Abstract
A mutant of herpes simplex virus type 1 lacking both glycoprotein M and glycoprotein E was marginally compromised in terms of its in vitro growth characteristics. This finding is in marked contrast to a similar mutant of the related alphaherpesvirus, pseudorabies virus (A. R. Brack, J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter, J. Virol. 73:5364-5372, 1999), and suggests that the glycoprotein requirements for virion assembly may vary among different members of this family of viruses.
All herpesviruses encode a number of membrane glycoproteins, many of which are nonessential for virus viability in vitro. While some of these molecules are unique to specific members of the herpesvirus subfamilies, it is notable that glycoprotein M (gM) and gN (which form a complex in a number of different herpesviruses analyzed to date [6, 7, 9, 10, 17]) are conserved in all alpha-, beta-, and gammaherpesviruses, suggesting that there may be conservation of function of these glycoproteins in some common aspect of the virus life cycle. Several groups have characterized the growth properties of mutant viruses which lack gM, and in herpes simplex virus type 1 (HSV1) (12), equine herpesvirus 1, (14) and pseudorabies virus (PRV) (3), the absence of functional gM results in only marginal phenotypic differences, a somewhat surprising finding given its conservation over a 200-million-year evolutionary period.
However, recent observations by Brack et al. (2) have highlighted the fact that the functions of some nonessential herpesvirus glycoproteins may be at least partially redundant and in some cases will only become apparent in the absence of two independent functional units. These workers noted that a triple mutant of PRV with deletions in the genes encoding the gEgI complex and gM was severely compromised at a late stage of virion morphogenesis; cells infected with this virus contained large numbers of unenveloped capsids in the cytoplasm and virtually no extracellular enveloped particles, whereas PRV mutants lacking either gEgI or gM alone produced more or less wild-type levels of mature virions. The phenotype of the triple mutant may be reversed by supplying either gM or gE in trans.
Given these findings, it was therefore of interest to determine whether deletion of the gM counterpart of another alphaherpesvirus, HSV1, in the context of a virus which also did not contain gE, resulted in a similar defect in virion morphogenesis to that reported for PRV.
HSV1 mutant viruses were derived from strain SC16 by standard procedures of homologous recombination. A gE-negative mutant (SCgE−luc) was constructed by the insertion of a luciferase expression cassette within the US8 gene at nucleotide 141612 of the HSV1 genome (13). A mutant virus lacking gM (SCgM−lacZ) was derived by the insertion of a lacZ expression cassette at codon 381 in the UL10 gene using plasmid pC78.1 (described by MacLean et al. [11]). We generated a double mutant containing insertions in both the gE and gM genes by cotransfection of SCgE−luc-infected cell DNA with pC78.1, followed by identification and purification of a plaque which stained blue in the presence of 5-bromo-4-chloro-3-β-d-galactopyranoside (X-Gal). Lack of expression of gE and gM was confirmed by immunoprecipitation (for gE) and immunofluorescence (for gM). This recombinant was named SCgE−M−. Stocks of all viruses were propagated in Vero cells, and purified virions were prepared by centrifugation on Ficoll gradients as described by Rodger et al. (15). Virus particle numbers were estimated by comparison with latex particles of known concentrations by using negatively stained preparations (16), and the particle-to-infectivity ratios of preparations of SC16, gE−, gM−, and gE−gM− were 45, 40, 97, and 78, respectively. Given the errors inherent to this type of analysis, the twofold range seen in these values is probably not significant, and the values fall within the range previously reported for wild-type strains of HSV1 (1, 5); these data suggest that deletion of gE alone, gM alone, or gE and gM in combination from HSV1 has no discernible effect on particle/PFU ratios.
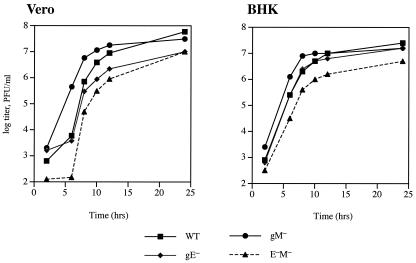
To assess the overall growth characteristics of the gE−, gM−, and gE−gM− viruses in vitro, single-step growth kinetics were examined in both Vero and BHK cells. These experiments were carried out as described by Balan et al. (1), and the results are shown in Fig. 1. In both cell types, we noticed that there were minor differences among the growth characteristics of the three mutant viruses compared to those of the parental strain SC16, but in all cases these differences did not result in more than a 10-fold reduction in the final yields obtained 24 h after infection. No revertant viruses were included in these experiments, and so we cannot rule out the possibility that mutations in other viral genes had arisen during the construction of these recombinants. However, all viruses were readily isolated following homologous recombination, plaque sizes were normal, and we did not have to undertake extensive plaque purification to obtain pure stocks of these viruses. All mutants also expressed wild-type levels of gB, gHL, gD, and gC (data not shown). These data therefore indicate that the phenotype of a gE−gM− mutant of HSV is strikingly different from that of a similar mutant of PRV, which was severely impaired in replication in vitro (2).
FIG. 1.
Single-step growth analysis of recombinant viruses. Monolayers of either Vero cells or BHK-21 cells were infected at 10 PFU/cell with either wild-type or recombinant viruses. The virus titers in the cells and medium combined were determined by plaque assay at various times after infection, and each point represents the mean value of duplicate samples.
The growth deficit of the PRV gE/gI/gM− triple mutant was attributed to a defect in some stage of virion assembly in the cytoplasm. Electron photomicrographs presented by Brack et al. (2) show the presence of large accumulations of nonenveloped virions in the cytoplasm of infected cells, and these appear to be associated with areas of electron-dense material which appear to represent tegument proteins. We therefore wanted to determine whether there was any evidence of similar structures in cells infected with the HSV mutants. Vero and BHK cells were therefore infected at 5 PFU/cell for 10 h with mutant or wild-type viruses and analyzed by electron microscopy. The infected cell monolayers were washed in 0.1 M HEPES and 0.1 M NaCl (pH 7.5) and were then fixed for 3 h at 4°C in 4% glutaraldehyde in 0.1 M HEPES, 2 mM CaCl2 (pH 7), and 0.3% H2O2. The cell pellets were resin embedded, sectioned, mounted on a 300-mesh copper grid, and stained with uranyl acetate and lead citrate. Fifty-nanometer sections were examined by using a CM100 transmission electron microscope. For each sample, 10 cells were analyzed, and the numbers of nuclear capsids, nonenveloped cytoplasmic virions, and enveloped virions in the cytoplasm were counted. All samples were counted blind by two independent observers. The mean numbers per cell of these species were determined, and these data, together with the ratios of nuclear capsids to unenveloped cytoplasmic virions and of nonenveloped to enveloped cytoplasmic virions, are shown in Table 1 and 2. Although there was some variation between the viruses in both cell types, these differences were small. We failed to observe any increase in the number of nonenveloped virions in the cytoplasm of any of the samples analyzed (as reported for PRV), and these data are consistent with the marginal phenotypes of these mutants obtained in one-step growth experiments. The data further highlight the difference between the characteristics of the PRV gE/gI/gM− triple mutant and those of a similar mutant of HSV1 and suggest that an HSV gE−gM− mutant is not compromised at a late stage of virion maturation, as has been shown for PRV.
TABLE 1.
Mean numbers of nonenveloped and enveloped particles per infected Vero cella
| Virus | No. of nuclear capsids | No. of nonenveloped cytoplasmic virions | No. of enveloped cytoplasmic virions | Ratio of nuclear capsids/nonenveloped cytoplasmic virions | Ratio of nonenveloped/enveloped cytoplasmic virions |
|---|---|---|---|---|---|
| Wild type | 24.8 (4.42) | 1.75 (0.34) | 1.75 (0.35) | 0.07 | 1.00 |
| gE− | 33.2 (7.30) | 4.20 (0.55) | 3.70 (1.18) | 0.08 | 1.14 |
| gM− | 48.0 (16.55) | 9.25 (2.68) | 5.75 (2.25) | 0.12 | 1.60 |
| gE− gM− | 51.8 (8.19) | 4.10 (1.58) | 4.00 (0.66) | 0.19 | 1.03 |
The numbers in parentheses represent the standard errors of the mean values.
TABLE 2.
Mean numbers of nonenveloped and enveloped particles per infected BHK cella
| Virus | No. of nuclear capsids | No. of nonenveloped cytoplasmic virions | No. of enveloped cytoplasmic virions | Ratio of nuclear capsids/nonenveloped cytoplasmic virions | Ratio of nonenveloped/enveloped cytoplasmic virions |
|---|---|---|---|---|---|
| Wild type | 23.6 (4.87) | 4.50 (1.52) | 4.00 (0.68) | 0.19 | 1.13 |
| gE− | 27.5 (9.93) | 8.50 (1.55) | 9.80 (1.48) | 0.30 | 0.87 |
| gM− | 16.6 (2.72) | 11.20 (3.13) | 3.20 (0.94) | 0.67 | 3.50 |
| gE− gM− | 21.3 (4.20) | 4.20 (1.25) | 3.50 (0.87) | 0.20 | 1.20 |
The numbers in parentheses represent the standard errors of the mean values.
Our results do not shed any light on the function of HSV gM. However, they highlight the fact that different herpesviruses may have different glycoprotein requirements for the process of virion morphogenesis and final envelopment. It is notable in this regard that gM has been proposed to play a role in the assembly and maturation of the gammaherpesvirus, Epstein-Barr virus (8). It remains a possibility that gM does play a role in HSV assembly but that the functional redundancy seen for PRV gM and gEgI is replaced by another glycoprotein in HSV, and the characterization of further double (or triple) mutant viruses may lead to the resolution of this issue. Indeed, recent studies by Johnson et al. (4), who constructed an HSV1 mutant which lacks both gD and the gEgI heterodimer, support this view; the HSV gD/gE/gI-negative virus shows an in vitro phenotype in terms of a defect in final envelopment which is very similar to that of the gE/gI/gM− triple mutant of PRV, implying that gD and gEgI act in a redundant fashion to mediate cytoplasmic envelopment of HSV. Furthermore, it is likely that future analyses of the phenotypes of such double or triple mutants in a wide range of different cell types will reveal further differences between the proteins required for assembly of the alphaherpesviruses.
Acknowledgments
We thank Jeremy Skepper and staff at the Multi-Imaging Centre, University of Cambridge, for assistance with electron microscopy.
This work was supported by the Wellcome Trust, London, United Kingdom, and by an MRC Cooperative group award.
REFERENCES
- 1.Balan, P., N. Davis-Poynter, S. Bell, H. Atkinson, H. Browne, and T. Minson. 1994. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoprotein gG, gE, gI or the putative gJ. J. Gen. Virol. 75:1245-1258. [DOI] [PubMed] [Google Scholar]
- 2.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dijkstra, J. M., V. Gerdts, B. G. Klupp, and T. C. Mettenleiter. 1997. Deletion of glycoprotein gM of pseudorabies virus results in attenuation for the natural host. J. Gen. Virol. 78:2147-2151. [DOI] [PubMed] [Google Scholar]
- 4.Farnsworth, A., K. Goldsmith, and D. C. Johnson. 2003. Herpes simplex virus glycoproteins gD and gE/gI serve essential but redundant functions during acquisition of the virion envelope in the cytoplasm. J. Virol. 77:8481-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths, A., S. Renfrey, and A. C. Minson. 1998. Glycoprotein-C deficient mutants of two strains of herpes simplex virus type 1 exhibit unaltered adsorption characteristics on polarised or non-polarised cells. J. Gen. Virol. 79:807-812. [DOI] [PubMed] [Google Scholar]
- 6.Jons, A., J. M. Dijkstra, and T. C. Mettenleiter. 1998. Glycoproteins M and N of pseudorabies virus form a disulfide-linked complex. J. Virol. 72:550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyano, S., E. C. Mar, F. R. Stamey, and N. Inoue. 2003. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 84:1485-1491. [DOI] [PubMed] [Google Scholar]
- 8.Lake, C. M., and L. M. Hutt-Fletcher. 2000. Epstein-Barr virus that lacks glycoprotein gN is impaired in assembly and infection. J. Virol. 74:11162-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lake, C. M., S. J. Molesworth, and L. M. Hutt-Fletcher. 1998. The Epstein-Barr virus (EBV) gN homolog BLRF1 encodes a 15-kilodalton glycoprotein that cannot be authentically processed unless it is coexpressed with the EBV gM homolog BBRF3. J. Virol. 72:5559-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mach, M., B. Kropff, P. Dal Monte, and W. Britt. 2000. Complex formation by human cytomegalovirus glycoproteins M (gpUL100) and N (gpUL73). J. Virol. 74:11881-11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLean, C. A., S. Efstathiou, M. L. Elliott, F. E. Jamieson, and D. J. McGeoch. 1991. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J. Gen. Virol. 72:897-906. [DOI] [PubMed] [Google Scholar]
- 12.MacLean, C. A., L. M. Robertson, and F. E. Jamieson. 1993. Characterisation of the UL10 gene product of herpes simplex virus type 1 and investigation of its role in vivo. J. Gen. Virol. 74:975-983. [DOI] [PubMed] [Google Scholar]
- 13.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. NcNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete sequence of the long unique region on the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 14.Osterrieder, N., A. Neubauer, C. Brandmuller, B. Braun, O. R. Kaaden, and J. D. Baines. 1996. The equine herpesvirus 1 glycoprotein gp21/22a, the herpes simplex virus type 1 gM homolog, is involved in virus penetration and cell-to-cell spread of virions. J. Virol. 70:4110-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodger, G., J. Boname, S. Bell, and T. Minson. 2001. The assembly and organisation of glycoproteins B, C, D, and H in herpes simplex virus type 1 particles lacking individual glycoproteins: no evidence for the formation of a complex of these molecules. J. Virol. 75:710-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson, D. H., W. C. Russell, and P. W. Wildy. 1963. Electron microscope particle counts on herpes virus using the phosphotungstate negative staining technique. Virology 19:250-260. [DOI] [PubMed] [Google Scholar]
- 17.Wu, S. X., X. P. Zhu, and G. J. Letchworth. 1998. Bovine herpesvirus 1 glycoprotein M forms a disulfide-linked heterodimer with the UL49.5 protein. J. Virol. 72:3029-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]