Abstract
We investigated the recently described colpodid ciliate Bromeliothrix metopoides in a series of laboratory experiments to reveal the environmental factors that constrain this species to its peculiar habitat, i.e. the tanks of bromeliads. Our results demonstrated that the various life stages of this ciliate (bacterivorous theronts and microstome trophonts, flagellate-feeding macrostomes) have specific demands in terms of food quality and quantity. Bromeliothrix required a high food threshold (>1.4 mg C L−1) in order to thrive. Food quality also affected resting cyst formation of B. metopoides when the experimental containers dried out. Its maximum growth rates (μmax = 4.71 d−1, i.e. 6.8 doublings d−1) belong to the highest ones recorded thus far for free-living ciliates. The pH niche of B. metopoides was relatively wide (pH ∼4 to >9) under optimal food conditions. However, its high sensitivity to unfavourable environmental conditions let the population collapse within several hours. We conclude that B. metopoides is a boom and bust ciliate that is specifically adapted to its peculiar habitat but virtually unviable in other environments.
Keywords: Bromeliothrix metopoides, Feeding threshold, Growth rates, pH response, Polytomella, Tank bromeliads
Introduction
Epiphytic plants of the family Bromeliaceae are wide-spread and species-rich in Central and South America. Many bromeliads are able to store water in a reservoir formed by the tightly overlapping bases of their rosette leaves. Such phytotelmata (Varga 1928), which are also known as tanks or cisterns, may contain up to 30 L of water; they represent a highly specialized aquatic habitat (Foissner et al. 2003; Schönborn 2003). Although the fauna and flora of those tanks is poorly explored and biased towards macroorganisms, many species, including over 400 metazoans, have already been recorded (summarized by Carrias et al. 2001; Kitching 2000; Schönborn 2003).
Recently, the ciliate fauna from bromeliad reservoirs has been studied in detail by Foissner and co-workers (Foissner 2003a,b, 2010, 2013; Foissner et al. 2003, 2011; Foissner and Wolf 2009; Omar and Foissner 2011, 2012). One of the most remarkable species is Bromeliothrix metopoides Foissner, 2010. This species has a complex life cycle (Fig. 1), with bacterivorous microstome theronts (dividing cells) and trophonts (usually 20–40 μm in length) and large mouthed, flagellate-feeding macrostomes (usually >45 μm in length). All morphs form resting cysts; another characteristic feature of this ciliate is the formation of division chains. This ciliate is wide-spread, occurring in bromeliads from Mexico to southern Chile (W. Foissner, unpubl. observations) and can be easily cultivated in an array of freshwater and soil media. The species was portrayed as an r-strategist, with doubling time ≤5 h. Food depletion induced cyst formation in both microstomes and macrostomes (Foissner 2010). However, the previous field and laboratory investigations could not explain why this ciliate is restricted to tank bromeliads and does not seem to occur in any other freshwater or soil environment (Foissner 2010). Foissner (2010) did not find B. metopoides in about 2000 soil and freshwater samples investigated globally, including several hundred samples from Central and South America. The only other habitat where it sporadically occurred was the water and mud accumulating in bamboo stumps (Foissner 2010). Similarly, Foissner et al. (2003) noted that several other new ciliate species occurring in bromelian tanks are endemics, although they are morphologically similar to those found in more common freshwater habitats.
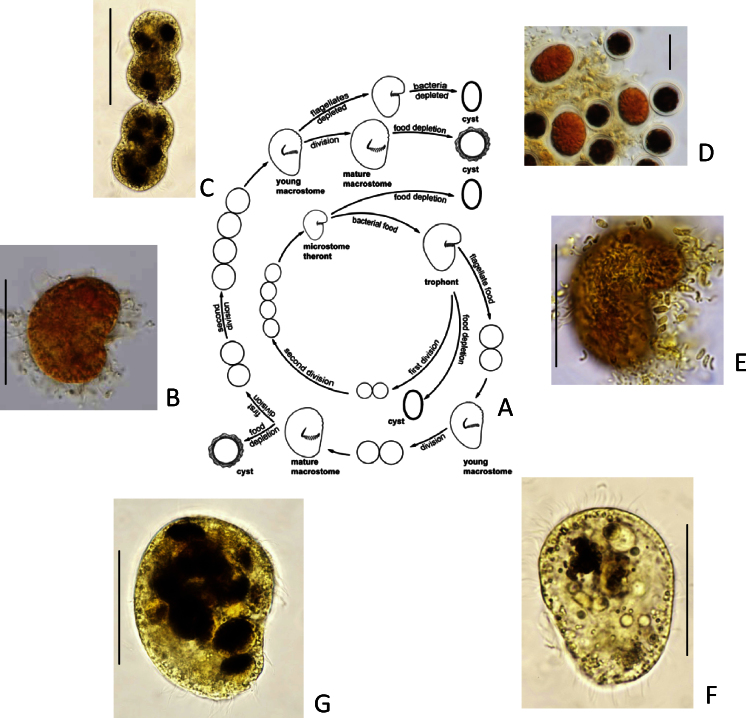
Fig. 1.
The life cycle of Bromeliothrix metopoides: (A) with its various stages after Lugol's fixation; (B) microstome theront; (C) macrostome division chain; (D) resting cysts of theronts (large and light brown) and the flagellate Polytomella sp. (small and dark brown); (E) trophont; (F) young macrostome with few food vacuoles; (G) mature macrostome packed with food vacuoles containing Polytomella sp. Scale bars: 30 μm (B, E, F), 40 μm (C, G), and 20 μm (D). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 1A reproduced from Foissner (2010).
The goal of the present study was to investigate why B. metopoides appears to be limited to such a peculiar habitat. Water volume (typically ∼0.01–3 L), light, pH (usually ranging from 4.0 to 7.0), nutrients and allochtonous organic input from leaf litter are the most important abiotic factors characterizing phytotelmata of tank bromeliads (Armbruster et al. 2002; Brouard et al. 2011, 2012; Laessle 1961; Lopez et al. 2009; Marino et al. 2011). However, detailed studies measuring environmental factors in tank bromeliads are rare. In the state of Rio de Janeiro, Brazil, temperature varies annually from 22 °C in July to 30 °C in January (Marino et al. 2011). These authors reported typical temperature variation in the tanks of bromeliads in this area in the range of 10 °C; pH was relatively variable, usually exceeding 2 pH units. A similar range of temperature (∼10 °C) and pH (2–3 units) is known from the tanks of Jamaican bromeliads (Laessle 1961). However, habitat size (i.e. water volume) appears to be the primary abiotic variable affecting biomass of protists and small metazoans in tank bromeliads (Armbruster et al. 2002; Marino et al. 2011). In accordance with these sparse data, we focused our investigation on the formation of macrostomes and cysts in response to food, pH, and desiccation. Together with competition, predation and parasitism, these are likely the environmental key variables determining the survival of ciliate populations. For logistic constraints, we could not investigate the effect of predation and parasitism on the life cycle of B. metopoides. Competition experiments with B. metopoides were performed in our laboratory together with Glaucomides bromelicola Foissner 2013, another new ciliate species common in tank bromeliads (Foissner 2013). Because the competition experiments required a different experimental design as used in the present study, results will be reported elsewhere (Weisse et al. submitted).
Since the goal of our study stated above does not represent a falsifiable hypothesis, we hypothesized more specifically that
-
•
larger food items would induce the formation of macrostomes,
-
•
adverse conditions other than food depletion (e.g. low pH) may also induce the formation of cysts,
-
•
the combination of several environmental factors narrows the realized ecological niche of B. metopoides.
Concerning the last hypothesis, it has recently been demonstrated for freshwater ciliates, flagellates and microeukaryotes that a combination of several environmental key variables such as food, temperature and pH may confine the ecological niche in situ to a relatively small range (Moser and Weisse 2011; Weisse 2006; Weisse et al. 2002, 2013a,b). By analogy, we assumed that the combination of food (quantity and quality), pH, and specific requirements related to cyst formation may restrict the ecological niche of B. metopoides to tank bromeliads.
Material and Methods
Origin and maintenance of stock cultures
All organisms used in this study were collected from tank water of small bromeliads growing on trees in subtropical forests of Brazil. Details of the origin of our study organisms have been reported by Foissner (2010). Cultures were established in Eau de Volvic (French table water) enriched with some sterilized crushed wheat grains to promote growth of indigenous bacteria and bacterivorous flagellates. Pure cultures were obtained by repeated dilution with Volvic and pipetting of individual target cells, respectively by removing unwanted predators or competitors. Stock cultures were maintained in ‘filter caps’ culture flasks (Biomedica, Vienna, Austria) with 50 mL of Eau de Volvic enriched with 1–2 wheat grains. The stock cultures were kept in an incubator at 22.5 °C under 14:10 h light:dark cycle; pH was ∼7.5. New cultures were inoculated once per week by transferring 25 mL of the aged culture to a new flask containing 25 mL Eau de Volvic and one new wheat grain.
Bromeliothrix metopoides Foissner, 2010 is a flexible (cell size <20–55 × 15–36 μm) ciliate of the order Colpodida (Fig. 1). We kept this ciliate in stock cultures with and without the flagellate Polytomella sp.; this flagellate, which was isolated together with B. metopoides, has four flagella at the basis of a distinct papilla, four contractile vacuoles, lacks an eyespot, and has the nucleus in the anterior body half (Foissner 2010). The genus Polytomella comprises several colourless species of nutritional versatile, auxotrophic flagellates that thrive on acetate and other organic acids, peptone, and yeast extract (de la Cruz and Gittleson 1981; Pringsheim 1955). The species used in this investigation is possibly undescribed and will be described elsewhere (Foissner et al. in preparation); this species occurs in two differently sized morphs (see section “Results”). We added two wheat grains to 50 mL of Eau de Volvic to provide a nutritious medium for our Polytomella sp. cultures.
Bacteria were always present in stock cultures. Due to ciliate grazing, bacterial abundance was usually lower in flagellate-free cultures (0.2–1.0 × 107 cells mL−1) than in cultures with Polytomella sp. (0.7–1.8 × 107 cells mL−1). This is because if Polytomella are present, macrostomes of B. metopoides ingest the flagellates (see section “Results”). Abundance of the latter ranged from 1–5 × 104 cells mL−1. Ciliate cell numbers in stock cultures usually ranged from 0.1–2.2 × 103 cells mL−1.
Growth experiments with different food organisms
We measured specific growth rate (μ) of Bromeliothrix metopoides in response to different food quality and quantity. If not specified, cell numbers of B. metopoides include microstomes and macrostomes. In asexually reproducing ciliates, μ is a direct proxy of their fitness (Weisse 2006). The ciliate was inoculated together with the respective food organism(s) into 50 mL culture flasks. Except for the first growth experiment (see legend of Fig. 2 for details), treatments with food organisms but without ciliates served as controls. Target food levels were obtained by diluting strongly growing cultures with Eau de Volvic. The initial ciliate abundance was 50–100 cells mL−1. All growth experiments were performed in the dark to prevent photoautotrophic food (the flagellate Cryptomonas sp.) from growing. Similarly, we removed remnants from wheat grains from the experimental containers to limit bacterial growth. Experimental duration lasted from several days to several weeks.
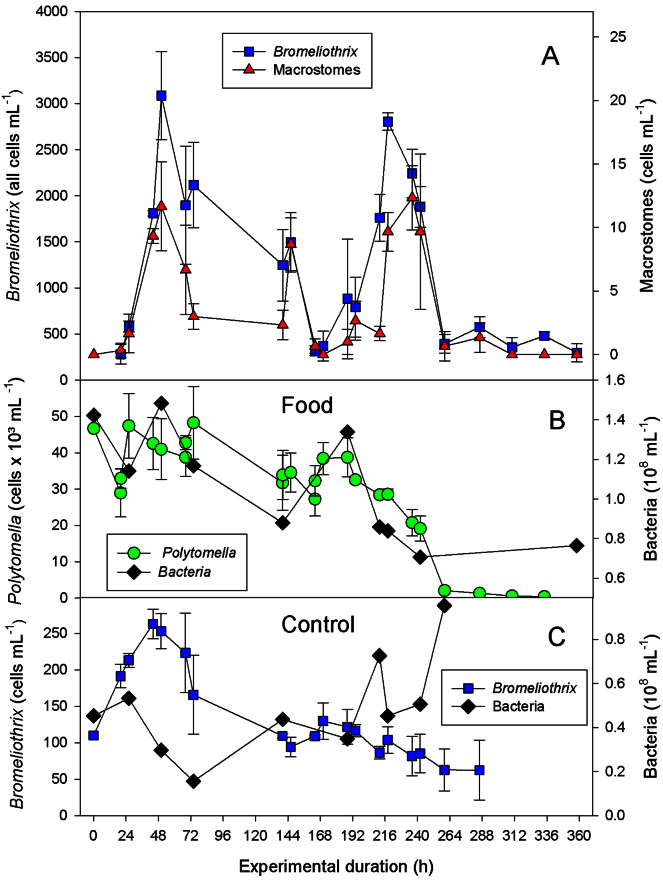
Fig. 2.
Population development of the ciliate Bromeliothrix metopoides; (A) total ciliate cell number and abundance of macrostomes fed with the flagellate Polytomella sp. and undefined heterotrophic bacteria (B); the bottom panel (C) shows the abundance of B. metopoides in control treatments without Polytomella, together with the bacterial abundance. Symbols denote mean values, error bars one standard deviation.
Samples (5 mL) were taken from the experimental containers at 24-h intervals and fixed with acid Lugol's iodine (final concentration 2%, vol/vol). Cell numbers of motile ciliates and their resting cysts were determined microscopically either in counting chambers of 3 mL volume or in Sedgewick Rafter chambers of 1 mL volume. Flagellate cell numbers were also counted microscopically, either together with the ciliates or separately in a 1-mL Sedgewick chamber. At higher abundance (>104 cells mL−1), flagellates were also counted and sized electronically by means of an automatic particle counter (CASY 1-model TTC, Schärfe System, Reutlingen, Germany; Weisse and Kirchhoff 1997). Bacterial levels were measured in Formalin-fixed (2%, vol/vol) samples (2 mL) taken together with the ciliate samples. Bacterial cell numbers were assessed by flow cytometry after staining with the green fluorescent nucleic acid stain SYTO-13 (Molecular Probes), using a FACSCalibur flow cytometer (Becton Dickinson, BD Biosciences, San Jose, USA) equipped with an argon ion laser emitting light at 488 nm.
Cell size of ciliates and flagellates was measured with Lugol's fixed material using an inverted microscope and a semi-automatic image analysis system (LUCIA version 4.51, Laboratory Imaging, Prague, Czech Republic). Flagellates were also sized in unfixed material. The automatic particle counter yielded an independent estimate of flagellate cell size, which was used mainly to determine the cell volume of living cells. Both methods yielded similar results.
The cell volume of the prey flagellates used in this study (Polytomella sp. and Cryptomonas sp.) was converted to carbon units assuming the allometric equation provided by Menden-Deuer and Lessard (2000), i.e. pg C cell−1 = 0.216 × cell volume0.939. To calculate carbon biomass of bacteria, we assumed a conservative estimate of 26 fg C cell−1 for our cultured bacteria (Troussellier et al. 1997).
Cell volume (V) of the ciliates was determined from length (l) and width (w) measurements, assuming a prolate spheroid shape:
| (1) |
where b is cell breadth (in μm, as l and w). Measurements were made on 50 ciliates each at the end of several experiments. Since we could not measure the third dimension, we assumed that b is equal to 0.7 × w. Cell size was highly variable in the different experiments, mainly depending on food and the percentage of macrostomes in the population. Assuming typical average dimensions of l = 34 μm and w = 25 μm and accounting for 10% shrinkage due to fixation, we calculated an average cell volume of B. metopoides of 8570 μm3. The assumption of 10% shrinkage results from comparing results obtained in the present study with in vivo measurements of B. metopoides reported by Foissner (2010).
Desiccation experiments
We studied the response of Bromeliothrix metopoides to desiccation by exposing ciliate cultures (50 mL) in open wide neck Erlenmeyer bottles to an experimental temperature of 25 °C and a 16:8 h light:dark cycle for several days. We used three different treatments: (A) B. metopoides with bacteria as sole food source and one wheat grain in each of the three replicates; (B) B. metopoides plus bacteria and Polytomella sp. with feeding 2 d after the beginning of the experiment; (C) B. metopoides plus bacteria and Polytomella sp. without feeding during the experiment. Bacteria and flagellates were provided in satiating amounts at the beginning of these experiments. The difference between treatments (B) and (C) was that 2 mL of a dense (4.3 × 104 cells mL−1) Polytomella sp. culture were added to the experimental flasks B to prevent the ciliates from becoming food limited during the experiment. Samples for the measurement of protist and bacterial cell numbers were taken once per day, as reported above. The experimental volume remaining in each container was measured on each sampling occasion. The volume removed at each subsampling was replaced with medium containing the respective target food concentrations.
To test for the viability of ciliate cysts produced in the course of the desiccation process, we repeated the desiccation experiment using a 12-well culture tissue plate. Four millilitres of a strongly growing B. metopoides culture were dispensed in each of the wells and incubated with open lid at 22.5 °C for 5 d. The wells were monitored several times per day over a period of five days, when all wells were completely dry. Three days thereafter, we added fresh Volvic medium with and without Cryptomonas sp. as food to each well. We used nine replicates with Cryptomonas sp. added and three without. Ciliates were monitored at least once per day during the first week and occasionally thereafter.
Response to pH
We investigated the growth and survival of Bromeliothrix metopoides over pH ranging from 4.0 to 9.0; pH was measured using a microprocessor pH-mV metre (WTW, Weilheim, Germany, model pH 526) to the nearest 0.01 unit. The pH sensor was 2-point calibrated with standard buffer solutions of pH = 6.87 and pH = 9.18 before each series of measurement. Ciliates and their prey (provided at satiating amounts) were acclimated to the experimental conditions in steps of 0.5 pH unit change d−1 for 2–5 d. We measured and adjusted the pH in each experimental container twice per day, i.e. if the pH differed by more than 0.2 from the target pH, it was adjusted by addition of small amounts (15–35 μL) of 0.1 mol L−1 NaOH or HCl (Weisse et al. 2007, 2013b; Weisse and Stadler 2006). The general experimental design followed that of the growth experiments.
Except for the experimental series with small and large Polytomella sp. as food organisms (results reported in Fig. 4), all experiments described above were performed in triplicate. Results reported in the text and figures are mean values ±1 standard deviation (SD).
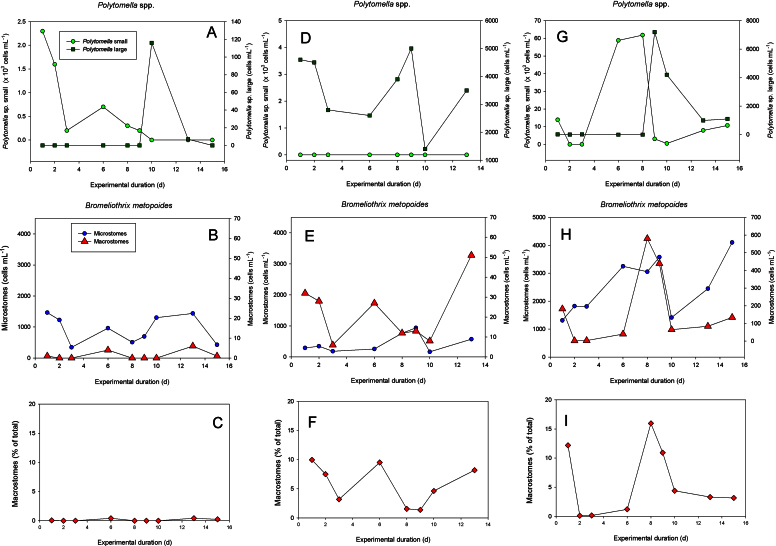
Fig. 4.
Population development of the ciliate Bromeliothrix metopoides fed with small and large forms of the flagellate Polytomella sp. in three different treatments (A–C, D–F, G–I); the treatments differed in prey concentration and the relative contribution of the two flagellate morphotypes. Panels A, D and G show the abundance of the two flagellate forms; graphs B, E and H show cell numbers of the ciliate with its two different sizes (microstomes and macrostomes). The bottom panels (C, F and I) report the occurrence of macrostomes as percentage of the total ciliate abundance.
Data analysis
Ciliate growth rate (μ) was calculated from changes in cell numbers, assuming exponential growth over the experimental period according to
| (2) |
where N0 and Nt are ciliate numbers at the beginning and end of the experimental period, respectively. Please note that ‘experimental period’ does not denote the total duration of an experiment, but specific periods of 1–4 d each during which the ciliate population increased exponentially. Details are reported in Results.
Ciliate growth rates were related to the geometric mean prey concentration (P) during the experimental period (Frost 1972; Heinbokel 1978) according to Eq. (3):
| (3) |
where P0 and Pt are the initial and final prey concentrations (cells L−1) during incubations.
Ciliate growth rates were fit to Eq. (4), which includes a positive x-axis intercept, using the Marquardt–Levenberg algorithm (SigmaPlot, SPSS Inc., Chicago, USA).
| (4) |
where μ = growth rate, μmax = maximum growth rate, P = prey concentration (Eq. (3)), k = a constant, x′ = the x-axis intercept (i.e. threshold concentration, where μ = 0). This equation is similar to the Michaelis–Menten model and Holling's type II functional response (Holling 1959), but assumes a positive x-axis intercept where population growth equals mortality (Weisse et al. 2002). This assumption was based upon our observations from maintenance cultures with B. metopoides that the ciliate needs relatively high food levels to thrive. The numerical response curve has the shape of a saturation curve, i.e. is characterized by an initial linear or nearly linear increase at low food concentrations (initial slope) that levels off asymptotically to the constant maximum growth rate. Our data mainly fell into the linear part of the curve; accordingly, we also calculated least-squares linear regression (Eq. (5)) to calculate the x-axis intercept:
| (5) |
where a is a constant and b is the slope of the regression.
Results
Response to flagellate and bacterial food – induction of macrostomes
The formation of macrostomes of Bromeliothrix metopoides can be induced by feeding with Polytomella sp. (Foissner 2010). Preliminary experiments had shown that the ciliate population (i) increases rapidly when small Polytomella sp. is offered as food in sufficient quantity (>2 × 104 cells mL−1) and (ii) B. metopoides cannot thrive if bacteria are the sole food and bacterial levels fall below ∼108 cells mL−1 (data not shown). Microstome theronts did not feed on Polytomella; we observed trophonts or young macrostomes (∼30–35 μm in length when Lugol's fixed) with single or, in rare cases, two flagellates ingested. Mature macrostomes (>45 μm), in contrast, contained up to ∼20 food vacuoles filled with flagellate cells (Fig. 1G).
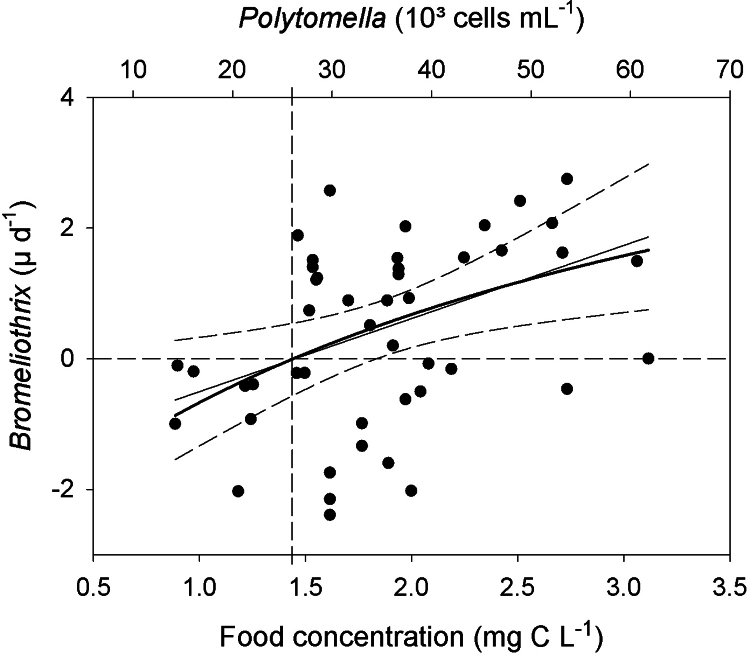
The first detailed growth experiment confirmed these preliminary results. We measured total cell numbers of B. metopoides, the formation of macrostomes, and the bacterial concentrations in the experimental flasks with Polytomella sp. as food and controls without flagellates (Fig. 2). The formation of macrostomes was significantly positively and linearly correlated (r2 = 0.648, n = 64) to the total Bromeliothrix population, i.e. macrostomes were always present in low numbers, corresponding to <1% of the total (Fig. 2A). We observed two peaks of Bromeliothrix at 48 h and 220 h after the beginning of the experiment. Growth rates calculated for the periods of maximum increase (μmax) were 2.4 d−1 (between 20 and 44 h), respectively 2.1 d−1 (between 194 and 218 h). Growth rates were positively related to Polytomella sp. biomass (Fig. 3). The non-linear fit (Eq. (4)) yielded a slightly better, but still poor overall curve fit than the least-squares linear regression calculated from Eq. (5) (r2 = 0.148, respectively r2 = 0.143). Both curve fits indicated a significant (P < 0.0001) threshold food level (where μ = 0) of 26,700 cells mL−1, corresponding to ∼1.4 mg C L−1; parameter estimates of the regression equations are reported in the supplementary Table S1.
Fig. 3.
Growth rate (μ) of Bromeliothrix metopoides vs. abundance (top x-axis), respectively biomass (bottom x-axis) of the prey flagellate Polytomella sp. Ciliate growth rates were fit to the non-linear Eq. (4) (thick solid curve) and to a linear regression (thin solid curve and 95% confidence intervals represented by long dashes). The horizontal dashed line indicates zero population growth (μ = 0); the intercept of the vertical dashed line with the x-axes indicates prey biomass (bottom), respectively prey abundance (top) at μ = 0.
The course of the bacterial cell numbers observed in the experimental bottles (Fig. 2B) and controls without flagellates (Fig. 2C) suggests that Bromeliothrix microstomes ingested bacteria and macrostomes fed on Polytomella (Fig. 2B). However, B. metopoides declined both in the experimental bottles (Fig. 2A) and in the controls without Polytomella (Fig. 2C) if bacterial levels fell below ∼0.9 × 108 cells mL−1 (Fig. 2B, C). This bacterial abundance corresponds to carbon levels of ∼2.3 mg C L−1, i.e. the threshold food level appeared to be higher when bacteria were used as the primary food. We did not observe macrostomes in the controls without flagellates, but cannot rule out that they were present in low numbers below the detection limit (1 cell mL−1).
We had observed in our routine cultures that the flagellate Polytomella sp. occurs in two morphs; a dominant small form (∼10 × 8 μm in length and width) used in the previous experiments and a distinctly larger form (up to ∼40 × 32 μm, Foissner 2010). The ratio between these two forms was variable in different treatments, but it also seemed to fluctuate with time in a single treatment. Accordingly, we monitored the abundance of B. metopoides in relation to the occurrence of both Polytomella sp. morphs in several experimental flasks over a period of two weeks (Fig. 4). In the first experimental treatment, the abundance of both Polytomella sp. morphs (Fig. 4A) and the ciliates (Fig. 4B) were relatively low, <2 × 103 cells mL−1, and food was kept at low levels due to the ciliate grazing. The average flagellate food level (i.e. without bacteria) was ∼0.1 mg C L−1 in this series. The Bromeliothrix population declined from initially 1464 cells mL−1 to 429 cells mL−1 recorded at the end of the experiment (Fig. 4B). The large Polytomella sp. morph was recorded only once, at 10 d after the beginning of the experiment (Fig. 4A). Macrostomes were present in low numbers (∼1% of the total ciliate population) throughout the experiment (Fig. 4C).
In the next experiment, Polytomella sp. was almost exclusively composed of the large form (Fig. 4D). Due to its larger cell size, the initial flagellate food concentration was ∼1.0 mg C L−1; except for the low level measured on the 10th day of the experiment, food remained >0.6 mg C L−1 throughout this experiment. Both the absolute number (Fig. 4E) and the ratio of macrostomes to microstomes of B. metopoides (Fig. 4F) were higher in this experiment than in the previous one. On average, macrostomes accounted for 5.7 ± 3.4 (SD) % of the total Bromeliothrix population in this experiment.
In a third experiment, the initial numerical ratio between small and large Polytomella sp. was approximately 10:1. We observed a rapid increase in the abundance of the small morph up to ∼60,000 cells mL−1 (∼3.2 mg C L−1), followed by a sudden shift from small to large Polytomella sp. (Fig. 4G); concurrently, cell numbers of B. metopoides macrostomes increased (Fig. 4H). Macrostomes reached 16% of the total B. metopoides population (Fig. 4I) during this period, the highest proportion of macrostomes that we recorded during all our experiments.
Some other food sources tested in our laboratories did not support sustainable growth of B. metopoides. Small (Colpoda steinii, Cyrtolophosis mucicola) and medium-sized (Colpoda maupasi) ciliates from bromeliads and the flagellate Chilomonas sp. were not ingested (Foissner 2010). Our initial attempts to rear B. metopoides with the common flagellate Cryptomonas sp., which is the preferred food of many freshwater ciliates (Weisse 2006), and with the small ciliate Urotricha furcata failed (this study; data not shown). In those experiments, we had tried to mimic dispersal of individual B. metopoides into new freshwater habitats. Individual cells of B. metopoides were transferred into wells of tissue culture plates and provided with flagellate or ciliate food at different levels ranging from ∼102 cells mL−1 to 5 × 104 cells mL−1. The latter corresponds to ∼1.4 mg C L−1, i.e. it is close to the threshold food level obtained in this study with Polytomella sp. as food. In a second step, we investigated the growth performance of B. metopoides with Cryptomonas sp. as food in the same culture flasks as used with Polytomella sp., using an inoculum of several mL (i.e. several hundred cells) of an exponentially growing Bromeliothrix culture. Bacterial abundance was relatively low (3.5–4.6 × 107 cells mL−1) in the experiments with Cryptomonas sp. and did not increase because Eau de Volvic is a poor medium for heterotrophic bacteria.
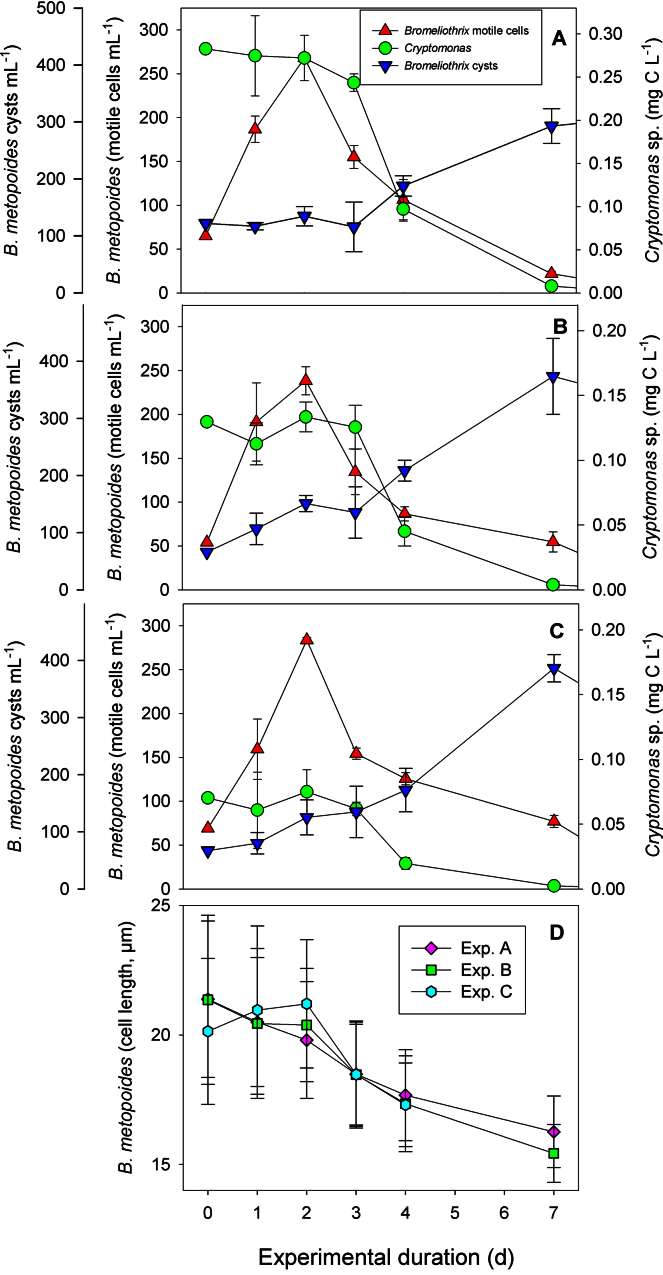
We observed the same pattern over a wide range of flagellate food levels (0.05–2.0 mg C L−1): cell numbers of B. metopoides increased during the first 2 d, then mass encystment began and motile cell numbers declined to zero levels within two weeks. At the lower food levels (0.05–0.3 mg C L−1, corresponding to 0.3–1.2 × 104 cells mL−1), which is typical for most oligotrophic to moderately eutrophic freshwater bodies, Cryptomonas sp. did not support survival of motile B. metopoides cells (Fig. 5A–C). Virtually all motile cells had encysted after one week, i.e. low to moderate Cryptomonas sp. food supply triggered mass encystment of the ciliate. The Cryptomonas sp. levels declined in all experimental containers and in the controls (data not shown) after three days because the experiments were conducted in the dark. Different from the experiments with Polytomella sp., macrostomes were not formed if algae were offered as prey. Another indication that Cryptomonas sp. was not a suitable food organism for B. metopoides is that the ciliate cell size significantly declined in the treatments with Cryptomonas sp. (Fig. 5D).
Fig. 5.
Population development and cell length of the ciliate Bromeliothrix metopoides fed with the flagellate Cryptomonas sp. at three different food levels (A–C). Graphs A–C show the abundance of motile ciliate cells and the occurrence of ciliate cysts, together with the flagellate cell numbers. Cell length of the ciliate in these three treatments is shown in the bottom panel (D).
Response to desiccation
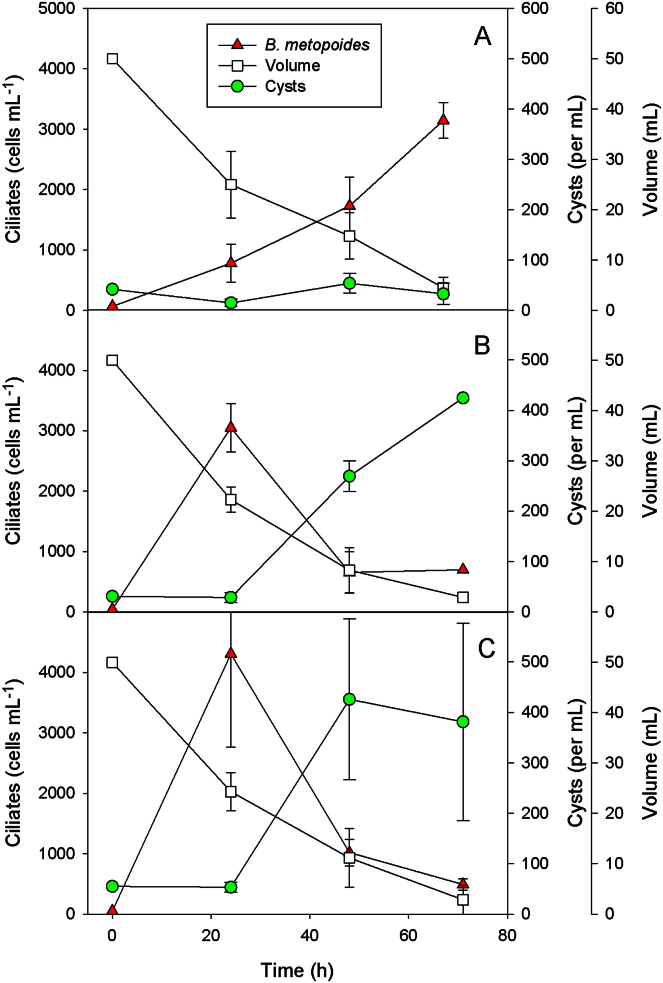
The small tanks of tree bromeliads may dry up occasionally. We, therefore, investigated the response to desiccation of Bromeliothrix metopoides in another experimental series. The initial experimental volume of 50 mL was rapidly reduced to <5 mL over a period of 72 h (Fig. 6). When bacteria were the sole food (treatment A), the ciliate population increased but did not respond with augmented cyst formation (Fig. 6A). Average bacterial concentrations in this experiment increased steadily from 0.22–2.01 × 108 cells mL−1 and were equivalent to >2.0 mg C L−1 during the final 2 d of the experiment (data not shown).
Fig. 6.
Population development of the ciliate Bromeliothrix metopoides fed with heterotrophic bacteria (A), respectively bacteria plus the flagellate Polytomella sp. (B, C) at continuously reduced culture volumes (mimicking desiccation) in three different treatments. Cell numbers of motile ciliates (triangles, left y-axis) and cysts (circles, right y-axis), and the experimental volume (squares, right y-axis offset) are reported in each graph; bacterial and flagellate levels are not shown. The difference between treatments B and C is that only in B the ciliate was fed with the flagellate in the course of the experiments.
With Polytomella sp. as additional food source (treatments B and C), ciliate cell numbers composed of theronts, trophonts and macrostomes increased rapidly during the first 24 h, but then declined (Fig. 6B, C) although food was present in satiating amounts (>2.0 mg C L−1). The treatments reported in Fig. 6B and C differed because only to the three replicates of the treatment shown in Fig. 6B small amounts of fresh Polytomella sp. were added during the experiments. However, because the flagellates grew at high rates and macrostomes were present in low numbers, ciliate feeding had little effect on the flagellate cell numbers. The initial flagellate cell numbers were 3.5 × 104 cells mL−1, final average cell numbers were >5 × 105 cells mL−1 in both experimental series. In contrast to the experimental series with only bacterial food (Fig. 6A), encystment was strongly stimulated, starting 24 h after the beginning of the experiment. The final average cyst number was close to 400 mL−1 (Fig. 6B, C), i.e. almost 50% of the ciliate population were encysted at the end of the experiment.
The ciliates reached high growth rates in the desiccation experiments. The average maximum growth rates (i.e. μmax averaged over the three replicates of each experimental series) recorded for B. metopoides were 2.44 d−1 with bacterial food only (Fig. 6A), respectively 4.26 d−1 and 4.48 d−1 with additional flagellate food. The maximum growth rate recorded in a single treatment was 4.71 d−1, corresponding to one cell division every 3.5 h, respectively 6.8 doublings d−1. Maximum growth rates of Polytomella sp. were lower, reaching 1.84 d−1 at the end of the experiment.
To test for the viability of the cysts, we repeated the desiccation experiment using a 12-well culture tissue plate. On the fourth day of the experiment, small drops remained in most wells. On day 5, all wells were dry. Three days thereafter, we added fresh medium with and without Cryptomonas sp. as food to each well. No ciliates excysted during the first 3 h after the addition of medium (Table 1). Two hours later, motile cells occurred in seven of the nine containers (78%) with food algae, whereas no ciliates had excysted in the treatments without food. Twenty hours after the beginning of this experiment, numerous ciliates swam in the containers with food algae, and motile cells were also encountered in each of the wells without flagellate food. We found the same pattern 24 h and 11 d after the beginning of the experiment. Since the tissue plates were not axenic, bacterial growth may have supported survival of excysted cells.
Table 1.
Total number and percentage of motile Bromeliothrix metopoides cells in 9 containers (wells) with medium and Cryptomonas sp. as food and 3 containers with medium only.
| Time after addition of medium (h) | With Cryptomonas sp. | Without Cryptomonas sp. |
|---|---|---|
| 2.75 | 0 (0%) | 0 (0%) |
| 5.1 | 15 (78%) | 0 (0%) |
| 20 | (100%)a | 24 (100%) |
| 24 | (100%)a | 30 (100%) |
| 264 | (100%)a | (100%)b |
Denotes >20 cells in each well.
Denotes >10 cells in each well.
Response to pH
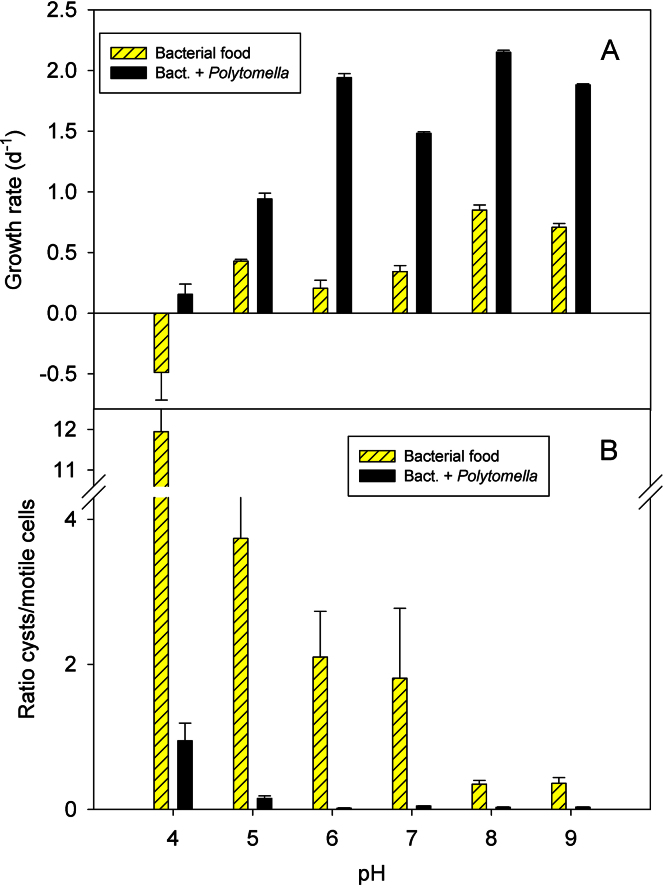
The experiments reported above were all conducted under circumneutral to moderately basic conditions (pH 7.1–8.3). To account for the effect of pH, we investigated the ciliate response to pH over a range of pH from 4 to 9 under satiating food conditions. Average growth rates of Bromeliothrix metopoides were highest at circumneutral conditions (Fig. 7A). Similar to the previous experiments, growth rates measured in the treatments with Polytomella sp. as food were higher than those recorded with bacteria as the sole food (Fig. 7A). However, in contrast to the desiccation experiment reported above, bacteria stimulated encystment (Fig. 7B). Under the most adverse conditions, i.e. a combination of low pH (pH 4) and suboptimal food (bacteria only), >90% of the ciliate population encysted in the course of the experiment. Similar to the previous experiments, the proportion of cysts was low if Polytomella sp. was present and pH was >5 (Fig. 7B).
Fig. 7.
Specific growth rate (A) and relative occurrence of cysts (B) of the ciliate Bromeliothrix metopoides vs. pH when fed with bacteria only or with a combination of flagellate (Polytomella sp.) and bacterial food.
Discussion
Bromeliothrix metopoides is an extreme r-strategist
Taken together, our experiments characterize Bromeliothrix metopoides as an extreme r-strategist, doubling more than six times per day under favourable conditions. Doubling time ≤5 h has already been recorded by Foissner (2010) for this species. Its growth rates belong to the fastest ones reported for free-living ciliates in the literature (reviewed by Fenchel 1987; Lynn 2008). The ciliate can increase its numerical population size more than tenfold within 24 h because of its peculiar life cycle: rapid cell divisions by microstome theronts, coupled with the formation of division chains, and the switch of bacterial feeding theronts to flagellate-feeding trophonts and macrostomes. We did not observe that B. metopoides divides in cysts, as it is known from other colpodid ciliates (Foissner 1993; Gutiérrez et al. 2001).
Growth rates presented in Fig. 3 were derived from time course experiments, i.e. they do not represent typical numerical response experiments where the predator was acclimated to prey for several generations (Weisse et al. 2002). Accordingly, nutritional history may have influenced the growth rate: if the ciliate had plenty of food the day before it might have grown faster than if exposed to the same mean prey concentration after acclimation. This bias may explain the high scatter around the threshold level (Fig. 3). Our attempts to acclimate B. metopoides to sub-saturating food levels below μmax failed because we could not keep the flagellate prey levels sufficiently constant. Accordingly, the lower growth rates obtained in the pH experiments at saturating food levels (Fig. 7) may more realistically represent sustainable μmax comparable to those obtained in numerical response experiments. On the other hand, the latter underestimate the ability of B. metopoides to rapidly increase its population size; the >5 cell divisions per day that we observed repeatedly are ecologically important. Shortest generation times recorded for the small terrestrial colpodeans Colpoda aspera and Grossglockneria acuta were 4.1 h, respectively 3.8 h (Lüftenegger et al. 1985), i.e. close to the shortest generation time presented in this study for B. metopoides (3.5 h).
Concerning the induction of macrostomes, our experimental results do not provide an unequivocal answer. The experimental series reported in Fig. 4 revealed that, in accordance with our initial hypothesis, the proportion of macrostomes is higher if larger food items (i.e., Polytomella sp., in particular the large morph) prevail. The results shown in Fig. 4G–I and our unpublished observations made during the routine culturing of B. metopoides with Polytomella sp. suggest that it may also be the flagellate that responds to the ciliate grazing pressure by forming large cells, which are inedible to the majority of the B. metopoides population. Accordingly, the presence of B. metopoides macrostomes and of the large Polytomella morph may both be indicative of heavy grazing pressure on the small Polytomella morph.
We observed repeatedly that the population size of B. metopoides may not only increase rapidly, but can also drastically decline within 24 h. For instance, in the experiment reported in Fig. 2A, the ciliate population was reduced twice by a factor of 5 within 24 h, equivalent to negative population growth rates of 2.1 d−1. This was not compensated by a switch to mass encystment. The desiccation experiments revealed that up to ∼40% of the total ciliate population may encyst within 24 h at circumneutral pH (Fig. 6B, C). Considering that we observed rapid declining of B. metopoides under food-replete conditions, reduction in its population size may be even stronger if food becomes limiting and/or other environmental stressors such as a shift to low pH deteriorate its habitat. We conclude that B. metopoides leads a boom and bust existence in its natural habitat.
Bromeliothrix metopoides has high food requirements typical of edaphic ciliates
Irrespective of the food source and its feeding mode (carnivorous macrostomes vs. bactivorous microstomes), our experimental results showed that B. metopoides has high threshold food concentrations for positive population growth. The results shown in Fig. 2 and similar results obtained in a preliminary experiment (data not shown) demonstrate that in the presence of moderate bacterial levels (<108 cells mL−1), the ciliate population needs >104 Polytomella sp. cells to increase in numbers. Based upon the measured size of the flagellate, we calculated an average cellular carbon content of 54 pg C for the small Polytomella (see section “Material and Methods”). Accordingly, the threshold food concentration is ∼1.4 mg C L−1 if the flagellates were the sole food. The question is, if large trophonts and macrostomes, which are the only stages in the life cycle of B. metopoides that feed upon Polytomella sp. (Foissner 2010; this study), can cause the decline in prey abundance observed in our experiments. If we assume that each macrostome contained, on average, eight flagellate cells in its food vacuoles and that the mean food vacuole life time is 2 h (Kenter et al. 1996; Verni and Gualtieri 1997; Verni and Rosati 1992), 500 macrostome B. metopoides mL−1 would ingest 48,000 Polytomella cells mL−1 d−1. Apparently, this calculation is in agreement with our experimental results (Fig. 4G, H)
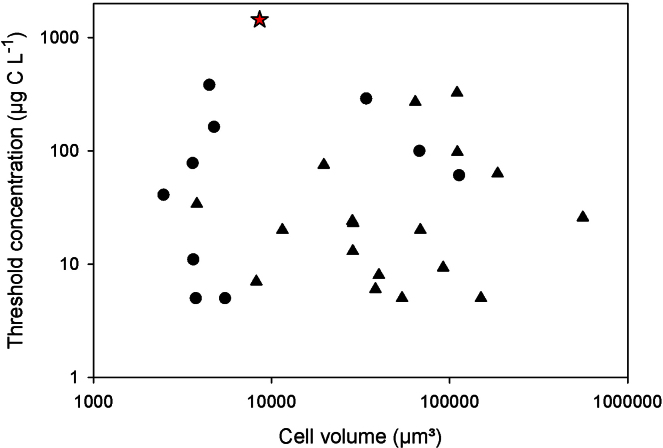
When bacteria were the only food, the threshold was close to 108 cells mL−1 (Fig. 2C). The bacterial threshold biomass of ∼2.3 mg C L−1 for B. metopoides microstomes feeding on bacteria is even higher than the threshold estimated above for macrostomes. Although the calculation of carbon levels from measured cellular abundances may provide only a crude approximation of the actual carbon concentration, these estimates are distinctly higher than any other food thresholds calculated for various aquatic ciliates under comparable experimental conditions (reviewed by Weisse 2006). Most threshold levels reported in the literature fall into the range <0.01–0.1 mg C L−1, and saturation is typically reached in the range 0.4–0.8 mg C L−1. None of the 28 case studies with marine and freshwater ciliates summarized by Weisse (2006) reported threshold food levels >1 mg C L−1 (Fig. 8). Notably, the threshold level of Glaucomides bromelicola, which is sympatric with B. metopoides (Dunthorn et al. 2012), falls into the ‘normal’ range reported for small and medium sized ciliates (Weisse et al. submitted).
Fig. 8.
Threshold food concentrations (i.e., where population growth rate is zero) of various freshwater (circles) and marine (triangles) ciliates (modified from Weisse 2006) and Bromeliothrix metopoides (star) used in this study.
We cannot rule out that there may be other bacteria than those used in our experiments that are more suitable food; however, the bacterial flora was enriched from the original habitat. Similarly, other flagellates than Polytomella sp. may sustain populations of B. metopoides in the tank bromeliads. We have demonstrated that, although Cryptomonas sp. may stimulate excystment (Tab. 1), it is not a suitable food source enabling sustainable growth of B. metopoides. The same applies to the flagellates Chilomonas sp. (Foissner 2010) and Goniomonas truncata (W. Foissner, unpubl.).
It remains to be investigated if B. metopoides is dependent on specific chemical properties that acetate flagellates such as Polytomella sp. (de la Cruz and Gittleson 1981) may possess and phototrophic flagellates such as Cryptomonas sp. do not.
The Neotropical phytotelmata ciliate fauna is characterized by the lack of typical and common freshwater genera such as Coleps, Colpidium, Frontonia, Glaucoma, Nassula, Paramecium, Stylonychia and Trithigmostoma (Dunthorn et al. 2012). Similarly, typical soil ciliates are rare in bromeliad tanks (Foissner et al. 2003). These authors concluded that the ciliate fauna in bromeliads evolved from cosmopolitan pond and moss biota. Several euryoecious species of the genus Colpoda were regularly found in bromeliad phytotelmata (Foissner et al. 2003). Colpodeans typically live in astatic humid environments associated with mosses, forest litters, soil and meadow puddles, and tree holes (reviewed by Lynn 2008), i.e. they are considered ‘terrestrial’ or ‘edaphic’ ciliates that may thrive in small water bodies. The smaller colpodeans are primary bacterivorous, fast growing r-strategists with doubling times <6 h (Chessa et al. 1983; Drake and Tsuchiya 1977; Lüftenegger et al. 1985). Colpoda steinii fed with Escherichia coli achieved maximum growth rates when bacterial abundance reached 1 × 108 cells mL−1 (Drake and Tsuchiya 1977); Kracht (1982, University of Hamburg, Germany, unpublished dissertation) measured maximum division rates of C. cucullus fed with E. coli at bacterial levels ranging from 4 × 108 cells mL−1 to 1.3 × 109 cells mL−1. Investigating patterns of colonization experimentally as a function of distance from a pond, Maguire (1963) observed that the genus Colpoda was quickly eliminated and prevented from reinvading the environment by Paramecium. Summarizing the scarce literature in his dissertation, Kracht concluded that the competitive ability of C. cucullus is low, relative to other common ciliates. Obviously, the growth and feeding characteristics that we described in this investigation for B. metopoides fits into this general scenario known from colpodean ciliates.
Survival in its natural habitat and competitive ability in other environments
The interpretation of the results presented in this study is hampered by the fact that the specific environmental conditions encountered in the tank bromeliads remain largely unknown. For instance, in the state of Rio de Janeiro, Brazil, Marino et al. (2011) reported typical seasonal temperature variation in the tanks of bromeliads of 10 °C; however, maximum temperature recorded was as high as 54.9 °C. These authors also concluded that each bromeliad species represents a set of physical and chemical characteristics. Considering bromeliad diversity in the vast area in Central and South America where Bromeliothrix metopoides occurred (Dunthorn et al. 2012; W. Foissner, unpubl. observations), a detailed survey of the environment was impossible during this investigation.
Although we have investigated the response of B. metopoides to only a limited number of the major environmental variables, severe constraints for the survival of this species became obvious. First, the earlier observation that this a ‘weed species’ that grows in all media containing bacteria and medium-sized flagellates (Foissner 2010), must be interpreted with some caution. Bacterial abundance ≥108 cells mL−1 is common in media rich in nutrients and organics, but is rarely found in natural freshwater habitats. The scarce data currently available point to mean bacterial levels ranging from 1 to 10 × 106 cells mL−1 in bromeliad phytotelmata (Brouard et al. 2011, 2012). However, Janetzky (1997, University of Oldenburg, Germany, unpublished dissertation) reported a bacterial peak abundance of 1.3 × 108 cells mL−1 from bromeliads in Jamaica. A burst in bacterial numbers may, for instance, follow the input of organic-rich material (due to decomposition of leaf litter, carcasses of insects and other small metazoans). Although the water in the reservoirs is primarily acidic and the nutrient supply indicative of impoverished rather than nutrient-replete conditions (Laessle 1961; Lopez et al. 2009; Marino et al. 2011), chlorophyll a maxima close to 40 μg L−1 have been reported (Marino et al. 2011). Accordingly, occasional inputs of large amounts of organic matter and nutrients leading to short-term eutrophic conditions appear likely (Lopez et al. 2009).
The rapid formation of cysts enables B. metopoides, similar to many other ciliates (Lynn 2008), to survive unfavourable environmental conditions. We tested its response to low pH and fast desiccation. Our results showed that 10–50% of the population may potentially survive as cysts if the reservoir dries up and pH is close to neutral conditions. Its pH response (Fig. 7A) characterizes B. metopoides as a neutrophil or acidotolerant species, which reaches high growth rates over the pH range commonly encountered (mostly moderately acidic; Laessle 1961) in the phytotelmata of bromeliads. Our results measured at circumneutral and basic (pH 8–9, Fig. 7A) conditions demonstrated that pH alone does not restrict the occurrence of B. metopoides to acidic conditions. Mass encystment, when >70% of the population encysted within 24 h, was triggered by unpalatable food (Cryptomonas sp., Fig. 5A–C) and by a combination of suboptimal food (bacteria) and low pH (Fig. 7B). This supports our initial hypothesis that, although food may be the most important trigger of (mass) encystment, other environmental clues contribute to the formation of cysts.
We observed regularly spontaneous excystment in our cultures and could stimulate excystment by adding growth medium to freshly produced cysts (Table 1). However, we can only speculate about how many of the cysts may be able to excyst in situ and build a new population once the original habitat returns to more favourable conditions or if the cysts are dispersed into a new, suitable environment. To mimic the latter situation, we offered culture medium with and without non-axenic Cryptomonas sp. as food to B. metopoides cysts that were formed when the culture dried up. Our observations on excystment of fresh (3–4 d old) cysts formed upon desiccation revealed that (i) the majority of cysts remained viable and (ii) B. metopoides may excyst even if in the presence of little food. Food supply is important to enable the excysted cells to proliferate. Recent experimental work with another colpodid ciliate, Colpoda inflata, yielded that 2–68% of the cysts may excyst after a period of six weeks of desiccation at a residual soil moisture of 9% (Müller et al. 2010). However, we did not try to stimulate excystment more systematically, because (i) too little is known about the rapidly changing environmental conditions in the tank bromeliads and (ii) laboratory investigations on the encystment/excystment cycle are generally of limited value to predict the situation in situ (Foissner 2006; Müller 2007; Müller et al. 2010).
Cannibalism, which may also be a successful strategy to survive shorter periods of food depletion, was never observed in B. metopoides (Foissner 2010; this study). Our results demonstrate that viability of motile cells is sensitive to changing environmental conditions. We conclude that cyst formation is the only option for this ciliate to survive in a given habitat with fluctuating food supply and to disperse to new habitats.
Conclusions
We conclude that the competitive ability of B. metopoides is heavily compromised in most natural environments because it needs threshold food concentrations that are rarely met in most habitats. Secondly, specific demands in terms of food quality may further restrict the occurrence of this species to its peculiar habitat. Thirdly, competition experiments performed in parallel to the present study (Weisse et al. submitted) suggest that B. metopoides is sensitive to competition at or above its food threshold. Even though vulnerability of this species to predation and parasitism needs to be explored, the results presented in this study support our third hypothesis: the ecological niche of this ciliate is unusually narrow. The ability of B. metopoides to form cysts enabled its survival only under some adverse conditions (poor food and low pH), while other unfavourable combinations (desiccation and poor food) did not trigger enhanced cyst formation. This species has reached a narrow peak along its fitness landscape (Gavrilets 2004; Wright 1932); any deviation from the optimum conditions will lead to lower fitness. Corollaries of our findings are that (i) B. metopoides is not viable in the vast majority of aquatic habitats and that (ii) the exceptionally high food concentrations needed by B. metopoides would be regularly met in the reservoir of tree bromeliads. More research is needed to test the latter conclusion.
Acknowledgements
This study was financially supported by the Austrian Science Fund (FWF, project P20360-B17). We thank in particular one anonymous reviewer for constructive comments on the article.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ejop.2013.02.001.
Appendix A. Supplementary data
The following are supplementary data to this article:
References
- Armbruster P., Hutchinson R.A., Cotgreave P. Factors influencing community structure in a South American tank bromeliad fauna. Oikos. 2002;96:225–234. [Google Scholar]
- Brouard O., Céréghino R., Corbara B., Leroy C., Pelozuelo L.t., Dejean A., Carrias J.-F. Understorey environments influence functional diversity in tank-bromeliad ecosystems. Freshwater Biol. 2012;57:815–823. [Google Scholar]
- Brouard O., Le Jeune A.-H., Leroy C., Cereghino R., Roux O., Pelozuelo L.t., Dejean A., Corbara B., Carrias J.-F. Are algae relevant to the detritus-based food web in tank-bromeliads? PLoS ONE. 2011;6:e20129. doi: 10.1371/journal.pone.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrias J.F., Cussac M.-E., Corbara B. A preliminary study of freshwater protozoa in tank bromeliads. J. Trop. Ecol. 2001;17:611–617. [Google Scholar]
- Chessa M.G., Zoncheddu A., Accomando R., Crippa Franceschi T. Sintesi di RNA e DNA nei nuclei isolati di Colpoda cucullus (protozoi ciliati) Bollet. Soc. Ital. Biol. Sperimentale. 1983;59:1141–1148. [PubMed] [Google Scholar]
- de la Cruz V.F., Gittleson S.M. The genus Polytomella: a review of classification, morphology, life cycle, metabolism, and motility. Arch. Protistenkd. 1981;124:1–28. [Google Scholar]
- Drake J.F., Tsuchiya H.M. Growth kinetics of Colpoda steinii on Escherichia coli. Appl. Environ. Microbiol. 1977;34:18–22. doi: 10.1128/aem.34.1.18-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunthorn M., Stoeck T., Wolf K.W., Breiner H.-W., Foissner W. Diversity and endemism of ciliates inhabiting Neotropical phytotelmata. Syst. Biodivers. 2012;10:195–205. [Google Scholar]
- Fenchel T. Science Tech./Springer; Madison, WI: 1987. Ecology of Protozoa. The Biology of Free-Living Phagotrophic Protists. [Google Scholar]
- Foissner W. G. Fischer; Stuttgart: 1993. Colpodea (Ciliophora) [Google Scholar]
- Foissner W. Morphology and ontogenesis of Bromeliophrya brasiliensis gen. n., sp. n., a new ciliate (Protozoa: Ciliophora) from Brazilian tank bromeliads (Bromeliaceae) Acta Protozool. 2003;42:55–70. [Google Scholar]
- Foissner W. Morphology and ontogenesis of Lambornella trichoglossa nov. spec., a new tetrahymenid ciliate (Protozoa, Ciliophora) from Brasilian tank bromeliads (Bromeliaceae) Eur. J. Protistol. 2003;39:63–82. [Google Scholar]
- Foissner W. Biogeography and dispersal of microorganisms: a review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- Foissner W. Life cycle, morphology, ontogenesis, and phylogeny of Bromeliothrix metopoides nov. gen., nov. spec., a peculiar ciliate (Protista, Colpodea) from tank bromeliads (Bromeliaceae) Acta Protozool. 2010;49:159–193. [PMC free article] [PubMed] [Google Scholar]
- Foissner W. Description of Glaucomides bromelicola n. gen., n. spec. (Ciliophora, Terahymenida), a macrostome forming inhabitant of bromeliads (Bromeliaceae), including redescriptions of Glaucoma scintillans and G. reniformis. J. Eukaryot. Microb. 2013;60:137–157. doi: 10.1111/jeu.12016. [DOI] [PubMed] [Google Scholar]
- Foissner W., Strüder-Kypke M., van der Staay G.W.M., Moon-van der Staay S.-Y., Hackstein J.H.P. Endemic ciliates (Protozoa, Ciliophora) from tank bromeliads (Bromeliaceae): a combined morphological, molecular, and ecological study. Eur. J. Protistol. 2003;39:365–372. [Google Scholar]
- Foissner W., Wolf K.W. Morphology and ontogenesis of Platyophrya bromelicola nov. spec.,a new macrostome-forming colpodid (Protists, Ciliophora) from tank bromeliads of Jamaica. Eur. J. Protistol. 2009;45:87–97. doi: 10.1016/j.ejop.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Foissner W., Wolf K.W., Yashchenko V., Stoeck T. Description of Leptopharynx bromelicola n. sp. and characterization of the genus Leptopharynx Mermod. 1914 (Protista, Ciliophora) J. Eukaryot. Microbiol. 2011;58:134–151. doi: 10.1111/j.1550-7408.2011.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B.W. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus. Limnol. Oceanogr. 1972;17:805–815. [Google Scholar]
- Gavrilets S. Princeton University Press; Princeton: 2004. Fitness Landscapes and the Origin of Species. [Google Scholar]
- Gutiérrez J.C., Callejas S., Borniquel S., Benítez L., Martín-González A. Ciliate cryptobiosis: a microbial strategy against environmental starvation. Int. Microbiol. 2001;4:151–157. doi: 10.1007/s10123-001-0030-3. [DOI] [PubMed] [Google Scholar]
- Heinbokel J.F. Studies on the functional role of tintinnids in the Southern California Bight. I. Grazing and growth rates in laboratory cultures. Mar. Biol. 1978;47:177–189. [Google Scholar]
- Holling C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959;91:293–320. [Google Scholar]
- Kenter U., Zimmermann U., Müller H. Grazing rates of the freshwater ciliate Balanion planctonicum determined by flow cytometry. J. Plankton Res. 1996;18:1047–1053. [Google Scholar]
- Kitching R.L. Cambridge University Press; Cambridge, UK: 2000. Food Webs and Container Habitats. [Google Scholar]
- Laessle A.M. A micro-limnological study of Jamaican bromeliads. Ecology. 1961;42:499–517. [Google Scholar]
- Lopez L.C.S., Da Nóbrega Alves R.R., Rios R.I. Micro-environmental factors and the endemism of bromeliad aquatic fauna. Hydrobiologia. 2009;625:151–156. [Google Scholar]
- Lüftenegger G., Foissner W., Adam H. r- and K-selection in soil ciliates: a field and experimental approach. Oecologia. 1985;66:574–579. doi: 10.1007/BF00379352. [DOI] [PubMed] [Google Scholar]
- Lynn D.H. 3rd ed. Springer; Dordrecht: 2008. The Ciliated Protozoa – Characterization, Classification, and Guide to the Literature. [Google Scholar]
- Maguire B.J. The exclusion of Colpoda (Ciliata) from superficially favorable habitats. Ecology. 1963;44:781–784. [Google Scholar]
- Marino N., Guariento R., Dib V., Azevedo F., Farjalla V. Habitat size determine algae biomass in tank-bromeliads. Hydrobiologia. 2011;678:191–199. [Google Scholar]
- Menden-Deuer S., Lessard E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000;45:569–579. [Google Scholar]
- Moser M., Weisse T. Combined stress effect of pH and temperature narrows the niche width of flagellates in acid mining lakes. J. Plankt. Res. 2011;33:1023–1032. doi: 10.1093/plankt/fbr014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. Live observation of excystment in the spirotrich ciliate Meseres corlissi. Eur. J. Protistol. 2007;43:95–100. doi: 10.1016/j.ejop.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Müller H., Achilles-Day U.E.M., Day J.G. Tolerance of the resting cysts of Colpoda inflata (Ciliophora, Colpodea) and Meseres corlissi (Ciliophora, Spirotrichea) to desiccation and freezing. Eur. J. Protistol. 2010;46:133–142. doi: 10.1016/j.ejop.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Omar A., Foissner W. Description of Leptopharynx bromeliophilus nov. spec. and Leptopharynx australiensis nov. spec. (Ciliophora, Nassulida) Acta Protozool. 2011;50:89–103. [Google Scholar]
- Omar A., Foissner W. Description of Leptopharynx brasiliensis nov. spec. and Leptopharynx costatus gonohymen nov. subspec. (Ciliophora, Microthoracida) Eur. J. Protistol. 2012;48:30–47. doi: 10.1016/j.ejop.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim E.G. The genus Polytomella. J. Protozool. 1955;2:137–145. [Google Scholar]
- Schönborn W. Schweizerbart; Stuttgart: 2003. Lehrbuch der Limnologie. [Google Scholar]
- Troussellier M., Bouvy M., Courties C., Dupuy C. Variation of carbon content among bacterial species under starvation condition. Aquat. Microb. Ecol. 1997;13:113–119. [Google Scholar]
- Varga L. Ein interessanter Biotop der Biocönose von Wasserorganismen. Biologisches Zentralblatt. 1928;48:143–162. [Google Scholar]
- Verni F., Gualtieri P. Feeding behaviour in ciliated protists. Micron. 1997;28:487–504. [Google Scholar]
- Verni F., Rosati G. A comparative study of digestion in a raptorial ciliate and in the facultative carnivorous form of a filter feeding ciliate. Tissue Cell. 1992;24:443–453. doi: 10.1016/0040-8166(92)90060-k. [DOI] [PubMed] [Google Scholar]
- Weisse T. Freshwater ciliates as ecophysiological model organisms - lessons from Daphnia, major achievements, and future perspectives. Arch. Hydrobiol. 2006;167:371–402. [Google Scholar]
- Weisse T., Kirchhoff B. Feeding of the heterotrophic freshwater dinoflagellate Peridiniopsis berolinense on cryptophytes: analysis by flow cytometry and electronic particle counting. Aquat. Microb. Ecol. 1997;12:153–164. [Google Scholar]
- Weisse, T., Laufenstein, N., Weithoff, G., 2013a. Multiple environmental stressors confine the ecological niche of the rotifer Cephalodella acidophila. Freshwater Biol. 58, http://dx.doi.org/10.1111/fwb.12104, in press. [DOI] [PMC free article] [PubMed]
- Weisse T., Moser M., Scheffel U., Stadler P., Berendonk T., Weithoff G., Berger H. Systematics and species-specific response to pH of Oxytricha acidotolerans sp. nov. and Urosomoida sp. (Ciliophora, Hypotricha) from acid mining lakes. Eur. J. Protistol. 2013;59:255–271. doi: 10.1016/j.ejop.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisse T., Scheffel U., Stadler P., Foissner W. Local adaptation among geographically distant clones of the cosmopolitan freshwater ciliate Meseres corlissi. II. Response to pH. Aquat. Microb. Ecol. 2007;47:289–297. [Google Scholar]
- Weisse T., Stadler P. Effect of pH on growth, cell volume, and production of freshwater ciliates, and implications for their distribution. Limnol. Oceanogr. 2006;51:1708–1715. [Google Scholar]
- Weisse T., Stadler P., Lindström E.S., Kimmance S.A., Montagnes D.J.S. Interactive effect of temperature and food concentration on growth rate: A test case using the small freshwater ciliate Urotricha farcta. Limnol. Oceanogr. 2002;47:1447–1455. [Google Scholar]
- Wright S. The roles of mutation, inbreeding, crossbreeding and selection in evolution. Int. Congr. Genetics. 1932;6:356–366. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.