Abstract
The dopamine (DA) transporter (DAT) and vesicular monoamine transporter (VMAT2) proteins interact as a biochemical complex to regulate dopaminergic neurotransmission. We have reported that HIV-1Tat1–86 decreases the specific [3H]DA uptake and [3H]WIN 35,428 binding sites without a change in total DAT immunoreactivity in rat striatum (Zhu et al., 2009b). The present study determined the effects of Tat on DAT phosphorylation and trafficking, and vesicular [3H]DA uptake. Pre-incubation of rat striatal synaptosomes with the protein kinase C (PKC) inhibitor bisindolylmaleimide I (1 µM) completely blocked Tat1–86 -induced reduction of [3H]DA uptake, indicating that Tat regulates DAT function through a PKC-dependent mechanism. After exposure of synaptosomes to Tat1–86 (1 µM), DAT immunoreactivity was decreased in plasma membrane enriched fractions (P3) and increased in vesicle-enriched fractions (P4) relative to controls without change in total synaptosomal fractions (P2), suggesting that Tat-induced inhibition of DA uptake is attributable to DAT internalization. Although both DAT and VMAT2 proteins are essential for the regulation of DA disposition in synapse and cytosol, Tat inhibited the specific [3H]DA uptake into vesicles (P4) and synaptosomes (P2) by 35% and 26%, respectively, inferring that the inhibitory effect of Tat was more profound in VMAT2 protein than in DAT protein. Taken together, the current study reveals that Tat inhibits DAT function through a PKC and trafficking-dependent mechanism and that Tat impacts the dopaminergic tone by regulating both DAT and VMAT2 proteins. These findings provide new insight into understanding the pharmacological mechanisms of HIV-1 viral protein-induced dysfunction of DA neurotransmission in HIV-infected patients.
Keywords: dopamine transporter, vesicular monoamine transporter, HIV-1 Tat, protein kinase C, uptake, trafficking
Introduction
The prevalence of HIV-1-associated neurocognitive disorders (HAND) in HIV-1 positive individuals remains high (∼50%), regardless of the success with treatments of anti-retroviral agents effectively controlling viral replication and dramatically improving longevity (Robertson et al., 2007; Tozzi et al., 2007; Ernst et al., 2009). Notably, the incidence and severity of HAND are greatly enhanced (∼70%) due to concomitant use of drugs of abuse such as cocaine (Norman et al., 2009), which has been postulated as a co-morbid factor in the susceptibility and progression of HAND (Larrat and Zierler, 1993; Fiala et al., 1998; Webber et al., 1999; Buch et al., 2011). While HIV-1 virus enters the brain and produces proviral DNA in the early stage of HIV-1 infection (Nath and Clements, 2011), anti-retroviral agents cannot prevent the production of HIV-1 viral proteins in infected brain cells (McArthur et al., 2010; Nath and Clements, 2011). HIV-1 viral proteins, such as Tat, have been detected in brains of patients with HIV-1 infection (Del Valle et al., 2000; Hudson et al., 2000; Lamers et al., 2010), and have been implicated in the pathophysiology of HAND (Li et al., 2009). Moreover, Tat protein has been found to synergize with psychostimulant drugs in producing profound neural and behavioral impairments in preclinical models (Ferris et al., 2008), consistent with the findings in the human HIV-1 positive drug abusing population (Larrat and Zierler, 1993). Unfortunately, there are no promising therapeutic approaches for such HIV-1-associated neurocognitive impairments.
The development of neurocognitive dysfunction in HAND patients is associated with perturbations of the central dopamine (DA) neurotransmission (Kumar et al., 2011; Meade et al., 2011a). The DA system is a clinically relevant HAND target as evidenced by recent human imaging (Chang et al., 2008; Meade et al., 2011a), neurocognitive (Kumar et al., 2011; Meade et al., 2011b), and post-mortem examinations (Silvers et al., 2007; Kumar et al., 2009), that HAND is associated with vulnerability of the DA system. Limited studies have reported that DA levels are decreased in DA-rich brain area (Sardar et al., 1996; Kumar et al., 2009), but increased in the CSF (Scheller et al., 2010) of HAND patients. The DA transporter (DAT) is critical for DA homeostasis, which is critical for neurocognitive function (Chudasama and Robbins, 2006). DAT is a target for HIV-1 viral proteins (Hu et al., 2009; Zhu et al., 2009b) and cocaine to impact the DA system (Zhu and Reith, 2008). We have demonstrated that Tat inhibits DA uptake in rat striatal synaptosomes and that influence of Tat on DAT ligand binding sites involves a protein-protein interaction (Zhu et al., 2009b). In particular, we have shown that Tat allosterically inhibits DAT function and modulates cocaine binding sites on DAT in rat striatal synaptosomes and heterologous cells expressing human DAT (Zhu et al., 2011).
The dynamic regulation of DAT function and cell surface localization of DAT are under the control of complex processes involving phosphorylation, protein-protein interaction, substrate pretreatment, and interaction with presynaptic receptors (Zhu and Reith, 2008). For example, activation of protein kinase C (PKC) results in reduced DA transport activity, decreased transporter recycling and DAT cell surface expression, thereby causing reduced DA uptake (Daniels and Amara, 1999; Melikian, 2004; Zahniser and Sorkin, 2004). On one hand, dynamic cell surface localization of DAT is regulated by cellular signaling pathways and endocytotic trafficking (Zhu and Reith, 2008). On the other hand, the DAT and vesicular monoamine transporter (VMAT2) proteins interact as a biochemical complex to regulate dopaminergic tone in response to motivationally relevant stimuli, including abused drugs (Vergo et al., 2007; Zhu and Reith, 2008; Egana et al., 2009).
This study was aimed to understand the molecular mechanisms underlying Tat-induced decrease in DAT reuptake activity. The primary hypothesis tested here was that Tat-induced reduction of DA transport is attributable to acceleration of DAT endocytosis through the dynamic-trafficking and phosphorylation-dependent mechanisms and that both DAT and VMAT-2 functions contribute to Tat-induced impairment of DA neurotransmission.
Material and Methods
Animals
Adult male Sprague – Dawley rats (225–250g body weight) were obtained from Harlan Laboratories, Inc. (Indianapolis, IN). Rats were housed in standard polyurethane cages and provided normal rodent food (ProLab Rat/Mouse/Hamster Chow 3000) and water ad libitum. The colony was maintained at 21±2 °C, 50±10% relative humidity and a 12L: 12D cycle with lights on at 0700 h (EST).The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina.
Drugs and Chemicals
[3H]DA (3,4-ethyl-2[N-3H]dihydroxyphenylethylamine; specific activity, 31 Ci/mmol) and [3H]WIN 35,428 (specific activity, 85 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Recombinant HIV-1 transactivator of transcription (Tat1–86, REP0002a) protein and its mutant protein Tat Cys22 (cysteine 22 was substituted to glycine, REP0032) were purchased from Diatheva (Fano, Italy). D-amphetamine, cocaine and Bisindolylmaleimide-I (BIM-I) were purchased from Tocris Biosciences (Ellisville, MO). Tetrabenazine (TBZ) was provided by NIMH Chemical Synthesis and Drug Supply Program (Bethesda, MD). Antibodies recognizing rat DAT (C-20; goat polyclonal antibody) and actin (C-2; mouse monoclonal antibody) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-goat IgG horseradish peroxidase was purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Goat anti-mouse IgG-horseradish peroxidase was purchased from Santa Cruz Biotechnology, Inc. D-Glucose, L-ascorbic acid, WIN35,428, L-leucine, L-lysine, bovine serum albumin, pyrocatechol, EDC, HEPES, isopropanol, nomifensine maleate, pargyline hydrochloride, polyethylene glycol, sucrose, and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of Synaptosomes, Subfractions and Synaptic Vesicles
Striata from individual rats were homogenized in 20 ml of ice-cold 0.32 M sucrose containing 5 mM NaHCO3, pH 7.4, with 16 up-and-down strokes using a Teflon pestle homogenizer (clearance, approximately 0.003 in.). Crude synaptosomal preparation was centrifuged at 2000g for 10 min at 4°C, and the resulting supernatants were then centrifuged at 20,000g for 15 min at 4°C. The resulting pellets (P2 fractions) were resuspended in 5 ml of ice-cold Krebs-Ringer-HEPES assay buffer (final concentration in mM: 125 NaCl, 5 KCl, 1.5 MgSO4, 1.25 CaCl2, 1.5 KH2PO4, 10 D-glucose, 25 HEPES, 0.1 EDTA, 0.1pargyline, and 0.1 L-ascorbic acid, saturated with 95% O2, 5% CO2; pH 7.4) for the synaptosomal [3H]DA uptake. To determine the distribution of DATs in subcellular fractions after exposure to Tat, striata from every two rats were pooled to achieve sufficient vesicles and the subcellular fractions were prepared using a previously published method with minor modifications (Middleton et al., 2007). Striata were homogenized in ice-cold 0.32 M sucrose buffer using a Teflon pestle homogenizer and then centrifuged at 800g for 12 min, 4°C. The resulting supernatant (S1) was centrifuged at 22,000g for 15 min at 4°C to yield a crude synaptosomal pellet (P2). The P2 pellet was then resuspended in DA uptake assay to obtain synaptosomes, and half of the synaptosomes were preincubated with Tat1–86 (1 µM) at 34°C for 15 min, and the other half were preincubated without Tat1–86 as control. This concentration of Tat1–86 was chosen based on our previous report that IC50 value for Tat1–86 inhibition of DA uptake was ∼ 3 µM (Zhu et al., 2009b). Subsequently, the synaptosomal samples were centrifuged at 22,000g for 15 min at 4°C and synaptosomes in the pellets (P2) were then lysed in ice-cold 25 mM HEPES, pH 7.5, and 100 mM potassium tartrate (pH 7.4) plus phosphatase inhibitor cocktails I (P2850, Sigma-Aldrich, St. Louis, MO) and protease inhibitor cocktail (P8340, Sigma-Aldrich, St. Louis, MO). After thorough mixing of all contents, the resulting mixture was centrifuged at 20,000g for 15 min at 4°C to get the plasma membrane enriched (P3) and vesicle-enriched supernatant (S3). The S3 was then centrifuged at 100,000g for 45 min at 4°C to yield the cytoplasmic vesicles pellet (P4). The three fractions (P2, P3, and P4) were used for Western blot assay. Protein concentrations were determined by the Bradford protein assay (Bradford, 1976) using bovine serum albumin as the standard (Bio-Rad Laboratories, Hercules, CA).
Synaptosomal [3H]DA Uptake
[3H]DA uptake was determined using previously described methods (Zhu et al., 2009b). Assays were performed in triplicate with a final volume of 1 mL. Aliquots of striatal synaptosomes were preincubated in assay buffer containing BIM-I (100 µM, final concentration) at 34 °C for 5 min in an oxygenated metabolic shaker, and then incubated with amphetamine (20 µM, final concentration) or Tat (0.7 µM) for an additional 15 min. The concentration of BIM-I was chosen based on the previous report showing that BIM-I (100 µM) alone did not show inhibitory effect on [3H]DA into rat striatal synaptosomes (Richards and Zahniser, 2009). Subsequently, the synaptosomes were centrifuged at 20,000g for 15 min and the resulting pellets were resuspended with ice cold assay buffer. Aliquots of the well-washed synaptosomes were incubated with 5 nM [3H]DA (final concentration) at 34 °C for 10 min. The reactions were terminated by the addition of 3 ml of ice-cold assay buffer. Nonspecific uptake was determined in duplicate at each [3H]DA concentration by including 10 µM nomifensine in the assay buffer. Samples were filtered through Whatman GF/B glass fiber filters (Whatman, Maidstone, UK), presoaked with assay buffer containing 1 mM pyrocatechol. Filters were washed three times with 3 ml of ice-cold assay buffer containing 1 mM pyrocatechol using a Brandel cell harvester (model M-48; Biochemical Research and Development Laboratories Inc., Gaithersburg, MD). Pyrocatechol (catechol) is a catechol-O-methyltransferase inhibitor. In the current study, pyrocatechol (1 mM) was included in the DA uptake assay buffer to prevent the degradation of [3H]DA during the processes of washes and harvesting (Zhu et al., 2004). Radioactivity was determined using liquid scintillation spectrometry (model Tri-Carb 2900TR; PerkinElmer Life and Analytical Sciences).
Vesicular [3H]DA Uptake
To determine inhibitory effects of Tat on VMAT-2 function, vesicular [3H]DA uptake was measured using previously described methods (Volz et al., 2007). In brief, assays were performed in duplicate with a final volume of 250 µl. For the competitive inhibition experiment, aliquots of P4 suspensions were preincubated in VMAT-2 assay buffer (final concentration in mM: 25 HEPES, 100 potassium tartrate, 0.05 EGTA, and 0.1 EDTA, 1.7 ascorbic acid, and 2 ATP-Mg2+, pH 7.5) containing Tat, Tat Cys22 or TBZ (final concentration, 0.1 nM-10 µM) at 34 °C for 15 min in an oxygenated metabolic shaker and subsequently incubated with a fixed concentration of [3H]DA (1 µM, final concentration) at 34 °C for additional 8 min. To determine if Tat differentially inhibits DAT and VMAT-2 function, in a separate experiment, synaptosomes were preincubated with 1 µM Tat at 34 °C for 15 min. Subsequently the synaptosomes were washed with fresh ice-cold assay buffer, and P2 and P4 fractions were then separated as described above. Aliquots of P4 fractions were incubated in the assay buffer with 1 to 8 concentrations of [3H]DA (0.03–5 µM) at 34 °C for 8 min. Nonspecific uptake was determined in the presence of 10 µM TBZ. Reactions were terminated by addition of 1 ml of cold wash buffer (assay buffer containing 2 mM MgSO4 substituted for the ATP-Mg2+, pH 7.5) and rapid filtration through Whatman GF/B filters soaked previously in 0.5% polyethylenimine. Filters were washed 3 times with the VMAT-2 assay buffer and radioactivity was measured using a liquid scintillation counter.
Western Blots
Proteins were extracted from each fraction (P2, P3 and P4) as described above with the Laemmli sample buffer (Sigma-Aldrich, St. Louis, MO) containing phosphatase inhibitor cocktail 1 (P2850, Sigma-Aldrich, St. Louis, MO) and protease inhibitors (P8340, Sigma-Aldrich, St. Louis, MO) and boiled for 5 min. To detect the immunoreactive DAT protein in these fractions, samples were subjected to gel electrophoresis and Western blotting. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis for 60 min at 150 V and transferred to Immobilon-P transfer membranes (Millipore, Billerica, MA) in transfer buffer (50 mM Tris, 250 mM glycine, and 3.5 mM SDS) using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad Laboratories) for 110 min at 72 V. Transfer membranes were incubated with blocking buffer (5% dry milk powder in phosphate-buffered saline containing 0.5% Tween 20) for 1 h at room temperature, followed by incubation with goat polyclonal DAT antibody (1 µg/ml in blocking buffer) overnight at 4°C. Transfer membranes were washed five times with wash buffer (phosphate-buffered saline containing 0.5% Tween 20) at room temperature and then incubated with rabbit anti-goat DAT antibody (1:2500 dilution in blocking buffer) for 1 h at 22°C. Blots on transfer membranes were detected using enhanced chemiluminescence and developed on Hyperfilm ECL-Plus (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). After detection and quantification of the DAT protein, each blot was stripped in 10% Re-blot plus mild antibody stripping solution (Millipore Bioscience Research Reagents, Temecula, CA) for 20 min at room temperature and reprobed for detection of actin. Actin was used as an intracellular control protein to monitor protein loading between samples and determined using a mouse monoclonal antibody (1:1000 dilution in blocking buffer). Multiple autoradiographs were obtained using different exposure time, and immunoreactive bands within the linear range of detection were quantified with densitometric scanning (Scion Image software; Scion Corporation, Frederick, MD). Band density measurements, expressed as relative optical density, were used to determine levels of the DAT immunoreactivity in synaptosomes.
[3H]WIN 35,428 Binding Assay
To determine whether Tat-induced inhibition of [3H]DA uptake was the result of alterations in the maximal number of binding sites (Bmax) or affinity (Kd) for [3H]WIN 35,428 binding in P3 fraction, kinetic analysis of [3H]WIN 35,428 binding was determined using a previously described method (Zhu et al., 2009b). After exposure of synaptosomes to Tat (1 µM) or vehicle (control) as described above, the P3 pellets were resuspended in ice-cold sodium-phosphate buffer (2.1 mM NaH2PO4, 7.3 mM Na2HPO47H2O, and 320 mM sucrose, pH 7.4). Aliquots of P3 fractions were incubated with one of the eight concentrations of [3H]WIN 35,428 (final concentration, 0.5–30 nM) on ice for 2 h. In parallel, nonspecific binding at each concentration of [3H]WIN 35,428 (in the presence of 30 µM cocaine, final concentration) was subtracted from total binding to calculate the specific binding. Assays were terminated by rapid filtration onto Whatman GF/B glass fiber filters, presoaked for 2 h with the assay buffer containing 0.5% polyethylenimine, using a Brandel cell harvester. Filters were rinsed three times with 3 ml of ice-cold assay buffer. Radioactivity remaining on the filters was determined by liquid scintillation spectrometry.
Data Analysis
Data are presented as mean ± S.E.M., and n represents the number of independent experiments for each experiment. The effect of BIM-I on Tat-induced changes in DA uptake was analyzed by one-way ANOVA. Student-Newman-Keuls comparisons were made for post hoc analyses. Separate paired Student’s t test was conducted on DAT immunoreactivity for comparisons between control and Tat treated samples. Kinetic parameters (Bmax and Kd) of [3H]WIN 35,428 binding were determined from saturation curves by nonlinear regression analysis using a one-site model with variable slope. For experiments involving comparisons between two paired samples, paired Student’s t test was used to determine the ability of Tat to alter the kinetic parameters [Km and Vmax for [3H]DA uptake; Kd and Bmax for [3H]WIN 35,428 compared with control (the absence of Tat)]; log-transformed values of Km or Kd were used for these statistical comparisons. IC50 values for Tat-induced inhibition in specific vesicular [3H]DA uptake were determined from inhibition curves by nonlinear regression analysis using a one-site model with variable slope. All statistical analyses were performed using SPSS, standard version 19.0 (SPSS Inc., Chicago, IL), and differences were considered significant at p < 0.05.
Results
Involvement of PKC in Tat-induced Down-regulation of DAT Function in Rat Striatal Synaptosomes
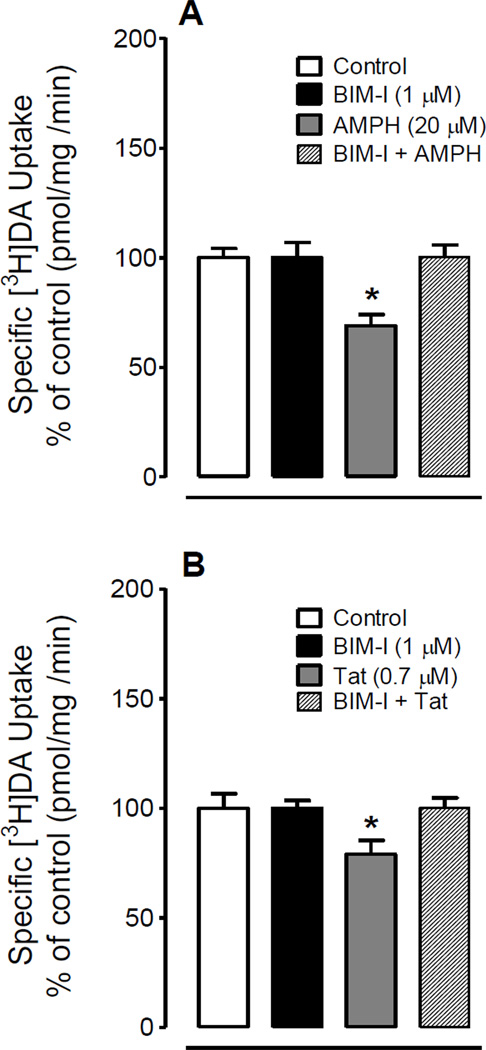
To determine whether the Tat-induced down-regulation of DAT function was mediated by activation of PKC, synaptosomes were preincubated with the PKC inhibitor BIM-I (1 µM) for 5 min prior to incubation with amphetamine (20 µM) or Tat (0.7 µM) for additional 15 min. Amphetamine was used as a positive control, because the previous report has shown that amphetamine-induced down-regulation of DAT activity was blocked by preincubation of BIM-I (Richards and Zahniser, 2009). As shown in Figure 1, amphetamine (F(3, 15) = 8.83, p < 0.01) or Tat (F(3, 15) = 8.28, p < 0.05) alone significantly reduced [3H]DA uptake, and preincubation of BIM-I completely blocked both amphetamine- and Tat-induced reductions.
Figure 1.
PKC inhibition attenuated Tat- and d-amphetamine (AMPH)-induced reduction of [3H]DA uptake in rat striatal synaptosomes. After pre-incubation of synaptosomes with 1 µM BIM-I for 5 min, AMPH (20 µM, A) or Tat (0.7 µM, B) were added for another 15 min and subsequently all reagents were washed off, specific uptake of 5 nM [3H]DA uptake was measured. * P < 0.05 versus AMPH or Tat only.
Tat Protein Decreased Cell Surface DAT Expression in Rat Striatal Synaptosomes
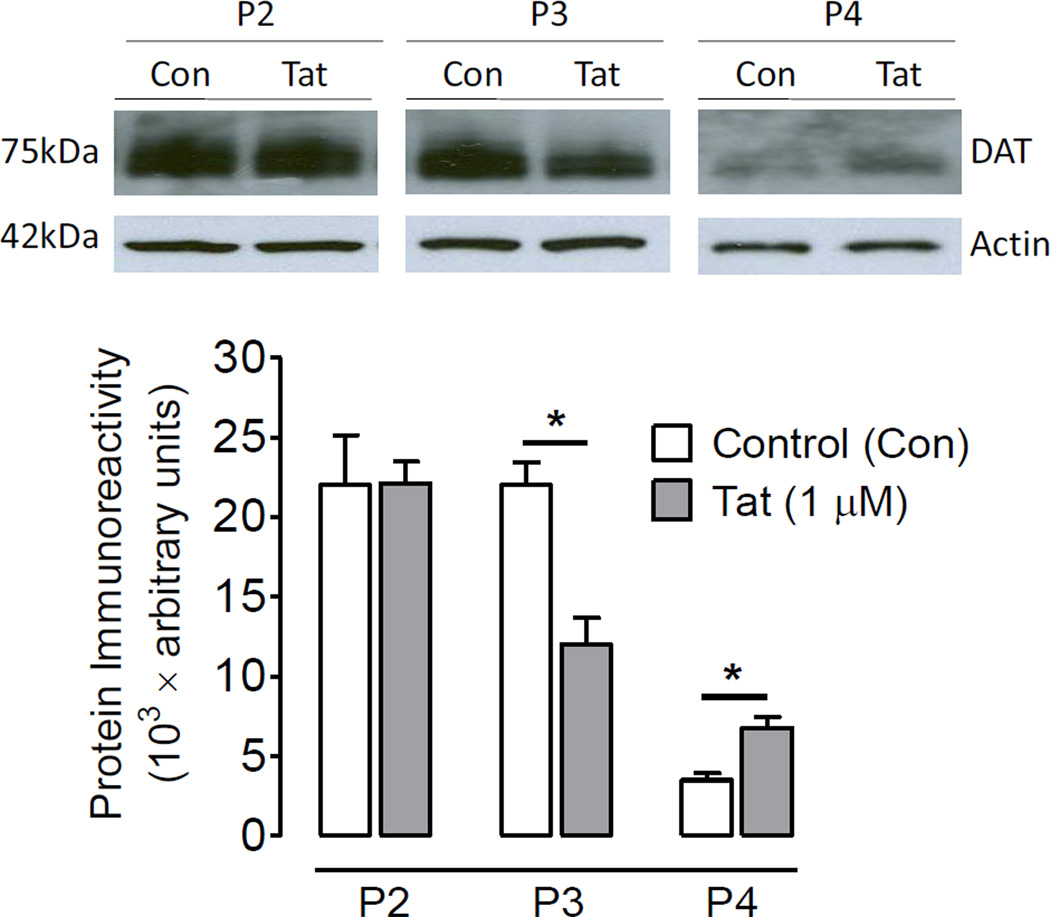
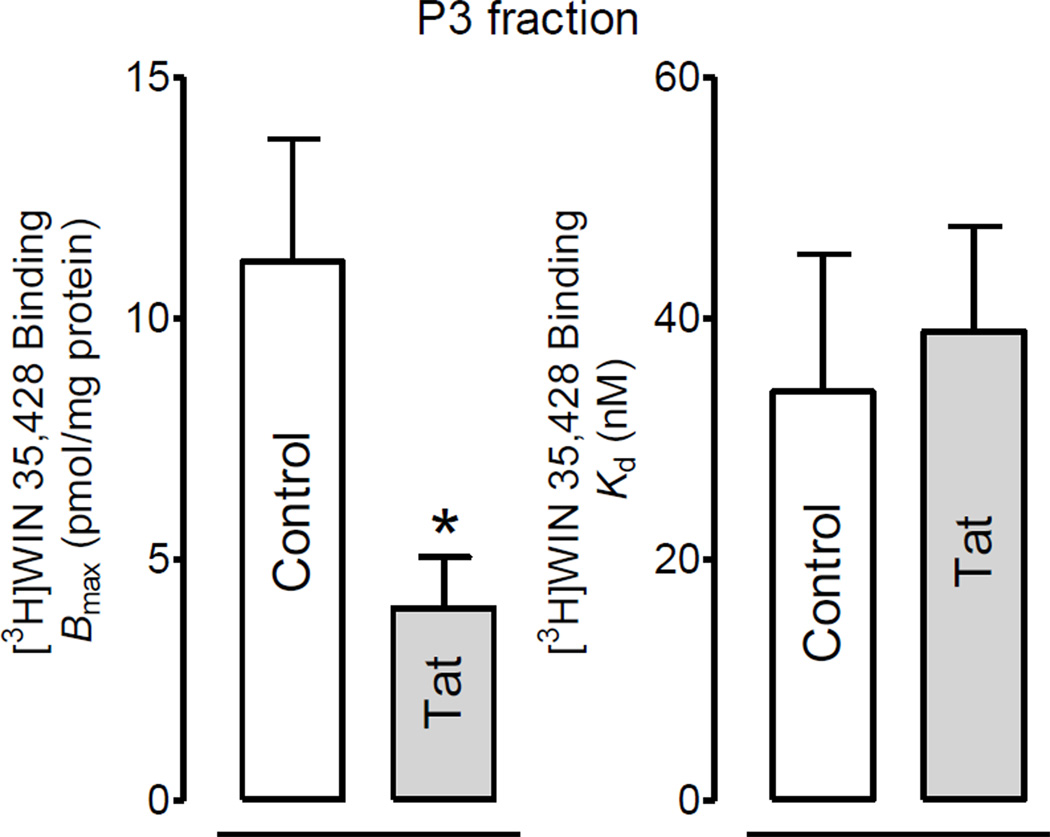
To determine if the Tat-induced decrease in [3H]DA uptake of DAT function was attributed to a reduction in the plasma membrane of the DATs, DAT expression in subfractions was examined. As shown in Figure 2, after exposure of synaptosomes to Tat (1 µM), DAT immunoreactivity was decreased by 46% in P3 fractions (t(3) = 3.22, p<0.05) and increased by 49% in P4 fractions (t(3) = 5.64, p<0.05) without changes in P2 fractions. To verify the Tat-induced decreases in P3 fractions, the Bmax of [3H]WIN 35,428 in P3 fractions was determined. Figure 3 shows that 15 min preincubation of synaptosomes with Tat (1 µM) led to a significant decrease of the Bmax value by 64% (3.99 ± 0.6 pmol/mg protein) compared with the control [11.2 ± 1.3 pmol/mg protein; t(3) = 5.6, p<0.05, paired Student’s t test]. There was no change in the Kd value between Tat-treated and control samples (33.9 ± 11.4 and 38.9 ± 8.7 nM).
Figure 2.
Tat protein decreased DAT cell surface expression. Rat striatal synaptosomes were incubated with or without Tat (1 µM). Subsequently, total synaptosomal fractions (P2), plasma membrane enriched fractions (P3), and vesicle-enriched fractions (P4) were prepared for western blot analysis. The same portion of P3 fraction were used in [3H]WIN 35,428 binding assay (see Figure 3). The levels of DAT immunoreactivity were increased in P4 and decreased in P3 without changes in P2 in Tat-exposed samples compared to the respective controls. * P < 0.05, paired Student’s t-test.
Figure 3.
Tat protein decreased the specific [3H]WIN35,428 binding in plasma membrane enriched fraction. Rat striatal synaptosomes were incubated with or without Tat (1 µM), and then total synaptosomal fractions (P2), plasma membrane enriched fractions (P3) and vesicle-enriched fractions (P4) were prepared and used for the specific [3H]WIN35,428 binding. * P <0.05, paired Student’s t-test.
Inhibitory Effects of Tat on VMAT-2 and DAT Function
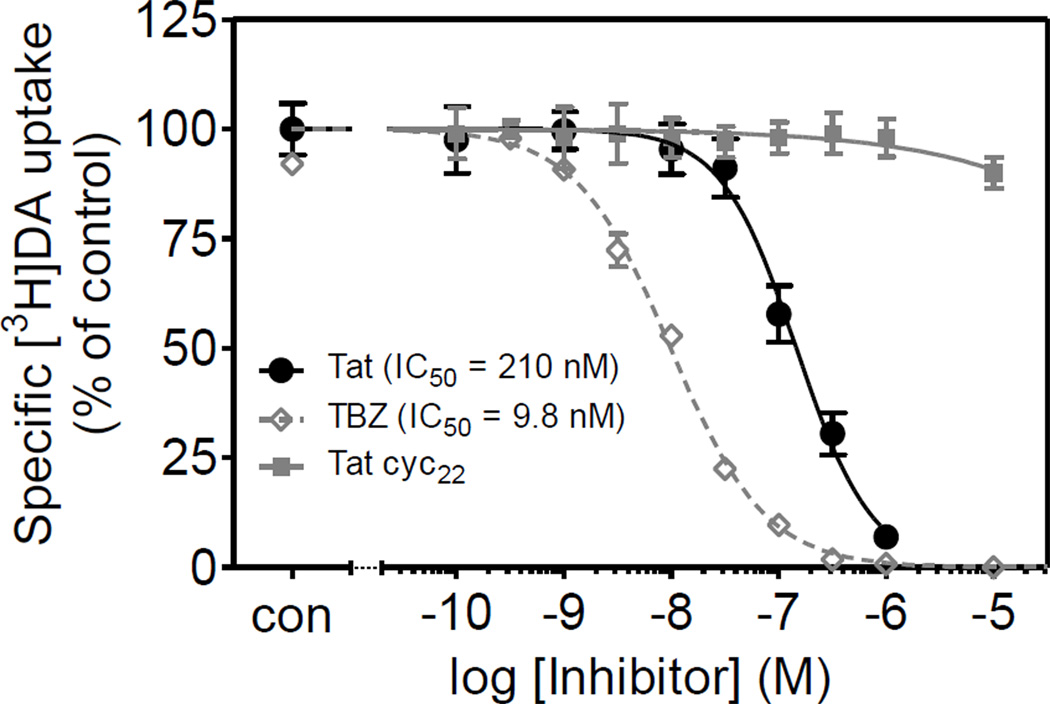
To determine whether Tat differentially inhibited DAT and VMAT-2 function, the ability of Tat protein to inhibit vesicular [3H]DA uptake or synaptosomal [3H]DA uptake was measured. As shown in Figure 4, 15 min preincubation of synaptosomes with Tat (1 µM) caused a 35 ± 1.8 and 26 ± 1.5% reduction in [3H]DA uptake into vesicles and synaptosomes, respectively. To determine the concentration-dependent inhibitory effect of Tat on specific [3H]DA uptake into striatal vesicles, vesicular [3H]DA uptake was examined in the presence of various concentrations of Tat. Specific [3H]DA uptake was substantially inhibited by Tat (IC50 = 210 ± 19 nM; Figure 5). TBZ was used as a positive control for the [3H]DA uptake assay and had an IC50 value of 9.8 ± 0.7 nM. Tat Cys22 was a negative control for the assay, which showed no inhibitory effect on vesicular [3H]DA uptake across the concentration range from 0.1 nM to 10 µM.
Figure 4.
Inhibitory effects of Tat on synaptosomal [3H]DA uptake and vesicular [3H]DA uptake in rat striatum. Rat striatal synaptosomes were preincubated with or without Tat (1 µM) for 15 min. Drug was then washed off, and specific synaptosomal and vesicular uptake of 0.1 µM [3H]DA uptake were measured. Tat inhibited the specific [3H]DA uptake into vesicles (A; via VMAT2) and synaptosomes (B; via DAT) by 35% and 26%, respectively, compared to the respective control values (*P <0.05, paired Student’s t-test).
Figure 5.
Pharmacological profiles of vesicular [3H]DA uptake in rat striatum in the presence of Tat1–86, Tat Cys22, or tetrabenazine (TBZ, a VMAT2 inhibitor). Striatal synaptosomes were preincubated with various concentrations of Tat1–86, Tat Cys22, or TBZ (0.1 nM–10 µM) at 34°C for 15 min followed by the addition of [3H]DA (final concentration, 0.1 µM) for 8 min. Tat Cys22 and TBZ were used as negative and positive controls, respectively. Data are expressed as mean ± S.E.M. as percentage of control (CON) values (28,205 ± 1965 dpm) from five independent experiments performed in duplicate. Nonspecific [3H]DA uptake was determined in the presence of 10 µM nomifensine. All curves were best fit to a single class of binding site and generated by nonlinear regression.
Discussion
We have reported previously that Tat protein induced decrease of [3H]DA uptake in rat striatal synaptosomes (Zhu et al., 2009b). The current study investigated the mechanism of this Tat-induced impairment of DAT activity. This study also determined the effect of Tat on VMAT-2 function. The results provide evidence that the Tat-induced decrease in DA uptake is ascribed to a DAT trafficking- and phosphorylation-dependent mechanism. Further, we demonstrated that Tat inhibited not only DAT activity but also VMAT-2 function. These findings provide new insight into understanding the molecular mechanisms of HIV-1 viral protein-induced dysfunction of DA neurotransmission in HIV-1 infected patients.
The present results are in agreement with previous work showing that in vitro exposure of rat striatal synaptosomes to Tat protein leads to a decrease in DAT uptake function (Wallace et al., 2006; Zhu et al., 2009b). In this study, while a PKC inhibitor, BIM-I, alone had no effect on DA uptake, Tat (0.7 µM)-induced decrease (21%) in specific [3H]DA uptake was ablated by BIM-I. Similarly, preincubation of the synaptosomes with BIM-I completely blocked amphetamine-induced decrease (31%) in [3H]DA uptake, which is consistent with a previous report (Richards and Zahniser, 2009). Therefore, compared to amphetamine, Tat produces a similar regulatory effect on DAT uptake function through a PKC-dependent mechanism. We have demonstrated that the influence of Tat on DAT function involves a protein-protein interaction between Tat and DAT (Zhu et al., 2009b). Moreover, Tat acts as an allosteric modulator of DAT rather than as either a reuptake inhibitor (e.g. cocaine) or a substrate releaser (e.g. amphetamine) (Zhu et al., 2011). Rapid regulation of DAT function by psychostimulants involves multiple processes including PKC-dependent and independent mechanisms. Amphetamine-stimulated DA efflux through reducing DAT function requires PKC activation (Kantor and Gnegy, 1998; Cowell et al., 2000; Johnson et al., 2005a). Allosterism has been shown to be responsible for the conformational transitions via substrate- and ligand-binding sites on the DAT (Shan et al., 2011). Therefore, Tat-induced inhibition of DA transporting may involve a change in the DAT conformation that requires DAT phosphorylation. Indeed, we have demonstrated that influence of Tat on DAT function is dependent on unique recognition residues of Tat that interact with the DAT (Zhu et al., 2009b). Future studies will identify the specific recognition binding residues in DAT responsible for the influence of Tat on PKC-induced DAT phosphorylation.
In the current study, exposure to Tat (0.7 µM) for 15 min decreased [3H]DA uptake activity in rat synaptosomes. In general, Tat-induced decrease of DA transport could be accomplished in several manners: increased DAT protein degradation, decreased turnover rates of DAT, or changes in DAT trafficking on the cell surface expression without changes in total DAT immunoreactivity. To validate these potential mechanisms, first, we have demonstrated that a 15-min exposure to Tat1–86 induced a rapid and reversible decrease in Vmax of [3H]DA uptake without changes in total DAT levels (Zhu et al., 2009b), suggesting that Tat-induced reduction in Vmax of DA uptake is not caused by DAT degradation. Second, transporter turnover rate, which reflects the number of DA molecules transported per second per site (Lin et al., 2000), was determined and shown that 15-min exposure of synaptosomes to 1 µM Tat did not alter the ratio of Vmax for [3H]DA uptake/Bmax for [3H]WIN 35,428 binding. This result provides evidence that Tat does not decrease the turnover rates of DAT (Zhu et al., 2009b). The current results display that the levels of DAT immunoreactivity were increased by 49% in vesicle-enriched fractions (P4) and decreased by 46% in plasma membrane enriched fractions (P3) without changes in total synaptosomal fractions (P2) in Tat-exposed samples compared to the respective controls. These data indicate that exposure to Tat results in a redistribution of DAT from the cell surface to intracellular compartments (i.e. internalization) and that loss of DAT from the plasma membrane is responsible for the decrease in Vmax observed after Tat exposure.
The efficiency of DA transport depends on the number of DAT molecules expressed on the plasma membrane, which is regulated by a dynamic-trafficking mechanism (Zhu and Reith, 2008). This has been consistently reported in a number of studies using cells expressing DAT (Saunders et al., 2000; Chi and Reith, 2003) and rat striatal synaptosomes (Chi and Reith, 2003; Zhu et al., 2005; Zhu et al., 2009a). Changes in DAT dynamics in plasma membranes can be detected using either biotinylation assay (Zhu et al., 2005; Zhu et al., 2009a) or subfractionation method (Middleton et al., 2007). In the current study, a 49% decrease in DAT surface expression (P3) was not comparable to the magnitude (26%) of the decrease in Vmax for [3H]DA uptake in rat striatal synaptosomes demonstrated in our previous report (Zhu et al., 2009b). This result is also supported by [3H]WIN 35,428 binding experiment showing that the Bmax was decreased in P3 of Tat-treated samples compared to controls. As reported in our previous studies, levels of the changes in DAT cell surface expression were less than (Zhu et al., 2009a) or similar (Zhu et al., 2005) to the magnitude of the changes in Vmax for [3H]DA uptake. Trafficking of plasma membrane transporters is associated with changes in posttranslational modifications of the DAT protein, including phosphorylation states and protein-protein interactions (Sager and Torres, 2011). Therefore, the current results infer that Tat-induced great decrease of DAT expression in plasma membranes could be regulated by both phosphorylation (i.e. PKC-dependent mechanism) and protein-protein interactions (e.g. allosteric modulation). In contradiction with our results, 30-min exposure of PC12 cells expressing human DAT to 120 nM Tat1–86 increased (24%) DA uptake using measurement of the fluorescence ASP+, which was accompanied by a profound increase (177%) in plasma membranes and a minor decrease (14%) in cytoplasmic membranes compared to respective controls (Perry et al., 2010). The contrasting results might be due to differences in DAT expression models (rat synaptosomes versus a human DAT cell line), Tat concentration, exposure time and methodology. Tat-induced changes in DAT surface levels could arise from increased PKC-mediated endocytosis, decreased DAT recycling rates, or both. Rat synaptosomes express DAT endogenously and have less surface DAT expression compared to DAT expressed in heterologous cells such as PC12 cells (Johnson et al., 2005b; Boudanova et al., 2008). In addition, synaptosomes exhibit fast DAT recycling rates for basal DAT levels in plasma membranes, whereas the PC12 cells overexpressing DAT have slow DAT recycling rates (Johnson et al., 2005b; Boudanova et al., 2008). These differences may, at least in part, contribute to the contrasting results between the current study and the previous report (Perry et al., 2010). Nevertheless, the results from Perry et al’s study demonstrate that increased membrane DAT may be a compensatory response to decreased transporting efficiency of individual DAT (Perry et al., 2010).
Another important finding from this study is that Tat inhibited not only DAT function and trafficking but also VMAT-2 function. Although both DAT and VMAT-2 proteins are essential for the regulation of DA disposition in the synapse and cytosol, our data show that the inhibitory effects of Tat are more profound in VMAT2 protein than in DAT protein. Moreover, the potency of Tat for inhibiting synaptosomal [3H]DA uptake (IC50 = 3.1 µM) (Zhu et al., 2009b) is 15-fold higher than that for inhibiting vesicular [3H]DA uptake (IC50 = 0.21 µM). Thus, VMAT-2 protein may play a more critical role in Tat-induced alterations of extracellular DA concentrations. In the current study, the affinity of TBZ, a highly selective compound for VMAT-2 (Erickson et al., 1996) was about 20-fold greater than that of Tat protein, whereas Tat Cys22Gly mutation showed no inhibitory effect on vesicular [3H]DA uptake. We have also demonstrated that Cys22Gly mutation of Tat eliminates the inhibitory activity of Tat against DAT (Zhu et al., 2009b). Therefore, our results provide evidence for inter-molecular interactions between VMAT-2 and Tat protein. In addition, this result also suggests that Tat protein may cause synergistic effects on impairing DA neurotransmission by inhibition of both DAT and VMAT-2 proteins. At the functional level, plasma membrane DAT translocates substrates through a sodium- and chloride-dependent mechanism (Rudnick and Clark, 1993; Gu et al., 1994), whereas vesicular transporters use the proton electrochemical gradient across the vesicular membrane to transport monoamines (Johnson, 1988). Many DAT inhibitors have an ability to rapidly regulate VMAT-2 localization and function (Fleckenstein et al., 2009). For example, methamphetamine leads to releases of DA from synaptic vesicles into the cytosol via an interaction with the VMAT2 and by disruption of the vesicular proton gradient (Sulzer et al., 1995; Fleckenstein et al., 2007). Subsequently, available cytosolic DA is reversely transported by the DAT into the extracellular space (Sulzer et al., 1995). Co-exposure to Tat and methamphetamine produces synergistic effects on impairing DA terminals (Theodore et al., 2006) and methamphetamine-mediated behavior (Kass et al., 2010). Thus, the current results provide a mechanistic basis to interpret the synergistic effects of Tat and methamphetamine.
In conclusion, Tat protein exposure led to changes of DAT and VMAT2 function and expression in rat striatal synaptosomes. The Tat-induced reduction of DAT uptake function is mediated by DAT trafficking- and PKC-dependent mechanisms. These findings may have important implication for preclinical studies of the role of DAT and VMAT-2 in drugs of abuse in HIV-1 infected individuals. Future studies will be necessary to investigate how DAT interacts with VMAT-2 to promote Tat-induced dysfunction of DA neurotransmission and which DAT residues are required for interactions of Tat and DAT.
Acknowledgements
This research was supported by grants from the National Institute on Drug Abuse to Jun Zhu (DA024275 and DA026721).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Boudanova E, Navaroli DM, Melikian HE. Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology. 2008;54:605–612. doi: 10.1016/j.neuropharm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Su TP, Wang J. Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J Neuroimmune Pharmacol. 2011;6:503–515. doi: 10.1007/s11481-011-9297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-Induced Trafficking of the Dopamine Transporter in Heterologously Expressing Cells and in Rat Striatal Synaptosomal Preparations. J Pharmacol Exp Ther. 2003;307:729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological psychology. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol. 2000;389:59–65. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- Daniels GM, Amara SG. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem. 1999;274:35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Egana LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, Pena K, Quiroz M, Hong WC, Dorostkar MM, Janz R, Sitte HH, Torres GE. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Schafer MK, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol. 2009;65:316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine's connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology. 2009;56(Suppl 1):133–138. doi: 10.1016/j.neuropharm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269:7124–7130. [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK, Rock RB. Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage. J Neurovirol. 2009;15:401–410. doi: 10.3109/13550280903296346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, Gnegy ME. Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem. 2005a;280:10914–10919. doi: 10.1074/jbc.M413887200. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005b;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Johnson RG., Jr Accumulation of biological amines into chromaffin granules: a model for hormone and neurotransmitter transport. Physiol Rev. 1988;68:232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:592–598. [PubMed] [Google Scholar]
- Kass MD, Liu X, Vigorito M, Chang L, Chang SL. Methamphetamine-Induced Behavioral and Physiological Effects in Adolescent and Adult HIV-1 Transgenic Rats. J Neuroimmune Pharmacol. 2010;5:566–573. doi: 10.1007/s11481-010-9221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15:257–274. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Salemi M, Galligan DC, Morris A, Gray R, Fogel G, Zhao L, McGrath MS. Human immunodeficiency virus-1 evolutionary patterns associated with pathogenic processes in the brain. J Neurovirol. 2010;16:230–241. doi: 10.3109/13550281003735709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrat EP, Zierler S. Entangled epidemics: cocaine use and HIV disease. J Psychoactive Drugs. 1993;25:207–221. doi: 10.1080/02791072.1993.10472272. [DOI] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Lin Z, Itokawa M, Uhl GR. Dopamine transporter proline mutations influence dopamine uptake, cocaine analog recognition, and expression. FASEB J. 2000;14:715–728. doi: 10.1096/fasebj.14.5.715. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J Behav Med. 2011a;34:128–138. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Lowen SB, MacLean RR, Key MD, Lukas SE. fMRI brain activation during a delay discounting task in HIV-positive adults with and without cocaine dependence. Psychiatry Res. 2011b;192:167–175. doi: 10.1016/j.pscychresns.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacology & Therapeutics. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Middleton LS, Apparsundaram S, King-Pospisil KA, Dwoskin LP. Nicotine increases dopamine transporter function in rat striatum through a trafficking-independent mechanism. Eur J Pharmacol. 2007;554:128–136. doi: 10.1016/j.ejphar.2006.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Clements JE. Eradication of HIV from the brain: reasons for pause. AIDS. 2011;25:577–580. doi: 10.1097/QAD.0b013e3283437d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman LR, Basso M, Kumar A, Malow R. Neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Current drug abuse reviews. 2009;2:143–156. doi: 10.2174/1874473710902020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SW, Barbieri J, Tong N, Polesskaya O, Pudasaini S, Stout A, Lu R, Kiebala M, Maggirwar SB, Gelbard HA. Human immunodeficiency virus-1 Tat activates calpain proteases via the ryanodine receptor to enhance surface dopamine transporter levels and increase transporter-specific uptake and Vmax. J Neurosci. 2010;30:14153–14164. doi: 10.1523/JNEUROSCI.1042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Zahniser NR. Rapid substrate-induced down-regulation in function and surface localization of dopamine transporters: rat dorsal striatum versus nucleus accumbens. J Neurochem. 2009;108:1575–1584. doi: 10.1111/j.1471-4159.2009.05910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Sager JJ, Torres GE. Proteins interacting with monoamine transporters: current state and future challenges. Biochemistry. 2011;50:7295–7310. doi: 10.1021/bi200405c. [DOI] [PubMed] [Google Scholar]
- Sardar AM, Czudek C, Reynolds GP. Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport. 1996;7:910–912. doi: 10.1097/00001756-199603220-00015. [DOI] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller C, Arendt G, Nolting T, Antke C, Sopper S, Maschke M, Obermann M, Angerer A, Husstedt IW, Meisner F, Neuen-Jacob E, Muller HW, Carey P, Ter Meulen V, Riederer P, Koutsilieri E. Increased dopaminergic neurotransmission in therapy-naive asymptomatic HIV patients is not associated with adaptive changes at the dopaminergic synapses. J Neural Transm. 2010;117:699–705. doi: 10.1007/s00702-010-0415-6. [DOI] [PubMed] [Google Scholar]
- Shan J, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS One. 2011;6:e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 Tat protein: involvement of D1 dopamine receptor. Neurotoxicology. 2007;28:1184–1190. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore S, Cass WA, Maragos WF. Methamphetamine and human immunodeficiency virus protein Tat synergize to destroy dopaminergic terminals in the rat striatum. Neuroscience. 2006;137:925–935. doi: 10.1016/j.neuroscience.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Vergo S, Johansen JL, Leist M, Lotharius J. Vesicular monoamine transporter 2 regulates the sensitivity of rat dopaminergic neurons to disturbed cytosolic dopamine levels. Brain Res. 2007;1185:18–32. doi: 10.1016/j.brainres.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, Fleckenstein AE. The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J Neurochem. 2007;101:883–888. doi: 10.1111/j.1471-4159.2006.04419.x. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47:80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Dwoskin LP. Nicotinic receptor activation increases [3H]dopamine uptake and cell surface expression of dopamine transporters in rat prefrontal cortex. J Pharmacol Exp Ther. 2009a;328:931–939. doi: 10.1124/jpet.108.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Green T, Bardo MT, Dwoskin LP. Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res. 2004;148:107–117. doi: 10.1016/s0166-4328(03)00190-6. [DOI] [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, Dwoskin LP. Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem. 2005;93:1434–1443. doi: 10.1111/j.1471-4159.2005.03130.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009b;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant HIV-1TAT(1–86) allosterically modulates dopamine transporter activity. Synapse. 2011 doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]