qualitatively discrete groups of psychopaths and non-psychopaths. To overcome these shortcomings we investigated the potentially differential associations between dimensional psychopathic traits and feedback processing.
Therefore, we used the PPI-R (Alpers & Eisenbarth, 2008), which is applicable also in the low and moderate range of psychopathy, enabling us to investigate potential etiological processes across a broader dimensional range in an undergraduate/graduate sample at the University of Vienna. Moreover, we investigated a female only sample to control for any gender differences that might occur and to enhance our knowledge about this less-studied population. Participants performed a modified time-estimation task (Miltner et al., 1997) and received negative and positive feedback in form of signs and emotional faces, resulting in four (2 × 2) experimental conditions.
According to the low-fear hypothesis of psychopathy (Lykken, 1957, 1995) behavioral adaptation deficits after punishment in psychopaths are due to a fundamental fear deficit. This suggests a link to PPI-R Fearless Dominance – a dimension reflecting among others low fear in terms of psychometric (Benning, Patrick, Bloningen, Hicks, & Iacono, 2005; Benning et al., 2003) and psychophysiological data such as inhibition of the fear-potentiated startle response (e.g. Anderson, Stanford, Wan, & Young, 2011; Benning, Patrick, & Iacono, 2005; Dindo & Fowles, 2011), reduced skin conductance response to aversive pictures (Benning, Patrick, & Iacono, 2005), and deficient fear-conditioning (López, Poy, Patrick, & Moltó, 2012). Since our behavior is crucially guided by feedback processes the question arises whether psychopathic traits affect this capacity and we hypothesize that in particular Fearless Dominance would be associated with impaired feedback processing indicated by reduced FRN amplitudes and reduced behavioral adaptation after negative feedback. Moreover, we expected decreased neuronal source activity in the rostral cingulate zone anterior (RCZa) for high Fearless Dominance due to the demonstrated link to feedback processing (Mars et al., 2005; Ullsperger & Von Cramon, 2003; van der Veen et al., 2011). Furthermore, feedback processing might be impaired to a greater degree when socio-emotional stimuli like faces are presented as compared to signs due to the social and affective deficits seen in psychopathy. Possible personality effects on P3 were explored without specific hypotheses.
2. Methods
2.1. Participants and measures
We investigated an undergraduate/graduate sample of 24 women. Two of them had to be excluded from further analysis due to movement and blink artifacts. One subject had to be excluded due to a history of social anxiety, depression and psychiatric treatment. The remaining 21 women were aged between 21 and 29 years (mean age = 24.29 years, SD = 1.95) and were enrolled as students or graduates of the University of Vienna. All participants were right-handed (Edinburgh Handedness Inventory; Oldfield, 1971) and had normal or corrected-to-normal vision. All participants gave written informed consent prior to the experiment. The study was conducted in accordance with the Declaration of Helsinki (World Medical Association, revised 2000) and local guidelines of the University of Vienna.
After EEG data collection, all subjects completed the German version of the Psychopathic Personality Inventory-Revised (PPI-R; Alpers & Eisenbarth, 2008).2 Furthermore, a shortened version of the SCID (Structural Clinical Interview for DSM-IV; Wittchen, Wunderlich, Gruschwitz, & Zaudig, 1996) was administered to screen for mental disorders. Participants did not receive any financial remuneration and participated voluntarily.
The PPI-R is a self-report questionnaire for measuring psychopathy. Internal consistency is satisfying with a reported Cronbach alpha of .85. The PPI-R consists of eight subscales, which form two higher-order factors (Benning, Patrick, Bloningen, et al., 2005). Scores for the higher-order factors were calculated as in Benning et al. (2003), Carlson and Thái (2010) and Carlson et al. (2009). The mean of the z-transformed scales Fearlessness, Social Influence, and Stress Immunity scores added up to the higher-order factor Fearless Dominance score, whereas the mean of the z-transformed scales Blame Externalization, Rebellious Nonconformitiy, Machiavellian Egocentricity, and Carefree Nonplanfulness scores added up to the higher-order factor Self-Centered Impulsivity score. The subscale Coldheartedness did not fit into this two-factor solution and was therefore not included.
In contrast to the interrelated PCL-R factors, the orthogonal nature of the PPI-R higher-order factors Fearless Dominance and Self-Centered Impulsivity (Benning et al., 2003) renders it an even more promising instrument to disentangle the different mechanisms potentially relevant in those etiological pathways (Patrick & Bernat, 2009) and core impairments typically seen in psychopathy.
Our sample showed considerable variability in PPI-R total score, in all subscales and in the higher-order factors Fearless Dominance and Self-Centered Impulsivity. The comparison of our sample with the norm sample of female students (n = 204) provided in the manual showed that PPI-R total scores ranged from percentile ranks 3.40% (score: 229) to 96.60% (score: 316) in our sample. Furthermore, the mean score of 273.90, which corresponds to a percentile rank of 48.50% indicating that our sample represents well the norm sample. However, in addition to students, the German version of the PPI-R has also been applied in a forensic sample, where higher scores were reported, as one would expect from a valid measure (Alpers & Eisenbarth, 2008). Fearless-Dominance scores ranged from −1.43 and 1.13 and Self-Centered Impulsivity scores from −1.56 and 1.52. These scores represent average z-scores of the respective subscales and thus indicate ranges from below to above one standard deviation of the raw scores and thereby sufficient variability.
2.2. Stimuli and paradigm
Participants sat comfortably about 70 cm in front of a 19-in. cathode ray tube monitor (Sony GDM-F520; 75 Hz refresh rate) in a sound-attenuated room. Stimulus presentation and EEG data collection (Pentium IV, 3.00 GHz) were synchronized by E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA).
A modified version of a time estimation task (Miltner et al., 1997) was used as experimental paradigm (Fig. 1). Each trial started with the presentation of a black fixation dot on a gray screen for 1000 ms. Subsequently, the dot was replaced by a black star which remained on the screen for 250 ms. The star indicated the starting point of the time estimation. Following the star, a blank gray screen was presented for max. 2000 ms. During this period, participants were asked to indicate the elapse of one second by pressing a keyboard button. Following the button press, the blank gray screen lasted another 600 ms, after which subjects were provided with feedback for 1000 ms indicating whether the estimation had been correct or incorrect. The inter-trial-interval varied between 400 and 600 ms. Feedback was provided performance-based, but task difficulty was adjusted to the individual performance level. Each participant started with the same criteria: positive feedback was provided if the button press fell within the time window of 900–1100 ms after star onset. The width of this time window was adjusted automatically based on the individual performance on the preceding trial (Johnson & Donchin, 1978; Miltner et al., 1997). Following a trial with positive feedback, the time window became narrower as 10 ms were subtracted; following a trial with negative feedback, the time window became wider as 10 ms were added. Thus, global probability of positive and negative feedback stimuli was approximately 50% during the whole experiment. Feedback stimuli were equiluminescent, comparable in size, and either emotional faces or signs (all 4 cm × 5 cm in size) were used. Facial feedback stimuli were photographs of a female poser of the Pictures of Facial Affect (Ekman & Friesen, 1976). The happy facial expression indicated positive feedback; the angry facial expression indicated negative feedback. Sign feedback stimuli were an X and an O. The assignment of X and O to positive and negative feedback was counterbalanced across participants. Feedback valence was explained in the instruction. Thus, there were four feedback conditions – negative face feedback, negative sign feedback, positive face feedback and positive sign feedback. The whole experiment consisted of 20 training trials and 400 experimental trials. The experimental trials were divided into four blocks: two blocks with facial feedback stimuli, and two with sign feedback stimuli. Blocks with facial and sign feedback stimuli were presented alternately. To recall the assignment of positive and negative feedback stimuli, detailed instruction was given prior to each block. Half the participants started with a facial feedback block, the other half with a sign feedback block. Data collection was paused every 50 trials to offer subjects a short rest. The whole EEG data collection lasted about 45 min.
Fig. 1.
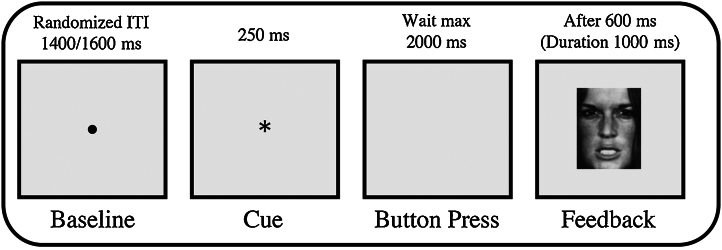
Trial time sequence.
2.3. Electrophysiological recording and preprocessing
Multi-channel EEG was recorded from 61 Ag/AgCl ring electrodes which were embedded equidistantly in an elastic cap (EASYCAP GmbH, Herrsching, Germany; model M10) with a sterno-clavicular reference (Stephenson & Gibbs, 1951). Vertical and horizontal electrooculogram (EOG) was recorded with a bipolar setting from electrodes placed on the outer canthi, 1 cm above and below the left eye for off-line eye-movement correction. Subject- and channel-specific parameters for eye-movement correction were obtained in two pre-experimental calibration trials (Bauer & Lauber, 1979). Furthermore, a template matching procedure was applied to minimize blink artifacts (cf. Lamm, Fischmeister, & Bauer, 2005). A skin scratching procedure (Picton & Hillyard, 1972) kept the electrode impedances below 2 kΩ, as measured with a manual impedance meter. Signals were amplified using an AC amplifier set-up with a time constant of 10 s (Ing. Kurt Zickler GmbH, Pfaffstätten, Austria). All signals were recorded within a frequency range of .016–125 Hz and sampled at 250 Hz for digital storage. In addition, individual three-dimensional electrode coordinates of 17 pre-defined electrode positions (referenced to nasion, inion, and the two preauricular electrodes) were measured for all participants with a photogrammetric scanner (3D-PHD; Bauer et al., 2000). Off-line, a standard head model was fit into these predefined locations, whereupon the remaining electrodes were interpolated using a radial basis function, based on the equidistant montage of the electrode cap.
EEGLAB 6.03b (Delorme & Makeig, 2004) was used for off-line data analysis. A low-pass filter with a cut-off frequency of 30 Hz (roll-off 6 dB/octave) was applied to the EEG data. Data were segmented into individual trials, starting 200 ms before feedback onset and lasting for 1100 ms. The 200 ms prior to feedback onset served as baseline interval. Artifact-afflicted trials that depicted voltage values exceeding ±75 μV or voltage drifts of more than 50 μV were discarded from further analysis. Extended infomax independent component analysis (ICA; Bell & Sejnowski, 1995; Lee, Girolami, & Sejnowski, 1999) was applied to single-subject data of two participants to detect and correct for residual eye movement-related activity (Delorme, Sejnowski, & Makeig, 2007).
2.4. Statistical analysis
Participants received negative and positive feedback in form of signs and emotional faces, resulting in the within-subject factors valence (negative vs. positive feedback) and form (face vs. sign feedback). For FRN analyses an additional within-subject factor electrode site was included (FCz vs. Cz). Fearless Dominance and Self-Centered Impulsivity served as between-subject factors. As dependent variables, behavioral data and brain electric activity by means of ERPs and source localization (sLORETA; Pascual-Marqui, 2002) were analyzed. The level of significance was set at p < .05 for all tests. Correlation coefficients (r) or partial eta-squared () are reported indicating the effect sizes (r < .10 and representing small effects, r < .30 and around .10 representing medium effects, and r > .50 and η2 > .20 representing large effects; Cohen, 1973, 1988). All statistical tests are two-tailed. Statistical analyses were performed using SPSS (version 19; SPSS, Inc., IBM Corporation, NY).
2.4.1. Questionnaire and behavioral data
Pearson intercorrelations between scores of the PPI-R subfactors, Fearless Dominance and Self-Centered Impulsivity were calculated. For the time estimation task, the overall number of correct responses was calculated. Then average time estimation was calculated per subject and condition as the mean interval between cue onset and button press. Subsequently, for each subject and separately for all four conditions (negative faces, negative signs, positive faces, and positive signs), the absolute trial-by-trial adjustment of time estimation was calculated (Miltner et al., 1997). Higher values indicate overall larger behavioral adaptation after feedback. For the behavioral data, the first trial of each block and after each rest between blocks was discarded from this analysis because of feedback change and rest.
The association between personality and time estimation was tested using a general linear model with the within-subject factors valence (positive vs. negative feedback) and form (face vs. sign feedback) as well as the between-subject factors Fearless Dominance and Self-Centered Impulsivity as continuous variables. Such a model provides within- and between-subject main effects and interactions as well as parameter estimates for the regression of the between-subject psychopathic traits on time estimation in all conditions. Regression parameter estimates should provide valid information about the unique contributions of Fearless Dominance and Self-Centered Impulsivity as no substantial collinearity is expected for these two PPI-R derived measures (and indeed was not found, see results). The same model was applied for the absolute trial-by-trial adjustment of time estimation as dependent variable.
2.4.2. ERP data
Artifact-free trials were averaged per subject and per feedback condition (negative faces, negative signs, positive faces, and positive signs). Subsequently, FRN amplitudes were scored at electrode sites FCz and Cz in all conditions as the peak-to-peak voltage difference between the most negative local peak and the voltage of the immediately preceding positive peak 140–350 ms after feedback onset, in line with previous peak detection methodology (e.g. with detection intervals beginning 150 or 160 ms post-stimulus; Hajcak, Moser, Holroyd, & Simons, 2006; Holroyd, Nieuwenhuis, Yeung, & Cohen, 2003). If no FRN peak was apparent, the difference score was set to zero. Electrode sites FCz and Cz were repeatedly used in previous literature (e.g. Holroyd & Coles, 2002; Holroyd et al., 2003; Yeung & Sanfey, 2004) and visual inspection showed pronounced ERP deflections at these electrode sites in the time range mentioned above. A difference wave approach (e.g. Miltner et al., 1997) was not undertaken as the FRN seems to be an integrative result of different processes in both the negative and positive feedback condition as described in the introduction (Baker & Holroyd, 2011; Holroyd et al., 2008). P3 amplitudes were scored at electrode site Pz as the most positive peak within 200–600 ms after feedback onset (Yeung & Sanfey, 2004).
FRN peak measures were subjected to a general linear model with the within subject factors electrode site (FCz vs. Cz), valence (negative vs. positive) and form (face vs. signs) and the between-subject factors Fearless Dominance and Self-Centered Impulsivity as regressors. In addition, we also analyzed FRN peak latencies with the same approach as well as FRN peak amplitudes with the total score of the PPI-R to compare the effects for the psychopathic traits (dual-process perspective) with those of the overall score (unitary-construct perspective). P3 peak measures were subjected to the same general linear model except for the within-subject factor electrode site (as there is only one, Pz). Although collinearity, i.e. highly correlated predictors, was not found for the PPI-R derived measures, zero-order correlations between Fearless Dominance as well as Self-Centered Impulsivity and FRN amplitudes were calculated in a second analysis. Moreover, in order to discuss potential differential effects of the psychopathic traits Fearless Dominance and Self-Centered Impulsivity, it is not sufficient to find a significant effect for one factor and a not significant results for the other (Gelman & Stern, 2006; Niewenhuis, Forstmann, & Wagenmakers, 2011). Consequently, we tested for differences between correlations for Fearless Dominance and Self-Centered Impulsivity in each feedback condition with four specific tests for dependent correlations per electrode site (T2 statistic; Steiger, 1980). The specificity of the FRN-related effects was also tested by applying the same general linear model to the ERP amplitudes to the positivity prior to the FRN and the P3 after the FRN at electrode sites FCz and Cz. In addition, the association between time estimation or behavioral adaptation and FRN amplitude in the respective conditions was tested with zero-order correlations.
2.4.3. Source activity
In order to corroborate our ERP findings and address our hypothesis on rostral cingulate zone anterior (RCZa) activity we applied source localization as an additional method to explore brain activity. Source localization was conducted by means of standardized low resolution brain electromagnetic tomography software (sLORETA; Pascual-Marqui, 2002). sLORETA provides a linear, minimum norm inverse solution that estimates the distribution of the electrical neuronal activity in three-dimensional space by assuming that neighboring neurons are simultaneously and synchronously activated, and produces images of electric neuronal activity without localization bias (Greenblatt, Ossadtchi, & Pflieger, 2005; Pascual-Marqui, 2002). sLORETA computes the electric activity at each voxel as the squared standardized magnitude of the estimated current density. The sLORETA solution space is restricted to cortical gray matter and hippocampus, defined via the MNI (Montreal Neurological Institute) reference brain and subdivided into 6239 voxels, with a spatial resolution of 5 mm × 5 mm × 5 mm. The sLORETA method has been validated in several simultaneous EEG/fMRI studies (e.g. Mobascher et al., 2009; Olbrich et al., 2009) and has also been applied to feedback processing (Santesso et al., 2011). The individual electrode positions which had been acquired with the photogrammetric scanner (Bauer et al., 2000) were cross-registered to the standard Talairach space (Talairach & Tournoux, 1988) and reconciled with the estimated cortical activation patterns. A regularization parameter of zero was used for transformation of electrode mean amplitudes into a three-dimensional distribution of cortical activation, thus achieving the smoothest of all possible solutions. Overall signal-to-noise-ratio was set to 100 during the transformation process.
We decided to use a region of interest (ROI) approach to address our specific hypothesis. In contrast to ROIs based on rather broad Brodmann areas as in Santesso et al. (2011), we created spherical regions of interest (ROIs) covering the anterior rostral cingulate zone for each hemisphere. The RCZa was repeatedly shown to be associated with error monitoring and feedback processing (Mars et al., 2005; Ullsperger & Von Cramon, 2003, 2004; van der Veen et al., 2011), Our ROIs were centered at ±8, 30, 32 mm, according to the stereotactic coordinates of Talairach and Tournoux (1988) as in Picard and Strick (1996) and in Mars et al. (2005), who found this area involved in error processing. In contrast to Mars et al. (2005), we used the nonlinear Yale MNI to Talairach converter algorithm (described in Lacadie, Fulbright, Rajeevan, Constable, & Papademetris, 2008; applet: http://www.bioimagesuite.org/Mni2Tal/index.html) to search for corresponding MNI coordinates, which are −8, 30, 35 and 9, 30, 35, respectively. As in Mars et al. (2005) the spheres had a radius of 8 mm. The location of the ROIs is illustrated in Fig. 2.
Fig. 2.
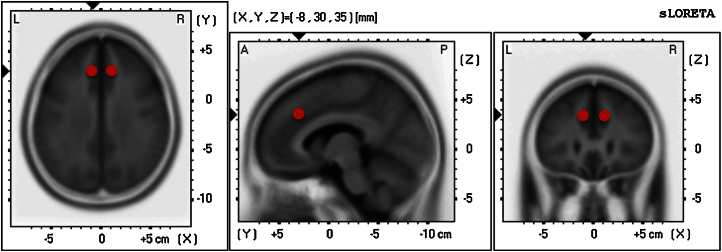
The red spots illustrate the two bilateral spherical seeds (8 mm) in the RCZa used for sLORETA ROI regression analysis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Zero-order correlations between the average electric activity in these ROIs between 140 and 300 ms post-stimulus and Fearless Dominance and Self-Centered Impulsivity were calculated for all feedback conditions. Differences between correlations for Fearless Dominance and Self-Centered Impulsivity in the four feedback conditions were tested with four specific tests for dependent correlations (T2 statistic; Steiger, 1980). This specific timeframe was chosen because of the early negativity onset visible in some individual ERP averages. When we tested for potential outliers by calculating Z-scores of the RCZa mean activity, one additional subject turned out as a biasing outlier (e.g. Z = 4.01 for negative faces) and had to be excluded from analysis. This was probably due to technical problems in peripheral electrodes visible in the scalp topography, which is not crucial for FRN and P3 analysis, but presumably for source analysis. Therefore source analysis was carried out on the 20 remaining subjects.
3. Results
3.1. Questionnaire and behavioral data
Pearson intercorrelations of all PPI-R scales and higher-order factors are provided in Table 1. The rather orthogonal nature of the PPI-R higher-order factors in contrast to PCL-R factors (Benning et al., 2003; Hare, 2003) was also evident in our sample, as there was no significant correlation between Fearless Dominance and Self-Centered Impulsivity (r = −.05, p = .83).
Table 1.
Means [and standard deviations] on the diagonal and bivariate zero-order correlations for PPI-R scales.
| PPI-R | FD | F | SI | StI | SCI | BE | CNP | RNC | ME | CH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPI-R Total Score | 273.90 [21.48] | .55* | .56* | .55* | .01 | .78* | .08 | .56* | .79* | .77* | .15 |
| PPI-R Fearless Dominance | .00 [.68] | .76* | .68* | .59* | −.05 | −.40 | −.25 | .28 | .23 | .06 | |
| PPI-R Fearlessness | 14.71 [4.79] | .38 | .18 | .17 | −.34 | .06 | .59* | .17 | −.23 | ||
| PPI-R Social Influence | 46.52 [5.38] | .01 | .20 | −.14 | −.05 | .38 | .36 | .04 | |||
| PPI-R Stress Immunity | 39.24 [7.02] | −.47* | −.34 | −.53* | −.39 | −.06 | .30 | ||||
| PPI-R Self-Centered Impulsivity | .00 [.70] | .49* | .86* | .76* | .71* | −.05 | |||||
| PPI-R Blame Externalization | 24.71 [6.40] | .40 | .00 | −.01 | −.38 | ||||||
| PPI-R Carefree Nonplanfullness | 29.19 [5.03] | .57* | .44* | .03 | |||||||
| PPI-R Rebellious Nonconformity | 54.57 [10.96] | .56* | −.22 | ||||||||
| PPI-R Machiavellian Egocentricity | 37.14 [3.86] | .43 | |||||||||
| PPI-R Coldheartedness | 27.81 [5.09] |
Note: Fearless Dominance and Self-Centered Impulsivity were the mean of the z-scores of the respective subscales. FD scales were Fearlessness, Social Influence, and Stress Immunity. SCI scales were Blame Externalization, Carefree Nonplanfulness, Rebellious Nonconformity, and Machiavellian Egocentricity.
Significantly different from 0 (p ≤ .05)
In the time estimation task, participants were correct on about half of the trials for face feedback (48.50%) and sign feedback (47.43%) as would be expected by applying the adaptive criterion for correctness of performance (see Section 2). Time estimation values were higher before negative than positive feedback, F (1,18) = 37.16, p < .01, , indicating that on average participants responded after too long time intervals in error trials. There was no significant difference between faces and signs, F (1,18) = .10, p = .76. Unexpectedly, there was a significant between-subjects effect for Self-Centered Impulsivity, F (1,18) = 14.46, p < .01, . Respective parameter estimates were significant for all conditions (all p < .05), indicating longer estimated time intervals for subjects with higher Self-Centered Impulsivity scores. In addition, there was a significant interaction effect between valence and Self-Centered Impulsivity, F (1,18) = 7.70, p = .01, , which was, however, qualified by substantially overlapping confidence intervals of the parameter estimates in the separate conditions across valence. Fearless Dominance was unrelated to time estimation, F (1,18) = .04, p = .84. No other effects were significant (all p > .08).
The absolute trial-by-trial adjustment in time estimation was larger after negative than after positive feedback, F (1,18) = 118.35, p < .001, , but there was no significant difference between faces and signs, F (1,18) = 2.28, p = .15. Although there was a significant interaction between valence and Self-Centered Impulsivity, F (1,19) = 5.73, p = .03, , this result was qualified by substantially overlapping confidence intervals of the parameter estimates in the separate conditions across valence. No other effects were significant (all p > .15). Means and standard deviations for time estimation and absolute trial-by-trial adjustment in time estimation data are presented in Table 2 for all conditions.
Table 2.
Means [and standard deviations] for time estimation and absolute trial-by-trial adjustment in time estimation (in ms).
| Measure | Condition | Mean [SD] |
|---|---|---|
| Time estimation | Negative faces | 1128.16 [196.94] |
| Negative signs | 1127.65 [184.00] | |
| Positive faces | 1039.32 [150.59] | |
| Positive signs | 1028.45 [111.66] | |
| Absolute trial-by-trial adjustment in time estimation | Negative faces | 169.24 [49.13] |
| Negative signs | 175.22 [50.97] | |
| Positive faces | 111.11 [29.56] | |
| Positive signs | 123.76 [39.81] | |
3.2. ERP data
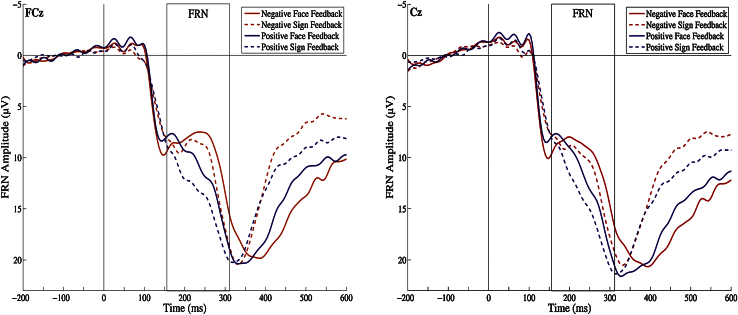
Regarding FRN amplitudes, the general linear model revealed significant effects for valence, F (1,18) = 18.97, p < .01, , indicating that the FRN amplitude was larger for negative than for positive feedback, and for form, F (1,18) = 66.89, p < .01, , indicating larger amplitudes for faces than for signs. There was no effect for electrode site, F (1,18) = .39, p = .54. Moreover, no interaction reached significance (all p > .06). Mean peak amplitudes, latencies, and standard deviations are displayed in Table 3. Grand averages for the four conditions are displayed in Fig. 3.
Table 3.
Means [and standard deviations] for FRN amplitudes.
| Measure | Condition | FCz | Cz |
|---|---|---|---|
| Mean [SD] | Mean [SD] | ||
| FRN amplitude | Negative faces | −6.44 [3.23] | −6.50 [2.82] |
| Negative signs | −4.51 [2.88] | −3.89 [2.93] | |
| Positive faces | −4.66 [2.04] | −4.76 [2.60] | |
| Positive signs | −2.06 [2.14] | −1.90 [2.04] | |
| FRN latency | Negative faces | 236.57 [37.78] | 211.52 [32.80] |
| Negative signs | 227.05 [36.00] | 212.76 [32.54] | |
| Positive faces | 231.62 [43.91] | 208.19 [34.79] | |
| Positive signs | 234.22 [27.69] | 214.53 [40.75] | |
Note: For FRN latency only trials with detectable negativity were included.
Fig. 3.
Grand averages for all feedback conditions (N = 21) at electrode sites FCz and Cz. Approximate FRN windows between peak minima (156 ms) and maxima (312 ms) are displayed.
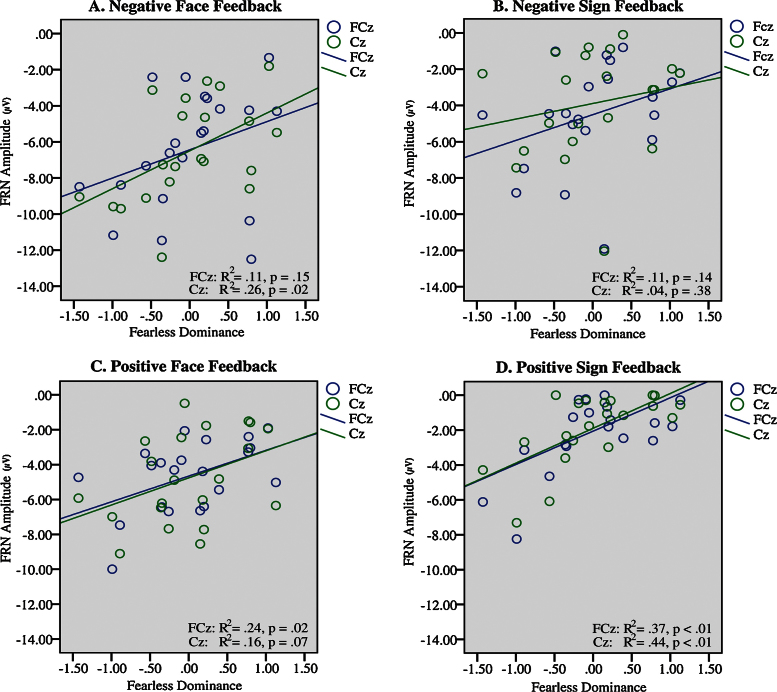
Regarding psychopathic traits, there was a between-subjects effect for Fearless Dominance, F (1,18) = 8.88, p < .01, . As hypothesized, parameter estimates (Table 4) indicate an inverse relation between Fearless Dominance and feedback processing in all feedback conditions at both electrode sites, i.e. higher scoring subjects displayed reduced FRN amplitudes, significant for negative faces and positive faces and positive signs at least at one electrode site, FCz or Cz. The confidence intervals of regression slopes were substantially overlapping and there were no significant interaction effects between Fearless Dominance and the within-subject factors electrode site, valence and form, (all p > .13). Together with the overall between-subject effect this indicates a more generalized pattern of reduced FRNs in higher-scoring subjects. There was no significant between-subject effect for Self-Centered Impulsivity, F (1,18) = .98, p = .34, indicating that this psychopathic trait is rather unrelated to feedback processing.
Table 4.
GLM regression parameter estimates (B) with confidence intervals (CI) and effect sizes (η2), as well as zero-order Pearson correlation coefficients (r) between PPI-R Fearless Dominance and Self-Centered Impulsivity and FRN amplitude in the four feedback conditions at electrode sites FCz and Cz.
| Condition | Fearless Dominance |
|||||||
|---|---|---|---|---|---|---|---|---|
| FRN (Fcz) |
FRN (Cz) |
|||||||
| B | CI | r | B | CI | r | |||
| Negative faces | 1.47 | [−.58, 3.53] | .11 | .33a | 2.06* | [.32, 3.81] | .26 | .51*,a |
| Negative signs | 1.40 | [−.57, 3.37] | .11 | .34 | .83 | [−1.24, 2.90] | .04 | .20 |
| Positive faces | 1.46* | [.17, 2.75] | .24 | .49* | 1.54 | [−.19, 3.28] | .16 | .41 |
| Positive signs | 1.90* | [.66, 3.13] | .37 | .61*,a | 2.01* | [.91, 3.12] | .45 | .66*,a |
| Condition | Self-Centered Impulsivity |
|||||||
|---|---|---|---|---|---|---|---|---|
| FRN (Fcz) |
FRN (Cz) |
|||||||
| B | CI | r | B | CI | r | |||
| Negative faces | −1.69 | [−3.67, .29] | .15 | −.39a | −.67 | [−2.35, 1.01] | .04 | −.19a |
| Negative signs | −.47 | [−2.37, 1.43] | .02 | −.13 | −.62 | [−2.62, 1.37] | .02 | −.16 |
| Positive faces | −.30 | [−1.54, .95] | .01 | −.13 | −.26 | [−1.93, 1.41] | .01 | −.09 |
| Positive signs | −.34 | [−1.53, .84] | .02 | −.14a | −.30 | [−.76, 1.37] | ||
Correlation coefficients of Fearless Dominance and Self-Centered Impulsivity are significantly different from each other in the respective condition (p ≤ .05).
Significantly different from 0 (p ≤ .05).
Zero-order Pearson correlation coefficients indicate that Fearless Dominance is associated with reduced FRN amplitudes (Table 4). Moreover, the zero-order correlation coefficients of Fearless Dominance and Self-Centered Impulsivity were significantly different for negative faces and positive signs when applying the T2 test statistic (Steiger, 1980) as illustrated also in Table 4. Descriptively, all the regression parameters and correlations pointed in different directions when comparing the two psychopathic traits. The relationship between Fearless Dominance and feedback processing is also illustrated by simple regression lines in Fig. 4.
Fig. 4.
Scatter plots and fitted regression lines with Fearless Dominance as independent variable and FRN amplitude as the dependent variable for electrode sites FCz and Cz illustrated separately for the four feedback conditions.
This effect for Fearless Dominance cannot be explained by differences in FRN latencies as there were no significant between-subject effect (p > .88) or parameter estimates in the four feedback conditions (p > .28), when running the same GLM for FRN peak latency as the dependent variable. Zero-order correlations were also not significant (all p > .24).
When applying a unitary construct perspective, running the same GLM for total PPI-R score did not reveal a significant between-subject effect (p = .65) nor significant parameter estimates for the separate conditions (all p > .06).
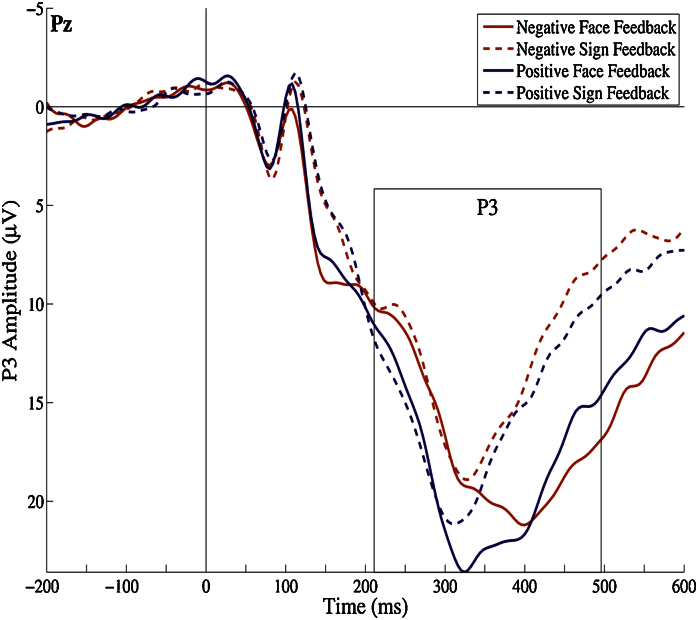
Regarding P3 amplitudes, the main effect for valence was not significant (p = .09), whereas we found a significant effect for form, F (1,18) = 6.15, p = .02, , indicating that P3 amplitudes were larger for faces. Grand averages for the four conditions are displayed in Fig. 5. The between-subject effects and all of the parameter estimates of Fearless Dominance and Self-Centered Impulsivity were not statistically significant (all p > .46). Moreover, no significant interactions emerged (all p ≥ .07). Zero-order Pearson correlation coefficients also indicate that Fearless Dominance and Self-Centered Impulsivity are unrelated with P3 amplitudes (all p > .43).
Fig. 5.
Grand averages for all feedback conditions (N = 21) at electrode site Pz. Approximate P3 windows between peak minima (212 ms) and maxima (496 ms) are displayed.
Therefore, effects seem to be specific for the FRN, also indicated by the non-significant main, interaction or between-subject effects applying the same GLM to the positivity prior to the FRN and the P3 after FRN at electrode sites FCz and Cz (all p > .10).
With regard to behavioral measures, there were no significant correlations between FRN amplitude at FCz and Cz and time estimation or absolute trial-by-trial adjustment of time estimation in the respective conditions (all p > .16).
3.3. Source analysis
We observed decreased RCZa activity for higher levels of Fearless Dominance in the FRN-time range for negative faces, whereas Self-Centered Impulsivity was unrelated to RCZa activity across all conditions (Table 5). No other effects were significant (all p > .07).
Table 5.
Zero-order Pearson correlation coefficients (r) between Fearless Dominance or Self-Centered Impulsivity and mean current source density (A/m2) in the RCZa (bilateral spherical ROIs, 8 mm) between 140 and 300 ms post-feedback in the four conditions.
| Condition | Fearless Dominance | Self-Centered Impulsivity |
|---|---|---|
| r | r | |
| Negative faces | −.45* | .08 |
| Negative signs | −.41 | .15 |
| Positive faces | −.39 | .09 |
| Positive signs | .03 | .01 |
Significantly different from 0 (p ≤ .05).
4. Discussion
Although there is a considerable body of evidence for behavioral and neural correlates of psychopathy, feedback processing has not been investigated with respect to specific psychopathic traits so far. Applying a dual-process perspective enabled us to find a relationship that previous studies focusing on a unitary construct of psychopathy could not establish. Considering the theoretically postulated role of low fear in behavioral deficits in psychopathy (Lykken, 1957, 1995), we hypothesized that in particular the psychopathic trait Fearless Dominance – an emotional-interpersonal factor associated with high dominance, low anxiety, venturesomeness and low fear-reactivity (Benning et al., 2003; Benning, Patrick, & Iacono, 2005) – would be associated with impaired feedback processing.
As expected, we found an overall between-subjects effect for Fearless Dominance as well as significant regression parameter estimates in the negative face, the positive face and positive signs condition (and descriptively the same relation for negative signs) in the GLM. There were no significant differential effects for faces and signs or negative and positive feedback. Together, this might indicate a rather generalized reduction of FRN amplitudes in higher Fearless Dominance. In contrast, Self-Centered Impulsivity as well as total PPI-R score were not significantly related to feedback processing. Moreover, zero-order correlation coefficients for Fearless Dominance partly differ from the coefficients for Self-Centered Impulsivity. Thus, our findings suggest that Fearless Dominance might uniquely contribute to impaired feedback processing.
Evidence for this specific relation between Fearless Dominance and brain activity associated with feedback processing was also found in our sLORETA results for negative faces. Fearless Dominance, but not Self-Centered Impulsivity, was negatively correlated with activity in the rostral cingulate zone anterior (RCZa) for negative face feedback, although for the other conditions no significant effects could be found. The RCZa is a key area for internal and external error monitoring (Mars et al., 2005; Ullsperger & Von Cramon, 2001, 2003, 2004).
4.1. Integration with theories of psychopathy
Our hypotheses were derived from dual process models (Fowles & Dindo, 2009; Patrick & Bernat, 2009) as well as from the low-fear hypothesis of psychopathy (Lykken, 1957, 1995). On the one hand, the neural dissociation between Fearless Dominance and Self-Centered Impulsivity observed in this study strongly supports the value of dual-process models of psychopathy – in line with previous studies focusing on different processes like executive functions related to the P3 component (Carlson & Thái, 2010; Carlson et al., 2009) or affect recognition (Gordon et al., 2004). On the other hand, our results support the low-fear hypothesis, which states that deficits in behavioral adaptation are the result of a fear deficit. Thus, we expected in particular a contribution of Fearless Dominance, a dimension of psychopathy incorporating among others low fear, to a reduced FRN, an ERP component, which has been associated with behavioral adaptation (Frank et al., 2007, 2005). This is what we have found. Theories emphasizing processing of the affective/motivational significance and subjective stimulus evaluation in error monitoring (Gehring & Willoughby, 2002; Pailing & Segalowitz, 2004; Yeung, Holroyd, & Cohen, 2005) would also predict impaired error monitoring in case of an affective deficit preventing the use of affective information to determine salience. From this perspective, feedback might have been less salient for subjects high in Fearless Dominance.
An alternative to affective-based theories of psychopathy is the response modulation theory (Newman & Lorenz, 2003). According to Newman and Baskin-Sommers (2011), response modulation is an early attentional process necessary for self-regulation and behavioral adaptation. The theory postulates that psychopaths are impaired in suspending a dominant response set (i.e. ongoing approach behavior), integrating contextual information, and shifting attention to the evaluation of the response, which might be reflected in impaired internal or external error monitoring. A deficiency in feedback processing might therefore indirectly reflect an impaired process at an earlier stage. However, the model does currently not offer an explanation why the socio-emotional dimension Fearless Dominance in contrast to Self-Centered Impulsivity would be specifically related to impaired feedback processing.
4.2. Integration with neuroscientific studies about feedback processing in psychopathy and related constructs
Our results extend the limited body of evidence on feedback processing in psychopathy and show that the latter is related to reduced FRN amplitudes – a relationship which has not been established by previous studies relying on a unitary construct of psychopathy. von Borries et al. (2010) found no difference in the FRN between psychopathic violent offenders and controls in a probabilistic learning task. Neither did Varlamov et al. (2010) find any difference in the FRN between psychopathic and non-psychopathic patients with comorbid personality disorder and controls in a visual Go/No Go task. Although Munro et al. (2007) and Varlamov et al. (2010) reported also correlational data between ERPs and PCL-R scores, they did not analyze the factor scores. Moreover, this analysis was restricted to violent offenders at a maximum security forensic hospital (Munro et al., 2007) or subjects with personality disorder detained at medium and high levels of security (Varlamov et al., 2010), potentially reducing the variation in underlying psychopathic dimensions and thus statistical power to detect more specific associations as reported for Fearless Dominance here. Another type of external error monitoring is reflected in the “observed ERN” (oERN), which is elicited when processing the action-outcomes of other observed individuals. This oERN was reduced in psychopathic violent offenders (Brazil et al., 2011). Although the authors found an oERN impairment in psychopaths, future studies might also investigate possible differential contributions of psychopathic traits.
As far as internal monitoring is concerned, von Borries et al. (2010) observed reduced ERN amplitudes in psychopathic offenders in a probabilistic learning task, as well as Dikman and Allen (2000) for the related construct of low socialization. In contrast, Munro et al. (2007) reported reduced ERN amplitudes in psychopaths in an emotional face flanker task, but not in a standard letter flanker task. Similarly, Brazil et al. (2009) found intact early error monitoring (ERN) but deficiencies in later stages of error monitoring/error awareness (Pe) in psychopaths and Brazil et al. (2011) observed similar ERNs in response to one's own action. Applying a dual-process perspective might also reveal differential contributions of psychopathic traits and offer an explanation for these inconsistent results.
A consistency check with studies investigating internal and external error monitoring in other personality or clinical constructs that have been related to psychopathy should provide additional information about the value of dual-process models. For instance, two of the three conceptual brain systems proposed by the Reinforcement Sensitivity Theory (Gray & McNaughton, 2000), the Behavioral Inhibition System (BIS) and the Behavioral Activation System (BAS) have been linked to psychopathy. Low BIS has been linked to the emotional-interpersonal dimension (i.e. “Trait Fearlessness) of psychopathy whereas high BAS to the second social-deviance dimension (i.e. “Externalizing Vulnerability”; Ross, Benning, Patrick, Thompson, & Thurston, 2009; Wallace, Malterer, & Newman, 2009). High BIS has been associated with larger ERN amplitudes in a Flanker Task (Boksem et al., 2006) and with larger FRN amplitudes in an instrumental Go/No-Go learning task (De Pascalis et al., 2010); which is consistent with our findings of reduced FRN amplitudes for higher Fearless Dominance. Another clinically relevant dimension, which has been specifically related to the social-deviance dimension of psychopathy, is externalizing psychopathology (Patrick et al., 2005). The latter has been associated with reduced ERN amplitudes in a flanker task (Bernat, Nelson, Steele, Gehring, & Patrick, 2011) but not with reduced FRN amplitudes after feedback in a gambling task (Bernat et al., 2011). Thus, one could hypothesize that Self-Centered Impulsivity is unrelated to the FRN amplitude variation. In fact, we found no significant relation between Self-Centered Impulsivity and FRN amplitudes. These overlapping results with regard to related constructs clearly emphasize the benefits of a dual-process perspective.
An additional remark to be made is that apart from a distinction of internal and external error monitoring, the latter might also be sub-divided on the basis of the kind of feedback involved. For instance, Pfabigan et al. (2011) found enhanced FRN amplitudes for more antisocial compared to less antisocial individuals after monetary but not after emotional-social feedback. Although this study did not investigate psychopathy or specific psychopathic traits, it demonstrates the importance of the type of reward used. In contrast to their results, we observed reduced FRN amplitudes for Fearless Dominance in the socio-emotional domain (facial feedback), whereas no monetary reward was included in our experiment–neither in the task nor in form of remuneration of participation. Hence, future studies might compare non-monetary with monetary reward/feedback in individuals with psychopathic traits.
4.3. Integration with neuroscientific models of feedback processing and psychopathy
On a neuro-computational level, our results give rise to the question how psychopathic traits are involved in the interacting mechanisms thought to be central to feedback processing. According to its recent formulation, Reinforcement Learning Theory (Baker & Holroyd, 2011; Holroyd et al., 2008) postulates that intrinsic activity of the ACC, which generates the N200 component, is suppressed by an extrinsic dopamine reward signal reflected by a component called feedback correct-related positivity (fCRP)/reward-positivity, resulting in the FRN. Thus, the FRN amplitude visible in difference waves (contrasting negative vs. positive feedback) or FRN amplitude differences between waves for negative and positive feedback (as measured separately in our study) might be the result of an interaction of these processes. Thus, future research should try to disentangle N200 and fCRP when investigating the association with specific psychopathic traits. The alteration in feedback processing associated with Fearless Dominance might be in the intrinsic activity of the ACC, associated with response conflict (Yeung, Botvinick, & Cohen, 2004) or in the extrinsic signals from the mesencephalic dopamine system. In addition to the analysis of event-related potentials, frequency-based methods might be a promising and complementary approach. For instance theta-activity (4–8 Hz) has been associated with response conflict, performance monitoring and affective processing (Luu, Tucker, & Makeig, 2004; Nigbur, Cohen, Ridderinkhof, & Stürmer, 2011; Nigbur, Ivanova, & Stürmer, 2011).
A different aspect of Reinforcement Learning Theory is that – when learning the action-outcome associations – also enhanced FRN amplitudes could in principle indicate impaired adaptation when back propagation (Holroyd & Coles, 2002) does not take place. However, the paradigm used ensured that feedback remained salient throughout the whole task due to the adaptive fashion of the feedback criteria (see Section 2). Thus, it is reasonable to assume that reduced FRN amplitudes indicate altered feedback processing.
Feedback processing is not specifically discussed in current neuroscientific models of psychopathy and associated brain areas like the RCZa have to be included in neuroscientific models of psychopathy. In contrast to Blair's (2005) integrated emotion system (IES) model of psychopathy, which focuses on the (orbito)frontal cortex and amygdala, Kiehl (2006) included the ACC in his extended paralimbic system dysfunction model of psychopathy. However, a detailed account for specific psychopathic traits is missing in his model. The dACC has connections with paralimbic and subcortical regions like the orbitofrontal cortex (Morecraft & Van Hoesen, 1998; van Hoesen, Morecraft, & Vogt, 1993) and the mesencephalic dopamine system (Crino, Morrison, & Hof, 1993). This points toward an interaction of affective/motivational and cognitive processes, which is consistent with effects of Fearless Dominance on feedback processing. Apart from our findings related to feedback processing and the dACC, other neuroscientific evidence should also be incorporated into a neurocognitive dual-process model of psychopathy. As already mentioned briefly, previous studies also found dissociations in executive functions (Carlson & Thái, 2010; Carlson et al., 2009) or affect recognition (Gordon et al., 2004). An integrative view also should include models not specifically created for psychopathy, e.g. Reinforcement Learning Theory (Baker & Holroyd, 2011; Holroyd & Coles, 2002; Holroyd et al., 2008), Reinforcement Sensitivity Theory (RST; Corr, 2010; Gray & McNaughton, 2000), and models of decision making (e.g. Rushworth et al., 2007).
4.4. Additional findings
Interestingly, Self-Centered Impulsivity was associated with higher values in time estimation (i.e. underestimation of the passage of time). This contrasts the time estimation literature on impulsivity (for a review see Wittmann & Paulus, 2008), which indicates lower values in time estimation (i.e. overestimation of the passage of time) in impulsive individuals. However, Wittmann and Paulus (2008) pointed out that this altered time perception seems to take place especially when subjects are not able to act on their impulsive urges, for example in situations of delayed reward and confrontation with the passage of time. In contrast our task did not present a mere passage of time (i.e. passive waiting), but an active time estimation, which might explain the differences. In addition, impulsivity and psychopathy are both multidimensional and only partly overlapping constructs (Poythress & Hall, 2011), which could account for the differences. This might also be true for findings of reduced FRNs in impulsive individuals (Onoda, Abe, & Yamaguchi, 2010).
Despite the significant impact of psychopathic traits on neural correlates of feedback processing, we observed no effects of psychopathy on absolute trial-to-trial adjustment of time estimation. This is what one would expect when considering that FRN amplitude and this behavioral adaptation measure were also not related, i.e. FRN amplitudes do not mediate behavioral change. Our measure might not perfectly indicate behavioral adaptation as only absolute trial-to-trial changes in estimation time irrespective of the direction of change were analyzed. Furthermore, for improving performance in the time-estimation task, feedback might need to provide additional directional information (Miltner et al., 1997).
Some further remarks have to be made about the main effects found. Both the FRN as well as the P3 are larger for emotional faces than for signs in both positive and negative feedback conditions, which could be due to differences in socio-emotional salience. However, differences between faces and signs could be equally due to other stimulus characteristics (e.g. differences in complexity).
4.5. Methodological considerations
Our study has some limitations to be considered. Especially the rather small sample size renders the present data as preliminary. Although our study had sufficient power to detect general effects of Fearless Dominance on feedback processing, detection of more subtle effects, especially interaction effects (e.g. concerning feedback condition factors), is more difficult given the reduced power. Further investigations are clearly needed if one wants to address for instance the question if feedback variants differentially modulate the relationship between Fearless Dominance and feedback processing. Another limitation is that only female participants were recruited for the present study. Therefore, our results add to the limited literature regarding psychopathic traits in healthy females but raise concerns regarding the generalizability with respect to the male population. However, as mentioned before, there is some overlap in structure and correlates of core features of psychopathy in men and women (Cale & Lilienfeld, 2002; Hare & Neumann, 2006; Salekin, Rogers, & Sewell, 1997; Vitacco, Neumann, & Jackson, 2005; Vitale & Newman, 2001). Differences were primarily found in aspects central to Self-Centered Impulsivity (e.g. impulsivity, disinhibition) and not Fearless Dominance (Verona & Vitale, 2006). This increases the probability that our main result can be generalized to the male population. Nevertheless, constructive replications with male or mixed gender participants would be needed.
As far as generalizability is concerned, one needs to be cautious in drawing inferences for a special segment on the psychopathy continuum, namely high-end psychopathy that is of most clinical interest in terms of diagnosis and intervention. An undergraduate/graduate sample does not capture the same variability in the high-end of psychopathic traits as for instance a psychopathic offender sample. Although our samples shows variability over a large range of the psychopathic traits, it is an open and interesting research question if the reported relationships also holds for high-end psychopathy. One would hypothesize this to be the case given the dimensionality of the construct. Thus, replications in incarcerated high-end psychopaths would be desirable, although possible differences might also be attributable to potentially confounding factors like institutionalization or substance abuse (Lilienfeld, 1996; Sellbom & Verona, 2007), which need to be addressed accordingly. A comparison of high- and low-end groups, should also be a powerful test for the relationship between Fearless Dominance and feedback processing as well as it would address generalizability. Although the conclusions that could be drawn for high-end psychopathy are preliminary, our study has higher generalizability for the general population, which is of special interest for personality research.
5. Conclusion
The present study demonstrated a neural dissociation between different aspects of psychopathic personality associated with recent dual-process models. Fearless Dominance, but not Self-Centered Impulsivity, was associated with impaired feedback processing, indicated by decreased FRN amplitudes. The current data strongly favor to incorporate the dual process perspective in (neuroscientific) models of psychopathy.
Acknowledgements
We thank our participants for their participation. We also thank Sascha Tamm for his advice for source analysis and Judith Kohlenberger for valuable comments on the manuscript. This study was supported by the Austrian Science Fund (FWF): P22813-B09. The funding source had no role in study design, data collection, analysis, interpretation, writing of report and submission.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
In addition, all subjects completed the German Version of the Liebowitz Social Anxiety Scale (LSAS; Stangier & Heidenreich, 2004) and the Experience of Emotions Scale (German: Skalen zum Erleben von Emotionen; Behr & Becker, 2004). Since these data fall beyond the scope of this article, they will not be presented in the present context.
References
- Alpers G.W., Eisenbarth H. Goettingen; Hogrefe: 2008. PPI-R. Psychopathic personality inventory-revised. (German version) [Google Scholar]
- Anderson N.E., Stanford M.S., Wan L., Young K.A. High psychopathic trait females exhibit reduced startle potentiation and increased P3 amplitude. Behavioral Sciences and The Law. 2011;29:649–666. doi: 10.1002/bsl.998. [DOI] [PubMed] [Google Scholar]
- Baker T.E., Holroyd C.B. Which way do I go? Neural activation in response to feedback and spatial processing in a virtual T-maze. Cerebral Cortex. 2009;19:1708–1722. doi: 10.1093/cercor/bhn223. [DOI] [PubMed] [Google Scholar]
- Baker T.E., Holroyd C.B. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biological Psychology. 2011;87:25–34. doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Bauer H., Lamm C., Holzreiter S., Holländer I., Leodolter U., Leodolter M. Measurement of 3D electrode coordinates by means of a 3D photogrammatic head digitizer (3D-PHD) Neuroimage. 2000;11:461. [Google Scholar]
- Bauer H., Lauber W. Operant conditioning of brain steady potential shifts in man. Biofeedback Self Regulation. 1979;4:145–154. doi: 10.1007/BF01007109. [DOI] [PubMed] [Google Scholar]
- Behr M., Becker M. [Experience of emotions scale]. Goettingen; Hogrefe: 2004. SEE. Skalen zum Erleben von Emotionen. [Google Scholar]
- Bell A.J., Sejnowski T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Benning S.D., Patrick C.J., Bloningen D.M., Hicks B.M., Iacono W.G. Estimating facets of psychopathy from normal personality traits: A step toward community-epidemiological investigations. Assessment. 2005;23:3–18. doi: 10.1177/1073191104271223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning S.D., Patrick C.J., Hicks B.M., Bloningen D.M., Krueger R.F. Factor structure of the Psychopathic Personality Inventory: Validity and implications for clinical assessment. Psychological Assessment. 2003;15:340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Benning S.D., Patrick C.J., Iacono W.G. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat E.M., Nelson L.D., Steele V.R., Gehring W.J., Patrick C.J. Externalizing psychopathology and gain/loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology. 2011;120:352–364. doi: 10.1037/a0022124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R. Applying a cognitive neuroscience perspective on the disorder of psychopathy. Development and Psychopathology. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Boksem M.A.S., Tops M., Wester A.E., Meijman T.F., Lorist M.M. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Brazil I.A., De Bruijn E.R.A., Bulten B.H., von Borries K.L., van Lankveld J.J.D.M., Buitelaar J.K. Early and late components of error monitoring in violent offenders with psychopathy. Biological Psychiatry. 2009;65:137–143. doi: 10.1016/j.biopsych.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Brazil I.A., Mars R.B., Bulten B.H., Buitelaar J.K., Verkes R.J., De Bruijn E.R.A. A neurophysiological dissociation between monitoring one's own and other's actions in psychopathy. Biological Psychiatry. 2011;69:693–699. doi: 10.1016/j.biopsych.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Cale E.M., Lilienfeld S.O. Sex differences in psychopathy and antisocial personality disorder: A review and integration. Clinical Psychology Review. 2002;22:1179–1207. doi: 10.1016/s0272-7358(01)00125-8. [DOI] [PubMed] [Google Scholar]
- Carlson S.R., Thái S. ERPs on a continous performance task and self-reported psychopathic traits: P3 and CNV augmentation are associated with Fearless Dominance. Biological Psychology. 2010;85:318–330. doi: 10.1016/j.biopsycho.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Carlson S.R., Thái S., McLarnon M.E. Visual P3 amplitude and self-reported psychopathic personality traits: Frontal reduction is associated with self-centered impulsivity. Psychophysiology. 2009;46:100–113. doi: 10.1111/j.1469-8986.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- Cleckley H. vol. 33. Mosby; St. Louis, MO: 1941. The mask of sanity; pp. 107–111. (Educational and psychological measurements). [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and Psychological Measurements. 1973;33:107–111. [Google Scholar]
- Cohen J. 2nd ed. Lawrence Erlbaum Associates; Hillsdale: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- Coles M., Smid H., Scheffers M., Otten L. Mental chronometry and the study of human information processing. In: Rugg M., Coles M., editors. Electrophysiology of the mind. Oxford University Press; New York: 1995. pp. 94–95. [Google Scholar]
- Corr P.J. The psychoticism-psychopathy continuum: A neuropsychological model of core deficits. Personality and Individual Differences. 2010;48:695–703. [Google Scholar]
- Crino P.B., Morrison J.H., Hof P.R. Monoaminergic innervation of cingulate cortex. In: Vogt B.A., Gabriel M., editors. Neurobiology of cingulate cortex and limbic thalamus: A comprehensive handbook. Birkhauser; Boston: 1993. pp. 285–310. [Google Scholar]
- Delorme A., Makeig S. EEGLAB: An open source toolbox for analysis of single trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A., Sejnowski T., Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34:1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalis V., Varriale V., D’Antuono L. Event-related components of the punishment and reward sensitivity. Clinical Neurophysiology. 2010;121:60–76. doi: 10.1016/j.clinph.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Dikman Z.V., Allen J.J. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Dindo L., Fowles D. Dual temperamental risk factors for psychopathic personality: Evidence from self-report and skin conductance. Journal of Personality and Social Psychology. 2011;100:557–566. doi: 10.1037/a0021848. [DOI] [PubMed] [Google Scholar]
- Dinn W.M., Harris C.L. Neurocognitive function in antisocial personality disorder. Psychiatry Research. 2000;97:173–190. doi: 10.1016/s0165-1781(00)00224-9. [DOI] [PubMed] [Google Scholar]
- Donchin E., Coles M.G.H. Is the P300 component a manifestation of context-updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Donchin E., Coles M.G.H. Context updating and the P300. Behavioral Brain Sciences. 1998;21:149–168. [Google Scholar]
- Edens J.F., Marcus D.K., Lilienfeld S.O., Poythress N.G. Psychopathic, not psychopath: Taxometric evidence for the dimensional structure of psychopathy. Journal of Abnormal Psychology. 2006;115:131–144. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W. Consulting Psychologists Press; Palo Alto: 1976. Pictures of facial affect. [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of crossmodal divided attention on late ERP components II: Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fowles D.C., Dindo L. Temperament and psychopathy: A dual-pathway model. Current Opinions in Psychological Science. 2009;18:179–183. [Google Scholar]
- Frank M.J., D’Lauro C., Curran T. Cross-task individual differences in error processing: Neural, electrophysiological, and genetic components. Cognitive Affective and Behavioral Neuroscience. 2007;7:297–308. doi: 10.3758/cabn.7.4.297. [DOI] [PubMed] [Google Scholar]
- Frank M.J., Woroch B.S., Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47:495–501. doi: 10.1016/j.neuron.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Gao Y., Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: A meta-analysis. Biological Psychology. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Gross B., Coles M.G.H., Meyer D.E., Donchin E. A neural system for error-detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;14:385–602. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gelman A., Stern H. The difference between significant and not significant is not itself statistically significant. The American Statistician. 2006;60:328–331. [Google Scholar]
- Gordon H.L., Baird A., End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56:516–521. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Gray J.A., McNaughton N. 2nd ed. Oxford University Press; Oxford: 2000. The neuropsychology of anxiety: An enquiry into the functions of the septohippocampal system. [Google Scholar]
- Greenblatt R.E., Ossadtchi A., Pflieger M.E. Local linear estimators for the bioelectromagnetic inverse problem. IEEE Transactions on Signal Processing. 2005;53:3403–3412. [Google Scholar]
- Gu R., Ge Y., Jiang Y., Luo Y.-j. Anxiety and outcome evaluation: The good, the bad and the ambiguous. Biological Psychology. 2010;85:200–206. doi: 10.1016/j.biopsycho.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., McDonald N., Simons R.F. Anxiety and error-related brain activity. Biological Psychology. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Holroyd C.B., Simons R.F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71:148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hall J.R., Benning S.D. The successful psychopath: Adaptive and subclinical manifestations of psychopathy in the general population. In: Patrick C.J., editor. Handbook of psychopathy. Guilford Press; New York: 2006. pp. 459–478. [Google Scholar]
- Hare R.D. 2nd ed. Multi-Health Syst.; Toronto, ON: 2003. The Hare Psychopathy Checklist-Revised. [Google Scholar]
- Hare R.D., Neumann C.S. The PCL-R assessment of psychopathy: Development, structural properties, and new directions. In: Patrick C.J., editor. Handbook of psychopathy. Guilford Press; New York: 2006. pp. 58–88. [Google Scholar]
- Hare R.D., Neumann C.S. Psychopathy as a clinical and empirical construct. Annual Review of Clinical Psychology. 2008;4:217–246. doi: 10.1146/annurev.clinpsy.3.022806.091452. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Nieuwenhuis S., Yeung N., Cohen J.D. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport. 2003;14:2481–2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Pakzad-Vaezi K.L., Krigolson O.E. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45:688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Israel J.B., Chesney G.L., Wickens C.D., Donchin E. P300 and tracking difficulty: Evidence for multiple resources in dual-task performance. Psychophysiology. 1980;17:259–273. doi: 10.1111/j.1469-8986.1980.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Johnson R., Donchin E. On how P300 amplitude varies with the utility of the eliciting stimuli. Electroencephalography and Clinical Neurophysiology. 1978;44:424–437. doi: 10.1016/0013-4694(78)90027-5. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Prentice Hall; Englewood-Cliffs: 1973. Attention and effort. [Google Scholar]
- Kiehl K.A. A cognitive neuroscience perspective on psychopathy: Evidence for a paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R.F. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Lacadie C.M., Fulbright R.K., Rajeevan N., Constable R.T., Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Lamm C., Fischmeister F., Bauer H. Individual differences in brain activity during visuo-spatial processing assessed by slow cortical potentials and low resolution electromagnetic tomography. Cognitive Brain Research. 2005;25:900–912. doi: 10.1016/j.cogbrainres.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Lee T.-W., Girolami M., Sejnowski T.J. Independent component analysis using an extended infomax algorithm and mixed subgaussian and supergaussian sources. Neural Computation. 1999;11:417–441. doi: 10.1162/089976699300016719. [DOI] [PubMed] [Google Scholar]
- Lilienfeld S.O. The MMPI-2 antisocial practices content scale: Construct validity and comparison with the psychopathic deviate scale. Psychological Assessment. 1996;8:281–293. [Google Scholar]
- Lilienfeld S.O., Andrews B.P. Development and preliminary validation of a self-report measure of psychopathic personality in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- López R., Poy R., Patrick C.J., Moltó J. Deficient fear conditioning and self-reported psychopathy: The role of fearless dominance. Psychophysiology. 2012 doi: 10.1111/j.1469-8986.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- Luu P., Tucker D.M., Makeig S. Frontal midline theta and error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Lykken D.D. A study of anxiety in the sociopathic personality. Journal of Abnormal and Social Psychology. 1957;55:6–10. doi: 10.1037/h0047232. [DOI] [PubMed] [Google Scholar]
- Lykken D.T. Erlbaum; Hillsdale, NJ: 1995. The antisocial personalities. [Google Scholar]
- MacCallum R.C., Zhang S., Preacher K.J., Rucker D.D. On the practice of dichotomization of quantitative variables. Psychological Methods 7. 2002:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- Marcus D.K., John S.L., Edens J.F. A taxometric analysis of psychopathic personality. Journal of Abnormal Psychology. 2004;113:626–635. doi: 10.1037/0021-843X.113.4.626. [DOI] [PubMed] [Google Scholar]
- Mars R.B., Coles M.G.H., Grol M.J., Holroyd C.B., Nieuwenhuis S., Hulstijn W. Neural dynamics of error processing in medial frontal cortex. NeuroImage. 2005;28:1007–1013. doi: 10.1016/j.neuroimage.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Miltner W.R., Braun C.H., Coles M.G. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a generic neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Mobascher A., Brinkmeyer J., Warbrick T., Musso F., Wittsack H.J., Stoermer R. Fluctuations in electrodermal activity reveal variations in single trial brain responses to painful laser stimuli–a fMRI/EEG study. NeuroImage. 2009;44:1081–1092. doi: 10.1016/j.neuroimage.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Morecraft R.J., Van Hoesen G.W. Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Research Bulletin. 1998;45:209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- Munro G.E., Dywan J., Harris G.T., McKee S., Unsal A., Segalowitz S.J. ERN varies with degree of psychopathy in an emotion discrimination task. Biological Psychology. 2007;76:31–42. doi: 10.1016/j.biopsycho.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Newman J.P., Baskin-Sommers A.R. Early selective attention abnormalities in psychopathy: Implications for self-regulation. In: Posner M.I., editor. Cognitive neuroscience of attention. 2nd ed. Guilford; New York: 2011. pp. 421–440. [Google Scholar]
- Newman J.P., Kosson D.S. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986;95:252–256. [PubMed] [Google Scholar]
- Newman J.P., Lorenz A.R. Response modulation and emotion processing: Implications for psychopathy and other dysregulatory psychopathology. In: Davidson R.J., Scherer K., Goldsmith H.H., editors. Handbook of affective sciences. Oxford University Press; Oxford: 2003. pp. 1043–1067. [Google Scholar]
- Newman J.P., Patterson C.M., Howland E.W., Nichols S.L. Passive avoidance in psychopaths: The effects of reward. Personality and Individual Differences. 1990;11:1101–1114. [Google Scholar]
- Niewenhuis S., Forstmann B.U., Wagenmakers E.-J. Erroneous analysis of interactions in neuroscience: A problem of significance. Nature Neuroscience. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Nigbur R., Cohen M.X., Ridderinkhof K.R., Stürmer B. Theta dynamics reveal domain-specific control over stimulus and response conflict. Journal of Cognitive Neuroscience. 2011;24:1264–1274. doi: 10.1162/jocn_a_00128. [DOI] [PubMed] [Google Scholar]
- Nigbur R., Ivanova G., Stürmer B. Theta power as a marker for cognitive interference. Clinical Neurophysiology. 2011;122:2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Olbrich S., Mulert C., Karch S., Trenner M., Leicht G., Pogarell O. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurements. NeuroImage. 2009;45:319–332. doi: 10.1016/j.neuroimage.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Onoda K., Abe S., Yamaguchi S. Feedback-related negativity is correlated with unplanned impulsivity. Neuroreport. 2010;21:736–739. doi: 10.1097/WNR.0b013e32833bfd36. [DOI] [PubMed] [Google Scholar]
- Pailing P.E., Segalowitz S.J. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low resolution brain electromagnetic tomography (sLORETA): Technical details. Methods and Findings in Experimental and Clinical Pharmacology. 2002;24:5–12. [PubMed] [Google Scholar]
- Patrick C.J., Bernat E.M. Neurobiology of psychopathy: A two-process theory. In: Berntson G.G., Cacioppo J.T., editors. Handbook of neuroscience for the behavioral sciences. John Wiley & Sons; New York: 2009. pp. 1110–1131. [Google Scholar]
- Patrick C.J., Hicks B.M., Krueger R.F., Lang A.R. Relations between psychopathy facets and externalizing in a criminal offender sample. Journal of Personality Disorders. 2005;19:339–356. doi: 10.1521/pedi.2005.19.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan D.M., Alexopoulus J., Bauer H., Lamm C., Sailer U. All about the money – External performance monitoring is affected by monetary, but not by socially conveyed feedback cues in more antisocial individuals. Frontiers in Human Neuroscience. 2011;5(100) doi: 10.3389/fnhum.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N., Strick P.L. Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picton C., Hillyard P. Cephalic skin potentials in electroencephalography. Electroencephalography and Clinical Neurophysiology. 1972;33:419–424. doi: 10.1016/0013-4694(72)90122-8. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poythress N.G., Hall J.R. Psychopathy and impulsivity reconsidered. Aggression and Violent Behavior. 2011;16:120–134. [Google Scholar]
- Ross S.R., Benning S.D., Patrick C.J., Thompson A., Thurston A. Factors of the Psychopathic Personality Inventory: Criterion-related validity and relationship to the BIS/BAS and Five-Factor models of personality. Assessment. 2009;16:71–87. doi: 10.1177/1073191108322207. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F.S., Behrens T.E.J., Rudebeck P.H., Walton M.E. Contrasting roles for cingulated and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Salekin R.T., Rogers R., Sewell K.W. Construct validity of psychopathy in a female offender sample: A multitrait-multimethod evaluation. Journal of Abnormal Psychology. 1997;106:576–585. doi: 10.1037//0021-843x.106.4.576. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Bogdan R., Birk J.L., Goetz E.L., Holmes A.J., Pizzagelli D.A. . Neural responses to negative feedback are related to negative emotionality in healthy adults. Social Cognitive and Affective Neuroscience. 2011 doi: 10.1093/scan/nsr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellbom M., Verona E. Neuropsychological correlates of psychopathic traits in a non-incarcerated sample. Journal of Research in Personality. 2007;41:276–294. [Google Scholar]
- Stangier U., Heidenreich T. Collegium Internationale Psychiatriae Scalaram (CIPS); Beltz, Weinheim: 2004. Liebowitz Soziale Angst-Skala. [Google Scholar]
- Steiger J.H. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Stephenson W., Gibbs F.A. A balanced non-cephalic reference electrode. Electroencephalography and Clinical Neurophysiology. 1951;3:237–240. doi: 10.1016/0013-4694(51)90017-x. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; Stuttgart: 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Ullsperger M., Von Cramon D.Y. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Ullsperger M., Von Cramon D.Y. Error monitoring using external feedback: Specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M., Von Cramon D.Y. Neuroimaging of performance monitoring: Error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- van der Veen F.M., Röder C.H., Mies G.W., van der Lugt A., Smits M. Remedial action and feedback processing in a time-estimation task: Evidence for a role of the rostral cingulate zone in behavioral adjustments without learning. Neuroimage. 2011;54:447–454. doi: 10.1016/j.neuroimage.2010.08.018. [DOI] [PubMed] [Google Scholar]
- van Hoesen G.W., Morecraft R.J., Vogt B.A. Connections of the monkey cingulate cortex. In: Vogt B.A., Gabriel M., editors. Neurobiology of cingulate cortex and limbic thalamus: A comprehensive handbook. Birkhauser; Boston: 1993. pp. 249–284. [Google Scholar]
- Varlamov A., Khalifa N., Liddle P., Duggan C., Howard R. Cortical correlates of impaired self-regulation in personality disordered patients with traits of psychopathy. Journal of Personality Disorders. 2010;25:75–88. doi: 10.1521/pedi.2011.25.1.75. [DOI] [PubMed] [Google Scholar]
- Verona E., Vitale J. Psychopathy in women: Assessment, manifestations, and etiology. In: Patrick C.J., editor. Handbook of psychopathy. Guilford Press; New York: 2006. pp. 415–436. [Google Scholar]
- Vitacco M.J., Neumann C.S., Jackson R.L. Testing a four-factor model of psychopathy and its association with ethnicity, gender, intelligence, and violence. Journal of Consulting and Clinical Psychology. 2005;73:466–476. doi: 10.1037/0022-006X.73.3.466. [DOI] [PubMed] [Google Scholar]
- Vitale J.E., Newman J.P. Using the Psychopathy Checklist-Revised with female samples: Reliability, validity, and implications for clinical utility. Clinical Psychology: Science and Practice. 2001;8:117–132. [Google Scholar]
- von Borries A.K.L., Brazil I.A., Bulten B.H., Buitelaar J.K., Verkes R.J., de Bruijn E.R.A. Neural correlates of error-related learning deficits in individuals with psychopathy. Psychological Medicine. 2010;40:1559–1568. doi: 10.1017/S0033291709992017. [DOI] [PubMed] [Google Scholar]
- Wallace J.F., Malterer M.B., Newman J.P. Mapping of Gray's BIS and BAS constructs onto Factor 1 and Factor 2 of Hare's Psychopathy Checklist – Revised. Personality and Individual Differences. 2009;42:812–816. doi: 10.1016/j.paid.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.-U., Wunderlich U., Gruschwitz S., Zaudig M. Beltz Test; Goettingen: 1996. Strukturiertes Klinisches Interview für DSM-IV (SKID) [Google Scholar]
- Wittmann M., Paulus M.P. Decision making, impulsivity and time perception. Trends in Cognitive Sciences. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- World Medical Association . World Medical Association; Helsinki: 2000. Declaration of Helsinki. [Google Scholar]
- Yeung N., Botvinick M.M., Cohen J.D. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Yeung N., Holroyd C.B., Cohen J.D. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cerebral Cortex. 2005;15:535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Yeung N., Sanfey A.G. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]