Abstract
Long lived organisms such as humans have evolved several intrinsic tumor suppressor mechanisms to combat the slew of oncogenic somatic mutations that constantly arise in proliferating stem cell compartments. One of these anti-cancer barriers is the telomere, a specialized nucleoprotein that caps the ends of eukaryotic chromosome. Impaired telomere function activates the canonical DNA damage response pathway that engages p53 to initiate apoptosis or replicative senescence. Here, we discuss how p53-dependent senescence induced by dysfunctional telomeres may be as potent as apoptosis in suppressing tumorigenesis in vivo.
More than 40 years ago, Hayflick and Moorhead discovered that normal human diploid fibroblasts (HDFs) cannot grow indefinitely in culture1. Rather, their proliferative capacities are intrinsically limited. After 60-80 population doublings in culture, HDFs stop dividing and adopt a phenotype characterized by large, flat cell size, a vacuolated morphology, inability to synthesize DNA, and the presence of the senescence-associated β-galactosidase (SA-β-gal) marker2. We now know that the endpoint of this proliferative limit, termed replicative senescence, is due largely to erosion of telomeres, protective structures that cap the end of all eukaryotic chromosomes. Confirmation of this came from studies in which telomerase, the enzyme that maintains telomeres, was ectopically expressed in normal human somatic cells3, 4. Activation of telomerase results in telomere elongation, abrogation of replicative senescence, a normal karyotype and cellular immortalization. These results clearly demonstrate that telomere length determines the proliferative lifespan of HDFs, and that upregulation of telomerase activity (hence telomere length) restores proliferative capacity.
A large body of experimental evidence has demonstrated that telomere attrition contributes to tumorigenesis by promoting genome instability5. However, in the setting of a competent p53 pathway, mouse models have recently shown that telomere shortening is also tumor suppressive by promoting replicative senescence to inhibit tumor formation. In this Perspective, we will discuss how telomeres shorten with cell replication and how this might initiate a DNA damage response to induce replicative senescence (and cell death) and ultimately prevent tumorigenesis.
Telomeres and telomerase
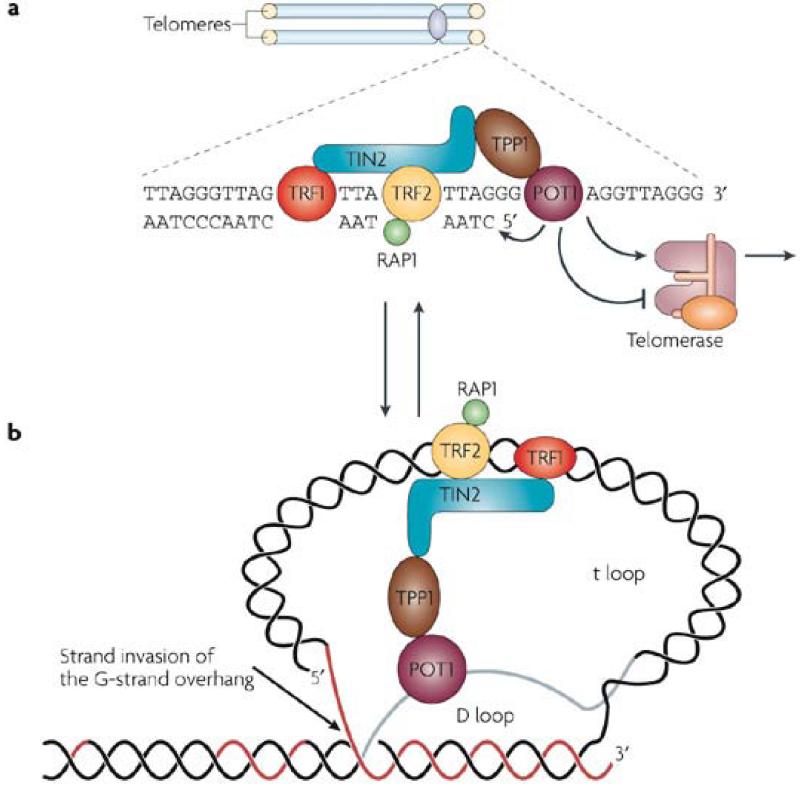
Telomeres are composed of TTAGGG repeats, oriented 5′-to-3′ towards the end of the chromosome, ending in an essential 3′ single-stranded G-rich overhang. They are maintained by telomerase, a specialized ribonucleoprotein complex that includes an RNA template (Terc) and a reverse transcriptase catalytic subunit (Tert) (Figure 1a). The G-rich overhang is generated by post-replicative processing of the C-rich strand, and is the substrate for telomerase-mediated telomere elongation. Telomerase expression is low or absent in most human somatic tissues6, while it is robust in early proliferative progenitor germ and stem cells 7, 8. In the absence of telomerase, each round of DNA replication is accompanied by telomere shortening due to the failure of DNA polymerase to synthesize fully the extreme terminus of the lagging DNA strand. A total lifetime loss of ~2-4 kb in average telomere length has been observed in human cells8-10. Considering that human telomeres are only 8-12 kb in length or less at birth, this degree of attrition is significant over the natural lifespan of humans, and may be responsible to elicit certain aspects of human aging phenotypes11.
Figure 1.
Analysis of telomeres from a diverse array of organisms revealed that the telomere 3′ single-stranded overhang can invade the double-stranded telomeric tracts, displacing the homologous strand of the same telomere. Consequently, a telomere forms a lasso-like structure, termed the ‘t-loop’, with a displacement (D) loop at the invasion site12, 13 (Figure 1b). It has been proposed that the t loop plays a crucial role in sequestering the 3′ end of telomeric DNA from being recognized by the DNA damage machinery as DNA double stranded breaks (DSBs) and therefore initiating inappropriate activation of DNA damage checkpoints.
Cytogenetic analyses of breakage and fusion of maize chromosomes provided the first evidence that telomeres maintain genome stability14. Mammalian telomeres associate with the shelterin complex of six core proteins — telomeric-repeat binding factor 1 (TRF1), TRF2, TRF1 interacting protein 2 (TIN2), protection of telomeres 1 (POT1), the POT1 and TIN2 interacting protein TPP1 and the transcriptional repressor/activator protein RAP115, 16 (Figure 1b, Box 1). Telomeres that can no longer exert end-protective functions are said to be dysfunctional, and these telomeres could arise either from progressive telomere attrition, or when components of the shelterin complex are perturbed, resulting in inappropriate chromosomal end-to-end fusions through the non-homologous end joining (NHEJ) or homologous recombination (HR) DNA repair pathways 17. One important cytogenetic distinction between these two repair processes is that the fusion points of chromosomes with naturally shortened telomeres do not normally posses FISH-detectable telomeric DNA, while fused chromosomes resulting from loss of shelterin components frequently posses intense telomeric signals at the sites of fusion. While NHEJ and HR pathways account for the vast majority of chromosomal fusions, other pathways of repair such as microhomology mediated telomere-telomere recombination may also be involved, especially when the classic repair pathways are compromised18-20.
Box 1. The shelterin complex.
The shelterin complex15 is composed of telomeric-repeat binding factor 1 (TRF1), TRF2, TRF1 interacting protein 2 (TIN2), protection of telomeres 1 (POT1), the POT1 and TIN2 interacting protein TPP1 and the transcriptional repressor/activator protein RAP1. Proteins that directly bind the double-stranded telomeric repeats include TRF1 and TRF2. TRF1 is a negative regulator of telomere length while TRF2 plays important roles in preventing a DNA damage response (DDR) at telomeres. POT1 belongs to a family of evolutionarily conserved oligosaccharide/oligonucleotide-binding (OB)-fold containing proteins and specifically recognizes the single-stranded G-overhang78-81. Shelterin components that do not bind telomeric DNA directly include TIN2, which associates with TRF1 and TRF2 and TPP1, which forms a heterodimer with POT182, 83. RAP1 is recruited to telomeres by TRF2 and negatively regulates telomere length. Depletion of endogenous TRF2 levels either by overexpression of dominant negative TRF2 (TRF2-DN) or through genetic knockout approaches in mouse cells with long telomeres results in massive chromosomal fusions with telomeric sequence at the sites of fusions. Mouse models in which shelterin components have been deleted experience telomere dysfunction, a DDR and chromosomal fusions resembling those observed in telomerase null mice79, 81, 84-88. These results suggest that both critically short telomeres and direct disruption of the shelterin structure can initiate telomere dysfunction, trigger a DDR and chromosomal fusions.
These fusions are the basis of genomic instability associated with telomere dysfunction as they lead to anaphase bridging, subsequent breakage and that requires further repair. Repetition of this fusion-bridge-breakage cycle leads to aneuploidy, further fusions and depending on how such DNA damage is resolved, it has been hypothesized that loss of heterozygosity or gene amplification could result in tumorigenesis5, 21.
Telomere dysfunction initiates a DNA damage response
Biochemical analyses revealed that HDFs that entered replicative senescence display molecular markers characteristic of cells bearing DNA double-strand breaks (DSBs), suggesting that dysfunctional telomeres elicit a potent DNA damage response (DDR)22. These markers of the DDR include phosphorylated γ-H2AX, 53BP1, NBS1, MDC1 and CHK2, among others. Many of these proteins localize directly to dysfunctional telomeres to form dysfunctional telomere-induced foci (TIFs), and their inactivation in senescent cells restores cell cycle progression into S-phase22, 23. These results suggest that dysfunctional telomeres are recognized as DSBs that impinge upon p53 and/or pRB tumor suppressor pathways, to induce expression of their regulators p21 and p16 Ink4a to initiate replicative senescence24-26.
Dysfunctional telomeres activate upstream checkpoint PI3 kinases, such as ATM (ataxia-telangiectasia mutated) or ATR (ataxia-telangiectasia and Rad3 related)22, 27-30 (Figure 2). Once activated, these kinases phosphorylate downstream factors including CHK1 and CHK2 that in turn phosphorylates p5329. Phosphorylation of p53 results in the displacement of the murine double minute 2 (MDM2) protein, liberating p53 from degradation and stimulation of the expression of the cyclin-dependent kinase inhibitor p21. p21 inhibits cell cycle progression by inhibiting cyclin-dependent kinases that phosphorylate and inactivate RB. In addition to promoting cell cycle arrest, dysfunctional telomeres can also activate p53-dependent apoptosis, since mice bearing dysfunctional telomeres show increased apoptosis in proliferative cells31-33. In the setting of a competent p53 pathway, dysfunctional telomeres appear to function as a potent tumor suppressor by engaging cellular pathways that activate replicative senescence and/or apoptosis to inhibit tumor formation. Therefore, it is not surprising that p53 loss results in a permissive environment that favors proliferation and survival of genomically damaged cells and the eventual progression to cancer. The role of p16Ink4a-Rb pathway in mediating the telomere DDR is less clear (Box2).
Figure 2.
Box 2. p16INK4A and a telomere-induced DNA damage response.
The role of p16Ink4a-RB pathway in mediating the telomere DDR is not clear. p16 is another CDKI that is markedly increased in senescent cells and results in RB hypophosphorylation89. Expression of hTERT in HDFs prevents p16Ink4a induction, suggesting that hTERT prevents formation of dysfunctional telomeres that would otherwise activate p16Ink4a 3. Support for this hypothesis comes from experiments in which treatment of HDF with TRF2-DN induces p16Ink4a protein levels and entry into senescence, suggesting that in addition to p53, p16Ink4a could be a second effector of the telomere DDR90. However, compared to the DDR-mediated p53 checkpoint, the kinetics of telomere DDR-mediated p16Ink4a is very slow. Since TIF formation occurs relatively transiently, this slow induction of a p16Ink4a -damage response could potentially explain why TIFs were not observed in senescent HDFs with elevated p16Ink4a levels91. Interestingly, p16 does not appear to be an important effector of the telomere DDR in mouse embryo fibroblasts (MEFs), since MEFs lacking p53 are completely refractory to the effects of TRF2-DN irrespective of p16 levels92. However, it is not clear whether this is a unique property of MEFs or a general property of DDR signal transduction wiring differences between mouse and man. Mouse models of p21 and p53 deficiency rescues some of the degenerative phenotypes of late generation telomerase null mice46, 61, Ink4a deficiency does not93. However, the Ink4a knockout mouse compromises the function of both p16Ink4a and p19ARF, resulting in impingement of both the p53 and pRb pathways. It would be important to generate a mouse model to examine how telomere initiated DDR is perturbed in the setting of p16 deficiency in vivo.
Inactivation of p53 and pRB by antisense oligonucleotides34 or by viral oncoproteins35 can extend replicative potential in HDFs, driving further telomere erosion and culminating in a period of massive cell death and rampant genomic instability termed ‘crisis’. Crisis serves as the second checkpoint in tumor suppression and is characterized by multiple chromosomal fusions36. Virally transformed human cells that eliminate p53 and/or pRB function can escape crisis at very low frequencies, and invariably adopt telomere-maintenance programs: 80-90% of human tumors possess some telomerase activity, while the remainder maintain telomeres via a recombination-mediated process termed ALT (for Alternative Lengthening of Telomeres)37-40. Together, these observations support the view that replicative senescence and crisis provide potent barriers to tumor development and, by extension, that proper telomere maintenance is an essential aspect of full malignant transformation.
Dysfunctional telomeres initiated-DDR-p53 pathway inhibits tumor initiation and progression
Telomere dysfunction has been proposed to fuel tumorigenesis, since depending on how dicentric chromosomes are resolved in the breakage-fusion-bridge cycle, loss of heterozygosity of tumor suppressors and/or amplification of oncogenes could result in a pro-cancer genotype5. The cloning of the gene encoding mTerc enabled the construction of the telomerase knockout mouse to formally test this hypothesis in vivo41, 42. The telomerase deficient mouse lacks the critical RNA subunit mTerc, is viable, fertile and has no significant morphological abnormalities42. To drive telomeres to shorter and potentially dysfunctional lengths in later generations, successive mTerc−/− intercrosses were instituted. By the sixth generation (G6), increased apoptosis and compromised function were observed in highly proliferative tissues including skin, hematopoietic and reproductive systems31, 42-44. Loss of hematopoietic function likely stems from accumulation of damaged DNA stemming from dysfunctional telomeres45. Importantly, removal of p53 function rescued many of the cellular defects despite the presence of critically short telomeres, including growth arrest in cell culture, testicular atrophy, intestinal and germ cell apoptosis46. Cell culture transformation assays showed that Trp53 null cells with critically shortened telomeres exhibited increased susceptibility to transformation by Myc and Ras. Similar findings were observed in vivo, where the progressive decline in telomere function correlated with increased tumor incidence and decreased survival. Therefore, in the absence of Trp53, telomere dysfunction and the resultant genomic instability promote tumorigenesis. As a further demonstration of the impact of p53 status on dysfunctional telomere-induced tumor growth, late generation mTerc−/− p53+/− mice emerged with a tumor spectrum that strikingly resembled those of aged humans: carcinomas of the skin, breast, and intestine with cytogenetic hallmarks of telomere dysfunction emerged as the largest group of tumors47. Together, these findings demonstrate that telomere dysfunction promotes the development of cancer in the setting of p53 deficiency, and further reinforce the importance of an intact p53 pathway in tumor inhibition in the setting of telomere dysfunction.
To further examine how dysfunctional telomeres cooperate with p53 activation to limit neoplastic growth in vivo, mouse models bearing deletions of various tumor suppressor genes were mated with telomerase knockout mice (Table 1). For example, the CDKN1A locus encodes the p16Ink4a and p19ARF tumor suppressors. Both genes share a common second exon that is deleted in the Ink4a−/− mouse48. These mice are highly prone to develop lymphomas and sarcomas. Importantly, the DDR that signals p53 is intact in the Ink4a−/− mouse25. Treatment of early generation mTerc−/−, Ink4a−/− mice with DMBA and UVB revealed that these mice are highly cancer prone. However, similar treatment of G6-mTerc−/−;Ink4a−/− mice with short dysfunctional telomeres yielded a dramatic reduction in tumor incidence (from 64% to 31%) and much longer survival49. Primary fibroblasts isolated from these embryos also exhibited resistance to transformation by Myc and Ras. A similar finding was observed in a skin carcinogenesis model, in which late generation mTerc−/− mice produced 20-fold fewer skin tumors upon chemical carcinogenesis treatment of the skin compared to wild-type controls with long telomeres50. In addition, up regulation of p53 was detected in late generation mTerc−/− papillomas. Taken together, these results suggest that dysfunctional telomeres inhibit tumor initiation in vivo in the setting of an intact DNA damage induced p53 signaling pathway, either by activating p53-dependent apoptosis or replicative senescence.
Table 1.
Dysfunctional telomeres (mTerc−/−) reduce tumor incidence in mouse models of cancer
| Genotype/Treatment | Tumor phenotypes | Effect of dysfunctional telomeres (phenotype in mTerc−/− background) |
Ref. |
|---|---|---|---|
| No mutations | Few tumors normally seen | Compared to wild-type and early generation telomerase null mice, aging late generation mice show an increase in the incidence of cancer. |
32, 42 |
| DMBA/TPA treatment |
Treatment with these carcinogens allow for the monitoring tumor initiation (papillomas) and progression to squamous cell carcinomas of the skin. |
Loss of telomerase (G1) resulted in decreased growth rate and size of papillomas, with a slight decrease in numbers. G5 mice with dysfunctional telomeres were almost completely resistant to papilloma formation. |
50 |
| Ink4a/Arf | Deletion of this locus results in loss of both p16Ink4a and p19ARF. The resulting mice develop lymphomas and sarcomas. |
Late generation double knockouts show decreased incidence of spontaneous and carcinogen-induced tumors, and increased tumor latency. |
49, 93 |
| APC min | 100% of mice with this mutation develop multiple intestinal neoplasias (MIN) that progress from micro- to macro-adenomas. |
Short telomeres led to increase in tumor initiation (microadenomas) but decreased size and number of macroscopic adenomas |
51 |
| Alb-uPA transgene or CCl4 or DEN treatment |
This transgenic mouse and the carcinogenic treatment are both effective ways of modeling hepatocellular carcinoma (HCC) |
Successive breeding of Alb-uPA onto a late generation telomerase null background or treatment of G3/G4 mice with CCl4, or DEN resulted in decreased number and size of liver nodules. |
53 |
| PMS2 | Deficiency of this mismatch repair genes leads to increased susceptibility for lymphomas, sarcomas, and colon carcinomas |
Progressively shortening telomeres reduced the incidence of all three tumors types. |
94 |
| Eμ-myc | Transgenic expression of c-myc in B-cells leads to potent formation of lymphoma in this model for Burkitt’s lymphoma. |
Formation of lymphoma was almost completely suppressed for two years in mice with dysfunctional telomeres (G5/G6), unlike wildtype and G1 mice that rapidly developed cancer within 6 months. |
57 |
| ATM | Thymic lymphoma |
Delayed onset and decreased incidence of thymic lymphomas |
95, 96 |
| P53 | Loss of this important tumor suppressor leads to rapid development of mainly lymphomas and soft tissue sarcomas. |
Combined homozygous loss of p53 and dysfunctional telomeres led to increased incidence of lymphomas. In addition, late generation mTerc−/− combined with heterozygous loss of p53 showed a shift in tumor spectrum from lymphomas to epithelial cancers (breast, GI, SCC) with non-reciprocal translocations. |
47 |
| P53 R172P | This point mutation commonly found in human tumors abolishes the ability of p53 to induce apoptosis and delays tumor formation for about 6 months. |
Mice from an intergenerational cross with mTerc−/− animals (iG1) have dysfunctional telomeres and show almost complete suppression of tumorigenesis. |
58 |
| ATM, P53 | T-cell lymphoma | Loss of p53 accelerated onset of lymphomas in mTerc−/− ATM−/− mice. |
97 |
| K5-Terf2 | Specific overexpression of TRF2 in the skin of these mice leads to development of spontaneous SCC on the skin. |
Increasing generations of telomerase deficient mice showed accelerated onset of spontaneous and UV-induced skin neoplasms. |
98 |
Note: APCmin, adenomatous polyposis coli min (multiple intestinal neoplasias) allele; ATM, ataxia telangiectasia mutated; Terc, telomerase RNA component; PMS2, postmeiotic segregation increased 2;
The link between telomere shortening and tumor suppression is further highlighted in the mTerc−/−ApcMin mouse51. ApcMin mice develop benign intestinal microadenomas and late stage macroadenomas upon loss of the wild type Apc allele52. In early generation mTerc−/−ApcMin mice with competent telomeres, early stage adenomas predominated, and many of these progressed into the more aggressive macroadenomas. In contrast, only microadenomas were present in G6 mTerc−/−ApcMin animals. These results suggest that progression from micro to macroadenomas was inhibited in G6 mTerc−/−ApcMin mice, presumably due to the activation of p53-dependent tumor suppressive pathways by dysfunctional telomeres. Similar results were observed in p53-competent mouse models of hepatocellular carcinoma, in which dysfunctional telomeres served to initiate tumor growth, but also limited the size of these tumors (progression) by activating apoptosis53.
Dysfunctional telomeres activate a p53-dependent senescence program to suppress tumorigenesis in vivo
Given that the aforementioned mouse models all possess wild-type p53 capable of inducing both apoptosis and senescence, it remained unclear how dysfunctional telomeres limit neoplastic growth in vivo. While apoptosis clearly has a tumor suppressive role in vivo, until recently it was not clear whether p53-dependent replicative senescence plays a role in tumor suppression in vivo. Several reasons account for why replicative senescence was not initially recognized as a tumor suppressor mechanism in vivo. First, since the laboratory mice normally possess very long telomeres, it was not even clear whether a replicative senescence mechanism exists in mice. Second, compared to apoptosis, it is relatively difficult to reliably detect the presence of senescence cells in vivo. Until recently, only the SA-β-galactosidase assay was available to mark senescent cells. However, there now exist several promising markers to detect both senescent mouse and human cells in vivo54-56. Two recent studies specifically examined the role of telomere-induced replicative senescence as a mechanism of tumor suppression in the telomerase knockout mice (Figure 3). The first study looked at lymphoma development in the context of telomere dysfunction using the established Eμ-myc transgenic model for Burkett’s lymphoma57. Tumorigenesis was markedly reduced in late generation mTerc−/−, Eμ-myc cohort: while wild type mice and early generation mTerc−/−, Eμ-myc mice developed B-cell lymphoma by 200 days, only 25% of mTerc−/−, Eμ-myc mice possessing dysfunctional telomeres succumbed to this cancer. Examination of tumors from late generation mice revealed increased end-to-end chromosomal fusion and non-reciprocal translocations, hallmarks of genomic instability due to telomere dysfunction. To examine the role of p53-dependent apoptosis in mediating tumor suppression, the anti-apoptotic gene Bcl-2 was overexpressed in hematopoietic stem cells harvested from mTerc−/− mice with dysfunctional telomeres. If p53-dependent apoptosis is the main driver of tumor suppression in the setting of telomere dysfunction, then its elimination by Bcl-2 overexpression should result in rapid development of lymphoma when transplanted into lethally irradiated recipients. Indeed, reconstitution of Bcl-2 expressing stem cells derived from wild type and G1 mTerc−/−, Eμ-myc bone marrows resulted in palpable tumors within 6 weeks post-transplantation. However, transplantation of G5/6 mTerc−/−, Eμ-myc, Bcl-2 bone marrow failed to produce any tumors 100 days post-transplantation. Examination of lymph nodes from these mice revealed the presence of small encapsulated tumors with a 3-fold decrease in mitotic index and positive staining for senescent markers SA-β-Gal, p16 and p15. These results suggest that dysfunctional telomeres could activate a cellular senescence pathway to suppress tumorigenesis in the absence of apoptosis to suppress tumorigenesis, while this pathway was not elicited in the tumors with competent telomeres.
Figure 3.
A second approach to examining the role of cellular senescence in tumorigenesis utilized a knock-in mouse with a single amino acid mutation (R172P) within the p53 protein58. Cells harboring this mutation (p53P/P) are incapable of activating p53-dependent apoptosis59, but the p53-dependent cellular senescence pathway is intact as induction of telomere dysfunction through overexpression of TRF2-DN in these cells led to dramatic reduction of cellular proliferation and the appearance of senescent cells that stain positive for SA-β-Gal58. To genetically dissect the contribution of p53-dependent apoptosis vs. cellular senescence to tumor suppression in the setting of telomere dysfunction in vivo, an intergenerational (iG) mating scheme was used to generate 4 cohorts of mice: mTerc+/− p53P/+ and mTerc+/− p53P/P mice with intact telomere function and iG1 mTerc−/− p53P/+ and iG1 mTerc−/− p53P/P mice with dysfunctional telomeres. Metaphase spreads of primary bone marrow and splenocyte cultures derived from telomerase competent, p53P/+ or p53P/P mice showed minimal structural chromosome abnormalities. In contrast, iG1 mTerc−/− p53P/+ and iG1 mTerc−/− p53P/P cells possessed a 6-8 fold increase in p-p arm fusions and a 3-fold increase in the formation of anaphase bridges, both hallmarks of telomere dysfunction. To determine whether p53-dependent apoptosis is required to suppress spontaneous tumorigenesis, tumor development was monitored in all 4 mouse cohorts over a 28-month period. While mTerc+/−p53P/+and mTerc+/−p53P/P cohorts readily developed lymphomas, the presence of dysfunctional telomeres was associated with a near complete suppression of tumor formation in both iG1 mTerc−/− p53P/+ (0/23 mice with tumors) and iG1 mTerc−/− p53P/P mice (1/9 mice with tumors). Immunohistochemical staining with antibodies against p21 and p53 revealed abundant immuno-positive cells in intestinal epithelium from iG1 mTerc−/− p53P/+ and iG1 mTerc−/− p53P/P mice, while minimal staining was observed for mTerc+/− p53P/+ and mTerc+/− p53P/P intestines. Robust SA-β-galactosidase staining was also detected in multiple organs from iG1 mTerc−/− p53P/P mice. Taken together, both studies suggest that p53-dependent apoptosis is dispensable in mediating telomere-dependent spontaneous tumor suppression in vivo. Instead, the p53-p21-dependent cellular senescence pathway is potently activated in mice bearing dysfunctional telomeres, and may be responsible for the tumor suppression observed in these animals.
One surprising result is the failure of the iG1 mTerc−/− p53P/P mouse to suppress tumorigenesis in a DMBA skin carcinogenesis model58. This is in sharp contrast to the strong tumor suppression observed in late generation mTerc−/− null mice with intact p53 function50. One explanation is that while telomere induced cellular senescence is capable of suppressing spontaneous tumorigenesis from mesenchymal tumors, it is insufficient to suppress cancer formation of epithelial tissues such as the skin. This notion that p53 is able to activate different checkpoint programs in different tissues is supported by the observation that restoration of endogenous p53 function in p53 null mice activates primarily an apoptotic response to inhibit T-cell lymphomas, while inhibition of sarcomas requires activation of a cellular senescence program60.
From the results presented above, one would hypothesize that telomere dysfunction in the absence of p21 would abrogate the senescence response in vivo, resulting in accelerated tumor formation. Surprisingly, this was not the case: late generation mTerc−/−, p21−/− mice do not show increased chromosomal instability nor do they succumb to increased tumorigenesis61. Instead, loss of p21 extended organismal lifespan and rescued cellular proliferative defects due to dysfunctional telomeres, presumably due to reduced entry of proliferative cells into cellular senescence. The fact that tumorigenesis is not increased in the context of p21 deficiency indicates that in this mouse model, p53-mediated apoptosis functions as a redundant anti-tumor barrier in response to short telomeres. Genetic abrogation of p21 in Terc-null, p53R172P mice will test this possibility, since loss of p21 would inhibit p53-mediated cellular senescence, while the presence of the p53R172P allele would abrogate p53-dependent apoptosis. This mouse should therefore resemble p53−/− mice and become very tumor prone. However, the age-dependent increases in apoptosis in these organs were not altered in mTerc−/−, p21−/− mice, suggesting it is possible that p53-dependent but senescence- and apoptosis-independent effects of dysfunctional telomeres might account for some of the observed tumor suppression in vivo. It would be interesting to test the hypothesis that p53-mediated autophagy contributes to tumor suppression in the setting of telomere dysfunction62.
Taken together, the above studies suggest that activation of either apoptotic or senescence pathway is sufficient to block tumorigenesis in most tissues. It also appears that when one anti-cancer pathway is selectively eliminated, the other one can serve as a back-up. In carcinomas, perhaps activation of both senescence and apoptotic pathways are required to enforce tumor suppression. How cells are ushered to undergo either apoptosis or/and cellular senescence in response to telomere dysfunction remains an important question to address in the future.
Future anti-cancer therapy: therapeutic strategies that target telomeres
The demonstration that dysfunctional telomeres could engage a senescence program to suppress tumorigenesis in vivo suggests possible future therapeutic applications63. The upregulation of telomerase in most human cancers and its requirement for proliferation makes anti-telomerase compounds a potential means to induce telomere shortening and initiation of a senescence program in tumor cells64. For example, treatment of HT1080 fibrosarcoma cell lines with the telomerase inhibitor BIBR1532 results in progressive telomere shortening, induction of a senescence phenotype and inhibition of tumor growth when transplanted into recipient mice65. GRN163L is a modified oligonucleotide complementary to hTERC and a potent and specific telomerase antagonist66. GRN163L effectively inhibits telomerase activity of various human cancer cell lines67-70, resulting in progressive telomere shortening and induction of cellular senescence to suppress tumor cell growth in vitro. Furthermore, administration of GRN163L is effective in preventing lung metastases in breast cancer xenograft animal models68.
In addition to telomerase, targeting components of the shelterin complex like TPP1 or POT1 to induce a DDR might also induce the onset of cellular senescence. The G-rich strand of telomeric DNA can fold into a 4-stranded G-quadruplex (G4), whose stabilization perturbs telomere function. The G4 inducing ligand RHPS4 triggers a potent DDR at telomeres specifically in transformed human fibroblasts and melanoma cells71. Interestingly, TIF formation correlated with delocalization of POT1 and was antagonized by overexpression of POT1 or TRF2. In mice, RHPS4 exerted its anti-tumor effect on xenografts of diverse human tumor cell lines. These data provide encouraging evidence that telomere dysfunction initiates a DDR in malignant cells to suppress tumorigenesis.
Conclusions
Understanding the molecular mechanisms that limit neoplastic growth could provide insights into novel anti-cancer therapies. Telomere-induced cellular senescence has been long hypothesized to contribute to tumor suppression72. However, this process is typically studied in cultured cells, and how it contributes to tumor suppression in vivo has been poorly defined. The mouse studies outlined above provide the first direct evidence that dysfunctional telomeres initiate p53-dependent cellular senescence to suppress spontaneous tumorigenesis in vivo. Surprisingly, it appears that p53-dependent apoptosis appears largely dispensable for spontaneous tumor suppression when the senescent pathway is activated, thereby placing cellular senescence on an equal footing with apoptosis in mediating tumor suppression.
Dysfunctional telomere-induced senescence was accompanied by increases in senescence markers, including p53, p21, p15 and SA-β-gal activity, suggesting that a DDR is activated by dysfunctional telomeres in vivo. Recent observations indicate that both telomere dysfunction73, 74 and the DDR75, 76 are activated at the earliest stages in many human carcinomas. The results presented would predict that activation of an intact DDR pathway by dysfunctional telomeres in premalignant lesions would engage cellular senescence or apoptotic pathways, suppressing further tumor progression. However, the increased genome instability in nascent tumor cells that stochastically inactivate components of the DDR pathways would promote tumor progression.
Although there is still much to learn about whether anti-telomerase therapy would be efficacious against human cancers, it is encouraging to see promising new drugs enter clinical trials. However, several limitations for this class of drugs remain. For anti-telomerase drugs to engage p53-dependent apoptotic and/or senescence pathways to suppress tumorigenesis, an intact p53 pathway is required to effectively inhibit cancer cell growth, a daunting scenario considering that p53 is mutated in approximately 50% of all human cancers. One must also be concerned about the possibility that tumor cells rendered senescent would yield secretory products that might itself be tumor promoting77. Finally, this therapy will not work in ALT tumors which lack functional telomerase. Further progress in our understanding of the basic biology of telomeres should enable us to circumvent these problems.
Glossary terms
- DICENTRIC CHROMOSOME
A chromosome that has two centromeres, formed by breakage and reunion of two chromosomes.
- BREAKAGE-FUSION-BRIDGE CYCLE
Chromosomal ends with critically shortened telomeres are highly recombinogenic, and undergo repeated cycles of end-to-end fusions, followed by randome breakage, and then subsequent fusions to generate loss of heterozygocity (LOH) or amplification of certain chromosomal loci.
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar AG, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 4.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–82. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 5.Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–9. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 6.Masutomi K, et al. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–53. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 7.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–9. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 9.Allsopp RC, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harley CB, et al. Telomerase, cell immortality, and cancer. Cold Spring Harb Symp Quant Biol. 1994;59:307–15. doi: 10.1101/sqb.1994.059.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–9. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 12.Griffith JD, et al. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–14. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 13.de Lange T. T-loops and the origin of telomeres. Nat Rev Mol Cell Biol. 2004;5:323–9. doi: 10.1038/nrm1359. [DOI] [PubMed] [Google Scholar]
- 14.McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941;26:234–82. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 16.Liu D, O’Connor MS, Qin J, Songyang Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J Biol Chem. 2004;279:51338–42. doi: 10.1074/jbc.M409293200. [DOI] [PubMed] [Google Scholar]
- 17.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–31. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 18.Corneo B, et al. Rag mutations reveal robust alternative end joining. Nature. 2007;449:483–6. doi: 10.1038/nature06168. [DOI] [PubMed] [Google Scholar]
- 19.Yan CT, et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–82. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 20.Capper R, et al. The nature of telomere fusion and a definition of the critical telomere length in human cells. Genes Dev. 2007;21:2495–508. doi: 10.1101/gad.439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DePinho RA, Polyak K. Cancer chromosomes in crisis. Nat Genet. 2004;36:932–4. doi: 10.1038/ng0904-932. [DOI] [PubMed] [Google Scholar]
- 22.d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–8. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 23.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol. 2003;13:1549–56. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 24.Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992;27:383–9. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- 25.Kamijo T, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–59. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 26.Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–8. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- 27.Guo X, et al. Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. Embo J. 2007;26:4709–19. doi: 10.1038/sj.emboj.7601893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Churikov D, Price CM. Pot1 and cell cycle progression cooperate in telomere length regulation. Nat Struct Mol Biol. 2008;15:79–84. doi: 10.1038/nsmb1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gire V, Roux P, Wynford-Thomas D, Brondello JM, Dulic V. DNA damage checkpoint kinase Chk2 triggers replicative senescence. Embo J. 2004;23:2554–63. doi: 10.1038/sj.emboj.7600259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–71. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 31.Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph KL, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–12. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 33.Rajaraman S, et al. Telomere uncapping in progenitor cells with critical telomere shortening is coupled to S-phase progression in vivo. Proc Natl Acad Sci U S A. 2007;104:17747–52. doi: 10.1073/pnas.0706485104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara E, Tsurui H, Shinozaki A, Nakada S, Oda K. Cooperative effect of antisense-Rb and antisense-p53 oligomers on the extension of life span in human diploid fibroblasts, TIG-1. Biochem Biophys Res Commun. 1991;179:528–34. doi: 10.1016/0006-291x(91)91403-y. [DOI] [PubMed] [Google Scholar]
- 35.Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–9. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- 36.Counter CM, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. Embo J. 1992;11:1921–9. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shay JW, Van Der Haegen BA, Ying Y, Wright WE. The frequency of immortalization of human fibroblasts and mammary epithelial cells transfected with SV40 large T-antigen. Exp Cell Res. 1993;209:45–52. doi: 10.1006/excr.1993.1283. [DOI] [PubMed] [Google Scholar]
- 38.Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 39.Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–4. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 40.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 41.Blasco MA, Funk W, Villeponteau B, Greider CW. Functional characterization and developmental regulation of mouse telomerase RNA. Science. 1995;269:1267–70. doi: 10.1126/science.7544492. [DOI] [PubMed] [Google Scholar]
- 42.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 43.Herrera E, Martinez AC, Blasco MA. Impaired germinal center reaction in mice with short telomeres. Embo J. 2000;19:472–81. doi: 10.1093/emboj/19.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flores I, et al. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22:654–67. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–9. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 46.Chin L, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–38. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 47.Artandi SE, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–5. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 48.Serrano M, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg RA, et al. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell. 1999;97:515–25. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Suarez E, Samper E, Flores JM, Blasco MA. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet. 2000;26:114–7. doi: 10.1038/79089. [DOI] [PubMed] [Google Scholar]
- 51.Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28:155–9. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- 52.Dove WF, et al. The intestinal epithelium and its neoplasms: genetic, cellular and tissue interactions. Philos Trans R Soc Lond B Biol Sci. 1998;353:915–23. doi: 10.1098/rstb.1998.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farazi PA, et al. Differential impact of telomere dysfunction on initiation and progression of hepatocellular carcinoma. Cancer Res. 2003;63:5021–7. [PubMed] [Google Scholar]
- 54.Collado M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 55.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–5. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 56.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11:461–9. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cosme-Blanco W, et al. Telomere dysfunction suppresses spontaneous tumorigenesis in vivo by initiating p53-dependent cellular senescence. EMBO Rep. 2007;8:497–503. doi: 10.1038/sj.embor.7400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu G, et al. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36:63–8. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- 60.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 61.Choudhury AR, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 62.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–74. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 63.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–79. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 64.Shay JW, Keith WN. Targeting telomerase for cancer therapeutics. Br J Cancer. 2008;98:677–83. doi: 10.1038/sj.bjc.6604209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Damm K, et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. Embo J. 2001;20:6958–68. doi: 10.1093/emboj/20.24.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dikmen ZG, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65:7866–73. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- 67.Djojosubroto MW, et al. Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology. 2005;42:1127–36. doi: 10.1002/hep.20822. [DOI] [PubMed] [Google Scholar]
- 68.Hochreiter AE, et al. Telomerase template antagonist GRN163L disrupts telomere maintenance, tumor growth, and metastasis of breast cancer. Clin Cancer Res. 2006;12:3184–92. doi: 10.1158/1078-0432.CCR-05-2760. [DOI] [PubMed] [Google Scholar]
- 69.Jackson SR, et al. Antiadhesive effects of GRN163L--an oligonucleotide N3′->P5′ thio-phosphoramidate targeting telomerase. Cancer Res. 2007;67:1121–9. doi: 10.1158/0008-5472.CAN-06-2306. [DOI] [PubMed] [Google Scholar]
- 70.Ozawa T, et al. Antitumor effects of specific telomerase inhibitor GRN163 in human glioblastoma xenografts. Neuro Oncol. 2004;6:218–26. doi: 10.1215/S1152851704000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salvati E, et al. Telomere damage induced by the G-quadruplex ligand RHPS4 has an antitumor effect. J Clin Invest. 2007;117:3236–47. doi: 10.1172/JCI32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Chin K, et al. In situ analyses of genome instability in breast cancer. Nat Genet. 2004;36:984–8. doi: 10.1038/ng1409. [DOI] [PubMed] [Google Scholar]
- 74.Meeker AK, et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–26. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 75.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 76.Gorgoulis VG, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–13. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 77.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–7. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baumann P, Cech TR. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–5. doi: 10.1126/science.1060036. [DOI] [PubMed] [Google Scholar]
- 79.He H, et al. POT1b protects telomeres from end-to-end chromosomal fusions and aberrant homologous recombination. Embo J. 2006;25:5180–90. doi: 10.1038/sj.emboj.7601294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loayza D, De Lange T. POT1 as a terminal transducer of TRF1 telomere length control. Nature. 2003;423:1013–8. doi: 10.1038/nature01688. [DOI] [PubMed] [Google Scholar]
- 81.Wu L, et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126:49–62. doi: 10.1016/j.cell.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 82.Wang F, et al. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–10. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 83.Xin H, et al. TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature. 2007;445:559–62. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- 84.Hockemeyer D, Daniels JP, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 85.Hockemeyer D, et al. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nat Struct Mol Biol. 2007;14:754–61. doi: 10.1038/nsmb1270. [DOI] [PubMed] [Google Scholar]
- 86.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–8. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 87.Chiang YJ, Kim SH, Tessarollo L, Campisi J, Hodes RJ. Telomere-associated protein TIN2 is essential for early embryonic development through a telomerase-independent pathway. Mol Cell Biol. 2004;24:6631–4. doi: 10.1128/MCB.24.15.6631-6634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munoz P, Blanco R, Blasco MA. Role of the TRF2 telomeric protein in cancer and ageing. Cell Cycle. 2006;5:718–21. doi: 10.4161/cc.5.7.2636. [DOI] [PubMed] [Google Scholar]
- 89.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–75. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Jacobs JJ, de Lange T. Significant role for p16INK4a in p53-independent telomere-directed senescence. Curr Biol. 2004;14:2302–8. doi: 10.1016/j.cub.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 91.Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–13. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 92.Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. Embo J. 2002;21:4338–48. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khoo CM, Carrasco DR, Bosenberg MW, Paik JH, Depinho RA. Ink4a/Arf tumor suppressor does not modulate the degenerative conditions or tumor spectrum of the telomerase-deficient mouse. Proc Natl Acad Sci U S A. 2007;104:3931–6. doi: 10.1073/pnas.0700093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siegl-Cachedenier I, Munoz P, Flores JM, Klatt P, Blasco MA. Deficient mismatch repair improves organismal fitness and survival of mice with dysfunctional telomeres. Genes Dev. 2007;21:2234–47. doi: 10.1101/gad.430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qi L, et al. Short telomeres and ataxia-telangiectasia mutated deficiency cooperatively increase telomere dysfunction and suppress tumorigenesis. Cancer Res. 2003;63:8188–96. [PubMed] [Google Scholar]
- 96.Wong KK, et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421:643–8. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- 97.Maser RS, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–71. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blanco R, Munoz P, Flores JM, Klatt P, Blasco MA. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev. 2007;21:206–20. doi: 10.1101/gad.406207. [DOI] [PMC free article] [PubMed] [Google Scholar]